Abstract
Currently, there are no reliably effective therapeutic options for metastatic pheochromocytoma (PCC) and paraganglioma. Moreover, there are no therapies that may prevent the onset or progression of tumors in patients with succinate dehydrogenase type B mutations, which are associated with very aggressive tumors. Therefore, we tested the approved and well-tolerated drugs lovastatin and 13-cis-retinoic acid (13cRA) in vitro in an aggressive PCC mouse cell line, mouse tumor tissue-derived (MTT) cells, and in vivo in a PCC allograft nude mouse model, in therapeutically relevant doses. Treatment was started 24 hours before sc tumor cell injection and continued for 30 more days. Tumor sizes were measured from outside by caliper and sizes of viable tumor mass by bioluminescence imaging. Lovastatin showed antiproliferative effects in vitro and led to significantly smaller tumor sizes in vivo compared with vehicle treatment. 13cRA promoted tumor cell growth in vitro and led to significantly larger viable tumor mass and significantly faster increase of viable tumor mass in vivo over time compared with vehicle, lovastatin, and combination treatment. However, when combined with lovastatin, 13cRA enhanced the antiproliferative effect of lovastatin in vivo. The combination-treated mice showed slowest tumor growth of all groups with significantly slower tumor growth compared with the vehicle-treated mice and significantly smaller tumor sizes. Moreover, the combination-treated group displayed the smallest size of viable tumor mass and the slowest increase in viable tumor mass over time of all groups, with a significant difference compared with the vehicle- and 13cRA-treated group. The combination-treated tumors showed highest extent of necrosis, lowest median microvessel density and highest expression of α-smooth muscle actin. The combination of high microvessel density and low α-smooth muscle actin is a predictor of poor prognosis in other tumor entities. Therefore, this drug combination may be a well-tolerated novel therapeutic or preventive option for malignant PCC.
Metastatic pheochromocytoma (PCC) and paraganglioma (PGL) are generally difficult to treat and resistant to most conventional therapies. Moreover, they may occur at a young age when they are frequently associated with germline mutations in succinate dehydrogenase type B, which are associated with more aggressive tumors. Even with conventional chemotherapy (cyclophosphamide, vincristine, and dacarbazine), any effect on overall survival has been difficult to demonstrate. Therefore, there is a substantial clinical need for novel effective therapeutic options. Over the last decade, an increasing number of PCC- and PGL-predisposing germline mutations have been identified, delineating the molecular pathways of the pathogenesis of these tumors. PCCs can be separated into 2 major groups (clusters), depending on their transcriptional pattern and underlying gene mutation(s), clusters 1 and 2, as recently reviewed (1). Cluster 1 and cluster 2 seem to follow 2 separate routes to tumorigenesis. Cluster 1 is associated with mutations in the succinate dehydrogenase subunit A[F2]/B/C/D (SDHx) genes or von Hippel–Lindau disease (VHL) gene and linked to pseudohypoxia, resulting primarily in decreased degradation and stabilization of hypoxia-inducible factor-a (HIF-α). To cluster 2 belong mutations in the neurofibromatosis type 1 (NF1) gene, rearranged during transfection (RET) gene, transmembrane protein 127 (TMEM127) gene, MYC-associated factor X (MAX) gene, and kinesin family member 1Bβ (KIF1Bβ) gene, which are associated with aberrant activation of kinase signaling pathways, such as phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase Akt/protein kinase B (AKT), ERK, and mammalian target of rapamycin complex 1/2 (mTORC1/2). Nevertheless, both these clusters converge on a hypoxia-inducible factor (HIF) signaling pathway, as recently suggested (1, 2).
We have shown in our in vitro studies that the established, well-tolerated, and approved drug lovastatin led to a dose- and time-dependent significant decrease in PCC cell survival in vitro and to significant inhibition of AKT and ERK signaling (3). Lovastatin induced G1-cell cycle arrest and apoptosis in our PCC cell line models (3). Lipophilic statins have been reported to have tumoristatic potential in vitro in several cell line models of different origin, as previously reviewed (4, 5). Moreover, lovastatin has shown antitumor potential in vivo, such as in a nude mouse anaplastic thyroid cancer xenograft model (6), in a mouse mammary carcinoma model, including prevention of lung metastases (7), and in colon and hepatic cancer mouse models (8, 9), as previously summarized (10). In a small number of clinical studies of statins used as monotherapy in cancer patients, however, the results have been less promising, with either mild or indeed no effect, as recently reviewed (4). Nevertheless, lovastatin and other statins remarkably increased the efficacy of different chemotherapeutics, such as doxorubicin and receptor tyrosine kinase (vascular endothelial growth factor receptor [VEGFR] and epidermal growth factor receptor [EGFR]) inhibitors, such as gefitinib and sorafenib in vitro and in vivo (5, 11–17). Moreover, a large retrospective study evaluated the influence of statin use before cancer diagnosis on cancer-related mortality and reported a significant (15%) reduction of all-cause and cancer-related mortality in statin users compared with those who had never used statins (18). Thus, statins may have potent antitumor potential in certain contexts.
13-cis-retinoic acid (13cRA), a vitamin A derivative, is another readily available and well-tolerated agent that has been reported to have antitumor potential in vitro in different types of cells (19). Treatment with all-trans-retinoic acid (ATRA) and 13cRA has been shown to induce neuroblastoma cell differentiation and inhibit neuroblastoma cell proliferation in vitro (20–22). Retinoic acid has also been described to induce apoptosis and inhibit angiogenesis (23). Neuroblastomas are also neuroendocrine tumors arising from the peripheral nervous system, similar to PGLs and PCCs, and therefore have similar features to PGLs and PCCs. Beneficial effects have also been reported with retinoic acid in the prevention of neuroblastoma recurrence in patients with minimal residual disease after completion of cytotoxic therapy, or the prevention of second primary head and neck squamous cell carcinomas at high doses, although it was ineffective at low doses (24–27). Moreover, the combination of 13cRA and α-tocopherol (vitamin E) has been reported to significantly reduce bronchial epithelial cell proliferation in former smokers (28).
The malignancies that are sensitive to the antiproliferative effects of 13cRA correspond to the subtypes of tumors that are sensitive to lovastatin-induced apoptosis (19, 29). Indeed, the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which is competitively inhibited by lovastatin (30), is a retinoic acid-repressed gene and also one of the mediators of the biological effects of retinoic acid (31, 32). Thus, there may be a potential synergistic activity of both drugs. Moreover, lovastatin may reduce the side effects of 13cRA: one of the major side effects of 13cRA is hyperlipidemia, particularly an increase of total cholesterol (33), which can be antagonized by lovastatin. 13cRA has been described to induce oxidative toxicity (33), whereas there is evidence that statin treatment reduces oxidative stress by lowering the prooxidant-antioxidant balance (34–36).
Some tumoristatic effects of lovastatin and 13cRA may be due to cellular interactions rather than particular effects on single cells. For this reason, we decided to study the effect of lovastatin and 13cRA on PCCs both in vitro in mouse tumor tissue (MTT) cells, and in vivo in an athymic nude mouse PCC allograft model, in terms of chemoprevention and therapy.
