Abstract
The Peroxisome Proliferator Activated Receptor alpha (PPARα) is a transcription factor that plays a major role in metabolic regulation. This review addresses the functional role of PPARα in intermediary metabolism and provides a detailed overview of metabolic genes targeted by PPARα, with a focus on liver. A distinction is made between the impact of PPARα on metabolism upon physiological, pharmacological, and nutritional activation. Low and high throughput gene expression analyses have allowed the creation of a comprehensive map illustrating the role of PPARα as master regulator of lipid metabolism via regulation of numerous genes. The map puts PPARα at the center of a regulatory hub impacting fatty acid uptake, fatty acid activation, intracellular fatty acid binding, mitochondrial and peroxisomal fatty acid oxidation, ketogenesis, triglyceride turnover, lipid droplet biology, gluconeogenesis, and bile synthesis/secretion. In addition, PPARα governs the expression of several secreted proteins that exert local and endocrine functions.
Keywords: PPARα, Liver, Transcriptional networks, Lipid metabolism, Expression profiling, Metabolic homeostasis, Systems biology
1. Introduction
PPARα was the first member to be cloned of a small subfamily of nuclear receptors called Peroxisome Proliferators Activated Receptors [1]. The other members of this subfamily are PPARδ, also referred to as PPARβ, and PPARγ [2]. Peroxisome proliferators encompass a diverse set of synthetic compounds that cause peroxisome proliferation and liver cancer in mice. Contradicting their name, PPARδ and PPARγ are not activated by peroxisome proliferators, in contrast to PPARα. The PPAR subfamily is part of the larger family of nuclear receptors that also includes receptors for fat soluble vitamins, steroid hormones, and sterols [3]. Nuclear receptors share a conserved modular structure consisting of a N-terminal domain involved in transcriptional activation, a DNA-binding domain containing a zinc-twist structure, a short hinge region, and a relatively spacious ligand binding domain, which accommodates the lipophilic ligands and also harbors a transcriptional activation function at the far C-terminus [4].
PPARs bind to DNA as a heterodimer with the Retinoid X Receptor RXR, and together they recognize specific DNA sequences in and around target genes referred to as PPAR response elements [5]. These PPAR response elements or PPREs consist of a direct repeat of the consensus hexanucleotide AGGTCA spaced by a single nucleotide. Agonist ligands for PPARs may promote the physical association of the PPAR–RXR heterodimer to DNA, but substantial binding of PPARs to DNA already occurs in the basal state [6]. In contrast to other nuclear receptor-RXR pairs, PPAR–RXR heterodimers are “permissive”, which means that they can be activated by either an RXR-selective ligand (“rexinoid”) or a PPAR ligand [7–9]. Binding of ligand leads to the dissociation of co-repressor proteins and the association of co-activator proteins, which can recruit or have intrinsic histone deacetylase and histone acetyltransferase activity, respectively, necessary for the assembly of the transcription initiation complex [10]. Readers are referred to another review for more detailed information on co-activators in PPAR-dependent gene regulation [11].
In addition to via direct binding to PPREs, PPARs and PPAR ligands also regulate gene expression by altering the activity of other transcription factors via direct protein–protein interactions. This action of PPARs generally inhibits the function of the other transcription factor and is referred to as transrepression. Transrepression predominantly accounts for the inhibitory effect of PPARs on inflammation related genes [12].
The most abundant natural ligands for PPARs encompass different types of (dietary) fatty acids and fatty-acid derived compounds, including various eicosanoids [13]. In addition, numerous dietary plant bioactive compounds have been suggested to serve as PPAR agonist, although the in vivo relevance of PPAR activation by these compounds remains uncertain. Finally, PPARs are the molecular target of different classes of drugs used in the treatment of diabetes and dyslipidemia.
All three PPARs are expressed in a variety of tissues [14,15]. Expression of PPARγ is most restrictive, showing high expression in white and brown adipocytes, macrophages and colonocytes, with lower expression in skeletal muscle and many other tissues. PPARδ is expressed in virtually all tissues and cell types examined, while expression of PPARα is highest in liver and brown adipose tissue, followed by small intestine, heart and kidney (http://biogps.org/). This review will concentrate on PPARα.
Since its discovery, PPARα has evolved from an intracellular receptor for synthetic peroxisome proliferators into one of the key transcriptional regulators of intermediary metabolism and an intermediate in the pathogenesis of numerous diseases [16]. Although most of our knowledge on PPARα is connected to its presence in the liver, studies on PPARα in other tissues, including heart [17,18], and small intestine [19] have indicated that the role of PPARα in metabolic homeostasis is relatively well conserved between different cell types. The first part of this review addresses the role of PPARα in intermediary metabolism in liver. A distinction is made between the metabolic function of PPARα upon physiological, pharmacological, and nutritional PPARα activation.
As a transcription factor, PPARα governs biological processes by altering the expression of numerous target genes. Accordingly, the functional role of PPARα is directly related to the biological function of its target genes. In the second part of the review, a comprehensive overview is provided of metabolic genes targeted by PPARα, organized into specific metabolic pathways.
2. Regulation of intermediary metabolism by PPARα upon physiological, nutritional and pharmacological activation
2.1. Physiological PPARα activation during fasting
2.1.1. Regulation of the adaptive response to fasting by PPARα
Throughout our ancestral history, fasting and starvation were common occurrences that posed a major threat to the survival of the human species. As a consequence, humans have developed an adaptive response mechanism to fasting that relies on two main pillars: 1) generation of a strong hunger sensation that triggers food-seeking behavior and 2) a shift in fuel utilization to exploit the abundant triglyceride stores in the adipose depot. A key event during fasting is activation of adipose tissue lipolysis, contributing to a gradual shift in whole-body fuel utilization from glucose and fatty acids in the fed state to almost exclusively fatty acids after a day of fasting [20]. The shift in fuel utilization is governed by changes in the production of the metabolic hormones insulin and glucagon, as well as by altered secretion of several gut- and adipose-tissue derived hormones. Within this complex network of metabolic adjustments, the liver plays a key role through its exclusive ability to synthesize glucose and catalyze the formation of ketone bodies [20]. Ample provision of glucose and ketone bodies is necessary to meet the needs of the metabolically active brain, which unlike other tissues and organs is unable to utilize fatty acids as fuel. Research in the past two decades has shown that PPARα is a master regulator of hepatic nutrient metabolism during fasting [21–23]. Specifically, PPARα induces hepatic fatty acid oxidation and ketogenesis and regulates hepatic glucose production, which are key events in the adaptive response to fasting (Figure 1). Additionally, PPARα governs hepatic amino acid metabolism. Elucidation of the role of PPARα during fasting has benefited immensely from the availability of PPARα−/− mice, which exhibit a striking fasting-induced phenotype [21–23].
Figure 1.
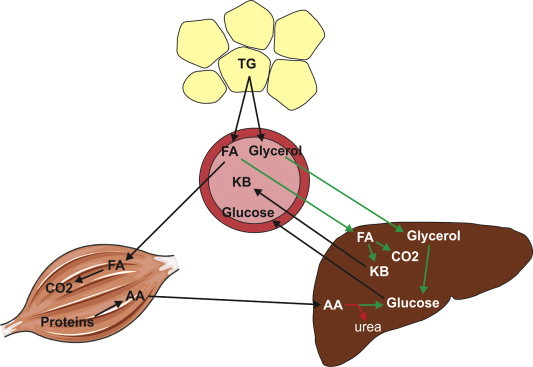
Overall role of PPARα in the adaptive response to fasting. Fasting is associated with activation of adipose tissue lipolysis, leading to the release of free fatty acids and glycerol into the circulation. Free fatty acids taken up by the liver are partially oxidized and converted into ketone bodies, or completely oxidized to CO2. Glycerol is converted into glucose, as are amino acids coming from skeletal muscle. The processes induced by PPARα are indicated by green arrows. The processes suppressed by PPARα are indicated by red arrow. TG = triglycerides, FA = fatty acids, KB = ketone bodies, AA = amino acids.
2.1.2. Regulation of hepatic lipid homeostasis during fasting by PPARα
As indicated above, a crucial event during fasting is activation of adipose tissue lipolysis, resulting in elevated circulating levels of glycerol and free fatty acids (FFA) and elevated flux of fatty acids into many tissues including liver. Increased hepatic uptake of fatty acids during fasting is associated with activation of a number of pathways, most prominently the activation of hepatic fatty acid oxidation and concomitant production of ketone bodies (Figure 2A). Metabolic data have unequivocally demonstrated the crucial role of PPARα in stimulation of hepatic fatty acid oxidation and ketogenesis during fasting [21–25]. Indeed, the increase in plasma ketone body levels during fasting is largely abolished in fasted mice lacking PPARα [26] (Figure 2B, Table 1), which is caused by blunted fasting-induced upregulation of numerous PPARα target genes involved in fatty acid oxidation (e.g. Cpt1a, Cpt2, Acadvl, Hadha) and ketogenesis (Hmgcs2, Hmgcl, Acat1). Furthermore, plasma levels of long chain acyl-carnitines are increased in fasted PPARα−/− mice, whereas plasma levels of medium and short chain acyl-carnitines and free carnitine are decreased, reflecting impaired fatty acid oxidation (Table 1) [21,27]. Decreased rates of hepatic fatty acid oxidation and ketogenesis were confirmed in primary hepatocytes of PPARα−/− mice compared with wildtype mice [28]. Due to a reduced capacity for fatty acid oxidation, a large share of the incoming fatty acids is diverted towards triglyceride synthesis via a pathway that does not seem to be noticeably affected by PPARα deletion. Consequently, liver triglyceride levels are markedly elevated in fasted PPARα−/− mice compared with fasted wildtype mice [21,22,26] (Figure 2, Table 1). In addition, plasma and liver concentrations of FFA are elevated in fasted PPARα−/− mice because of impaired fatty acid oxidation [22,26,29]. For reasons that are unclear, plasma triglyceride levels are elevated in PPARα−/− mice only in the fed but not fasted state [21,26]. An overview of the metabolic changes in plasma and liver of PPARα−/− mice is provided in Table 1. The markedly disturbed response to fasting in PPARα−/− mice thus illustrates the profound importance of PPARα in governing hepatic lipid metabolism during fasting.
Figure 2.
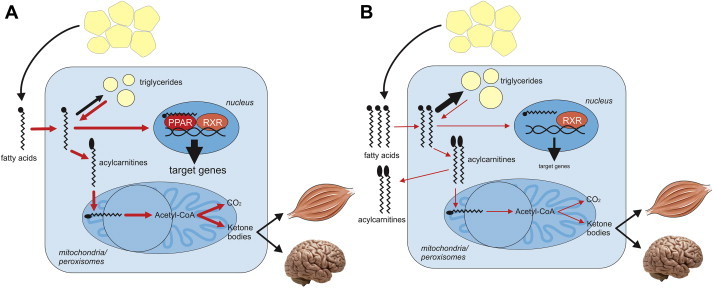
Role of PPARα in hepatic lipid metabolism. Free fatty acids released by adipose tissue lipolysis are taken up by hepatocytes and either converted into triglycerides or, after conversion to acyl-carnitine, are oxidized in the mitochondria. The metabolic steps or pathways under transcriptional control of PPARα are indicated in red. A) The normal situation in fasted wildtype mice. B) Consequences of PPARα deletion on metabolic pathways and specific intra- and extracellular metabolites in the fasted state.
Table 1.
