Abstract
Metabolic characteristics of polycystic ovary syndrome women and polycystic ovary syndrome-like, prenatally androgenized (PA) female monkeys worsen with age, with altered adipogenesis of sc abdominal adipose potentially contributing to age-related adverse effects on metabolism. This study examines whether adipocyte morphology and gene expression in sc abdominal adipose differ between late reproductive-aged PA female rhesus monkeys compared with age-matched controls (C). Subcutaneous abdominal adipose of both groups was obtained for histological imaging and mRNA determination of zinc finger protein 423 (Zfp423) as a marker of adipose stem cell commitment to preadipocytes, and CCAAT/enhancer binding protein (C/EBP)α/peroxisome proliferator-activated receptor (PPAR)δ as well as C/EBPα/PPARγ as respective markers of early- and late-stage differentiation of preadipocytes to adipocytes. In all females combined, serum testosterone (T) levels positively correlated with fasting serum levels of total free fatty acid (r2 = 0.73, P < .002). PA females had a greater population of small adipocytes vs C (P < .001) in the presence of increased Zfp423 (P < .025 vs C females) and decreased C/EBPα (P < .003, vs C females) mRNA expression. Moreover, Zfp423 mRNA expression positively correlated with circulating total free fatty acid levels during iv glucose tolerance testing (P < .004, r2 = 0.66), whereas C/EBPα mRNA expression negatively correlated with serum T levels (P < .02, r2 = 0.43). Gene expression of PPARδ and PPARγ were comparable between groups (P = .723 and P = .18, respectively). Early-to-mid gestational T excess in female rhesus monkeys impairs adult preadipocyte differentiation to adipocytes in sc abdominal adipose and may constrain the ability of this adipose depot to safely store fat with age.
Increased adiposity enhances the severity of polycystic ovary syndrome (PCOS) (1, 2), beginning in adolescence (3, 4) and increasing with age. Women with PCOS develop greater abdominal adiposity than normal women of comparable body mass index (BMI), which positively correlates with insulin resistance, altered adipose function, and glucose dysregulation (5). Moreover, sc abdominal adipocyte morphology appears altered in PCOS women (6), potentially linking altered adipocyte morphology with a perturbed capacity of sc adipose to safely store fat (7, 8). This phenomenon is important, because metabolic dysfunction in humans likely results from ectopic lipid accumulation in nonadipose cells, causing lipotoxicity (9). Thus, when energy intake exceeds the capacity of normal sc adipose to safely store fat, excess free fatty acids (FFAs) become deposited in abnormal locations, such as muscle and liver, where they induce oxidative/endoplasmic reticulum stress tightly linked with insulin resistance and inflammation (6–10).
Altered adipose function also occurs in prenatally androgenized (PA) adult female monkeys exposed during early-to-mid gestation to male levels of testosterone (T) (11). Similar to PCOS women, PA monkeys express ovarian hyperandrogenism, ovulatory dysfunction and polycystic ovaries (12–14), and progressively manifest metabolic dysfunction with age (15), thereby increasing abdominal adiposity in combination with hyperlipidemia, insulin resistance, and type 2 diabetes (5, 11, 16).
These findings suggest that gestational T excess in female nonhuman primates may alter adipogenesis at any of its 3 stages of development: 1) adipose stem cell (ASC) commitment to preadipocytes via bone morphogenetic protein 4 (BMP4)-associated zinc finger protein 423 (Zfp423) activation; 2) early-stage preadipocyte differentiation via CCAAT/enhancer binding protein (C/EBP)β and C/EBPδ activation and peroxisome proliferator-activated receptor (PPAR)δ and C/EBPα induction; and/or 3) late-stage preadipocyte differentiation to mature adipocytes through further activation of PPARγ, C/EBPα, and other transcriptional factors (17–22). In support of this, exposure of cultured human ASCs (hASCs) from sc abdominal adipose of normal women to androgen impairs their commitment to preadipocytes and subsequent early-stage preadipocyte differentiation through androgen receptor action (23). To determine whether gestational T excess similarly affects sc abdominal adipogenesis in vivo, the present study examines whether changes in sc abdominal adipose morphology and gene expression occur in relation to metabolic and reproduction dysfunction in PA female monkeys.
Materials and Methods
Ethics statement
The Institutional Animal Care and Use Committee of the Graduate School of the University of Wisconsin-Madison approved all procedures used in the study, and the care and housing of the monkeys was in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and Animal Welfare Act with its subsequent amendments.