Materials and Methods
Reagents
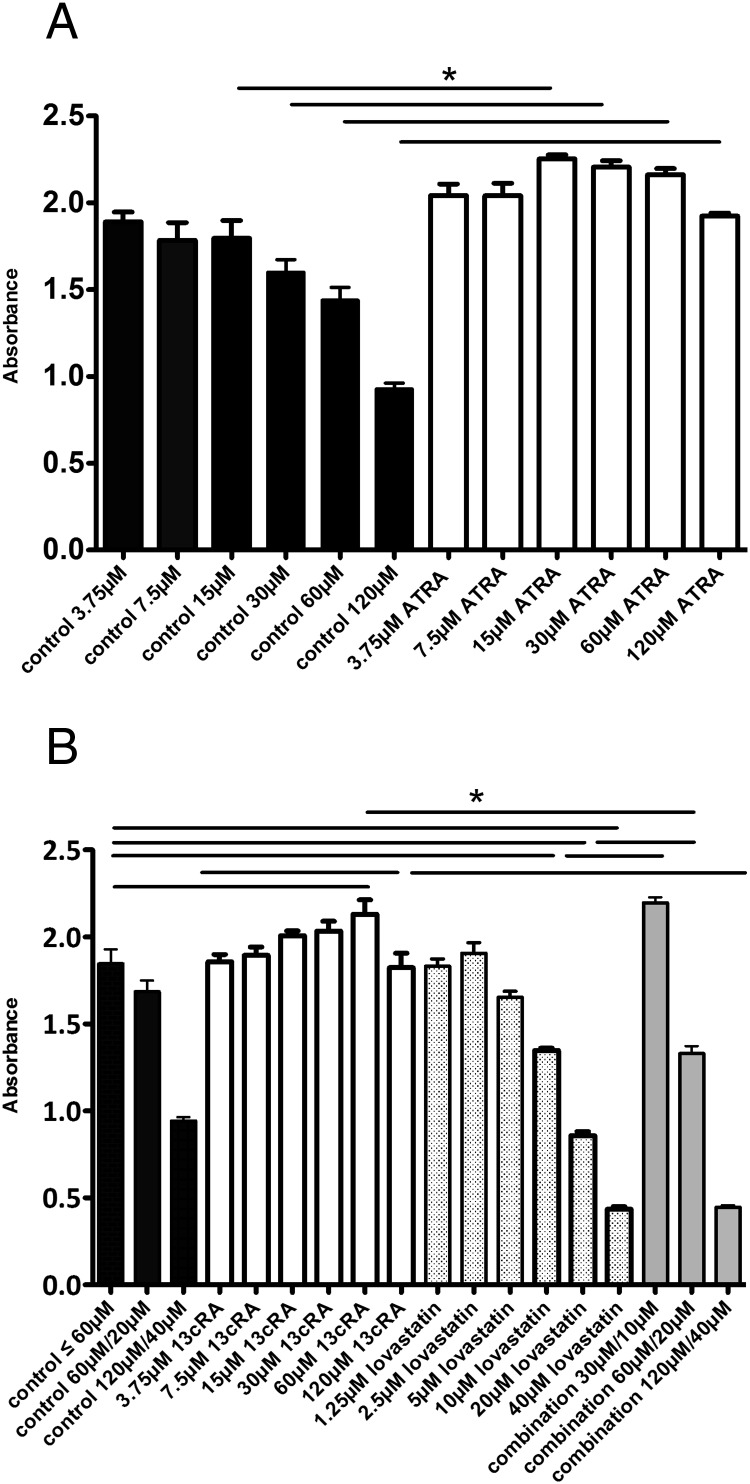
For cell culture experiments, lovastatin (1530; Tocris Bioscience) and 13cRA (R3255; Sigma) were dissolved in dimethylsulfoxide (DMSO) (10mM stock solution, D8418; Sigma), which was used at the appropriate dilution as the vehicle. For our in vitro experiments, we have chosen the lovastatin doses (effective doses 5μM–40μM) according to the dose-response curve of our previous in vitro studies (3). Drug levels up to 12.3μM are tolerated in humans without dose-limiting toxicity (5). Therefore, lovastatin doses of 5μM–10μM, which are effective in vitro, are also therapeutically relevant. For 13cRA, the dose-response curve is shown in Figure 1.
Figure 1.
Treatment of MTT cells. A, Treatment of MTT cells with ATRA for 72 hours. Treatment with 15μM and higher concentrations of ATRA significantly increased MTT cell viability. B, Treatment of MTT cells with 13cRA, lovastatin, and the combination for 72 hours. Treatment with 60μM and higher concentrations of 13cRA significantly increased MTT cell viability. Combination treatment with 10μM (or 20μM) lovastatin and 30μM (or 60μM) 13cRA significantly increased cell viability compared with treatment with 10μM (or 20μM) lovastatin alone, and significantly decreased cell viability compared with treatment with 60μM 13cRA alone. There was no significant difference between the combination treatment of 10μM lovastatin with 30μM 13cRA and single treatment with 30μM 13cRA. Combination treatment with 40μM lovastatin and 120μM 13cRA significantly decreased MTT cell viability compared with treatment with 120μM 13cRA alone and showed no significant difference compared with treatment with 40μM lovastatin alone.
For in vivo experiments, lovastatin and 13cRA were applied by oral gavage on 5 days a week. For oral application, lovastatin was dissolved in 100% ethanol (50mM corresponding to 20 mg/mL stock solution, 459836; Sigma) and 13cRA in corn oil (25mM corresponding to 7.5 mg/mL stock solution, C8267; Sigma). For our in vivo studies, we used therapeutically relevant doses of lovastatin and 13cRA. According to the guidance of the National Cancer Institute we converted the human standard doses of the established drugs lovastatin and 13cRA (mg/kg) to mouse doses (mg/kg) by multiplying by 12.3 (37). Each mouse from the lovastatin group and from the combination group was supplied with 16 mg/kg lovastatin in a total volume of 200 μL corn oil containing 10% ethanol per day on 5 days a week (corresponding to a daily human standard dose of 80 mg lovastatin assuming an average human body weight of 60 kg [ie, 1.3 mg/kg] and converted to an equivalent mouse dose). Each mouse from the 13cRA group and from the combination group was supplied with 53 mg/kg 13cRA in a total volume of 200 μL corn oil containing 10% ethanol per day on 5 days a week (corresponding to a daily standard dose in young adults of 4.32 mg/kg 13cRA used in studies for prevention of recurrence after curative neuroblastoma surgery and converted to an equivalent mouse dose). Each mouse from the vehicle group was supplied with 200 μL corn oil containing 10% ethanol per day on 5 days a week. Stock solutions were stored at −20°C and thawed before use. All mice were treated transiently with amoxicillin, because of skin hyperkeratosis, in a uniform manner.
Cell culture
The mouse PCC cell line MTT (38) generated from heterozygous NF1 knockout mice was cultured in DMEM (11965-084; Gibco, Life Technologies) supplemented with 10% fetal bovine serum (10082; Gibco), 5% horse serum (26050; Gibco), and antibiotic-antimycotic (15240; Gibco). Cells were grown until 80% confluence, afterwards detached using 0.05% trypsin-EDTA (25300; Gibco), incubated for 3 minutes at 37°C, resuspended, and counted to obtain the desired concentration.
Cell proliferation assays
Cell proliferation was determined by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) Cell Proliferation/Viability Assay (M5655; Sigma). MTT cells (20 × 103) were grown in 96-well plates (3596, Costar; Corning) in a final volume of 200 μL for 24 hours before treatment with the indicated concentrations of lovastatin, 13cRA, or the combination, for 72 hours in DMEM containing 10% fetal bovine serum and antibiotic-antimycotic: 20 μL of MTT solution (1 mg/mL) were added, and plates were incubated at 37°C for 3 hours before washing with DMSO and measuring absorbance at 562 nm (BioTek Instruments).
Animal experiments and bioluminescence imaging
All animal studies were conducted in accordance to the principles and procedures outlined in the National Institutes of Health (NIH) Guide for the Animal Care and Use Committee (ACUC) and approved by the NIH ACUC. A total of 1.5 × 106 MTT-luciferase cells (luciferase-expressing MTT cells) were injected sc in the right flank of female athymic nude mice (Taconic) (spontaneous metastasis model) (39). Experimental groups consisted of 8-week-old mice (n = 8–9) housed in a pathogen-free facility. The animals were imaged weekly by bioluminescence (described below). The experiments were performed in the NIH Mouse Image Facility in accordance with ACUC regulations. Bioluminescent data were collected and analyzed with a Xenogen IVIS Imaging System 100 Series (Xenogen, Caliper LifeSciences). On the indicated days, 150 mg/kg D-luciferin (760504; Xenogen, Caliper LifeSciences) in PBS (RGF-3210; KD Medical) was injected ip 15 minutes before measuring luciferase activity on anesthetized animals (1%–2% isoflurane). The mice were placed inside a camera box with 4 different levels (level A–D) under continuous exposure to 1%–2% isoflurane. Level A is closest to the camera and level D furthest away from the camera. All imaging variables were equalized. Photographic and bioluminescent images were collected after different luminescent exposure times (1, 5, 20, and 60 s on level C and 1 s on level D) and at different time points for each animal. The bioluminescence data are presented visually as a color overlay on the photographic image. For data analysis, Living Image software (Xenogen, Vivovision Systems) was used. A region of interest was drawn around tumor sites of interest, and photons/s were quantified after a luminescent exposure time of 1 second on level D. At day 30, anesthetized animals (1%–2% isoflurane) were killed by cervical dislocation. Afterwards, liver and lung were extracted and subjected to bioluminescence imaging to evaluate liver and lung metastases. Three randomly chosen tumors of each group were extracted for immunohistochemical staining (see below).