Metabolites changes in PPARα−/− mice.
| Intracellular | |
|---|---|
| Increased | Decreased |
| Triglycerides | Short chain acyl-carnitines |
| Long chain acyl-carnitines | Free carnitine |
| Free fatty acids | Glucose |
| Glycogen (fasted state) | Glycogen |
| Blood plasma | |
|---|---|
| Increased | Decreased |
| Triglycerides (in fed state) | Short chain acyl-carnitines |
| Long chain acyl-carnitines | Free carnitine |
| Free fatty acids | Ketone bodies |
| Urea | Glucose |
| Arginine | |
| Tyrosine | |
| Alanine | |
2.1.3. Regulation of hepatic glucose homeostasis during fasting by PPARα
Besides leading to dramatic alterations in lipid metabolism, deletion or knock-down of PPARα also causes severe fasting-induced hypoglycemia and reduces intra-hepatic glucose levels, indicating a major impact of PPARα on glucose homeostasis [21–23,29–31]. Several explanations for the hypoglycemia have been put forward. First, hepatic glycogen level are lower in (re)-fed PPARα−/− mice [22,29,31–33], likely leading to reduced glycogen breakdown and glucose release during early fasting. Metabolic changes detected in the liver of PPARα−/− mice are consistent with reduced glucose storage and glucose production by gluconeogenesis (Table 1) [29]. The lower glycogen levels may be due to reduced expression of Gys2, leading to a diminished rate of glycogen formation upon (re)feeding [25,31,32]. Alternatively, it was suggested that impaired fatty acid oxidation and resultant ATP and NADH shortage in livers of PPARα−/− mice may disable gluconeogenesis [22]. However, the stimulatory effect of fatty acid oxidation on gluconeogenesis appears to be minimal [34]. Another potential explanation for the fasting hypoglycemia in PPARα−/− mice is decreased hepatic gluconeogenesis from glycerol, uptake and conversion of which is under direct control of PPARα [24]. However, tracer studies have not supported this notion. One tracer study found that total hepatic glucose production and hepatic glucose production from glycerol were increased in PPARα−/− mice in the fasted state, whereas hepatic glucose production from lactate was reduced [31]. Another study reported that after a short-term fast the gluconeogenic flux in PPARα−/− mice is directed more towards glycogen, leading to a decrease in hepatic glucose output [32]. Despite the ambiguity emerging from tracer studies, the observation that the fasting-induced increase in expression of several genes involved in the gluconeogenic pathway (Pcx, Fbp1, Gpd1, Gyk, Slc16a1), many of which represent PPARα target genes, is abolished in PPARα−/− mice provides strong evidence that gluconeogenesis is directly targeted by PPARα [35]. This notion is further supported be reduced rates of glucose synthesis from lactate/pyruvate in primary hepatocytes from PPARα−/− mice compared to wildtype mice [28]. Other gluconeogenic genes that have been reported to be reduced in PPARα−/− mice include G6pc (glucose 6 phosphatase) and Pck1 (phosphoenolpyruvate carboxykinase) [31,32,36]. However, others have not been able to confirm these findings.
Finally, apart from changes in glucose production, there is ample evidence that the fasting hypoglycemia is at least partially caused by elevated peripheral glucose utilization, likely as a result of impaired fatty acid oxidation and a reduced supply of ketone bodies [36,37].
2.1.4. Regulation of hepatic amino acid metabolism during fasting by PPARα
PPARα not only is the master regulator of hepatic lipid and glucose homeostasis but also has a profound impact on amino acid metabolism. Specifically, PPARα downregulates the expression of numerous enzymes involved in amino acid degradation, transamination and the urea cycle (Got1, Prodh, Gls2, Pah, Sds, Asl, Ass1, Cps1) [35,38]. In agreement with enhanced amino acid degradation and urea synthesis, plasma urea levels are elevated in PPARα−/− mice, whereas plasma levels of several amino acids (arginine, tyrosine, alanine) are reduced [27,38]. These data demonstrate that during fasting PPARα minimizes amino acid degradation and ureagenesis, which together with induction of fatty acid oxidation is part of an adaptive mechanism aimed at relying maximally on stored triglycerides as fuel, as opposed to breaking down valuable muscle tissue and oxidizing amino acids [27]. The molecular mechanisms underlying downregulation of amino acid metabolism by PPARα remain elusive.
2.1.5. Mechanism of activation of PPARα during fasting
Induction of most of the above described genes by fasting is dependent on PPARα, which suggests that PPARα in liver is activated during fasting. Activation of PPARα during fasting is supported by experiments using in vivo PPAR-reporter mice [39]. Since fatty acids are ligands for PPARα [40], and plasma FFA increase during fasting, it has been tempting to attribute the activation of hepatic PPARα during fasting to increased plasma FFA uptake into the liver [22]. However, studies suggest that plasma FFA cannot activate PPARα in liver [41,42]. An alternative mechanism for PPARα activation during fasting is via induction of PPARα mRNA [22]. In line with major regulation at the mRNA level, PPARα mRNA follows a pronounced circadian rhythm that is inversely related to feeding status [43,44]. In addition, circadian transcription of genes encoding acyl-CoA thioesterases may lead to rhythmic changes in the intracellular level of fatty acid ligands [44]. Other potential mechanisms for PPARα activation during fasting include induction of co-activator proteins such as PGC1α [42], and intracellular generation of PPARα ligands via lipolysis of locally stored triglycerides [45,46]. Recently, evidence was provided that induction of the PGC1α–PPARα—target genes axis during fasting in liver is mediated by the basic helix–loop–helix (bHLH) leucine zipper transcription factor TFEB, which itself is induced by fasting [47].
2.2. Pharmacological and toxicological PPARα activation
2.2.1. Fibrates are high affinity PPARα activators
Besides binding a range of endogenous ligands, PPARα also serves as receptor for a diverse array of synthetic compounds collectively referred to as peroxisome proliferators that include phthalates, insecticides, herbicides, surfactants, organic solvents, and hypolipidemic fibrate drugs [1,48]. The latter group encompasses the compounds Wy14643, fenofibrate, clofibrate, ciprofibrate, bexafibrate and gemfibrozil. Synthetic PPARα agonists cause hepatomegaly and peroxisome proliferation in mice but not in humans [49] (Table 2). In humans, fibrates raise plasma HDL and reduce plasma triglycerides and therefore carry therapeutic value in the treatment of dyslipidemia [50]. Accordingly, most of the literature on fibrates is related to their clinical and pre-clinical effects on circulating cardiovascular disease risk factors and lipid metabolic parameters [51]. Chronic administration of peroxisome proliferators to rodents leads to hepatic carcinogenesis via PPARα [52], which has led to concern about the industrial use of peroxisome proliferators and the potential carcinogenicity of these compounds in humans [53].
Table 2.
Differences in PPARα biology between rodents and humans
| Feature | Rodent | Human |
|---|---|---|
| Peroxisome proliferation in response to PPARα agonists | + | − |
| Hepatomegaly in response to PPARα agonists | + | − |
| Plasma TG lowering by PPARα agonists | + | + |
| Effect PPARα agonist on liver transaminase | ↓ | ↑ |
| Highest expression of PPARα | Liver, heart, brown adipose tissue, heart | Liver, heart, brown adipose tissue?, small intestine |
| Robust common target genes | Cpt1a, Fgf21, Acox1 Hmgcs2, Angptl4 |
CPT1A. FGF21, ACOX1 HMGCS2, ANGPTL4 |
| Mouse-specific targets | Mgll, Bbox1, Decr1, Fbp2 | |
| Human-specific targets | PCK1, MBL2, APOA2 |
2.2.2. Rexinoids activate PPARα–RXR heterodimers
Consistent with PPARα–RXR functioning as a permissive heterodimer, RXR activation using the synthetic rexinoid LG1069 leads to upregulation of a large number of PPARα target genes in liver and heart [54,55], a response that is abolished in mice lacking PPARα [56,57]. Comparison of the effect of LG1069 and WY14643 in rodent liver reveals a marked resemblance in gene regulation by the two compounds, with many genes being induced by both agonists, including well established PPARα targets such as Acot1, Acaa1, Ehhadh, Cpt2 and Plin2 [35,55]. By contrast, we found that LG1069 activates a distinct set of genes in primary human hepatocytes as compared with fenofibrate and WY14643, with minimal overlap (our unpublished data).
2.2.3. Regulation of hepatic lipid homeostasis by synthetic PPARα agonists
Numerous in vitro studies in cultured hepatocytes have demonstrated a stimulatory effect of synthetic PPARα agonists on peroxisomal and mitochondrial fatty acid oxidation [58–61]. Similarly, treatment of rodents with synthetic PPARα agonists markedly induces peroxisomal and mitochondrial fatty acid oxidation in liver homogenates and isolated hepatocytes, concurrent with an increase in size and number of peroxisomes, as well as increased activity of fatty acid oxidative enzymes such as carnitine palmitoyl-transferase, 2,4-dienoyl-CoA reductase, peroxisomal 3-ketoacyl-CoA thiolase, and acyl-CoA dehydrogenase [61–69]. Induction of fatty acid oxidative enzyme activity by PPARα agonists is paralleled by marked upregulation of the corresponding genes Cpt1a, Decr2, Acaa1a, and Acadvl [35,70–72], as well as numerous other genes participating in fatty acid oxidation. The stimulatory effect of in vivo fibrate treatment on fatty acid oxidation and oxidative enzymes can be detected in isolated mitochondria and peroxisomes [73–76]. Besides mitochondrial and peroxisomal fatty acid oxidation, fibrates also stimulate hepatic microsomal omega-hydroxylation of fatty acids [75,77–79]. In addition, fibrates markedly induce ketogenesis in cultured hepatocytes and rodent livers [68,73,80–82].
Stimulation of fatty acid oxidation likely accounts for the beneficial effect of synthetic PPARα agonists on fatty liver in rats and mice [83–91], and may contribute to lowering of VLDL-triglyceride secretion [83], giving rise to their hypotriglyceridemic effect. It should be mentioned that in humans synthetic PPARα agonists primarily lower circulating triglycerides by stimulating plasma triglyceride clearance [83,92].
Intriguingly, pharmacological PPARα activation also stimulates hepatic de novo lipogenesis and chain elongation, as determined using stable isotopes [93]. Induction of lipogenesis by (chronic) fenofibrate treatment depends on sterol regulatory element-binding protein 1c (SREBP-1c) but not carbohydrate response element-binding protein (ChREBP) [93]. In contrast to de novo lipogenesis, which is thus regulated via an indirect mechanism, several genes involved in fatty acid elongation are under direct transcriptional control of PPARα (see below).
2.2.4. Regulation of hepatic glucose homeostasis by synthetic PPARα agonists
There is limited evidence linking pharmacological PPARα activation to regulation of glucose homeostasis. Whereas clofibrate lowers fasting glucose and insulin level and improves glucose tolerance and utilization in rodents [86,94–96] and humans [97–100], indicating enhancement of insulin sensitivity, fenofibrate does not have any effect on insulin sensitivity in humans [92,101–104]. It can thus be argued that the effect of clofibrate may be independent of PPARα activation. Alternatively, the differential impact of clofibrate and fenofibrate on glucose homeostasis may be linked to selective PPAR modulation (SPPARM), based on the notion that different PPAR agonists may induce differential gene expression patterns via selective receptor–coregulator interactions.
At the intracellular level, fenofibrate treatment was found to reduce the contribution of glycolysis to acetyl-CoA production [93], which may be mediated by PPARα-mediated induction of pyruvate dehydrogenase kinase 4 (Pdk4) [105]. Pdk4 phosphorylates and inactivates pyruvate dehydrogenase, thereby limiting carbon flux through glycolysis. Additional information about the impact of pharmacological PPARα activation on intracellular glucose metabolism in liver and relevant mechanisms is lacking.
2.2.5. Regulation of hepatic amino acid metabolism by synthetic PPARα agonists
Pharmacological PPARα activation has a significant influence on amino acid metabolism. Specifically, hepatic gene expression of numerous enzymes involved in amino acid metabolism and urea synthesis is markedly decreased upon treatment of rodents with the PPARα agonists Wy14643 or perfluorooctanoic acid (PFOA), including Got1, Asl, Ass1, Gls2, and Otc [38,106]. Downregulation of the corresponding proteins by synthetic PPARα agonists in mice [107], rats [108], and dogs [109], is supported by proteomics analysis. In agreement with impaired ureagenesis, plasma ammonia levels were elevated in rats fed Wy14643 [110]. For reasons that are unclear, plasma urea levels were increased by Wy14643 as well [110].
In contrast to what is observed in rodents, PPARα agonists directly upregulate expression of aspartate aminotransferase (Got1) and alanine aminotransferase (Gpt) in human primary hepatocytes and human HepG2 hepatoma cells (Table 2) [111–113], which may explain the elevated serum aspartate and alanine aminotransferase activities in PPARα agonists-treated subjects that do not show further signs of liver injury. In fact, there is evidence that Gpt is a direct PPARα target in human hepatocytes [112]. So far, the molecular basis for differential regulation of aminotransferases between mouse and human has remained elusive.
2.3. Nutritional PPARα activation
2.3.1. Fatty acids and various fatty acid derivatives serve as PPARα agonists
Several studies have demonstrated that PPARα is able to bind fatty acids with a general preference for long-chain poly-unsaturated fatty acids (PUFAs) [40,48,114–120]. Furthermore, numerous fatty acid-derived compounds and compounds showing a structural resemblance to fatty acids, including oxidized fatty acids, eicosanoids, endocannabinoids, and phytanic acid, are able to activate PPARα [121–126]. In addition, biochemical studies indicate that fatty acyl-CoAs can bind PPARα. Interestingly, it was found that they oppose the effects of WY14643 and fatty acids on PPARα conformation, DNA binding, and co-activator interaction, suggesting fatty acyl-CoAs serve as PPARα antagonists. However, the in vivo relevance of acyl-CoAs as PPARα ligands remains uncertain [125,127,128]. The combined evidence strongly suggests that PPARα serves as general fatty acid sensor with a limited degree of ligand selectively. In addition, there are reports postulating that PPARα may be activated by specific phospholipid species, including the phosphatidylcholines PC(16:0/18:1) or PC(18:0/18:1) [129,130]. Because phosphatidylcholines are abundant in any cell, and likely fluctuate very little, it is unclear how activation of PPARα by phosphatidylcholines fits into the notion of PPARα being a metabolic sensor.