Animals
The 12 adult female rhesus monkeys (Macaca mulatta) used in this study were maintained at Wisconsin National Primate Research Center (WNPRC), according to standard protocol (24, 25). They were fed Purina monkey chow (product number 5038; Ralston Purina) with occasional supplementation of fresh fruits. This formulation of monkey chow provides 70% of calories as carbohydrate, 13% as fat, and 17% as protein. Age and BMI (body weight [kg]/crown-rump length [m2]) (26) of both female groups were comparable (Table 1). Six of the female monkeys were exposed to gestational T excess by sc injection of their dams with 10-mg T propionate starting on gestational days 40–44 for 15–35 consecutive days (early-to-mid gestation, PA). Only 6 of the 14 previously described PA monkeys were studied (27), because they exhibited high T levels and intermittent menstrual cycles (16) compared with controls (C) (Table 1) and provided appropriate quality tissue at necropsy. The 6 C female monkeys in this study were not exposed to exogenous T excess in utero and were selected from monkeys not otherwise manipulated during gestation by other investigators or colony management at the WNPRC.
Table 1.
Selected Parameters of Study Monkeys
| Parameter | C Monkeys | PA Monkeys |
|---|---|---|
| Age/somatometric | ||
| Age (y) | 24.0 ± 0.9 | 25.8 ± 0.7 |
| BMI (kg/m2) | 41.9 ± 2.2 | 38.1 ± 2.9 |
| % Abdominal fat | 48.6 ± 3.8 | 33.3 ± 3.1a |
| Subcutaneous abdominal fat (cm3) | 816 ± 362 | 346 ± 84 |
| Visceral fat (cm3) | 1066 ± 98 | 630 ± 99b |
| Ratio sc abdominal/visceral fat | 0.7 ± 0.3 | 0.7 ± 0.2 |
| Metabolic physiology | ||
| Basal total FFAs (mM/L) | 522 ± 84 | 1052 ± 380c |
| Area-under-the curve total FFAs (mM/L per 180 min) | 75.7 ± 0.9 | 104.1 ± 15.2 |
| Basal insulin (μU/mL) | 52.7 ± 12.7 | 19.3 ± 36.7 |
| Basal glucose (mg/dL) | 57.9 ± 1.4 | 73.5 ± 10.2 |
| Insulin sensitivity (SI) | 1.4 ± 1.0 | 3.4 ± 2.7 |
| Glycosylated hemoglobin (%) | 8.0 ± 0.4 | 9.6 ± 1.6 |
| Reproductive physiology | ||
| Basal T (ng/mL) | 0.2 ± 0.1 | 0.4 ± 0.1d |
| % Intermittent/absent menstrual cycles | 0 | 67e |
| % Polycystic ovaries | 0 | 83f |
| Ratio of mRNA expression | ||
| Zfp423/C/EBPα | 0.12 ± 0.01 | 0.35 ± 0.02g |
| Zfp423/PPARλ | 0.40 ± 0.03 | 0.41 ± 0.03 |
| Zfp423/PPARδ | 0.57 ± 0.07 | 0.62 ± 0.02 |
| Positive correlation with basal T levels (ng/mL) | ||
| Basal insulin (μU/mL) | r2 = 0.65, P < .029 (All female monkeys combined) | |
| Glycosylated hemoglobin (%) | r2 = 0.62, P < .035 (All female monkeys combined) | |
| Total FFAs (μM/L) | r2 = 0.73, P < .002 (All female monkeys combined) | |
Data are mean ± SEM.
P < .021 vs C.
P < .029 vs C.
P < .059 vs C.
P < .020 vs C.
P < .062 vs C.
P < .016 vs C.
P < .001 vs C.
In some of the female monkeys in this study, somatometric measures, basal serum T levels, menstrual cycle duration, and ovarian morphology were previously reported but are included here to provide appropriate context for analyses of adipose tissue (12, 13, 16, 27–31). Blood samples providing serum for hormone analyses were obtained from animals accustomed to using a tabletop restraint without anesthesia (29).
Hormone assays
Serum T levels were determined by enzyme-immunoassay, plasma insulin concentrations were measured by RIA (Linco Research, Inc), and plasma glucose concentrations were measured by the glucose oxidase method (Yellow Springs Instruments) in the WNPRC/Institute of Clinical Translational Research Hormone Assay Services Laboratory, as previously reported (30, 32). T measurements were performed after diethyl ether extraction of serum and solvent fraction separation by celite chromatography. Intra- and interassay coefficient of variation (CVs) for quality control (QC) preparation values were, respectively, T, 3.5% and 14.0%; insulin, 8.1 and 4.8%; and glucose, 4.0 and 2.9%. Because T values have a wide range of variability, we adjusted them within the range of values observed in the study samples (QC preparation value [n = 57]: 0.18 ± 0.04 ng/mL, mean ± SD), with intra- and interassay CVs for QC values of 4.9% and 21.1%, respectively. Glycosylated hemoglobin was assayed by the Glyco-Teck affinity column method, as previously established for rhesus monkeys at WNPRC (33, 34).