Measurement by caliper (40)
Tumors were measured with a caliper 3 times a week by the same investigator. In order to determine tumor area by external caliper, the greatest longitudinal diameter (length) and the greatest transverse diameter (width) were determined. Tumor areas based on caliper measurements were calculated by the formula: length × width. Moreover, we calculated tumor volumes by the formula: tumor volume = 1/2 (length × width2) (data not shown). Because the results of both formulae were highly correlated (Spearman correlation coefficient r = 0.99), and length and width are the only measurable parameters by caliper, we decided for convenience to use the tumor areas (length × width) for all further calculations. Treated to vehicle (T/V) ratios of tumor growth were calculated using the formula T/V = (T2 − T1)/(V2 − V1). T1 is the median initial tumor area in treated mice on day 22, T2 is the median final treated tumor area on day 30, V1 is the median initial tumor area in vehicle mice, and V2 is the median final vehicle tumor area.
Statistical analysis
Histograms suggested a strong deviation from normal distribution with regard to luminescence as well as tumor size. Thus, all groups were compared using the Kruskal-Wallis test. P ≤ .05 was considered significant. If a significant difference in the overall test was found, groups were compared pairwise with the Mann-Whitney-Wilcoxon test. For these tests, mice were sorted and ranks were attributed, starting with the rank of 1 for the smallest observation. Boxplots denote median (thick line), quartiles (upper and lower margin of the box), maximum and minimum (whiskers), and outliers that are more than the 1.5-fold interquartile range away from the quartiles (single dots). For the luminescence data, a logarithmic scale was used. All analyses were conducted with R 2.12.2 (GNU General public license). Due to the exploratory character of this work, all P values have to be interpreted descriptively. An adjustment of P values was not performed.
Histopathology and immunohistochemistry
At day 30, anesthetized animals (1%–2% isoflurane) were euthanized by cervical dislocation. A subset of allografts (n = 3 per group) were fixed in 10% buffered neutral formalin, paraffin embedded, and sectioned at 5 μm. Slides were stained with hematoxylin and eosin and with antibodies for α-smooth muscle actin (α-SMA) (Sigma) as a differentiation marker of smooth muscle cells, CD34 (a cluster of differentiation molecule) (eBioscience) as a measure of microvascular density, and chromogranin A (CgA) (ImmunoStar) and tyrosine hydroxylase (TH) (Millipore) as parameters of the catecholamine secretion pathway. Tumor slides were examined microscopically by a board-certified veterinary pathologist. Lesions in hematoxylin and eosin sections included necrosis and angiectasis (ectasia of capillary-sized vessels lined by well-differentiated endothelial cells). These lesions and some immunohistochemical reactivity were graded as minimal, mild, moderate, or severe. For quantitation of microvessels (CD34), slides were digitally imaged with the Aperio Scanscope and analyzed using the Aperio Image Analysis Toolbox.
Results
High doses of 13cRA and ATRA, respectively, promote MTT cell survival in vitro
Treatment with 15μM or higher doses of ATRA and with 60μM or higher doses of 13cRA, respectively, for 72 hours significantly increased MTT cell survival compared with the control group (P ≤ .05) (Figure 1, A and B).
Therapeutically relevant doses of lovastatin significantly reduce MTT cell survival in vitro
Treatment with 10μM and higher doses of lovastatin for 72 hours significantly reduced MTT cell survival (P ≤ .001) compared with the control group (Figure 1B).
In vitro combination of lovastatin and 13cRA is not synergistic
Combination treatment with 40μM lovastatin and 120μM 13cRA for 72 hours showed no significant difference compared with treatment with 40μM lovastatin alone but significantly decreased cell survival compared with treatment with 120μM 13cRA alone (P ≤ .001) (Figure 1B). However, combination treatment with 20μM (or 10μM) lovastatin and 60μM (or 30μM) 13cRA significantly increased cell survival compared with treatment with 20μM (or 10μM) lovastatin alone (P ≤ .001) but significantly decreased cell survival compared with treatment with 60μM 13cRA alone (P ≤ .001) (Figure 1B). There was no significant difference between the combination treatment of 10μM lovastatin with 30μM 13cRA and single treatment with 30μM 13cRA.
Inhibition of PCC allograft tumor growth in vivo by the combination of 13cRA and lovastatin
To investigate the effects of 13cRA, lovastatin, and the combination of both drugs in vivo, 8-week-old female athymic nude mice were injected sc with luciferase-expressing MTT cells. Mice were treated orally once a day on 5 days a week either with vehicle (n = 8), 13cRA (n = 8), lovastatin (n = 9), or the combination of 13cRA and lovastatin (n = 9). To investigate potential chemopreventive effects of the drugs, treatment was started 24 hours before tumor cell injection and continued for 30 days after tumor cell injection. Three mice of the 13cRA group died from dehydration, inanition, and hyperkeratosis on days 12 and 13, and 1 mouse of the 13cRA group had to be euthanized because of suffering from inanition, dehydration, and hyperkeratosis on day 16 after the beginning of treatment (4 of 8 died, 50%). One mouse of the vehicle group died from dehydration, gastrointestinal bleeding, inanition, and hyperkeratosis on day 15 after beginning of treatment (1 of 8 died, 13%). One mouse of the lovastatin group died from inanition and hyperkeratosis on day 19 after beginning of treatment (1 of 9 died, 11%). None of mice in the combination-treated group died during the treatment period (0%). Tumor areas (sizes) were measured by caliper from outside, and active tumor, ie, size of viable, not necrotic, or apoptotic tumor mass, was measured by bioluminescence imaging.
Measured tumor areas by caliper. Lovastatin-treated and combination-treated mice show significantly smaller final tumor sizes compared with the vehicle group
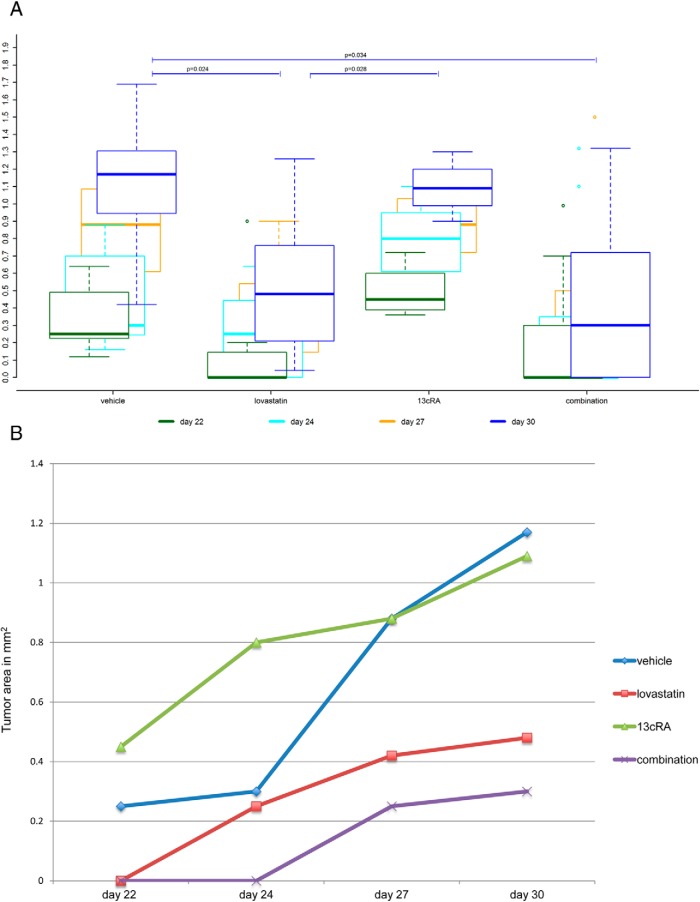
Tumor areas were measurable by external caliper from a minimum length and width of 0.2 cm. On day 22 after tumor cell injection, tumor areas were measurable in all 7 mice (100%) of the vehicle-treated group and in all 4 mice (100%) of the 13cRA-treated group. In contrast, at this time point, tumors were measurable in only 3/8 (38%) of the lovastatin-treated mice and in only 4/9 (44%) of the combination-treated mice. Figure 2A shows the boxplots of the tumor areas of the 4 different groups determined 3 times a week from day 22 to 30 after tumor cell injection. On day 30 after tumor cell injection, the study was terminated. We found significant differences in the final tumor size between the 4 groups on day 30 (Kruskal-Wallis test, P = .026) (Figure 2A). Lovastatin-treated mice (median tumor area, 0.480 cm2; range, 0.040–1.260 cm2) and the combination-treated mice (median tumor area, 0.300 cm2; range, 0–1.320 cm2), respectively, showed significantly smaller final tumor areas compared with vehicle-treated mice (median tumor area, 1.170 cm2; range, 0.420–1.690 cm2) (P = .024 and P = .034). The 13cRA-treated group (median tumor area, 1.090 cm2; range, 0.900–1.300 cm2) showed significantly larger final tumor areas compared with the lovastatin-treated group (P = .028). Moreover, the 13cRA-treated group showed larger final tumor areas compared with the combination-treated group, although the difference was not statistically significant (P = .103). There was neither significant difference in the final tumor areas between the vehicle- and the 13cRA-treated group, nor between the lovastatin- and the combination-treated group, respectively (P = .850 and P = .595, respectively).