Activation of PPARα by fatty acids and fatty acid derivatives not only depends on their absolute concentration in the cell but also by the abundance of fatty acid-binding proteins such as FABP1. FABP1 was found to transfer fatty acids to the nucleus and co-localize with PPARα [131,132]. Subsequent studies revealed that FABP1 physically interacts with PPARα and enhances PPARα-dependent gene regulation [133–135].
2.3.2. Activation of PPARα by high fat ketogenic diets
Studies using in vivo PPAR-reporter mice have suggested that PPAR is activated by chronic high fat feeding [39]. Gene expression analysis of PPARα target genes has confirmed modest activation of PPARα in response to high fat feeding [136–140]. A complicating factor is that chronic high fat feeding also invariably causes hepatic steatosis, which is associated with induction of PPARγ and may therefore lead to induction of PPAR target genes via PPARγ [137,141–143]. An extreme form of high fat diet that potently induces expression of PPARα target genes in liver, including Fgf21, Cd36, Pdk4, Acadm, and Pex11a, is the ketogenic diet [144,145]. A ketogenic diet is almost entirely devoid of carbohydrate and elicits a metabolic state of low insulin, high plasma free fatty acids, and enhanced ketogenesis, that resembles fasting. Similar to what is observed during fasting, the metabolic phenotype of PPARα−/− mice becomes much more prominent upon feeding a ketogenic diet, illustrated by pronounced hepatic steatosis, hypoglycemia and elevated plasma FFA [144]. Overall, the extent of PPARα activation by high fat feeding will likely depend on three main variables: the amount of fat in the diet in comparison with the control diet, the amount of sucrose in the diet in comparison with the control diet, and the fatty acid composition of the diet. The lower the amount of sucrose and the higher the proportion of poly-unsaturated fatty acids, the stronger the activation of PPARα.
2.3.3. Preferential activation of PPARα by long chain poly-unsaturated fatty acids
As indicated above, diets rich in poly-unsaturated fatty acids lead to more pronounced PPARα activation in liver compared to diets rich in saturated or mono-unsaturated fatty acids, as determined by measurement of enzyme activity or mRNA expression of target genes. Strongest activation of PPARα is observed when feeding fish oil [90,146–150]. The potency of fish oil towards PPARα is corroborated by oral lipid loading experiments in which mice received a single bolus of triglyceride composed of one single fatty acid [119]. A single oral fat load, which mimics a high fat meal low in carbohydrate, markedly and very specifically activates PPARα in liver, with docosahexanoic acid being the most potent fatty acid, followed by linolenic acid, linoleic acid and oleic acid. The dietary fatty acids mostly copy the effects of high affinity synthetic PPARα agonists, except that they are much less potent [119]. These findings demonstrate that dietary fatty acids are able to activate hepatic PPARα after their delivery to the liver as part of chylomicron remnants.
The large hydrophobic binding cavity allows PPARs to interact with a variety of structurally related and unrelated compounds [151]. Indeed, apart from fatty acids, numerous other dietary components have been proposed to serve as PPARα agonists, including but not limited to the flavanoid cyanidin [152], the carotenoid astaxanthin [153], the plant triterpenoid ursolic acid [154], the plant stilbenoid pterostilbene [155], isohumulones in hops [156], soy isoflavones [157], and conjugated linoleic acid [158]. However, it is highly questionable whether intake of any of these compounds is high enough to lead to PPARα activation in vivo, especially taking into account the abundance of fatty acids in our diet and in the cell.
3. Target genes and pathways of PPARα
3.1. Overview of PPARα-mediated gene regulation
3.1.1. Pharmacological versus physiological PPARα target genes
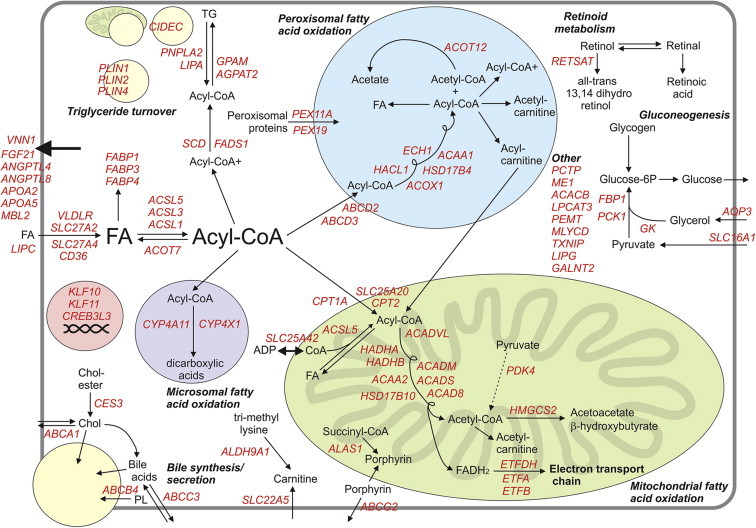
PPARα governs biological processes by altering the expression of a large number of target genes. Gene expression profiling studies have indicated that PPARα target genes number in the hundreds [35,119,159], which is similar to other liver-enriched nuclear receptors such as LXR [160]. Accordingly, the functional role of PPARα is directly related to the biological function of its target genes. However, it should be realized that the influence of PPARα on gene regulation is dependent on whether PPARα is activated pharmacologically, physiologically, or nutritionally. Fibrates not only bind to PPARα more avidly compared with fatty acids [48,161], but likely also cause a slightly different conformational change in the PPARα protein as dictated by the SPPARM concept [119], together leading to slightly altered and generally more robust induction of target genes by fibrates. Furthermore, the endocrine profile and thereby the general chromatin and transcriptional landscape supporting PPARα-dependent gene regulation can be different depending on feeding status. Nevertheless, for the most part the genes induced by fibrates are the same genes that are reduced in PPARα−/− mice in the fasted state [35] (Figure 3). The similarity in gene regulation by PPARα upon pharmacological and physiological activation is well illustrated by gene set enrichment analysis (GSEA), showing large overlap in enriched gene sets between the two conditions (Figure 4). Major exceptions are genes involved in bile synthesis and secretion, many of which are markedly downregulated in PPARα−/− mice in the fasted state yet are not induced by fibrates (Figure 3). The same is true for several genes involved in retinoid metabolism. The exclusive regulation of retinol metabolism and bile secretion by PPARα during fasting is confirmed by GSEA (Figure 4).
Figure 3.
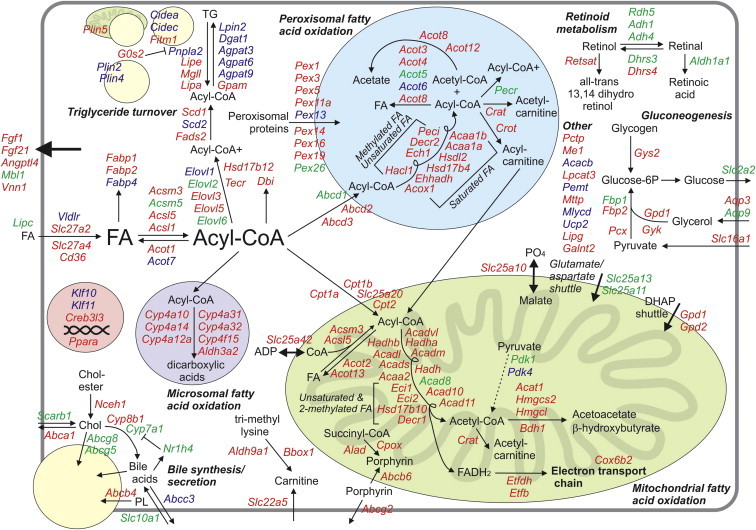
Detailed overview map of metabolic genes upregulated by PPARα in mouse liver. The map is based on the published literature combined with transcriptomics analysis of liver of wildtype and PPARα−/− mice. Colors indicate whether expression of a gene is governed by PPARα during fasting (In green: significantly decreased expression in fasted PPARα−/− mice compared with fasted wildtype mice, representing physiological regulation), and/or by synthetic PPARα agonists (In blue: significantly increased expression in wildtype mice treated with Wy14643 or fenofibrate compared with vehicle-treated wildtype mice, representing pharmacological/toxicological regulation). Genes shown in red are upregulated by PPARα during fasting and are upregulated by Wy14643/fenofibrate. Genes were excluded if they were only upregulated upon chronic administration of Wy14643/fenofibrate, which likely reflects indirect regulation. Genes in this category included Mecr, Fads1, and Lpl.
Figure 4.
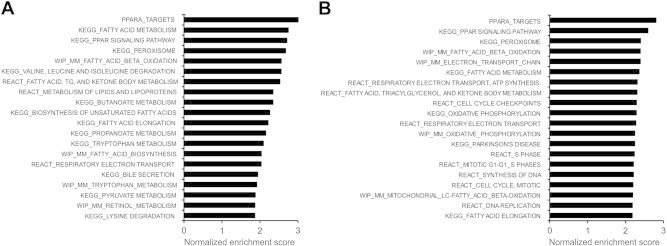
Pathways upregulated by PPARα during fasting or by Wy14643. Gene set enrichment analysis was performed on transcriptomics data comparing livers from 24 h fasted PPARα+/+ and 24 h fasted PPARα−/− mice (A), or livers from wildtype mice treated with Wy14643 or treated with vehicle for 5 days (B). The top 20 most significant positively enriched (upregulated) gene sets are shown ranked according to normalized enrichment score.
It is possible that this pattern of gene regulation may reflect indirect regulation, e.g. genes that are reduced in fasted PPARα−/− mice but that are not induced by synthetic PPARα agonists may not be direct targets of PPARα. The opposite is also observed: a number of genes are significantly induced by fibrates but are not decreased in PPARα−/− mice in the fasted state [42]. Several potential explanations for this type of regulation can be put forward. A) Induction of the gene by fibrates reflects regulation in Kupffer cells [162]. In contrast to hepatocytes, PPARα in Kupffer cells is most likely not activated by fasting. For example, LPL is specifically expressed in Kupffer cells and is markedly induced by fibrates, yet expression is unchanged by PPARα deletion in the fasted state. B) The elevated plasma FFA levels in fasted PPARα−/− mice lead to induction of other transcription factors, such as PPARδ, obscuring a potential role of PPARα in regulation of these genes in the fasted state [42]. Genes in this category include Lpin2, Plin3, and Pdk4. C) As mentioned above, high affinity activation of PPARα may cause differential recruitment of co-factors (SPPARM) compared with physiological PPARα activation, resulting in subtle differences in gene regulation. D) Certain genes are relatively weak targets of PPARα characterized by low affinity PPREs and therefore require a fully activated PPARα to show any mRNA induction.
3.1.2. Detailed map of PPARα-mediated gene regulation
Gene expression analysis of livers of PPARα−/− mice at the level of individual genes or the entire genome via transcriptomics has indicated that PPARα governs many aspects of hepatic lipid metabolism, providing a molecular basis for the pronounced metabolic disturbances in fasted PPARα−/− mice and explaining the marked impact of synthetic PPARα agonist on lipid metabolism [163,164]. To capture the scope of PPARα activity in liver and to illustrate the role of PPARα in transcriptional regulation of liver (lipid) metabolism, a detailed regulatory map was created of metabolic genes upregulated by PPARα, separated according to metabolic pathway (Figure 3). A distinction is made between genes regulated by PPARα during fasting (in green), by synthetic PPARα agonist (in blue), or both (in red). Many of the genes shown in Figure 3 and described in the text below are direct PPARα target genes based on the presence of a functional PPRE, as determined by transactivation assay, gel shift, and/or genomic DNA binding by chromatin immunoprecipitation. However, this criteria was not applied very strictly was assigning PPARα target gene status, under the assumption that genes showing strong functional resemblance to known PPARα targets and that are significantly upregulated upon PPARα activation and/or significantly downregulated upon PPARα deletion most likely represent direct target genes, despite the fact that a functional PPRE has not (yet) been located. Genes in that category include Acadvl, Hadha, and Etfdh. Because of these relatively loose criteria, it cannot be excluded that some of the genes shown are indirectly regulated by PPARα. Indeed, several putative targets of PPARα are also regulated by CREB3L3, a transcription factor that is under strong transcriptional control of PPARα, including Fgf21, Gys2, Elovl2, Elovl5, Bdh1, G0s2, Mgll, Decr2, and Cidec [159,165]. Some of these genes have previously been identified as direct PPARα targets based on the presence of a functional PPRE, including Fgf21, Gys2, and G0s2 [33,166–168], suggesting possible dual regulation by PPARα and CREB3L3. In fact, recent data suggest that CREB3L3 and PPARα form a transcriptional complex, which binds to an integrated CRE–PPRE binding motif in the FGF21 gene promoter [169]. Whether CREB3L3 is more broadly involved in target gene regulation by PPARα is a very intriguing question that deserves further exploration. Other transcription factors that cooperate with PPARα or are under transcriptional control of PPARα, and thus may mediate effects of PPARα on gene expression include KLF11 (Cpt1a, Cyp4a10, Cyp4a14, Acadm) and KLF10 (Cyp4a14, Gpam, Acot3, Retsat) [159,170,171].