Ovarian morphology
During laparoscopic assessment of ovarian dimensions (35, 36) while the animals were sedated with ketamine HCl (10 mg/kg, im), photographic images were taken of transilluminated ovaries (37) at their largest diameter during the early follicular phase (menstrual cycle d 1–5) or an anovulatory interval. Ovarian images with more than 10, approximately 1–3 mm in diameter follicles were scored as polyfollicular (Table 1), a criterion modified from the prevailing ultrasonographic determination of polycystic ovaries in women (38) before the Rotterdam consensus (39), contemporary with ovarian observation.
Frequently sampled iv glucose tolerance test (FSIGT)
Monkeys underwent a single, 3-hour, FSIGT starting at 7–9 am after an overnight fast, according to the tolbutamide-modified minimal model of Bergman and at approximately 4 weeks before dual-x-ray absorptiometry (DXA) and computed tomography scans, as previously reported (31). All FSIGTs were performed during the early follicular phase of a menstrual cycle or an anovulatory period and provided the glucose, insulin, and total FFA data for parameters presented in Table 1.
Abdominal fat determination
Crown-rump length measurements for BMI determination were obtained from each animal while anesthetized for DXA scan.
For DXA, DXA scanner model DPX-L (GE/Lunar Corp) had a precision (CV%) of less than 4% for total body and regional composition in rhesus monkeys. Animals were placed in a supine position for scanning, and images were acquired and analyzed with pediatric software (versions 1.5e and 4.0a, respectively) to determine total body and abdominal lean and fat mass, as reported previously (11). The abdominal region of interest was defined by area between the thoracic 12-lumbar (L) interspace and the L6-L7 interspace.
Computed tomography was performed within 7–8 days of the DXA scan, as previously reported (11), using a HiLight Advantage scanner (GE) at 120 kV and 200 mA with a scan time of 2 seconds and slice thickness of 0.5 cm. The abdominal region was analyzed using SliceOMatic (version 4.3 rev0h; Tomovision).
Preparation and morphological assessment of sc abdominal fat
Subcutaneous fat was collected after monkeys were killed. Monkeys were put through timed injection with ketamine and barbiturate before necropsy. During the necropsy protocol, abdominal fat was obtained from sc depots adjacent to the umbilicus. Subcutaneous fat was placed into sterile cryovials, which were immediately placed into liquid nitrogen and transported to a −80°C freezer soon afterwards.
For cell sizing studies, approximately 0.5-g frozen sc abdominal fat was embedded in optimal cutting temperature medium, cut into 10-μm sections, stained with hematoxylin and eosin following standard protocols, and digitally imaged using Aperio ScanScope. Six sections were cut from each sample, and a minimum of 100 cells per section was analyzed. Adipocyte area (AA) and circumference were determined from the digital images using Aperio ImageScope software, which is internally calibrated (50 000 pixels per inch, 0.5 μm per pixel).
mRNA expression
Total cellular RNA from frozen tissue samples was isolated using an RNeasy kit (QIAGEN) according to manufacturer's protocol. mRNA expression of Zfp423, PPARδ, C/EBPβ, C/EBPα, and PPARγ was determined by real-time quantitative RT-PCR (qRT-PCR) under standard conditions. All primers were provided by A&B Applied Biosystems. Human primer for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to detect GAPDH mRNA as an internal C housekeeping gene (Life Technologies Corp). The relative expression of target genes was measured using the comparative critical threshold (Ct) method. The results were expressed as mRNA expression relative to GAPDH using the formula 2−δCt(δCt is the difference between the mean Ct values of genes of interest and the mean Ct value GAPDH). The results were expressed as fold changes (PA vs C). As a negative C, first strand DNA synthesis was performed in the absence of mRNA followed by qRT-PCR.
Statistical analyses
All results were expressed as mean ± SEM. Adipocyte size was transformed using the square root transformation for display purposes. The empirical cumulative distribution function was calculated and plotted for each group (treated/untreated). The empirical cumulative distribution function was constructed by finding the probability that the adipocyte size was less than or equal to the given value. The treated and untreated distributions were compared using the two-sample Kolmogorov-Smirnov test. For qRT-PCR studies, each sample was subjected to at least 3 independent experiments performed in triplicates. Statistical significance was determined as P < .05. Regression analysis was used to examine the associations between gene expression and area under the curve (AUC) for total FFA or circulating T levels. Similar analysis was performed to determine the association between basal total FFA and circulating T levels.
Results
Monkey baseline parameters
Although the percentages of abdominal and visceral fat content were lower in PA than C monkeys, both female groups had a similar approximately 0.7 ratio of sc abdominal to visceral fat (Table 1). Glucoregulatory parameters were similar between female groups, except for a trend for an increase in basal (fasting) circulating concentrations of total FFA in PA monkeys.