Figure 2.
Tumor areas. A, Tumor areas in mm2 (tumor area: l*w) at 4 different time points from day 22 to 30 after tumor cell injection. Boxplots are denoting median (thick line), quartiles (upper and lower margin of the box), maximum and minimum (whiskers), and outliers that are more than the 1.5-fold interquartile range away from the quartiles (single dots). The lovastatin- and the combination-treated mice, respectively, showed significantly smaller final tumor areas than the vehicle-treated mice (P = .024 and P = .034). The 13cRA-treated mice showed significantly larger final tumor areas compared with the lovastatin-treated ones (P = .028). Moreover, the 13cRA-treated group showed larger final tumor areas compared with the combination-treated group, although the difference was not statistically significant (P = .103). There was neither significant difference in the final tumor areas between the vehicle- and the 13cRA-treated group, nor between the lovastatin- and the combination-treated group, respectively (P = .850 and P = .595, respectively). B, Median tumor areas in mm2 (tumor area: l*w) at 4 different time points from day 22 to 30 after tumor cell injection. From day 22 to 30, the combination-treated tumors showed highly significantly slower growth than the vehicle-treated ones (P = .008) and significantly slower growth than the 13cRA-treated ones (P = .036). There were nonsignificant trends for a slower growth of the lovastatin-treated tumors, compared with the vehicle-treated ones, and for a slower growth of the combination-treated tumors, compared with the lovastatin-treated ones (P = .105 and P = .059, respectively).
Tumor growth. Tumors of the combination-treated mice grew significantly more slowly compared with those of the vehicle-treated group and with those of the 13cRA only-treated group
We observed significant differences in tumor growth (increase in measurable tumor mass over time) between the 4 groups from day 22 to 30 after tumor cell injection (Kruskal-Wallis test, P = .032). The median tumor areas of the 4 groups at the different time points are shown in Figure 2B. From day 22 to 30, the tumors of the combination-treated mice grew highly significantly more slowly than those of the vehicle-treated mice (P = .008) and significantly more slowly than those of the mice treated with 13cRA alone (P = .036). There was a trend for the lovastatin-treated group to be more slowly growing compared with the vehicle-treated mice, but this was not statistically significant (P = .105). The calculated T/V ratios (tumor growth rates referred to the vehicle) for lovastatin, 13cRA, and the combination were 0.426, 0.797, and 0.324, respectively, reflecting slowest tumor growth in the combination-treated group, followed by the lovastatin-treated group and fastest tumor growth in the group treated with 13cRA alone. In 4/9 (44%) of the combination-treated mice, the tumor was hardly visible until the very end of the study, in contrast to all other groups (Figure 3). There was a clear trend, although statistically not significant, for a slower tumor growth in the combination-treated group, compared with the lovastatin-treated group (P = .059).
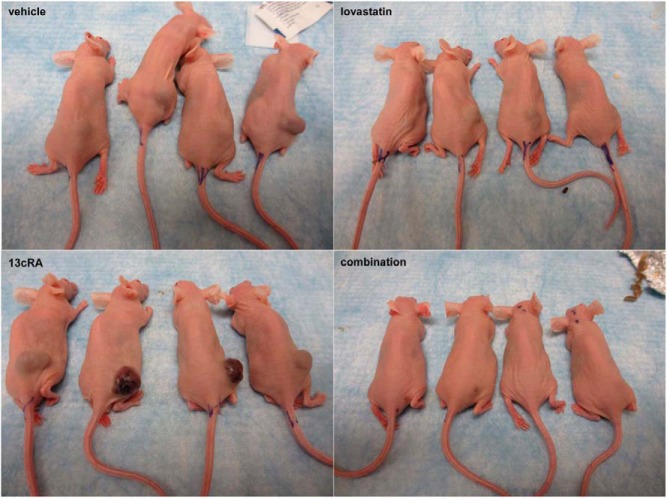
Figure 3.
Mice on day 30 after tumor cell injection. The photos show 4 mice of each group on day 30 at the end of the treatment course. It is clearly visible that the 4 surviving mice of the 13cRA-treated group show the largest tumors with a reddish surface consisting of pronounced angiectasis. In 4 mice of the combination-treated group, the tumors were hardly visible until the end of the treatment course. The tumors of the lovastatin-treated mice were clearly smaller than those of the vehicle- and the 13cRA-treated ones.
Luminescence tumor imaging
The combination-treated group shows the smallest viable tumor mass of all groups
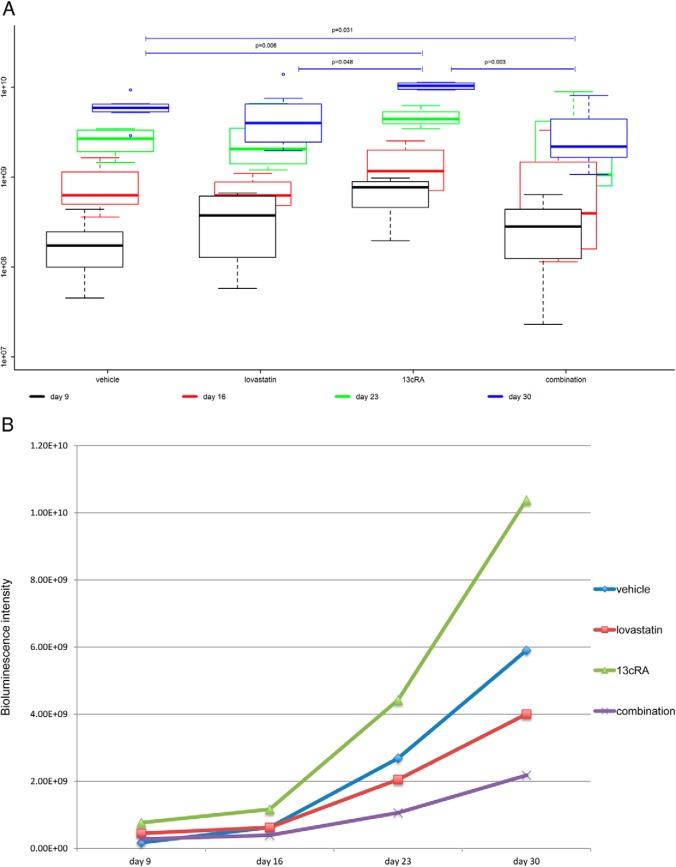
Tumor imaging was performed weekly from day 9 after tumor cell injection. Bioluminescence intensity reflects the size of viable, not necrotic or apoptotic, tumor mass (active tumor). Figure 4A shows the boxplots for the luminescence intensities of the primary tumors of the 4 groups measured weekly from day 9 to 30. We found significant differences in the bioluminescence intensity between the 4 groups reflecting a significant difference in primary tumor viability (Kruskal-Wallis test, P = .006) (Figure 4A). The combination-treated primary tumors (median luminescence intensity, 2.18 × 109; range, 1.08 × 109 to 8.05 × 109) showed the lowest luminescence intensity of all groups with significantly lower luminescence intensity compared with those of the vehicle-treated group (median luminescence intensity, 5.91 × 109; range, 2.90 × 109 to 9.34 × 109) (P = .031) and highly significantly lower luminescence intensity compared with those of the 13cRA only-treated group (median luminescence intensity, 1.04 × 1010; range, 9.34 × 109 to 1.13 × 1010) (P = .003). The lovastatin-treated tumors (median luminescence intensity, 4.00 × 109; range, 1.96 × 109 to 1.39 × 1010) and the vehicle-treated tumors, respectively, also showed significantly and highly significantly lower luminescence intensity compared with the 13cRA-treated ones (P = .048 and P = .006). There was a tendency for lower luminescence activity in the combination-treated group compared with the lovastatin-treated group (P = .200). Moreover, there was a nonsignificant trend between the lovastatin only-treated group and the vehicle-treated group with lower luminescence intensity in the lovastatin-treated group (P = .232).