In the above situations, PPARα indirectly upregulates gene expression without binding to a canonical PPRE. Other PPRE-independent mechanisms of gene regulation by PPARα include physical interaction with other transcription factors, which can either be stimulatory or inhibitory (transrepression), and physical interactions with co-activators leading to reduced availability of those co-activators for other transcription factor pathways (squelching, inhibitory) [172].
3.1.3. PPARα cistrome
The PPARα cistrome is defined as the in vivo genome-wide location of PPARα binding-sites, as determined using ChIP-SEQ and ChIP-on-CHIP. Interestingly, these analyses have found relatively little overlap between induction of gene expression by the PPARα agonist GW7647 and the GW7647-induced binding of PPARα to a PPRE in the proximity of the gene, at least in primary human hepatocytes and HepG2 cells [173,174]. These data suggest significant uncoupling between gene induction by PPARα and the binding of PPARα to the gene, thus pointing at other mechanisms of gene (up)regulation by PPARα. However, this conclusion may be partially confounded by the likely flawed assumption that binding of ligand enhances PPARα binding to DNA and that PPAR binding sites are located within a certain distance of the transcriptional start site. Profiling of genomic binding sites of PPARα in human HepG2 cells by ChIP-on-CHIP analysis raised the possibility that PPARα may be recruited to certain gene promoters via direct physical interaction with other transcription factors [174]. In agreement with this notion, ChIP-SEQ revealed extensive overlap in genomic binding sites of LXR and PPARα [175]. The consequences of the LXR and PPARα overlap with respect to gene regulation seem to be determined by the context of the genomic binding site(s) and the activity of the individual receptors at the particular sites, suggesting that cross talk is context dependent and probably not due to competition for RXR [175].
Despite the overall usefulness of information on PPARα cistrome towards enhancing our understanding of the determinants of genomic binding by PPARα, its value for specific gene regulation by PPARα and assigning PPARα target gene status has so far been limited.
3.2. Target genes of PPARα in hepatic lipid metabolism
3.2.1. Fatty acid uptake, binding and activation
The mechanism of fatty acid uptake into hepatocytes remains poorly defined but a (partial) role for the fatty acid translocase Cd36 is likely. Cd36 is upregulated by PPARα [176], as are the putative fatty acid transporters Fatp2 and Fatp4 [35,176,177]. Because they are localized to the ER and thus cannot mediate membrane fatty acid transport directly, Fatp2 and Fatp4 seem to stimulate fatty acid uptake into hepatocytes indirectly via their acyl-CoA synthetase activity [178]. Acyl-Co synthetase activity is necessary to convert fatty acids to their acyl-CoA derivatives via thio-esterification, which is an essential step for directing fatty acids towards specific metabolic pathways. Several cytosolic acyl-Co synthetases are under transcriptional control of PPARα, including Acsl1 and Acsl5 [35,179–181]. Interestingly, expression of the cytosolic Acyl-coenzyme A thio-esterases Acot1 and Acot7, which catalyze the reverse reaction and together with acyl-Co synthetases determine the balance between cytoplasmic concentrations of acyl-CoAs, free CoA and fatty acids, is also upregulated by PPARα [182].
The FABP gene family comprises a group of high-affinity intracellular fatty acid-binding proteins. Fabp1 (L-FABP) was one of the first PPARα target genes identified [183–185], and is highly abundant in liver. Fabp1 is likely involved in partitioning of fatty acids to specific lipid metabolic pathways [186]. Other Fabp genes induced by PPARα activation in mouse liver include Fabp2 (I-FABP) and Fabp4 (A-FABP, aP2), whose role in liver is uncertain.
3.2.2. Peroxisomal fatty acid oxidation
The first identified target gene of PPARα was Acyl-CoA oxidase (Acox1), which encodes the first enzyme in peroxisomal long-chain fatty acid oxidation [2,5]. Peroxisomes are involved in many lipid metabolic pathways, including synthesis of bile acids and plasmalogens, synthesis of cholesterol and isoprenoids, alpha-oxidation, glyoxylate and H2O2 metabolism, and β-oxidation of very-long-straight-chain or branched-chain acyl-CoAs. Many aspects of peroxisomal fatty acid metabolism are under transcriptional control of PPARα including peroxisomal fatty acid uptake (Abcd1, Abcd2 and Abcd3) [187–189], conversion of acyl-CoA/acetyl-CoA to acyl-carnitine/acetyl-carnitine (Crot/Crat) [35], and conversion of acyl-CoAs back to fatty acids via numerous thioesterases (Acots) [35,190]. Thioesterase and acylcarnitine transferase activities are likely required for transporting fatty acids of various chain length out of the peroxisomes for further oxidation in mitochondria. Apart from Acox1, the enzymes operating downstream from Acox1 in peroxisomal β-oxidation of acyl-CoAs are also all target genes of PPARα. These enzymes carry enoyl-CoA-hydratase and 3-hydroxyacyl-CoA dehydrogenase activity (L-bifunctional enzyme, Ehhadh; D-bifunctional enzyme, Hsd17b4), and peroxisomal 3-ketoacyl-CoA thiolase activity (Acaa1a, Acaa1b) [179,191–193]. Finally, expression of numerous genes involved in import of peroxisomal proteins and thus peroxisome assembly, referred to as the Pex family, are transcriptionally regulated by PPARα [159,194]. Induction of the above mentioned genes accounts for the massive proliferation of peroxisomes observed in rodents exposed to synthetic PPARα agonists.
3.2.3. Mitochondrial fatty acid oxidation
Almost every single enzymatic step in mitochondrial uptake and subsequent oxidative breakdown of acyl-CoAs to acetyl-CoA is regulated by PPARα. Specifically, PPARα stimulates acyl-CoA import into the mitochondria by upregulating expression of carnitine palmitoyl-transferases 1a, 1b and 2 (Cpt1a, Cpt1b, Cpt2), and the acyl-carnitine translocase (Slc25a20) [195,196]. Furthermore, PPARα upregulates expression of genes involved in cellular uptake and biosynthesis of carnitine (Aldh9a1, Bbox1, Slc22a5) [35,197,198]. Decreased expression of carnitine biosynthetic genes explains the reduced total carnitine levels in plasma and liver of PPARα−/− mice [27].
In the first step of β-oxidation, acyl-CoA is dehydrogenated in a PPARα-induced reaction catalyzed by a family of four closely related and chain length-specific enzymes, the acyl-CoA dehydrogenases (Acadvl, Acadl, Acadm, Acads) [179,199]. The three subsequent steps in β-oxidation leading to chain shortening and release of acetyl-CoA are catalyzed by the mitochondrial trifunctional enzyme (Hadha and Hadhb). Upon progressive chain shortening, two other enzymes (Hadh, Acaa2) take over the function of trifunctional enzyme, both of which are PPARα targets, as are the enzymes required to convert unsaturated and 2-methylated acyl-CoAs into intermediates of β-oxidation (Eci1, Eci2, Decr1, Hsd17b10) [119,179]. Other highly suspected PPARα target genes linked to fat oxidation include a set of poorly characterized acyl-CoA dehydrogenases (Acad8, Acad10 and Acad11), and electron transfer flavoprotein beta (Etfb) and electron transfer flavoprotein dehydrogenase (Etfdh), which shuttle electrons from acyl-CoA dehydrogenases to the electron transport chain [35,200].
Under certain metabolic conditions such as fasting, excess acetyl-CoA generated via β-oxidation feeds into ketogenesis, a pathway reliant on four enzymes (Acat1, Hmgcs2, Hmgcl, Bdh1). All are transcriptional targets of PPARα, with Hmgcs2 as the best described representative [201].
3.2.4. Microsomal fatty acid oxidation
Cytochrome P450 monoxygenase (CYP4A) enzymes involved in microsomal ω-hydroxylation of fatty acids are among the most sensitive target genes of PPARα [202,203]. Studies using PPARα−/− mice have shown that hepatic expression of Cyp4a genes is almost completely abolished in the absence of PPARα (Cyp4a10, Cyp4a12, Cyp4a14 in mice, Cyp4a11 in human) [137]. The Cyp4A family members exhibit highest activity for lauric acid, with activity decreasing with increasing fatty acid chain length, whereas the Cyp4F family is active towards eicosanoids and xenobiotics [204].
3.2.5. Lipid storage and hydrolysis
Activation of fatty acid oxidation by PPARα is well established but the stimulatory effect of PPARα on several other lipid metabolic pathways is less well recognized. Compelling evidence from microarrays indicates that pharmacological and/or physiological activation of PPARα leads to upregulation of numerous genes involved in fatty acid elongation (Elovl family) and desaturation (Scd1) [35,205]. In addition, PPARα induces expression of a number of enzymes in the triglyceride biosynthesis pathway (Gpam, Lpin2, Dgat1), albeit primarily upon pharmacological activation of the receptor [35,42]. Concurrent with induction of triglyceride synthesis, enzymes required for intracellular triglyceride hydrolysis are upregulated by PPARα as well, including monoglyceride lipase Mgll, the triglyceride lipase Pnpla2 and the Pnpla2 inhibitor G0s2 [35,168,206,207].
Finally, several proteins that are physically and functionally associated with lipid droplets are direct targets of PPARα in liver. These include Plin2 [208,209], Plin4 [42,210], Plin5 [211,212], Fitm1 [213], Cidea [214], and Cidec [35,173,215]. Most of the genes encoding lipid-droplet associated proteins are specifically induced by pharmacological PPARα activation and show no regulation by PPARα during fasting. Detailed insight into the biochemical role of the various lipid-droplet associated proteins is currently lacking, rendering assessment of the functional impact of their regulation by PPARα challenging.
It is well recognized that loss of PPARα worsens hepatic steatosis, particularly during fasting and high fat feeding [21,22,216,217], whereas PPARα activation reduces steatosis [83–91]. In general, it is difficult to tie the diverse effects of PPARα on lipases, lipid droplet associated proteins, elongases, and enzymes involved in triglyceride synthesis together into a coherent functional model that aligns with the PPARα-induced changes in intracellular lipid storage. Furthermore, the overarching physiological rationale for activation of these pathways by PPARα remains ambiguous. It can be hypothesized that induction of genes involved in triglyceride synthesis such as Gpam by PPARα during fasting may reflect a broader role of PPARα in metabolizing and neutralizing large amounts of incoming adipose tissue-derived free fatty acids.
3.2.6. Bile synthesis and secretion
Numerous genes involved in bile synthesis and secretion are reduced in fasted PPARα−/− mice compared with fasted wildtype mice. This includes genes involved in phospholipid synthesis (Lpcat3) [218,219], secretion of cholesterol and phospholipids into bile (Abcg5, Abcg8, Abcb4) [35,220,221], genes mediating bile acid uptake (Slc10a1 = Ntcp) and excretion (Abcc3 = Mrp3), and genes promoting bile acid synthesis (Cyp7a1, Cyp8b1, Nr1h4 = Fxr) [168,220,222]. Furthermore, PPARα regulates cholesterol uptake and export via Scarb1 and Abca1, respectively [220,223]. Most of these genes are uniquely regulated by PPARα during fasting, with the exception of Nceh1, Abca1 and Abcb4, which are also induced upon pharmacological PPARα activation. Despite being a direct PPARα target gene in macrophages [224,225], Scarb1 protein and mRNA levels are significantly decreased by fibrates in liver [226].
Expression of Cyp7a1, encoding the purported rate-limiting enzyme in bile acid synthesis, is markedly downregulated in PPARα−/− mice during fasting [222]. Paradoxically, Cyp7a1 mRNA and activity are also markedly reduced by synthetic PPARα agonists in rodents and humans [227–232]. Consistent with an important role of PPARα in the regulation of many genes implicated in bile acid metabolism, plasma and liver levels of bile acids are elevated in PPARα−/− mice when challenged with dietary cholic acid [233]. A detailed review of the impact of PPARα on bile acid homeostasis is provided elsewhere [234]. Overall, what is clear is that unlike for the peroxisomal and mitochondrial fatty acid oxidation pathway, the direction of the impact of PPARα on genes involved in bile acid homeostasis is highly dependent on the mode of PPARα activation.
3.2.7. Retinoid metabolism
Conversion of retinol to retinal and retinoic acid is catalyzed by a number of enzymes, of which the corresponding genes are subject to PPARα stimulation during fasting (Dhrs3, Dhrs4, Adh1, Adh4, Aldh1a1) [235]. 9-cis retinoic acid serves as a ligand for the PPARα permissive binding partner RXR, suggesting that stimulation of retinoic acid synthesis by PPARα may further increase transcriptional activation by PPARα. Conversion of retinol to all-trans 13,14 dehydroretinol is catalyzed by retinol saturase (Retsat), a direct target gene of PPARα [236]. Currently, the actual function of Retsat in liver is not clear.