Reproductive parameters distinguished the 2 female groups, with increases in circulating T levels, and incidences of oligo-ovulation and polycystic ovaries in PA monkeys (Table 1). In all females combined, circulating T levels positively correlated with overnight basal circulating levels of total FFA, insulin, and glycosylated hemoglobin (Table 1; also, see figure 3 below), with PA monkeys contributing both high T concentrations and increased total FFA, insulin, and glycosylated hemoglobin levels.
Adipocyte morphology
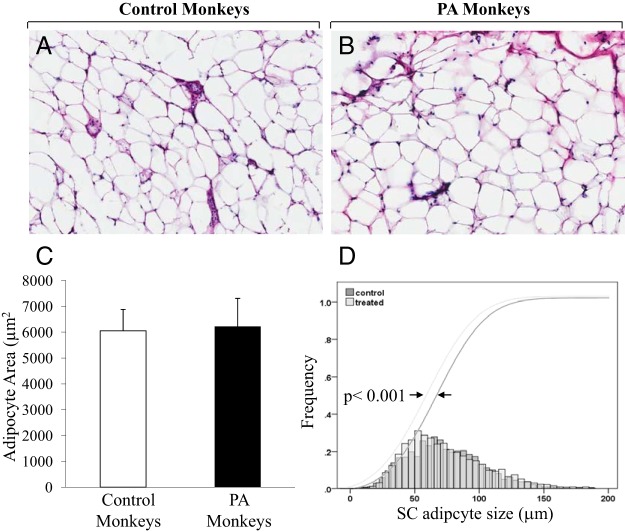
A similar number of cells was analyzed between C and PA females. No significant differences in adipocyte morphology, mean AA (C = 6052 ± 827 μm2 vs PA = 6224 ± 1085 μm2), mean adipocyte circumference (C = 331 ± 45 μm vs PA = 332 ± 75 μm), or ratio of AA/adipocyte circumference (C = 15.6 ± 3.8 vs PA = 16.0 ± 3.8) were observed between female groups (Figure 1, A–C). The cell size distribution analysis, however, showed a significant shift (P < .001) toward smaller sc adipocyte diameter in the PA compared with C females (Figure 1D).
Figure 1.
Quantification of adipocyte cell size in PA vs C adult female rhesus monkeys. Representative images of sc abdominal adipocyte samples stained with hematoxylin and eosin (H&E) in C (A) and PA monkeys (B). C, Graphical representation of the sc AA (mean ± SEM) in PA (n = 6) vs C (n = 6) monkeys. D, Subcutaneous adipocyte size distributions shows a significant shift toward smaller sc adipocyte diameter in PA vs C monkeys; *, P < .001 vs C.
Gene expression analysis
mRNA expression of Zfp423 in sc adipose of PA monkeys was increased (2.77 ± 0.17-fold change vs C; P < .02) (Figure 2A), whereas C/EBPα mRNA expression was reciprocally decreased (0.26 ± 0.05-fold change vs C; P < .002) (Figure 2C). No female-type differences were detected in sc adipose of PPARδ (1.15 ± 0.04-fold change vs C; P = .723) (Figure 2B) or PPARγ (2.05 ± 0.50-fold change vs C; P = .18) mRNA expression (Figure 2D). Furthermore, in a subset of PA and C females combined, sc adipose Zfp423 mRNA expression (relative to GAPDH) positively correlated with AUC total FFA levels during an FSIGT (P < .004, r2=0.66) (Figure 3A). Subcutaneous adipose C/EBPα mRNA expression (relative to GAPDH) negatively correlated with circulating T levels (P < .02, r2 = 0.43, all animals combined) (Figure 3B). Subcutaneous abdominal adipose Zfp423, C/EBPα, PPARδ, and PPARγ mRNA expression was not associated with adipocyte morphology, glucoregulatory parameters, BMI, or age.
Figure 2.