Figure 4.
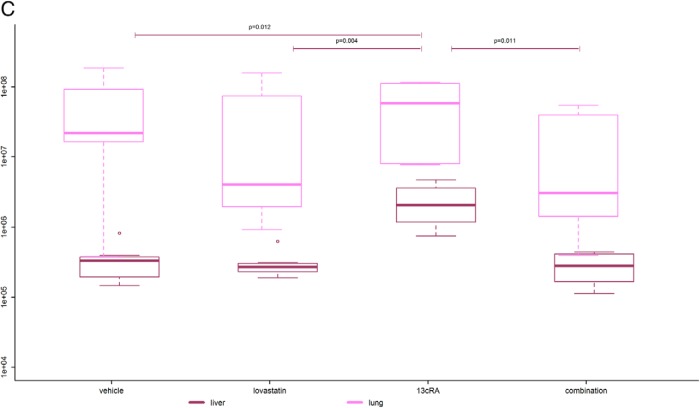
Bioluminescence tumor imaging. A, Primary tumor luminescence. Boxplots are denoting median (thick line), quartiles (upper and lower margin of the box), maximum and minimum (whiskers), and outliers that are more than the 1.5-fold interquartile range away from the quartiles (single dots). Primary tumor luminescence intensities of the 4 groups measured weekly from day 9 to 30 are shown. We found significant differences in the bioluminescence intensity between the 4 groups reflecting a significant difference in primary tumor viability (P = .006). The combination-treated tumors showed the lowest luminescence intensity of all groups with significantly lower luminescence intensity compared with the vehicle-treated tumors (P = .031) and highly significantly lower luminescence intensity compared with the 13cRA only-treated ones (P = .003). The 13cRA-treated tumors showed significantly and highly significantly higher luminescence intensity compared with the lovastatin- and vehicle-treated tumors (P = .048 and P = .006, respectively). B, Median tumor luminescence at 4 different time points from day 9 to 30 after tumor cell injection. The median tumor luminescence intensities of the 4 groups at 4 different time points are shown. There was a significantly slower increase of tumor activity over time in the combination-treated group compared with the vehicle-treated group (P = .023). Moreover, there was a highly significantly slower increase of tumor activity over time in the combination-treated group than in the 13cRA only-treated group (P = .003). The tumor activity of the lovastatin- and vehicle-treated mice also increased significantly slower over time than those of the 13cRA only-treated mice (P = .048 and P = .012, respectively). The lovastatin-treated group showed a (not statistically significantly) faster increase of tumor activity over time compared with the combination-treated group (P = .200) and a (not statistically significantly) slower increase of tumor activity over time compared with the vehicle-treated group (P = .232). C, Luminescence of lung and liver metastases at day 30. The 13cRA only-treated mice showed a significantly higher tumor activity in the liver than all the other groups (P ≤ .05). There was no obvious difference concerning liver metastases between all other groups. The combination-treated mice showed lowest tumor activity in the lung compared with all other groups. In the lovastatin- and the combination-treated group, there was a tendency of less tumor activity in the lung compared with the vehicle- and the 13cRA-treated group.
The slowest increase in viable tumor mass over time was observed in the combination-treated group
We observed highly significant differences in the increase in viable tumor mass (active tumor) over time between the 4 groups from day 9 to 30 after tumor cell injection (Kruskal-Wallis test, P = .002). The median tumor luminescence intensities of the 4 groups at the different time points are shown in Figure 4B. Consistent with the tumor growth measured by caliper, the bioluminescence data showed a significantly slower increase in viable tumor over time in the combination-treated group compared with the vehicle-treated group from day 9 to 30 after tumor cell injection (P = .023). Moreover, there was a highly significantly slower increase in viable tumor over time in the combination-treated group compared with the 13cRA only-treated group (P = .003). The viable tumor mass of the lovastatin- and vehicle-treated mice also increased significantly more slowly over time compared with those of the 13cRA only-treated mice (P = .048 and P = .012, respectively). The lovastatin-treated group showed a nonstatistically significantly faster increase in tumor activity over time compared with the combination-treated group (P = .200) and a (not statistically significantly) slower increase in tumor activity over time compared with the vehicle-treated group (P = .232).
Lung and liver metastases at day 30
The 13cRA only-treated animals showed a significantly higher tumor activity in the liver (median luminescence intensity, 20.4 × 105) compared with the lovastatin-treated (median luminescence intensity, 2.69 × 105), the combination-treated (median luminescence intensity, 2.80 × 105), and the vehicle-treated animals (median luminescence intensity, 3.32 × 105) (P ≤ .05). The combination-treated mice showed lowest tumor activity in the lung (median luminescence intensity, 0.303 × 107) compared with all other groups. There was a tendency for less tumor activity in the lung in the lovastatin-treated (median luminescence intensity, 0.401 × 107) and the combination-treated group compared with the vehicle-treated (median luminescence intensity, 2.17 × 107) and 13cRA-treated (median luminescence intensity, 5.81 × 107) group (Figure 4C).
Histopathology and immunohistochemistry
Three tumors of each group were randomly chosen and analyzed by immunohistochemistry.
Angiectasis. Highest levels of angiectasis after 13cRA only treatment
Angiectasis was most prominent in the 13cRA-treated group. Two of 3 randomly analyzed tumors from the 13cRA only-treated group showed moderate angiectasis (+++), whereas none of the 3 vehicle-treated tumors analyzed showed angiectasis. In 2 of the 3 randomly chosen lovastatin-treated tumors and in 1 of the 3 randomly chosen combination-treated tumors, only mild angiectasis (++) was found, and no angiectasis was found in the remaining ones.
Microvessel density (MVD). Lowest median MVD in the combination-treated tumors and highest median MVD in the lovastatin only-treated tumors
The MVD of 3 randomly chosen tumors from each group was assessed by immunohistochemical staining for CD34. The highest median MVD was found in the lovastatin only-treated tumors (1.54 × 10−4 μm2). The vehicle- and the 13cRA only-treated tumors showed a lower median MVD (1.13 × 10−4 and 1.12 × 10−4 μm2, respectively). The lowest median MVD was detected in the combination-treated tumors (0.827 × 10−4 μm2).
Necrosis. Severe necrosis in the combination-treated group
Tumor necrosis was present at the highest grade in the combination-treated group. The 3 randomly chosen combination-treated tumors showed the highest level of necrosis of all groups. Moderate necrosis (+++) was found in 1 of the 3 combination-treated tumors and severe necrosis (++++) in 2 of the 3 combination-treated tumors. In contrast, in the lovastatin-treated group as well as in the 13cRA-treated group, 1 tumor showed minimal (+), 1 mild (++), and 1 moderate necrosis (+++). In the vehicle-treated group, 1 tumor displayed no necrosis and 2 tumors moderate necrosis (+++).
α-SMA. Highest percentage of α-SMA positive cells in the combination-treated group
The percentage of α-SMA positive cells was highest in the 3 combination-treated tumors (16%, 33%, and 46%, respectively; median, 33%; mean, 32%). The lowest percentage of α-SMA positive cells was detected in the vehicle-treated tumors (11%, 18%, and 20%, respectively; median, 18%; mean, 16%). Both the 13cRA only- and the lovastatin only-treated tumors showed a lower percentage of α-SMA positive cells than the combination- and higher percentage of α-SMA positive cells than the vehicle-treated tumors (13cRA: 12%, 24%, and 25%, respectively; median, 24%; mean, 20%; lovastatin: 16%, 23%, and 36%, respectively; median, 23%; mean, 25%).