3.3. Target genes of PPARα in other metabolic pathways
3.3.1. Glucose metabolism
As described previously, PPARα activation and deletion are associated with major changes in glucose metabolism. A number of genes in the hepatic gluconeogenesis pathway are known or very likely PPARα targets, including pyruvate carboxylase (Pcx), and fructose bisphosphatase 1 [35,237,238]. Phosphoenol pyruvate carboxykinase (Pck1) appears to be specifically regulated by PPARα in human but not mouse hepatocytes [24,159].
Adipose tissue lipolysis leads to elevated circulating levels of glycerol, which is processed in the liver. Importantly, numerous genes involved in the metabolic conversion of glycerol in liver such as Gpd1, Gpd2, Gyk, Aqp3, and Aqp9, are targets of PPARα [24].
Besides governing glucose production, PPARα may also alter glucose utilization in numerous tissues via induction of pyruvate dehydrogenase kinase isoform 1 and 4 (Pdk1, Pdk4) [25,239]. Finally, glycogen synthesis in liver is targeted by PPARα via regulation of Gys2, the liver and adipose tissue-specific glycogen synthase isoform [33].
3.3.2. Secreted factors
Apart from direct regulation of genes encoding key enzymes in fatty acid oxidation and ketogenesis, it has been suggested that PPARα may partially stimulate these pathways indirectly via upregulation of the sensitive PPARα target and hormone FGF21 [144,166,167]. Importantly, many classical PPARα targets, including Acot1-4, Ehhadh, Cpt1a, Cpt1b, Cpt2, Hmgcs2, and Hadha, can be induced by PPARα activation in cultured mouse liver slices in the absence of any changes in FGF21, indicating that FGF21 is not essential for induction of genes involved in fatty acid oxidation and ketogenesis by PPARα. How FGF21 induces these pathways remains unclear but its effects are thus clearly distinct from direct gene regulation by PPARα and likely involve extra-hepatic tissues [240,241]. Other secreted factors that may mediate extra-hepatic actions of PPARα during fasting include Angiopoietin-like 4 (ANGPTL4), Mannose Binding Lectin (MBL2), and Fibroblast Growth Factor (FGF1) [242–244]. Fgf1 was recently identified as target of PPARγ in adipose tissue but is also transcriptionally regulated by PPARα in liver [242]. ANGPTL4 is an endogenous inhibitor of lipoprotein lipase that regulates tissue uptake of plasma TG-derived fatty acids [245], whereas MBL2 is a soluble mediator of innate immunity and primary component of the lectin branch of the complement system [244,246].
3.3.3. Vanin-1
One of the most highly induced genes upon PPARα activation in liver is Vanin-1 (Vnn1), which encodes a glycosylphosphatidylinositol-linked membrane-associated pantetheinase that catalyzes the hydrolysis of pantetheine into pantothenic acid (vitamin B5) and cysteamine. Multiples lines of experimentation clearly demonstrate that Vnn1 is a direct PPARα target gene [164,247]. Vanin-1 is present in plasma and determination of Vanin-1 activity and protein abundance was found to accurately reflect PPARα activation in liver, indicating that serum Vanin-1 may be used as a reliable reporter of hepatic PPARα activity in mice and humans [247,248].
3.4. Similarity in gene regulation by PPARα between mouse and human
Befitting their name, peroxisome proliferators and other synthetic PPARα agonists cause proliferation of peroxisomes and hepatomegaly in rats and mice in a PPARα-dependent manner [49]. In contrast, neither response is observed in humans (Table 2), which together with the observed lack of effect of PPARα agonists on peroxisomal fatty acid oxidation in humans [249], has led to suggestions that the function of PPARα is fundamentally different between mice and humans, and, partly due to the presumed low expression of PPARα in human liver, that the role of PPARα in human liver is relatively insignificant [250]. Subsequent studies have dispelled those notions, showing that a) PPARα expression is similar in mouse and human liver, b) regulation of lipid metabolic pathways and genes, including peroxisomal fatty acid oxidation, is well conserved between mice and humans [159,173]. In addition, the clinical efficacy of synthetic PPARα agonists attests to the functional importance of PPARα in governing lipid metabolism in human. Nevertheless, a number of metabolic genes appear to be specifically regulated by PPARα in rodents and not humans (Figure 5). Putative rodent-specific PPARα target genes include Mgll, Bbox1, Decr1, and Fbp2 [159]. It has been suggested that the first PPARα target Acyl-CoA oxidase is also a rodent-specific PPARα target, presumably due to the absence of a functional PPRE in the human ACOX1 gene [251]. However, gene expression profiling studies indicate that ACOX1 is one of the mostly significantly induced genes in human hepatocytes treated with synthetic PPARα agonists [159,173]. Conversely, a very small number of genes may be exclusively induced by PPARα in human liver cells, including PCK1, MBL2, APOA2 and possibly APOA5 [244,246,252]. With respect to APOA5, the human APOA5 gene has clearly been demonstrated to be a direct PPARα target characterized by a functional PPRE in its promoter [253]. Consistent with this finding, APOA5 mRNA is induced by PPARα activation in human and monkey hepatocytes, and APOA5 plasma levels are increased by treatment of monkeys with a synthetic PPARα agonist [173,253,254]. In contrast, Apoa5 upregulation is not observed in mouse liver or in cultured mouse hepatocytes, presumably due to a degenerate and non-functional PPRE in the mouse Apoa5 promoter [255]. Surprisingly, we found that Apoa5 mRNA is markedly and reproducibly induced by Wy14643 in mouse liver slices (our unpublished data), suggesting that Apoa5 may be a PPARα target in mouse after all, perhaps via the use of an alternative PPAR response element.
Figure 5.
Detailed overview map of metabolic genes upregulated by PPARα in human hepatocytes. The map is based on the published literature, including transcriptomics analysis of primary human hepatocytes treated with synthetic PPARα agonists.
Full understanding of the differences in PPARα-mediated gene regulation between mice and humans is hampered by practical limitations in the form of lack of (fibrate-treated) human livers. One of our future ambitions is to perform a whole genome comparison of the response to PPARα agonist in mouse and human cultured precision-cut liver slices, under the assumption that cultured liver tissue slices are superior to primary human hepatocytes for studying liver metabolism and gene regulation.
4. Conclusion
According to the traditional view, PPARα governs fatty acid oxidation and ketogenesis in liver. However, gene expression analysis at the level of individual genes or the entire genome via transcriptomics has indicated that the role of PPARα extends to numerous other metabolic pathways, providing a molecular basis for the pronounced metabolic disturbances in fasted PPARα−/− mice and explaining the marked impact of synthetic PPARα agonists on lipid metabolism in rodents and humans. It is now evident that PPARα functions at the center of a regulatory hub impacting fatty acid uptake, fatty acid activation, intracellular fatty acid binding, mitochondrial and peroxisomal fatty acid oxidation, ketogenesis, triglyceride turnover, lipid droplet biology, gluconeogenesis, and bile synthesis/secretion. In addition, PPARα governs the expression of several secreted proteins that exert local and endocrine functions. Hence, PPARα can aptly be described as a master regulator of hepatic lipid metabolism.
Conflict of interest
None declared.
References
- 1.Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 2.Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 3.Chawla A., Repa J.J., Evans R.M., Mangelsdorf D.J. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 4.Gronemeyer H., Laudet V. Transcription factors 3: nuclear receptors. Protein Profile. 1995;2:1173–1308. [PubMed] [Google Scholar]
- 5.Tugwood J.D., Issemann I., Anderson R.G., Bundell K.R., McPheat W.L., Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO Journal. 1992;11:433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefterova M.I., Zhang Y., Steger D.J., Schupp M., Schug J., Cristancho A. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes & Development. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiRenzo J., Soderstrom M., Kurokawa R., Ogliastro M.H., Ricote M., Ingrey S. Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Molecular and Cellular Biology. 1997;17:2166–2176. doi: 10.1128/mcb.17.4.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee R., Davies P.J., Crombie D.L., Bischoff E.D., Cesario R.M., Jow L. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386:407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 9.Schulman I.G., Shao G., Heyman R.A. Transactivation by retinoid X receptor-peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimers: intermolecular synergy requires only the PPARgamma hormone-dependent activation function. Molecular and Cellular Biology. 1998;18:3483–3494. doi: 10.1128/mcb.18.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu S., Reddy J.K. Transcription coactivators for peroxisome proliferator-activated receptors. Biochimica et Biophysica Acta. 2007;1771:936–951. doi: 10.1016/j.bbalip.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Viswakarma N., Jia Y., Bai L., Vluggens A., Borensztajn J., Xu J. Coactivators in PPAR-regulated gene expression. PPAR Research. 2010;2010 doi: 10.1155/2010/250126. pii: 250126. http://dx.doi.org/10.1155/2010/250126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venteclef N., Jakobsson T., Steffensen K.R., Treuter E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends in Endocrinology and Metabolism: TEM. 2011;22:333–343. doi: 10.1016/j.tem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Georgiadi A., Kersten S. Mechanisms of gene regulation by fatty acids. Advances in Nutrition. 2012;3:127–134. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bookout A.L., Jeong Y., Downes M., Yu R.T., Evans R.M., Mangelsdorf D.J. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escher P., Braissant O., Basu-Modak S., Michalik L., Wahli W., Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142:4195–4202. doi: 10.1210/endo.142.10.8458. [DOI] [PubMed] [Google Scholar]
- 16.Kersten S., Desvergne B., Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 17.Georgiadi A., Boekschoten M.V., Muller M., Kersten S. Detailed transcriptomics analysis of the effect of dietary fatty acids on gene expression in the heart. Physiological Genomics. 2012;44:352–361. doi: 10.1152/physiolgenomics.00115.2011. [DOI] [PubMed] [Google Scholar]
- 18.Madrazo J.A., Kelly D.P. The PPAR trio: regulators of myocardial energy metabolism in health and disease. Journal of Molecular and Cellular Cardiology. 2008;44:968–975. doi: 10.1016/j.yjmcc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Bunger M., van den Bosch H.M., van der Meijde J., Kersten S., Hooiveld G.J., Muller M. Genome-wide analysis of PPARalpha activation in murine small intestine. Physiological Genomics. 2007;30:192–204. doi: 10.1152/physiolgenomics.00198.2006. [DOI] [PubMed] [Google Scholar]
- 20.Cahill G.F., Jr. Fuel metabolism in starvation. Annual Review of Nutrition. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto T., Cook W.S., Qi C., Yeldandi A.V., Reddy J.K., Rao M.S. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. Journal of Biological Chemistry. 2000;275:28918–28928. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- 22.Kersten S., Seydoux J., Peters J.M., Gonzalez F.J., Desvergne B., Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. Journal of Clinical Investigation. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone T.C., Weinheimer C.J., Kelly D.P. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patsouris D., Mandard S., Voshol P.J., Escher P., Tan N.S., Havekes L.M. PPARalpha governs glycerol metabolism. Journal of Clinical Investigation. 2004;114:94–103. doi: 10.1172/JCI20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugden M.C., Bulmer K., Gibbons G.F., Knight B.L., Holness M.J. Peroxisome-proliferator-activated receptor-alpha (PPARalpha) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochemical Journal. 2002;364:361–368. doi: 10.1042/BJ20011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanderson L.M., Boekschoten M.V., Desvergne B., Muller M., Kersten S. Transcriptional profiling reveals divergent roles of PPARalpha and PPARbeta/delta in regulation of gene expression in mouse liver. Physiological Genomics. 2010;41:42–52. doi: 10.1152/physiolgenomics.00127.2009. [DOI] [PubMed] [Google Scholar]