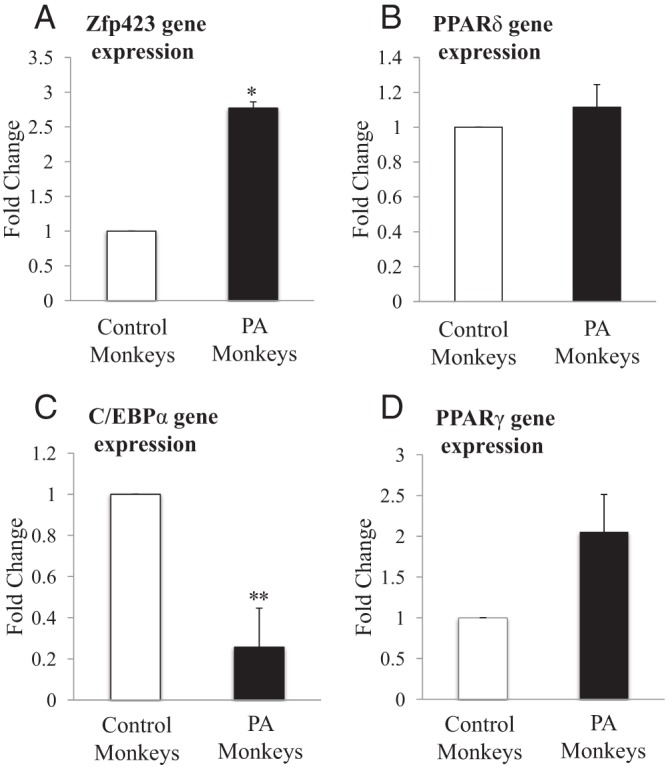
mRNA expression of adipogenic genes in PA vs C adult female rhesus monkeys. Subcutaneous abdominal adipose tissue collected from 6 PA and 6 C was immediately placed into liquid nitrogen after animals were killed. mRNA extraction and qRT-PCR were performed as indicated in Materials and Methods. Zfp423 (A), PPARδ (B), CEBPα (C), and PPARγ (D) mRNA levels were quantified by qRT-PCR relative to the housekeeping gene GAPDH. Results represent fold changes in PA (n = 6) vs C (n = 6) monkeys; *, P < .020; **, P < .001 vs C.
Figure 3.
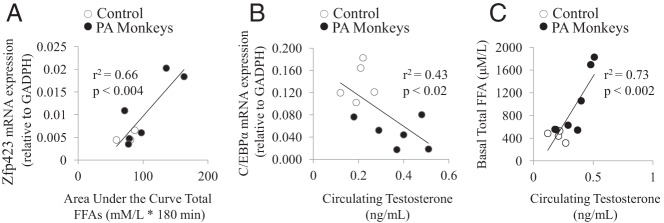
Correlation between selected parameters of all monkeys combined. Regression analysis was performed in PA (black) vs C (no fill) monkeys to determine (A) Zfp423 mRNA expression vs AUC total FFA, C (n = 3), PA (n = 6); (B) C/EBPα mRNA expression vs circulating T, C (n = 5), PA (n = 6); and (C) basal total FFA vs circulating T, C (n = 5), PA (n = 5). mRNA expression of specific genes relative to the housekeeping gene GAPDH was determined by qRT-PCR; circulating T and total FFA were determined by RIA and radiochemical assay, respectively. FFAs were measured throughout the FSIGT, and the basal measurements presented represent the first measurements taken during the test.
Discussion
Altered metabolic function accompanied by increased abdominal adiposity is a salient characteristic of PCOS women (14, 40) and PCOS-like, PA female monkeys alike (14, 15), implicating possible abnormalities of adipogenesis from ASC commitment to preadipocytes to subsequent differentiation of preadipocytes to adipocytes (Figure 4). Using several adipogenic gene markers, the present study shows in sc abdominal adipose of aging female rhesus monkeys that gestational T excess impairs preadipocyte differentiation to mature adipocytes while promoting earlier ASC commitment to preadipocytes, as evidenced by a reciprocal C/EBPα decrease and Zfp423 increase in mRNA expression, respectively. These findings support, in part, our previous observation in normal women that androgens reduce preadipocyte differentiation to mature adipocytes, perhaps limiting adipocyte numbers and fat storage in sc abdominal adipose (23).
Figure 4.
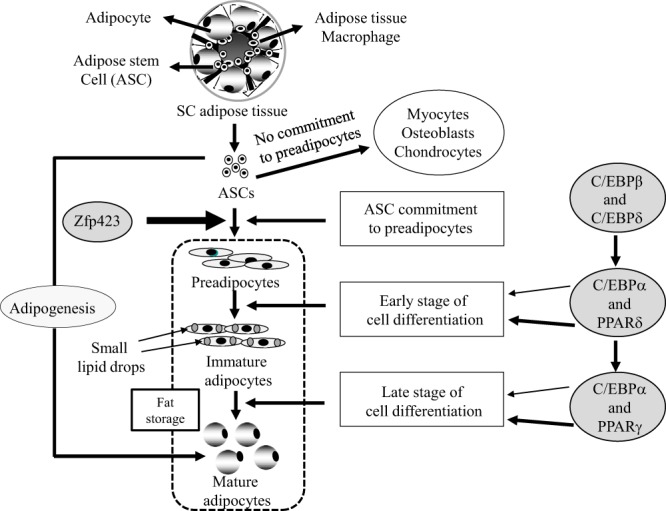
Schematic representation of adipogenesis. ASC commitment to preadipocytes is governed by Zfp423 mRNA expression. Activation of C/EBPβ and PPARδ, and C/EBPα and PPARγ regulates early and late stage preadipocyte differentiation to mature adipocytes, respectively.