CgA and TH. CgA and TH positive cells were similar in all 4 groups
There was no difference in the median percentage of CgA and TH positive cells among all 4 groups (99% of CgA positive cells in the vehicle-, lovastatin-, and combination-treated group and 98% of CgA positive cells in the 13cRA-treated group, and 98% of TH positive cells in all 4 groups).
Discussion
In the present study, we used an allograft PCC mouse model (38) to investigate the effect of the established and approved drugs lovastatin and 13cRA alone and in combination in therapeutically relevant doses on metastatic PCCs. Although the development of a human PCC cell line has been reported recently (41), in the present study, the highly malignant mouse MTT cell line was used for the allograft PCC mouse model, as well as for in vitro studies. MTT cells were initially generated from the more benign mouse pheochromocytma cell (MPC) cells by tail-vein injection into mice modifying the number of cells injected, length of trypsin pretreatment, incubation temperature, and duration for the MPC cells before injection and by serial passage and reselection of tumors exhibiting the metastatic phenotype (38). Subcutaneously and iv injected MTT cells spread quickly to other organs and simulate a very aggressive PCC phenotype (38). Therefore, currently, this PCC mouse model is the most suitable in vivo model to study the potential therapeutic effects of different drugs on malignant PCCs. The recently developed human PCC cell line should be used in the future to validate some results and conclusions of the present study.
In the cell line studies, we found that, as previously demonstrated (3), lovastatin has marked antiproliferative effects; in this context, 13cRA was proproliferative. By contrast, when used in athymic nude mice, the antiproliferative effects of lovastatin were confirmed but were not antagonized by 13cRA and indeed may even have been enhanced by its presence. Both the lovastatin only- and the combination-treated groups showed significantly smaller tumor sizes compared with the vehicle-treated mice at the end of the study. The combination-treated tumors grew slowest with significantly slower tumor growth compared with the vehicle-treated group. Moreover, the combination-treated group displayed the smallest size of viable tumor mass (lowest amount of active tumor) and the slowest increase in viable tumor mass over time of all groups, with a significant difference compared with the vehicle- and 13cRA only-treated group at the end of the treatment course. The lovastatin group also showed a remarkable trend of slower tumor growth compared with the vehicle-treated group. In contrast, a single application of 13cRA led to significantly larger tumors compared with the lovastatin only-treated tumors, a significantly higher mass of active tumor and significantly faster increase in viable tumor mass over time compared with all other groups. Furthermore, it is remarkable that 50% (4 of 8) of the 13cRA only-treated mice died from inanition and dehydration during the treatment course, whereas just 11% (1 of 9) of the lovastatin only-treated mice, 13% (1 of 8) of the vehicle-treated mice, and none (0%) of the combination-treated mice died. This may on the one hand be due to faster and more aggressive tumor growth in the 13cRA only-treated group, but on the other hand, it may be due to the direct effect of 13cRA inducing severe dehydration itself, which might be alleviated by the addition of lovastatin.
To evaluate the influence of the drugs on the catecholamine secretion pathway, immonhistochemical staining of the surrogate parameters for catecholamine secretion CgA and TH was performed on tumor tissue. There was no difference in the median percentage of CgA and TH positive cells among all 4 groups, indicating a drug effect rather on cell number than on the catecholamine secretion pathway or cell phenotype. Nevertheless, a decrease of catecholamine secretion in the lovastatin- and combination-treated group would be expected due to a reduction in total cell number.
Lovastatin
The lovastatin-pretreated mice showed significantly smaller tumor sizes compared with the vehicle-treated ones at the end of the treatment course, although there was no statistically significant difference regarding viable tumor mass. We have previously shown in vitro in PCC (MPC and MTT) cells that treatment with lovastatin led to intracellular AKT and ERK inhibition and induced apoptosis, indicating a possible antitumor effect at a cellular level (3), although this effect may be of lesser significance in vivo, where tumor viability and induction of necrosis depend predominantly on vascularization. Accordingly, in our present study, the lovastatin-treated tumors showed the highest median MVD. This may also partly explain the relatively low efficacy of a single treatment with lovastatin in human malignancies (5). In our experimental setting, we pretreated the mice with lovastatin 24 hours before tumor cell injection. Thus, the mice were already under statin therapy at the beginning of tumor development. Accordingly, the significantly smaller tumor sizes that we measured in the lovastatin-treated animals may reflect a chemopreventive effect of lovastatin, which has also been suggested by the study reporting lower cancer-associated mortality in patients developing cancer under statin therapy (18). There was no remarkable difference regarding active liver metastases between the lovastatin-, combination-, and vehicle-treated animals; possibly, a longer treatment time would have revealed higher differences. However, there was a tendency for less severe metastatic spread to lungs in the lovastatin-treated animals compared with the vehicle-treated ones. This finding may argue for a chemopreventive effect, including metastatic spread.
13cRA
13cRA was proproliferative in our cell line model and also enhanced tumor growth when used alone in vivo. Angiectasis was pronounced in the 13cRA-treated group. However, addition of an antioxidative statin (34, 35) to 13cRA treatment may potentially block the cancer-promoting effect and reveal the active anticancer potential of 13cRA, as discussed below.
Interestingly, opposing effects with regard to chemoprevention have been reported between current smokers and former-or-never smokers (28). Although in current smokers high doses of 13cRA could not reverse bronchial metaplasia, a trend towards reversion was found in former smokers (28). In current smokers, retinoic acid treatment was associated with increased lung cancer incidence, whereas in former-or-never smokers, a slightly decreased incidence was observed (28). These opposite effects between current smokers and former-or-never smokers argue against a major direct antitumor effect of 13cRA at the single cell level and indicate a negative correlation of the antitumor effect of retinoic acid with oxidative stress in the tumor cell environment. Thus, the presence of the antioxidative statin (34, 35) may possibly enable the antitumor potential of 13cRA to be realized in a hypoxic environment in vivo.
Accordingly, we found significantly higher metastatic tumor activity in livers of the 13cRA only-treated mice compared with all other groups, and this metastasis promoting effect was abrogated by addition of lovastatin. The 13cRA only-treated mice also showed highest metastatic tumor activity in the lung. However, this unfavorable effect was not just abolished by lovastatin but even turned into a chemopreventive effect by lovastatin, as shown by the lowest metastatic tumor activity in the lung after combination treatment.
Combination of lovastatin and 13cRA
Although the drug combination did not show a synergistic or additive effect in vitro, the possible synergism of the drug combination in vivo would appear to be based on a more complex mechanism, including cellular interactions, hypoxia, antioxidant effects, and angiogenesis rather than effects on single cells. The combination-treated group showed the slowest tumor growth, the lowest tumor viability, and the highest extent of necrosis; 2 of 3 analyzed combination-treated tumors showed severe necrosis, whereas the tumors of the other groups only showed minimal to moderate necrosis. Immunohistochemical staining of CD34 revealed the lowest median MVD in the combination-treated tumors (0.827 × 10−4 μm2). Interestingly, the highest median MVD was detected in the lovastatin only-treated group (1.54 × 10−4 μm2). The vehicle- and the 13cRA only-treated group showed higher median MVD compared with the combination-treated group and lower median MVD compared with the lovastatin only-treated group. Thus, we might speculate that the combination treatment may lead to the induction of severe necrosis, possibly in part through down-regulation of MVD and induction of hypoxia in tumor tissue addicted to a higher blood supply due to additional treatment with the antioxidant (34–36) drug lovastatin.