- 27.Makowski L., Noland R.C., Koves T.R., Xing W., Ilkayeva O.R., Muehlbauer M.J. Metabolic profiling of PPARalpha-/- mice reveals defects in carnitine and amino acid homeostasis that are partially reversed by oral carnitine supplementation. FASEB Journal. 2009;23:586–604. doi: 10.1096/fj.08-119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le May C., Pineau T., Bigot K., Kohl C., Girard J., Pegorier J.P. Reduced hepatic fatty acid oxidation in fasting PPARalpha null mice is due to impaired mitochondrial hydroxymethylglutaryl-CoA synthase gene expression. FEBS Letters. 2000;475:163–166. doi: 10.1016/s0014-5793(00)01648-3. [DOI] [PubMed] [Google Scholar]
- 29.Atherton H.J., Gulston M.K., Bailey N.J., Cheng K.K., Zhang W., Clarke K. Metabolomics of the interaction between PPAR-alpha and age in the PPAR-alpha-null mouse. Molecular Systems Biology. 2009;5:259. doi: 10.1038/msb.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Souza A.T., Dai X., Spencer A.G., Reppen T., Menzie A., Roesch P.L. Transcriptional and phenotypic comparisons of Ppara knockout and siRNA knockdown mice. Nucleic Acids Research. 2006;34:4486–4494. doi: 10.1093/nar/gkl609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J., Xiao G., Trujillo C., Chang V., Blanco L., Joseph S.B. Peroxisome proliferator-activated receptor alpha (PPARalpha) influences substrate utilization for hepatic glucose production. Journal of Biological Chemistry. 2002;277:50237–50244. doi: 10.1074/jbc.M201208200. [DOI] [PubMed] [Google Scholar]
- 32.Bandsma R.H., Van Dijk T.H., Harmsel At A., Kok T., Reijngoud D.J., Staels B. Hepatic de novo synthesis of glucose 6-phosphate is not affected in peroxisome proliferator-activated receptor alpha-deficient mice but is preferentially directed toward hepatic glycogen stores after a short term fast. Journal of Biological Chemistry. 2004;279:8930–8937. doi: 10.1074/jbc.M310067200. [DOI] [PubMed] [Google Scholar]
- 33.Mandard S., Stienstra R., Escher P., Tan N.S., Kim I., Gonzalez F.J. Glycogen synthase 2 is a novel target gene of peroxisome proliferator-activated receptors. Cellular and Molecular Life Sciences. 2007;64:1145–1157. doi: 10.1007/s00018-007-7006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derks T.G., van Dijk T.H., Grefhorst A., Rake J.P., Smit G.P., Kuipers F. Inhibition of mitochondrial fatty acid oxidation in vivo only slightly suppresses gluconeogenesis but enhances clearance of glucose in mice. Hepatology. 2008;47:1032–1042. doi: 10.1002/hep.22101. [DOI] [PubMed] [Google Scholar]
- 35.Rakhshandehroo M., Sanderson L.M., Matilainen M., Stienstra R., Carlberg C., de Groot P.J. Comprehensive analysis of PPARalpha-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Research. 2007;2007:26839. doi: 10.1155/2007/26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J., Chang V., Joseph S.B., Trujillo C., Bassilian S., Saad M.F. Peroxisomal proliferator-activated receptor alpha deficiency diminishes insulin-responsiveness of gluconeogenic/glycolytic/pentose gene expression and substrate cycle flux. Endocrinology. 2004;145:1087–1095. doi: 10.1210/en.2003-1173. [DOI] [PubMed] [Google Scholar]
- 37.Knauf C., Rieusset J., Foretz M., Cani P.D., Uldry M., Hosokawa M. Peroxisome proliferator-activated receptor-alpha-null mice have increased white adipose tissue glucose utilization, GLUT4, and fat mass: role in liver and brain. Endocrinology. 2006;147:4067–4078. doi: 10.1210/en.2005-1536. [DOI] [PubMed] [Google Scholar]
- 38.Kersten S., Mandard S., Escher P., Gonzalez F.J., Tafuri S., Desvergne B. The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. FASEB Journal. 2001;15:1971–1978. doi: 10.1096/fj.01-0147com. [DOI] [PubMed] [Google Scholar]
- 39.Ciana P., Biserni A., Tatangelo L., Tiveron C., Sciarroni A.F., Ottobrini L. A novel peroxisome proliferator-activated receptor responsive element-luciferase reporter mouse reveals gender specificity of peroxisome proliferator-activated receptor activity in liver. Molecular Endocrinology. 2007;21:388–400. doi: 10.1210/me.2006-0152. [DOI] [PubMed] [Google Scholar]
- 40.Gottlicher M., Widmark E., Li Q., Gustafsson J.A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakravarthy M.V., Pan Z., Zhu Y., Tordjman K., Schneider J.G., Coleman T. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metabolism. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Sanderson L.M., Degenhardt T., Koppen A., Kalkhoven E., Desvergne B., Muller M. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Molecular and Cellular Biology. 2009;29:6257–6267. doi: 10.1128/MCB.00370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemberger T., Saladin R., Vazquez M., Assimacopoulos F., Staels B., Desvergne B. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. Journal of Biological Chemistry. 1996;271:1764–1769. doi: 10.1074/jbc.271.3.1764. [DOI] [PubMed] [Google Scholar]
- 44.Gachon F., Leuenberger N., Claudel T., Gos P., Jouffe C., Fleury Olela F. Proline- and acidic amino acid-rich basic leucine zipper proteins modulate peroxisome proliferator-activated receptor alpha (PPARalpha) activity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4794–4799. doi: 10.1073/pnas.1002862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nature Medicine. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong K.T., Mashek M.T., Bu S.Y., Greenberg A.S., Mashek D.G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Settembre C., De Cegli R., Mansueto G., Saha P.K., Vetrini F., Visvikis O. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nature Cell Biology. 2013;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forman B.M., Chen J., Evans R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S.S., Pineau T., Drago J., Lee E.J., Owens J.W., Kroetz D.L. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Molecular and Cellular Biology. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duval C., Muller M., Kersten S. PPARalpha and dyslipidemia. Biochimica et Biophysica Acta. 2007;1771:961–971. doi: 10.1016/j.bbalip.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Staels B., Maes M., Zambon A. Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nature clinical practice. Cardiovascular Medicine. 2008;5:542–553. doi: 10.1038/ncpcardio1278. [DOI] [PubMed] [Google Scholar]
- 52.Peters J.M., Cattley R.C., Gonzalez F.J. Role of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis. 1997;18:2029–2033. doi: 10.1093/carcin/18.11.2029. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez F.J. The peroxisome proliferator-activated receptor alpha (PPARalpha): role in hepatocarcinogenesis. Molecular and Cellular Endocrinology. 2002;193:71–79. doi: 10.1016/s0303-7207(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 54.Mukherjee R., Strasser J., Jow L., Hoener P., Paterniti J.R., Jr., Heyman R.A. RXR agonists activate PPARalpha-inducible genes, lower triglycerides, and raise HDL levels in vivo. Arteriosclerosis Thrombosis and Vascular Biology. 1998;18:272–276. doi: 10.1161/01.atv.18.2.272. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Yao R., Maciag A., Grubbs C.J., Lubet R.A., You M. Organ-specific expression profiles of rat mammary gland, liver, and lung tissues treated with targretin, 9-cis retinoic acid, and 4-hydroxyphenylretinamide. Molecular Cancer Therapeutics. 2006;5:1060–1072. doi: 10.1158/1535-7163.MCT-05-0322. [DOI] [PubMed] [Google Scholar]
- 56.Martin P.G., Lasserre F., Calleja C., Van Es A., Roulet A., Concordet D. Transcriptional modulations by RXR agonists are only partially subordinated to PPARalpha signaling and attest additional, organ-specific, molecular cross-talks. Gene Expression. 2005;12:177–192. doi: 10.3727/000000005783992098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouamrane L., Larrieu G., Gauthier B., Pineau T. RXR activators molecular signalling: involvement of a PPAR alpha-dependent pathway in the liver and kidney, evidence for an alternative pathway in the heart. British Journal of Pharmacology. 2003;138:845–854. doi: 10.1038/sj.bjp.0705113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cornu-Chagnon M.C., Dupont H., Edgar A. Fenofibrate: metabolism and species differences for peroxisome proliferation in cultured hepatocytes. Fundamental and Applied Toxicology: Official Journal of the Society of Toxicology. 1995;26:63–74. doi: 10.1006/faat.1995.1075. [DOI] [PubMed] [Google Scholar]
- 59.Gray T.J., Lake B.G., Beamand J.A., Foster J.R., Gangolli S.D. Peroxisome proliferation in primary cultures of rat hepatocytes. Toxicology and Applied Pharmacology. 1983;67:15–25. doi: 10.1016/0041-008x(83)90240-5. [DOI] [PubMed] [Google Scholar]
- 60.Lake B.G., Gray T.J., Pels Rijcken W.R., Beamand J.A., Gangolli S.D. The effect of hypolipidaemic agents on peroxisomal beta-oxidation and mixed-function oxidase activities in primary cultures of rat hepatocytes. Relationship between induction of palmitoyl-CoA oxidation and lauric acid hydroxylation. Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 1984;14:269–276. doi: 10.3109/00498258409151411. [DOI] [PubMed] [Google Scholar]
- 61.Paul H.S., Adibi S.A. Paradoxical effects of clofibrate on liver and muscle metabolism in rats. Induction of myotonia and alteration of fatty acid and glucose oxidation. Journal of Clinical Investigation. 1979;64:405–412. doi: 10.1172/JCI109476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furuta S., Miyazawa S., Hashimoto T. Induction of acyl-CoA dehydrogenases and electron transfer flavoprotein and their roles in fatty acid oxidation in rat liver mitochondria. Journal of Biochemistry. 1981;90:1751–1756. doi: 10.1093/oxfordjournals.jbchem.a133652. [DOI] [PubMed] [Google Scholar]
- 63.Glatz J.F., Wagenmakers A.J., Veerkamp J.H., van Moerkerk H.T. Effect of clofibrate feeding on palmitate and branched-chain 2-oxo acid oxidation in rat liver and muscle. Biochemical Pharmacology. 1983;32:2489–2493. doi: 10.1016/0006-2952(83)90007-2. [DOI] [PubMed] [Google Scholar]
- 64.Kahonen M.T., Ylikahri R.H. Effect of clofibrate and gemfibrozil on the activities of mitochondrial carnitine acyltransferases in rat liver. Dose–response relations. Atherosclerosis. 1979;32:47–56. doi: 10.1016/0021-9150(79)90146-1. [DOI] [PubMed] [Google Scholar]
- 65.Kawashima Y., Katoh H., Watanuki H., Takegishi M., Kozuka H. Effects of long-term administration of clofibric acid on peroxisomal beta-oxidation, fatty acid-binding protein and cytosolic long-chain acyl-CoA hydrolases in rat liver. Biochemical Pharmacology. 1985;34:325–329. doi: 10.1016/0006-2952(85)90039-5. [DOI] [PubMed] [Google Scholar]
- 66.Mannaerts G.P., Debeer L.J., Thomas J., De Schepper P.J. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats. Journal of Biological Chemistry. 1979;254:4585–4595. [PubMed] [Google Scholar]
- 67.Mortensen P.B., Rasmussen K. Beta-oxidation of C-6-C-10 fatty acids in rat liver homogenates measured by selected ion monitoring: effects of cyanide and clofibrate. Biomedical Mass Spectrometry. 1983;10:528–533. doi: 10.1002/bms.1200100908. [DOI] [PubMed] [Google Scholar]
- 68.Pande S.V., Parvin R. Clofibrate enhancement of mitochondrial carnitine transport system of rat liver and augmentation of liver carnitine and gamma-butyrobetaine hydroxylase activity by thyroxine. Biochimica et Biophysica Acta. 1980;617:363–370. doi: 10.1016/0005-2760(80)90002-8. [DOI] [PubMed] [Google Scholar]
- 69.Reddy M.K., Lalwani N.D., Qureshi S.A., Reddy J.K. Induction of hamster hepatic peroxisomal beta-oxidation and peroxisome proliferation-associated 80000 mol. wt. polypeptide by hypolipidemic drugs. Human Toxicology. 1982;1:135–147. doi: 10.1177/096032718200100205. [DOI] [PubMed] [Google Scholar]
- 70.Bodnar A.G., Rachubinski R.A. Cloning and sequence determination of cDNA encoding a second rat liver peroxisomal 3-ketoacyl-CoA thiolase. Gene. 1990;91:193–199. doi: 10.1016/0378-1119(90)90088-9. [DOI] [PubMed] [Google Scholar]
- 71.Ozasa H., Miyazawa S., Furuta S., Osumi T., Hashimoto T. Induction of peroxisomal beta-oxidation enzymes in primary cultured rat hepatocytes by clofibric acid. Journal of Biochemistry. 1985;97:1273–1278. doi: 10.1093/oxfordjournals.jbchem.a135178. [DOI] [PubMed] [Google Scholar]
- 72.Reddy J.K., Goel S.K., Nemali M.R., Carrino J.J., Laffler T.G., Reddy M.K. Transcription regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:1747–1751. doi: 10.1073/pnas.83.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kahonen M. Effect of clofibrate treatment on acylcarnitine oxidation in isolated rat liver mitochondria. Medical Biology. 1979;57:58–65. [PubMed] [Google Scholar]