In support of this, PA monkeys had a greater population of small sc adipocytes compared with C, suggesting a developmental programming role for T in constraining adipocyte size, as previously shown in female sheep exposed to excess T during gestation (41). Such a phenomenon in PA monkeys may also diminish sc abdominal storage of lipid and, therefore, explain why visceral fat, as an alternative fat depot, increases in amount with BMI in PA, but not C, female monkeys (11). Moreover, an increase in the number of small sc adipocytes is linked with human metabolic dysfunction (42), as is an alternative increase in sc adipocyte size in some PCOS women (6), implying that a change in either direction away from the optimal size of sc adipocytes might have metabolic consequences.
A strength of our study is the determination of adipocyte size using fixed histological sections of adipose tissue instead of using freshly isolated adipocytes obtained by collagenase digestion, which could lyse larger adipocytes leading to inaccurate measurements of the size population of adipocytes (43).
Along with androgen-induced impairment of preadipocyte differentiation as a function of constrained sc abdominal adipose storage capacity, circulating T levels negatively correlated with sc abdominal adipose C/EBPα gene expression while simultaneously positively correlating with total FFA levels, in all monkeys combined, agreeing with PCOS women showing several defects in lipid metabolism (44–46). Moreover, increased FFA release during FSIGT positively correlated with elevated sc abdominal adipose Zfp423 mRNA expression, presumably to enhance ASC commitment to preadipocytes from impaired onward differentiation of preadipocytes to adipocytes.
PA monkeys have reduced numbers of mature sc adipocytes (this study), along with a defect in the ability to increase sc lipid storage in proportion to BMI (11). They also exhibit dyslipidemia, an abdominal fat volume that positively correlates with basal insulin levels (11, 16), and a subtle impairment in insulin sensitivity in some (11), but not all (16), studies that increases the incidence of type 2 diabetes compared with age- and BMI-similar C (15). Our present findings may therefore have identified a molecular component(s) underpinning how prenatal androgen excess constrains the ability of the sc abdominal adipose depot to safely store fat, causing lipotoxicity from ectopic lipid accumulation in nonadipose cells in PCOS-like monkeys.
Our present data also show similarities and differences to those of other reports of T-regulated adipogenic genes. Although Zfp423 gene expression is up-regulated in PA female monkeys, cultured hASCs from women show T inhibition of Zfp423-BMP4-mediated gene expression (23). This discrepancy may be attributed to the differences in species (human vs monkey), study design (in vitro vs in vivo), supraphysiological vs pharmacological concentrations, and/or stimulation techniques (in vitro T and BMP4 stimulation vs in vivo gestational T excess).
Similar to decreased C/EBPα mRNA expression in sc abdominal adipose of PA females, exposure of cultured hASCs and mouse preadipocytes to T also inhibits C/EBPα mRNA expression (23, 47). In contrast, however, to the inhibitory effect of T on PPARγ gene expression in hASCs (23), PPARγ mRNA levels were not affected in sc abdominal adipose of PA monkeys. These unexpected results challenge contemporary understanding that C/EBPα and PPARγ gene expression engage concurrently during late-stage preadipocyte differentiation to mature adipocytes. One possible explanation of the present monkey data might be that PA treatment epigenetically reprograms the androgen receptor-chromodomain helicase DNA-binding complex (48), leading to preferential inactivation of C/EBPα rather than PPARγ (49). Developmental programming of PA monkeys could also select for specific microRNAs critical for adipogenesis such as microRNA-31, which primarily down-regulates C/EBPα expression but only secondarily associates with PPARγ expression (50, 51). Whether developmental programming in PA monkeys affects the earliest stage of preadipocyte differentiation to adipocytes is unclear, because C/EBPβ, a critical gene involved during this process, was undetectable.
An important limitation of our study is its inability to distinguish the long-term effect of prenatal androgen exposure from the short-term action of high circulating androgen levels on sc abdominal adipose function, particularly because androgen inhibits sc abdominal lipoprotein lipase activity in women (52). As a result, this confounding action of circulating androgen levels on sc abdominal adipose function, apart from the earlier developmental programming effect of prenatal androgen exposure, may also vary with age, because the T to estradiol ratio in the circulation of late-reproductive-aged women increases across the perimenopausal transition (53). Moreover, visceral adipose volume increases in hyperandrogenic anovulatory women after pituitary desensitization to GnRH, whereas sc abdominal adipose does not (54), making extrapolation of androgen action on sc abdominal to visceral adipose unwarranted. Equally relevant, the negative impact of androgen on adipogenesis and lipid synthesis, although similar in direction by sex type, differs in magnitude between men and women (52), so that additional studies are required to understand the developmental consequences of prenatal androgen exposure in men on preadipocyte differentiation in sc abdominal adipose.