Possibly, it is the effect of the antioxidant (34–36) drug lovastatin, which has realized the antitumor potential of 13cRA. This is consistent with the observation of a slightly decreased lung cancer incidence in former-or-never smokers under retinoic acid treatment but an increased incidence in current smokers (28), because, as expected, smoking is associated with chronic oxidative stress. It is possible that 13cRA requires the presence of oxidative stress in (tumor) tissue to be antagonized in order to unmask its antitumor effect. In hypoxia-adapted (tumor) tissue under chronic oxidative stress, 13cRA alone may even support the tumor-promoting effect of chronic oxidative stress by enhancing this (33). In fast growing malignancies, there is usually chronic oxidative stress, and VHL- and SDHx-mutated PCCs are associated with pseudohypoxia, as recently reviewed (1). Accordingly, the combination of 13cRA with the antioxidant vitamin E (α-tocopherol) has been reported to significantly reduce bronchial epithelial cell proliferation in former smokers (a significant decrease in subjects with a high Ki-67 labeling in the parabasal layer) (28). Vitamin E has also shown synergistic chemopreventive activity with retinoids in an animal model (42) and in some clinical trials in patients with head and neck squamous cell carcinomas (43, 44) and has been found to reduce the toxic side effects of 13cRA, such as mucocutaneous toxicity, liver function abnormalities, and hypertriglyceridemia (27, 45). It would appear that 13cRA needs to be combined with an antioxidant to fully reveal its antitumor potential, especially under chronic oxidative stress. Moreover, interestingly, both lovastatin and 13cRA are inhibitors of the enzyme HMG-CoA reductase, lovastatin as a competitive inhibitor of the enzyme at the protein level (30) and 13cRA at the level of the gene as a repressor of the HMG-CoA reductase gene (31, 32). This may indicate that the antitumor effect of lovastatin may be enhanced by 13cRA via a synergistic action. However, possibly the inhibitory effect of 13cRA alone at the gene level is not potent enough to show antitumor potential, and thus, its proproliferative characteristics predominate under chronic oxidative stress.
A correlation between necrosis, MVD, and hypoxia has already been observed in computed tomography (CT)-enhanced images and is used as a prognostic criterion; lower relative CT values have been associated with more significant tumor necrosis and lower MVD (46). High MVD has been shown to be an independent negative prognostic factor for overall and recurrence-free survival and be associated with microvascular invasion in hepatocellular and pancreatic carcinoma (47). Necrosis induction in part through low MVD and relative hypoxia may explain the lowest detectable tumor viability in the combination-treated animals compared with those of all other groups at the end of the treatment course. Consistently for neuroendocrine tumors, it has already been postulated that treatment with VEGF receptor inhibitors, such as sunitinib, induces few if any changes in tumor size but rather a significant decrease in tumor density on CT imaging corresponding to lower tumor viability and more severe necrosis (48). These authors postulated that the Choi criteria considering both the size and the density of the tumor as parameters for response to targeted therapies could be an alternative or even superior to the Response Evaluation Criteria in Solid Tumors, which focus only on the largest diameters (48). Therefore, the critical prognostic factor to evaluate treatment response is probably not only tumor size itself but rather residual tumor viability. After combination treatment with lovastatin and 13cRA, all 3 criteria indicative of a good treatment response were fulfilled: significantly smaller tumor size, significantly lower viable tumor mass, and more severe necrosis compared with the vehicle.
Moreover, the combination of low α-SMA and high MVD was a predictor of the worst prognosis for pancreatic and hepatocellular carcinoma and had a higher predictive value for death and early recurrence than MVD alone (47). Interestingly, there was a tendency of higher expression of α-SMA in the combination-treated tumors (median percentage of α-SMA positive cells 33%), compared with clearly less α-SMA expression in the vehicle-treated tumors (median percentage of α-SMA positive cells 18%). The α-SMA expression in the 13cRA-treated and in the lovastatin-treated group was higher compared with the vehicle-treated but lower compared with the combination-treated group. Therefore, treatment with 13cRA in addition to lovastatin may lead to the beneficial results of lower MVD and higher α-SMA expression. Poor microvessel integrity, as indicated by high MVD, together with low perivascular α-SMA positive cell coverage, has been reported to be associated with early recurrence, unfavorable metastasis, and short survival after tumor resection (47).
Conclusions
We have previously shown that lovastatin has antiproliferative activity in mouse PCC cell lines (3). We now show that lovastatin treatment alone seems to have cancer-preventive potential in vivo in our PCC allograft model and may lead to significantly smaller tumor sizes compared with the vehicle, although no significant change was observed in tumor viability. 13cRA alone was proproliferative in the cell line study and promoted tumor growth in our PCC allograft model resulting in significantly larger tumor sizes and significantly larger viable tumor masses, possibly through a prooxidant potential associated with a higher level of angiectasis. However, the combination of lovastatin and 13cRA significantly inhibited tumor growth and was associated with significantly smaller tumor sizes and significantly lower tumor viability, compared with the vehicle- and 13cRA-treated tumors. Thus, the combination treatment seems to be cancer preventive with regards to tumor size, growth, and viability. This may in part be due to lower MVD, higher α-SMA expression, and induction of more severe necrosis. We speculate that the antioxidant statin may reveal the antitumor potential of the prooxidant drug 13cRA under hypoxic conditions in tumor tissue less adapted to hypoxia and more addicted to oxygen due to lower MVD. Both drugs have been tested in therapeutically relevant doses in this study (calculated from human to mouse dose), are already approved for clinical use, and are well tolerated. This makes consideration of translation from this animal study to a human clinical trial practicable.
Acknowledgments
We thank Celinia Ondeck for animal care supervision and Ghassan Alusi for original intellectual contribution.
This work was supported in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract number HSN261200800001E and in part by the German Research Foundation (Deutsche Forschungsgemeinschaft).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACUC
- Animal Care and Use Committee
- AKT
- serine/threonine protein kinase Akt/protein kinase B
- ATRA
- all-trans-retinoic acid
- CD34
- a cluster of differentiation molecule and a measure of microvessel density
- CgA
- chromogranin A
- CT
- computed tomography
- 13cRA
- 13-cis-retinoic acid
- HMG-CoA
- 3-hydroxy-3-methylglutaryl-coenzyme A
- MPC
- mouse pheochromocytma cell
- MTT
- mouse tumor tissue
- MVD
- microvessel density
- NIH
- National Institutes of Health
- PCC
- pheochromocytoma
- PGL
- paraganglioma
- α-SMA
- α-smooth muscle actin
- TH
- tyrosine hydroxylase
- T/V
- treated to vehicle.