- 74.Lazarow P.B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osmundsen H., Cervenka J., Bremer J. A role for 2,4-enoyl-CoA reductase in mitochondrial beta-oxidation of polyunsaturated fatty acids. Effects of treatment with clofibrate on oxidation of polyunsaturated acylcarnitines by isolated rat liver mitochondria. Biochemical Journal. 1982;208:749–757. doi: 10.1042/bj2080749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veitch K., Draye J.P., Van Hoof F., Sherratt H.S. Effects of riboflavin deficiency and clofibrate treatment on the five acyl-CoA dehydrogenases in rat liver mitochondria. Biochemical Journal. 1988;254:477–481. doi: 10.1042/bj2540477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orton T.C., Parker G.L. The effect of hypolipidemic agents on the hepatic microsomal drug-metabolizing enzyme system of the rat. Induction of cytochrome(s) P-450 with specificity toward terminal hydroxylation of lauric acid. Drug Metabolism and Disposition: the Biological Fate of Chemicals. 1982;10:110–115. [PubMed] [Google Scholar]
- 78.Sharma R., Lake B.G., Gibson G.G. Co-induction of microsomal cytochrome P-452 and the peroxisomal fatty acid beta-oxidation pathway in the rat by clofibrate and di-(2-ethylhexyl)phthalate. Dose-response studies. Biochemical Pharmacology. 1988;37:1203–1206. doi: 10.1016/0006-2952(88)90771-x. [DOI] [PubMed] [Google Scholar]
- 79.Sharma R.K., Lake B.G., Makowski R., Bradshaw T., Earnshaw D., Dale J.W. Differential induction of peroxisomal and microsomal fatty-acid-oxidising enzymes by peroxisome proliferators in rat liver and kidney. Characterisation of a renal cytochrome P-450 and implications for peroxisome proliferation. European Journal of Biochemistry. 1989;184:69–78. doi: 10.1111/j.1432-1033.1989.tb14991.x. [DOI] [PubMed] [Google Scholar]
- 80.Bergseth S., Christiansen E.N., Bremer J. The effect of feeding fish oils, vegetable oils and clofibrate on the ketogenesis from long chain fatty acids in hepatocytes. Lipids. 1986;21:508–514. doi: 10.1007/BF02535638. [DOI] [PubMed] [Google Scholar]
- 81.el Kebbaj M.H., Cherkaoui Malki M., Latruffe N. Effect of peroxisomes proliferators and hypolipemic agents on mitochondrial inner membrane linked D-3-hydroxybutyrate dehydrogenase (BDH) Biochemistry and Molecular Biology International. 1995;35:65–77. [PubMed] [Google Scholar]
- 82.Krueger R.G., Staneck L.D., Boehlecke J.M. Tumor-associated antigens in human myeloma. Journal of the National Cancer Institute. 1976;56:711–715. doi: 10.1093/jnci/56.4.711. [DOI] [PubMed] [Google Scholar]
- 83.Kersten S. Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Research. 2008;2008:132960. doi: 10.1155/2008/132960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haluzik M.M., Lacinova Z., Dolinkova M., Haluzikova D., Housa D., Horinek A. Improvement of insulin sensitivity after peroxisome proliferator-activated receptor-alpha agonist treatment is accompanied by paradoxical increase of circulating resistin levels. Endocrinology. 2006;147:4517–4524. doi: 10.1210/en.2005-1624. [DOI] [PubMed] [Google Scholar]
- 85.Nagasawa T., Inada Y., Nakano S., Tamura T., Takahashi T., Maruyama K. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. European Journal of Pharmacology. 2006;536:182–191. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 86.Chan S.M., Sun R.Q., Zeng X.Y., Choong Z.H., Wang H., Watt M.J. Activation of PPARalpha ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased ER stress. Diabetes. 2013;62:2095–2105. doi: 10.2337/db12-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang B., Wu P., Harris R.A. Additive effects of clofibric acid and pyruvate dehydrogenase kinase isoenzyme 4 (PDK4) deficiency on hepatic steatosis in mice fed a high saturated fat diet. FEBS Journal. 2012;279:1883–1893. doi: 10.1111/j.1742-4658.2012.08569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sasaki Y., Shimada T., Iizuka S., Suzuki W., Makihara H., Teraoka R. Effects of bezafibrate in nonalcoholic steatohepatitis model mice with monosodium glutamate-induced metabolic syndrome. European Journal of Pharmacology. 2011;662:1–8. doi: 10.1016/j.ejphar.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 89.Tailleux A., Wouters K., Staels B. Roles of PPARs in NAFLD: potential therapeutic targets. Biochimica et Biophysica Acta. 2012;1821:809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 90.Thomassen M.S., Christiansen E.N., Norum K.R. Characterization of the stimulatory effect of high-fat diets on peroxisomal beta-oxidation in rat liver. Biochemical Journal. 1982;206:195–202. doi: 10.1042/bj2060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ye J.M., Doyle P.J., Iglesias M.A., Watson D.G., Cooney G.J., Kraegen E.W. Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
- 92.Fabbrini E., Mohammed B.S., Korenblat K.M., Magkos F., McCrea J., Patterson B.W. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. Journal of Clinical Endocrinology and Metabolism. 2010;95:2727–2735. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oosterveer M.H., Grefhorst A., van Dijk T.H., Havinga R., Staels B., Kuipers F. Fenofibrate simultaneously induces hepatic fatty acid oxidation, synthesis, and elongation in mice. Journal of Biological Chemistry. 2009;284:34036–34044. doi: 10.1074/jbc.M109.051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X., Matthews J., Zhou L., Pelton P., Liang Y., Xu J. Improvement of dyslipidemia, insulin sensitivity, and energy balance by a peroxisome proliferator-activated receptor alpha agonist. Metabolism. 2008;57:1516–1525. doi: 10.1016/j.metabol.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 95.Guerre-Millo M., Gervois P., Raspe E., Madsen L., Poulain P., Derudas B. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. Journal of Biological Chemistry. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- 96.Schafer H.L., Linz W., Falk E., Glien M., Glombik H., Korn M. AVE8134, a novel potent PPARalpha agonist, improves lipid profile and glucose metabolism in dyslipidemic mice and type 2 diabetic rats. Acta pharmacologica Sinica. 2012;33:82–90. doi: 10.1038/aps.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Enger S.C., Johnsen V., Samuelsen A., Laws E.A. The effect of clofibrate on glucose tolerance, insulin secretion, triglycerides and fibrinogen in patients with coronary heart disease. Acta Medica Scandinavica. 1977;201:563–566. doi: 10.1111/j.0954-6820.1977.tb15748.x. [DOI] [PubMed] [Google Scholar]
- 98.Ferrari C., Frezzati S., Romussi M., Bertazzoni A., Testori G.P., Antonini S. Effects of short-term clofibrate administration on glucose tolerance and insulin secretion in patients with chemical diabetes or hypertriglyceridemia. Metabolism. 1977;26:129–139. doi: 10.1016/0026-0495(77)90048-8. [DOI] [PubMed] [Google Scholar]
- 99.Ferrari C., Testori G.P., Bertazzoni A., Romussi M., Caldara R., Frezzati S. Increased glucose disappearance rate after short-term clofibrate administration in normal subjects and in patients with chemical diabetes. Hormone and Metabolic Research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1978;10:4–6. doi: 10.1055/s-0028-1093469. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi M., Shigeta Y., Hirata Y., Omori Y., Sakamoto N., Nambu S. Improvement of glucose tolerance in NIDDM by clofibrate. Randomized double-blind study. Diabetes Care. 1988;11:495–499. doi: 10.2337/diacare.11.6.495. [DOI] [PubMed] [Google Scholar]
- 101.Anderlova K., Dolezalova R., Housova J., Bosanska L., Haluzikova D., Kremen J. Influence of PPAR-alpha agonist fenofibrate on insulin sensitivity and selected adipose tissue-derived hormones in obese women with type 2 diabetes. Physiological Research. 2007;56:579–586. doi: 10.33549/physiolres.931058. [DOI] [PubMed] [Google Scholar]
- 102.Belfort R., Berria R., Cornell J., Cusi K. Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. Journal of Clinical Endocrinology and Metabolism. 2010;95:829–836. doi: 10.1210/jc.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perreault L., Bergman B.C., Hunerdosse D.M., Howard D.J., Eckel R.H. Fenofibrate administration does not affect muscle triglyceride concentration or insulin sensitivity in humans. Metabolism. 2011;60:1107–1114. doi: 10.1016/j.metabol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Subramanian S., DeRosa M.A., Bernal-Mizrachi C., Laffely N., Cade W.T., Yarasheski K.E. PPARalpha activation elevates blood pressure and does not correct glucocorticoid-induced insulin resistance in humans. American Journal of Physiology – Endocrinology and Metabolism. 2006;291:E1365–E1371. doi: 10.1152/ajpendo.00230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Motojima K., Seto K. Fibrates and statins rapidly and synergistically induce pyruvate dehydrogenase kinase 4 mRNA in the liver and muscles of mice. Biological and Pharmaceutical Bulletin. 2003;26:954–958. doi: 10.1248/bpb.26.954. [DOI] [PubMed] [Google Scholar]
- 106.Walters M.W., Wallace K.B. Urea cycle gene expression is suppressed by PFOA treatment in rats. Toxicology Letters. 2010;197:46–50. doi: 10.1016/j.toxlet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 107.Edvardsson U., von Lowenhielm H.B., Panfilov O., Nystrom A.C., Nilsson F., Dahllof B. Hepatic protein expression of lean mice and obese diabetic mice treated with peroxisome proliferator-activated receptor activators. Proteomics. 2003;3:468–478. doi: 10.1002/pmic.200390061. [DOI] [PubMed] [Google Scholar]
- 108.Leonard J.F., Courcol M., Mariet C., Charbonnier A., Boitier E., Duchesne M. Proteomic characterization of the effects of clofibrate on protein expression in rat liver. Proteomics. 2006;6:1915–1933. doi: 10.1002/pmic.200500251. [DOI] [PubMed] [Google Scholar]
- 109.Makino T., Kinoshita J., Arakawa S., Ito K., Ando Y., Yamoto T. Comprehensive analysis of hepatic gene and protein expression profiles on phenobarbital- or clofibrate-induced hepatic hypertrophy in dogs. Journal of Toxicological Sciences. 2009;34:647–661. doi: 10.2131/jts.34.647. [DOI] [PubMed] [Google Scholar]
- 110.Sheikh K., Camejo G., Lanne B., Halvarsson T., Landergren M.R., Oakes N.D. Beyond lipids, pharmacological PPARalpha activation has important effects on amino acid metabolism as studied in the rat. American Journal of Physiology – Endocrinology and Metabolism. 2007;292:E1157–E1165. doi: 10.1152/ajpendo.00254.2006. [DOI] [PubMed] [Google Scholar]
- 111.Edgar A.D., Tomkiewicz C., Costet P., Legendre C., Aggerbeck M., Bouguet J. Fenofibrate modifies transaminase gene expression via a peroxisome proliferator activated receptor alpha-dependent pathway. Toxicology Letters. 1998;98:13–23. doi: 10.1016/s0378-4274(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 112.Thulin P., Rafter I., Stockling K., Tomkiewicz C., Norjavaara E., Aggerbeck M. PPARalpha regulates the hepatotoxic biomarker alanine aminotransferase (ALT1) gene expression in human hepatocytes. Toxicology and Applied Pharmacology. 2008;231:1–9. doi: 10.1016/j.taap.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 113.Tomkiewicz C., Muzeau F., Edgar A.D., Barouki R., Aggerbeck M. Opposite regulation of the rat and human cytosolic aspartate aminotransferase genes by fibrates. Biochemical Pharmacology. 2004;67:213–225. doi: 10.1016/j.bcp.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 114.Keller H., Dreyer C., Medin J., Mahfoudi A., Ozato K., Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kliewer S.A., Sundseth S.S., Jones S.A., Brown P.J., Wisely G.B., Koble C.S. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krey G., Braissant O., L'Horset F., Kalkhoven E., Perroud M., Parker M.G. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Molecular Endocrinology. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 117.Lin Q., Ruuska S.E., Shaw N.S., Dong D., Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor alpha. Biochemistry. 1999;38:185–190. doi: 10.1021/bi9816094. [DOI] [PubMed] [Google Scholar]
- 118.Murakami K., Ide T., Suzuki M., Mochizuki T., Kadowaki T. Evidence for direct binding of fatty acids and eicosanoids to human peroxisome proliferators-activated receptor alpha. Biochemical and Biophysical Research Communications. 1999;260:609–613. doi: 10.1006/bbrc.1999.0951. [DOI] [PubMed] [Google Scholar]
- 119.Sanderson L.M., de Groot P.J., Hooiveld G.J., Koppen A., Kalkhoven E., Muller M. Effect of synthetic dietary triglycerides: a novel research paradigm for nutrigenomics. PLoS One. 2008;3:e1681. doi: 10.1371/journal.pone.0001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oswal D.P., Balanarasimha M., Loyer J.K., Bedi S., Soman F.L., Rider S.D., Jr. Divergence between human and murine peroxisome proliferator-activated receptor alpha ligand specificities. Journal of Lipid Research. 2013;54:2354–2365. doi: 10.1194/jlr.M035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fang X., Hu S., Xu B., Snyder G.D., Harmon S., Yao J. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. American Journal of Physiology – Heart and Circulatory Physiology. 2006;290:H55–H63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- 122.Fu J., Gaetani S., Oveisi F., Lo Verme J., Serrano A., Rodriguez De Fonseca F. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 123.Muga S.J., Thuillier P., Pavone A., Rundhaug J.E., Boeglin W.E., Jisaka M. 8S-lipoxygenase products activate peroxisome proliferator-activated receptor alpha and induce differentiation in murine keratinocytes. Cell Growth & Differentiation. 2000;11:447–454. [PubMed] [Google Scholar]