The collective data suggest that early-to-mid gestation exposure to T excess in adult female rhesus monkeys impairs preadipocyte differentiation and reduces adipocyte size in sc abdominal adipose. Dysfunctional adipocyte maturation may thus constrain the capacity of this adipose depot to safely store fat, promoting insulin resistance through lipotoxicity.
Acknowledgments
We thank Amber Edwards for technical support and the animal care and veterinary staff at the Wisconsin National Primate Research Center for their care and support of the monkeys.
This work was supported in part by National Institutes of Health Grants R01 RR013635 (to D.H.A.), U01 HD044650 (to D.A.D.), and P51 RR000167 (Wisconsin National Primate Research Center) and by the Department of Obstetrics and Gynecology at University of California Los Angeles and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health through Cooperative Agreement U54 HD071836 (to D.A.D.). Statistical analyses were funded by National Institutes of Health/National Center for Advancing Translational Sciences/University of California, Los Angeles Clinical and Translational Science Institute Grant UL1TR000124.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- adipocyte area
- ASC
- adipose stem cell
- AUC
- area under the curve
- BMI
- body mass index
- BMP4
- bone morphogenetic protein 4
- C
- controls
- C/EBP
- CCAAT/enhancer binding protein
- Ct
- comparative critical threshold
- CV
- coefficient of variation
- DXA
- dual-x-ray absorptiometry
- FFA
- free fatty acid
- FSIGT
- frequently sampled iv glucose tolerance test
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- hASC
- human ASC
- L
- lumbar
- PA
- prenatally androgenized
- PCOS
- polycystic ovary syndrome
- PPAR
- peroxisome proliferator-activated receptor
- QC
- quality C
- qRT-PCR
- real-time quantitative RT-PCR
- WNPRC
- Wisconsin National Primate Research Center
- Zfp423
- zinc finger protein 423.
References
- 1. Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107–2111 [DOI] [PubMed] [Google Scholar]
- 2. Legro RS. The genetics of obesity. Lessons for polycystic ovary syndrome. Ann NY Acad Sci. 2000;900:193–202 [DOI] [PubMed] [Google Scholar]
- 3. Trottier A, Battista MC, Geller DH, et al. Adipose tissue insulin resistance in peripubertal girls with first-degree family history of polycystic ovary syndrome. Fertil Steril. 2012;98:1627–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maliqueo M, Galgani JE, Pérez-Bravo F, et al. Relationship of serum adipocyte-derived proteins with insulin sensitivity and reproductive features in pre-pubertal and pubertal daughters of polycystic ovary syndrome women. Eur J Obstet Gynecol Reprod Biol. 2012;161:56–61 [DOI] [PubMed] [Google Scholar]
- 5. Barber TM, Franks S. Adipocyte biology in polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:68–76 [DOI] [PubMed] [Google Scholar]
- 6. Mannerås-Holm L, Leonhardt H, Kullberg J, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304–E311 [DOI] [PubMed] [Google Scholar]
- 7. de Zegher F, Lopez-Bermejo A, Ibáñez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab. 2009;20:418–423 [DOI] [PubMed] [Google Scholar]
- 8. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome–an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349 [DOI] [PubMed] [Google Scholar]
- 9. Sørensen TI, Virtue S, Vidal-Puig A. Obesity as a clinical and public health problem: is there a need for a new definition based on lipotoxicity effects? Biochim Biophys Acta. 2010;1801:400–404 [DOI] [PubMed] [Google Scholar]
- 10. Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75 [DOI] [PubMed] [Google Scholar]
- 11. Bruns CM, Baum ST, Colman RJ, et al. Prenatal androgen excess negatively impacts body fat distribution in a nonhuman primate model of polycystic ovary syndrome. Int J Obes (Lond). 2007;31:1579–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374 [DOI] [PubMed] [Google Scholar]
- 13. Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62–67 [DOI] [PubMed] [Google Scholar]
- 14. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e25 [DOI] [PubMed] [Google Scholar]
- 15. Abbott DH, Nicol LE, Levine JE, Xu N, Goodarzi MO, Dumesic DA. Nonhuman primate models of polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou R, Bruns CM, Bird IM, et al. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol. 2007;23:438–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254 [DOI] [PubMed] [Google Scholar]
- 21. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896 [DOI] [PubMed] [Google Scholar]
- 22. Siersbæk R, Mandrup S. Transcriptional networks controlling adipocyte differentiation. Cold Spring Harb Symp Quant Biol. 2011;76:247–255 [DOI] [PubMed] [Google Scholar]