References
- 1. Nölting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr Pathol. 2012;23(1):21–33 [DOI] [PubMed] [Google Scholar]
- 2. Jochmanová I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105(17):1270–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nölting S, Garcia E, Alusi G, et al. Combined blockade of signalling pathways shows marked anti-tumour potential in phaeochromocytoma cell lines. J Mol Endocrinol. 2012;49(2):79–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osmak M. Statins and cancer: current and future prospects. Cancer Lett. 2012;324(1):1–12 [DOI] [PubMed] [Google Scholar]
- 5. Holstein SA, Knapp HR, Clamon GH, Murry DJ, Hohl RJ. Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother Pharmacol. 2006;57(2):155–164 [DOI] [PubMed] [Google Scholar]
- 6. Wang CY, Shui HA, Chang TC. In vivo evidence of duality effects for lovastatin in a nude mouse cancer model. Int J Cancer. 2010;126(2):578–582 [DOI] [PubMed] [Google Scholar]
- 7. Shibata MA, Ito Y, Morimoto J, Otsuki Y. Lovastatin inhibits tumor growth and lung metastasis in mouse mammary carcinoma model: a p53-independent mitochondrial-mediated apoptotic mechanism. Carcinogenesis. 2004;25(10):1887–1898 [DOI] [PubMed] [Google Scholar]
- 8. Narisawa T, Fukaura Y, Terada K, et al. Prevention of 1,2-dimethylhydrazine-induced colon tumorigenesis by HMG-CoA reductase inhibitors, pravastatin and simvastatin, in ICR mice. Carcinogenesis. 1994;15(9):2045–2048 [DOI] [PubMed] [Google Scholar]
- 9. Tatsuta M, Iishi H, Baba M, et al. Suppression by pravastatin, an inhibitor of p21ras isoprenylation, of hepatocarcinogenesis induced by N-nitrosomorpholine in Sprague-Dawley rats. Br J Cancer. 1998;77(4):581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006;11(3):306–315 [DOI] [PubMed] [Google Scholar]
- 11. Bil J, Zapala L, Nowis D, Jakobisiak M, Golab J. Statins potentiate cytostatic/cytotoxic activity of sorafenib but not sunitinib against tumor cell lines in vitro. Cancer Lett. 2010;288(1):57–67 [DOI] [PubMed] [Google Scholar]
- 12. Mantha AJ, Hanson JE, Goss G, Lagarde AE, Lorimer IA, Dimitroulakos J. Targeting the mevalonate pathway inhibits the function of the epidermal growth factor receptor. Clin Cancer Res. 2005;11(6):2398–2407 [DOI] [PubMed] [Google Scholar]
- 13. Dimitroulakos J, Lorimer IA, Goss G. Strategies to enhance epidermal growth factor inhibition: targeting the mevalonate pathway 1. Clin Cancer Res. 2006;12(14 pt 2):4426s–4431s [DOI] [PubMed] [Google Scholar]
- 14. Cemeus C, Zhao TT, Barrett GM, Lorimer IA, Dimitroulakos J. Lovastatin enhances gefitinib activity in glioblastoma cells irrespective of EGFRvIII and PTEN status. J Neurooncol. 2008;90(1):9–17 [DOI] [PubMed] [Google Scholar]
- 15. Park IH, Kim JY, Jung JI, Han JY. Lovastatin overcomes gefitinib resistance in human non-small cell lung cancer cells with K-Ras mutations. Invest New Drugs. 2010;28(6):791–799 [DOI] [PubMed] [Google Scholar]
- 16. Zhao TT, Trinh D, Addison CL, Dimitroulakos J. Lovastatin inhibits VEGFR and AKT activation: synergistic cytotoxicity in combination with VEGFR inhibitors 1. PLoS One. 2010;5(9):e12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rozados VR, Hinrichsen LI, Binda MM, et al. Lovastatin enhances the antitumoral and apoptotic activity of doxorubicin in murine tumor models. Oncol Rep. 2008;19(5):1205–1211 [PubMed] [Google Scholar]
- 18. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2013;368(6):576–577 [DOI] [PubMed] [Google Scholar]
- 19. Siddikuzzaman, Guruvayoorappan C, Berlin Grace VM. All trans retinoic acid and cancer. Immunopharmacol Immunotoxicol. 2011;33(2):241–249 [DOI] [PubMed] [Google Scholar]
- 20. Reynolds CP, Lemons RS. Retinoid therapy of childhood cancer. Hematol Oncol Clin North Am. 2001;15(5):867–910 [DOI] [PubMed] [Google Scholar]
- 21. Påhlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14(2):135–144 [DOI] [PubMed] [Google Scholar]
- 22. Sidell N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982;68(4):589–596 [PubMed] [Google Scholar]
- 23. Miller WH., Jr The emerging role of retinoids and retinoic acid metabolism blocking agents in the treatment of cancer. Cancer. 1998;83(8):1471–1482 [DOI] [PubMed] [Google Scholar]
- 24. Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341(16):1165–1173 [DOI] [PubMed] [Google Scholar]
- 25. Benner SE, Pajak TF, Lippman SM, Earley C, Hong WK. Prevention of second primary tumors with isotretinoin in patients with squamous cell carcinoma of the head and neck: long-term follow-up. J Natl Cancer Inst. 1994;86(2):140–141 [DOI] [PubMed] [Google Scholar]
- 26. Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795–801 [DOI] [PubMed] [Google Scholar]
- 27. Kurie JM. The biologic basis for the use of retinoids in cancer prevention and treatment. Curr Opin Oncol. 1999;11(6):497–502 [DOI] [PubMed] [Google Scholar]
- 28. Hittelman WN, Liu DD, Kurie JM, et al. Proliferative changes in the bronchial epithelium of former smokers treated with retinoids. J Natl Cancer Inst. 2007;99(21):1603–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dimitroulakos J, Ye LY, Benzaquen M, et al. Differential sensitivity of various pediatric cancers and squamous cell carcinomas to lovastatin-induced apoptosis: therapeutic implications. Clin Cancer Res. 2001;7(1):158–167 [PubMed] [Google Scholar]
- 30. Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 1995;31(1):9–27 [DOI] [PubMed] [Google Scholar]
- 31. Dimitroulakos J, Pienkowska M, Sun P, et al. Identification of a novel zinc finger gene, zf5-3, as a potential mediator of neuroblastoma differentiation. Int J Cancer. 1999;81(6):970–978 [DOI] [PubMed] [Google Scholar]
- 32. Dimitroulakos J, Yeger H. HMG-CoA reductase mediates the biological effects of retinoic acid on human neuroblastoma cells: lovastatin specifically targets P-glycoprotein-expressing cells. Nat Med. 1996;2(3):326–333 [DOI] [PubMed] [Google Scholar]
- 33. Erturan I, Naziroglu M, Akkaya VB. Isotretinoin treatment induces oxidative toxicity in blood of patients with acne vulgaris: a clinical pilot study. Cell Biochem Funct. 2012;30(7):552–557 [DOI] [PubMed] [Google Scholar]
- 34. Huang W, Shang WL, Li DH, Wu WW, Hou SX. Simvastatin protects osteoblast against H2O2-induced oxidative damage via inhibiting the upregulation of Nox4. Mol Cell Biochem. 2012;360(1–2):71–77 [DOI] [PubMed] [Google Scholar]
- 35. Parizadeh SM, Azarpazhooh MR, Moohebati M, et al. Simvastatin therapy reduces prooxidant-antioxidant balance: results of a placebo-controlled cross-over trial. Lipids. 2011;46(4):333–340 [DOI] [PubMed] [Google Scholar]
- 36. Altintas ND, Atilla P, Iskit AB, Topeli A. Long-term simvastatin attenuates lung injury and oxidative stress in murine acute lung injury models induced by oleic acid and endotoxin. Respir Care. 2011;56(8):1156–1163 [DOI] [PubMed] [Google Scholar]
- 37. Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50(4):219–244 [PubMed] [Google Scholar]
- 38. Martiniova L, Lai EW, Elkahloun AG, et al. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin Exp Metastasis. 2009;26(3):239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giubellino A, Woldemichael GM, Sourbier C, et al. Characterization of two mouse models of metastatic pheochromocytoma using bioluminescence imaging. Cancer Lett. 2012;316(1):46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31(4):229–234 [DOI] [PubMed] [Google Scholar]
- 41. Ghayee HK, Bhagwandin VJ, Stastny V, et al. Progenitor cell line (hPheo1) derived from a human pheochromocytoma tumor. PLoS One. 2013;8(6):e65624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calhoun KH, Stanley D, Stiernberg CM, Ahmed AE. Vitamins A and E do protect against oral carcinoma. Arch Otolaryngol Head Neck Surg. 1989;115(4):484–488 [DOI] [PubMed] [Google Scholar]
- 43. Papadimitrakopoulou VA, Clayman GL, Shin DM, et al. Biochemoprevention for dysplastic lesions of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg. 1999;125(10):1083–1089 [DOI] [PubMed] [Google Scholar]
- 44. Seixas-Silva JA, Jr, Richards T, Khuri FR, et al. Phase 2 bioadjuvant study of interferon alfa-2a, isotretinoin, and vitamin E in locally advanced squamous cell carcinoma of the head and neck: long-term follow-up. Arch Otolaryngol Head Neck Surg. 2005;131(4):304–307 [DOI] [PubMed] [Google Scholar]
- 45. Besa EC, Abrahm JL, Bartholomew MJ, Hyzinski M, Nowell PC. Treatment with 13-cis-retinoic acid in transfusion-dependent patients with myelodysplastic syndrome and decreased toxicity with addition of α-tocopherol. Am J Med. 1990;89(6):739–747 [DOI] [PubMed] [Google Scholar]
- 46. Shan X, Wang D, Chen J, et al. Necrosis degree displayed in computed tomography images correlated with hypoxia and angiogenesis in breast cancer. J Comput Assist Tomogr. 2013;37(1):22–28 [DOI] [PubMed] [Google Scholar]
- 47. Wang WQ, Liu L, Xu HX, et al. Intratumoral α-SMA enhances the prognostic potency of CD34 associated with maintenance of microvessel integrity in hepatocellular carcinoma and pancreatic cancer. PLoS One. 2013;8(8):e71189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Faivre S, Ronot M, Dreyer C, et al. Imaging response in neuroendocrine tumors treated with targeted therapies: the experience of sunitinib. Target Oncol. 2012;7(2):127–133 [DOI] [PubMed] [Google Scholar]