- 23. Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78:920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varlamov O, White AE, Carroll JM, et al. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153:3100–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goy RW, Robinson JA. Prenatal exposure of rhesus monkeys to patent androgens: morphological, behavioral, and physiological consequences. In: Hunt VR, Smith MK, Worth D, eds. Banbury Report II: Environmental Factors in Human Growth and Development. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1982:355–378 [Google Scholar]
- 26. Kemnitz JW, Elson DF, Roecker EB, Baum ST, Bergman RN, Meglasson MD. Pioglitazone increases insulin sensitivity, reduces blood glucose, insulin, and lipid levels, and lowers blood pressure, in obese, insulin-resistant rhesus monkeys. Diabetes. 1994;43:204–211 [DOI] [PubMed] [Google Scholar]
- 27. Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71:776–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol. 2002;174:1–5 [DOI] [PubMed] [Google Scholar]
- 29. Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67:155–163 [DOI] [PubMed] [Google Scholar]
- 30. Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77:167–172 [DOI] [PubMed] [Google Scholar]
- 31. Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206–1210 [DOI] [PubMed] [Google Scholar]
- 32. Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:6630–6637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–E765 [DOI] [PubMed] [Google Scholar]
- 34. Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540–E547 [DOI] [PubMed] [Google Scholar]
- 35. Dumesic DA, Schramm RD, Bird IM, et al. Reduced intrafollicular androstenedione and estradiol levels in early-treated prenatally androgenized female rhesus monkeys receiving follicle-stimulating hormone therapy for in vitro fertilization. Biol Reprod. 2003;69:1213–1219 [DOI] [PubMed] [Google Scholar]
- 36. Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119 [DOI] [PubMed] [Google Scholar]
- 37. Dierschke DJ, Clark JR. Laparoscopy in Macaca mulatta: specialized equipment employed and initial observations. J Med Primatol. 1976;5:100–110 [DOI] [PubMed] [Google Scholar]
- 38. Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed). 1986;293:355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25 [DOI] [PubMed] [Google Scholar]
- 40. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231 [DOI] [PubMed] [Google Scholar]
- 41. Veiga-Lopez A, Moeller J, Patel D, et al. Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology. 2013;154:1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McLaughlin T, Lamendola C, Coghlan N, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity (Silver Spring). 2014;22:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen HC, Farese RV., Jr Determination of adipocyte size by computer image analysis. J Lipid Res. 2002;43:986–989 [PubMed] [Google Scholar]
- 44. Di Sarra D, Tosi F, Bonin C, et al. Metabolic inflexibility is a feature of women with polycystic ovary syndrome and is associated with both insulin resistance and hyperandrogenism. J Clin Endocrinol Metab. 2013;98:2581–2588 [DOI] [PubMed] [Google Scholar]
- 45. Macut D, Panidis D, Glisi B, et al. Lipid and lipoprotein profile in women with polycystic ovary syndrome. Can J Physiol Pharmacol. 2008;86:199–204 [DOI] [PubMed] [Google Scholar]
- 46. Whigham LD, Butz DE, Dashti H, et al. Metabolic evidence of diminished lipid oxidation in women with polycystic ovary syndrome. Curr Metabololomics. 2014;2:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singh R, Artaza JN, Taylor WE, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with β-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Menon T, Yates JA, Bochar DA. Regulation of androgen-responsive transcription by the chromatin remodeling factor CHD8. Mol Endocrinol. 2010;24:1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takada I, Mihara M, Suzawa M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat Cell Biol. 2007;9:1273–1285 [DOI] [PubMed] [Google Scholar]
- 50. Eeckhoute J, Oger F, Staels B, Lefebvre P. Coordinated regulation of PPARγ expression and activity through control of chromatin structure in adipogenesis and obesity. PPAR Res. 2012;2012:164140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun F, Wang J, Pan Q, et al. Characterization of function and regulation of miR-24-1 and miR-31. Biochem Biophys Res Commun. 2009;380:660–665 [DOI] [PubMed] [Google Scholar]
- 52. Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009;301:97–103 [DOI] [PubMed] [Google Scholar]
- 53. Yasui T, Matsui S, Tani A, Kunimi K, Yamamoto S, Irahara M. Androgen in postmenopausal women. J Med Invest. 2012;59:12–27 [DOI] [PubMed] [Google Scholar]
- 54. Dumesic DA, Abbott DH, Eisner JR, et al. Pituitary desensitization to gonadotropin-releasing hormone increases abdominal adiposity in hyperandrogenic anovulatory women. Fertil Steril. 1998;70:94–101 [DOI] [PubMed] [Google Scholar]