- 124.Zomer A.W., van Der Burg B., Jansen G.A., Wanders R.J., Poll-The B.T., van Der Saag P.T. Pristanic acid and phytanic acid: naturally occurring ligands for the nuclear receptor peroxisome proliferator-activated receptor alpha. Journal of Lipid Research. 2000;41:1801–1807. [PubMed] [Google Scholar]
- 125.Hostetler H.A., Petrescu A.D., Kier A.B., Schroeder F. Peroxisome proliferator-activated receptor alpha interacts with high affinity and is conformationally responsive to endogenous ligands. Journal of Biological Chemistry. 2005;280:18667–18682. doi: 10.1074/jbc.M412062200. [DOI] [PubMed] [Google Scholar]
- 126.Sun Y., Alexander S.P., Garle M.J., Gibson C.L., Hewitt K., Murphy S.P. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. British Journal of Pharmacology. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.JØrgensen C., Krogsdam A.M., Kratchmarova I., Willson T.M., Knudsen J., Mandrup S. Opposing effects of fatty acids and acyl-CoA esters on conformation and cofactor recruitment of peroxisome proliferator-activated receptors. Annals of the New York Academy of Sciences. 2002;967:431–439. doi: 10.1111/j.1749-6632.2002.tb04299.x. [DOI] [PubMed] [Google Scholar]
- 128.Elholm M., Dam I., Jorgensen C., Krogsdam A.M., Holst D., Kratchmarova I. Acyl-CoA esters antagonize the effects of ligands on peroxisome proliferator-activated receptor alpha conformation, DNA binding, and interaction with co-factors. Journal of Biological Chemistry. 2001;276:21410–21416. doi: 10.1074/jbc.M101073200. [DOI] [PubMed] [Google Scholar]
- 129.Chakravarthy M.V., Lodhi I.J., Yin L., Malapaka R.R., Xu H.E., Turk J. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu S., Brown J.D., Stanya K.J., Homan E., Leidl M., Inouye K. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502:550–554. doi: 10.1038/nature12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang H., Starodub O., McIntosh A., Atshaves B.P., Woldegiorgis G., Kier A.B. Liver fatty acid-binding protein colocalizes with peroxisome proliferator activated receptor alpha and enhances ligand distribution to nuclei of living cells. Biochemistry. 2004;43:2484–2500. doi: 10.1021/bi0352318. [DOI] [PubMed] [Google Scholar]
- 132.Huang H., Starodub O., McIntosh A., Kier A.B., Schroeder F. Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. Journal of Biological Chemistry. 2002;277:29139–29151. doi: 10.1074/jbc.M202923200. [DOI] [PubMed] [Google Scholar]
- 133.Hostetler H.A., McIntosh A.L., Atshaves B.P., Storey S.M., Payne H.R., Kier A.B. L-FABP directly interacts with PPARalpha in cultured primary hepatocytes. Journal of Lipid Research. 2009;50:1663–1675. doi: 10.1194/jlr.M900058-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McIntosh A.L., Atshaves B.P., Hostetler H.A., Huang H., Davis J., Lyuksyutova O.I. Liver type fatty acid binding protein (L-FABP) gene ablation reduces nuclear ligand distribution and peroxisome proliferator-activated receptor-alpha activity in cultured primary hepatocytes. Archives of Biochemistry and Biophysics. 2009;485:160–173. doi: 10.1016/j.abb.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wolfrum C., Borrmann C.M., Borchers T., Spener F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha- and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2323–2328. doi: 10.1073/pnas.051619898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kozawa S., Honda A., Kajiwara N., Takemoto Y., Nagase T., Nikami H. Induction of peroxisomal lipid metabolism in mice fed a high-fat diet. Molecular Medicine Reports. 2011;4:1157–1162. doi: 10.3892/mmr.2011.560. [DOI] [PubMed] [Google Scholar]
- 137.Patsouris D., Reddy J.K., Muller M., Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 2006;147:1508–1516. doi: 10.1210/en.2005-1132. [DOI] [PubMed] [Google Scholar]
- 138.Sunny N.E., Satapati S., Fu X., He T., Mehdibeigi R., Spring-Robinson C. Progressive adaptation of hepatic ketogenesis in mice fed a high-fat diet. American Journal of Physiology – Endocrinology and Metabolism. 2010;298:E1226–1235. doi: 10.1152/ajpendo.00033.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brady P.S., Marine K.A., Brady L.J., Ramsay R.R. Co-ordinate induction of hepatic mitochondrial and peroxisomal carnitine acyltransferase synthesis by diet and drugs. Biochemical Journal. 1989;260:93–100. doi: 10.1042/bj2600093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ouali F., Djouadi F., Merlet-Benichou C., Riveau B., Bastin J. Regulation of fatty acid transport protein and mitochondrial and peroxisomal beta-oxidation gene expression by fatty acids in developing rats. Pediatric Research. 2000;48:691–696. doi: 10.1203/00006450-200011000-00023. [DOI] [PubMed] [Google Scholar]
- 141.Duval C., Thissen U., Keshtkar S., Accart B., Stienstra R., Boekschoten M.V. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57BL/6 mice. Diabetes. 2010;59:3181–3191. doi: 10.2337/db10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gavrilova O., Haluzik M., Matsusue K., Cutson J.J., Johnson L., Dietz K.R. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. Journal of Biological Chemistry. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 143.Inoue M., Ohtake T., Motomura W., Takahashi N., Hosoki Y., Miyoshi S. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochemical and Biophysical Research Communications. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 144.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 145.Garbow J.R., Doherty J.M., Schugar R.C., Travers S., Weber M.L., Wentz A.E. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2011;300:G956–967. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martin P.G., Guillou H., Lasserre F., Dejean S., Lan A., Pascussi J.M. Novel aspects of PPARalpha-mediated regulation of lipid and xenobiotic metabolism revealed through a nutrigenomic study. Hepatology. 2007;45:767–777. doi: 10.1002/hep.21510. [DOI] [PubMed] [Google Scholar]
- 147.Ren B., Thelen A.P., Peters J.M., Gonzalez F.J., Jump D.B. Polyunsaturated fatty acid suppression of hepatic fatty acid synthase and S14 gene expression does not require peroxisome proliferator-activated receptor alpha. Journal of Biological Chemistry. 1997;272:26827–26832. doi: 10.1074/jbc.272.43.26827. [DOI] [PubMed] [Google Scholar]
- 148.Lu Y., Boekschoten M.V., Wopereis S., Muller M., Kersten S. Comparative transcriptomic and metabolomic analysis of fenofibrate and fish oil treatments in mice. Physiological Genomics. 2011;43:1307–1318. doi: 10.1152/physiolgenomics.00100.2011. [DOI] [PubMed] [Google Scholar]
- 149.Nilsson A., Arey H., Pedersen J.I., Christiansen E.N. The effect of high-fat diets on microsomal lauric acid hydroxylation in rat liver. Biochimica et Biophysica Acta. 1986;879:209–214. doi: 10.1016/0005-2760(86)90104-9. [DOI] [PubMed] [Google Scholar]
- 150.Yamazaki R.K., Shen T., Schade G.B. A diet rich in (n-3) fatty acids increases peroxisomal beta-oxidation activity and lowers plasma triacylglycerols without inhibiting glutathione-dependent detoxication activities in the rat liver. Biochimica et Biophysica Acta. 1987;920:62–67. doi: 10.1016/0005-2760(87)90311-0. [DOI] [PubMed] [Google Scholar]
- 151.Xu H.E., Lambert M.H., Montana V.G., Parks D.J., Blanchard S.G., Brown P.J. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Molecular Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 152.Jia Y., Kim J.Y., Jun H.J., Kim S.J., Lee J.H., Hoang M.H. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochimica et Biophysica Acta. 2013;1831:698–708. doi: 10.1016/j.bbalip.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 153.Jia Y., Kim J.Y., Jun H.J., Kim S.J., Lee J.H., Hoang M.H. The natural carotenoid astaxanthin, a PPAR-alpha agonist and PPAR-gamma antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Molecular Nutrition & Food Research. 2012;56:878–888. doi: 10.1002/mnfr.201100798. [DOI] [PubMed] [Google Scholar]
- 154.Jia Y., Bhuiyan M.J., Jun H.J., Lee J.H., Hoang M.H., Lee H.J. Ursolic acid is a PPAR-alpha agonist that regulates hepatic lipid metabolism. Bioorganic and Medicinal Chemistry Letters. 2011;21:5876–5880. doi: 10.1016/j.bmcl.2011.07.095. [DOI] [PubMed] [Google Scholar]
- 155.Rimando A.M., Nagmani R., Feller D.R., Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. Journal of Agricultural and Food Chemistry. 2005;53:3403–3407. doi: 10.1021/jf0580364. [DOI] [PubMed] [Google Scholar]
- 156.Shimura M., Hasumi A., Minato T., Hosono M., Miura Y., Mizutani S. Isohumulones modulate blood lipid status through the activation of PPAR alpha. Biochimica et Biophysica Acta. 2005;1736:51–60. doi: 10.1016/j.bbalip.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 157.Mezei O., Li Y., Mullen E., Ross-Viola J.S., Shay N.F. Dietary isoflavone supplementation modulates lipid metabolism via PPARalpha-dependent and -independent mechanisms. Physiological Genomics. 2006;26:8–14. doi: 10.1152/physiolgenomics.00155.2005. [DOI] [PubMed] [Google Scholar]
- 158.Moya-Camarena S.Y., Vanden Heuvel J.P., Blanchard S.G., Leesnitzer L.A., Belury M.A. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. Journal of Lipid Research. 1999;40:1426–1433. [PubMed] [Google Scholar]
- 159.Rakhshandehroo M., Hooiveld G., Muller M., Kersten S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS One. 2009;4:e6796. doi: 10.1371/journal.pone.0006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ducheix S., Podechard N., Lasserre F., Polizzi A., Pommier A., Murzilli S. A systems biology approach to the hepatic role of the oxysterol receptor LXR in the regulation of lipogenesis highlights a cross-talk with PPARalpha. Biochimie. 2013;95:556–567. doi: 10.1016/j.biochi.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 161.Willson T.M., Brown P.J., Sternbach D.D., Henke B.R. The PPARs: from orphan receptors to drug discovery. Journal of Medicinal Chemistry. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 162.Hoekstra M., Kruijt J.K., Van Eck M., Van Berkel T.J. Specific gene expression of ATP-binding cassette transporters and nuclear hormone receptors in rat liver parenchymal, endothelial, and Kupffer cells. Journal of Biological Chemistry. 2003;278:25448–25453. doi: 10.1074/jbc.M301189200. [DOI] [PubMed] [Google Scholar]
- 163.Mandard S., Muller M., Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cellular and Molecular Life Sciences. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rakhshandehroo M., Knoch B., Muller M., Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Research. 2010;2010 doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee J.H., Giannikopoulos P., Duncan S.A., Wang J., Johansen C.T., Brown J.D. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nature Medicine. 2011;17:812–815. doi: 10.1038/nm.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 167.Lundasen T., Hunt M.C., Nilsson L.M., Sanyal S., Angelin B., Alexson S.E. PPARalpha is a key regulator of hepatic FGF21. Biochemical and Biophysical Research Communications. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 168.Zandbergen F., Mandard S., Escher P., Tan N.S., Patsouris D., Jatkoe T. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochemical Journal. 2005;392:313–324. doi: 10.1042/BJ20050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim H.B., Mendez R., Zheng Z., Chang L., Cai J., Zhang R. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor alpha to regulate metabolic hormone FGF21. Endocrinology. 2014;155:769–782. doi: 10.1210/en.2013-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guillaumond F., Grechez-Cassiau A., Subramaniam M., Brangolo S., Peteri-Brunback B., Staels B. Kruppel-like factor KLF10 is a link between the circadian clock and metabolism in liver. Molecular and Cellular Biology. 2010;30:3059–3070. doi: 10.1128/MCB.01141-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang H., Chen Q., Yang M., Zhu B., Cui Y., Xue Y. Mouse KLF11 regulates hepatic lipid metabolism. Journal of Hepatology. 2012;58:763–770. doi: 10.1016/j.jhep.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 172.Gill G., Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988;334:721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- 173.McMullen P.D., Bhattacharya S., Woods C.G., Sun B., Yarborough K., Ross S.M. A map of the PPARalpha transcription regulatory network for primary human hepatocytes. Chemico-biological Interactions. 2013;209C:14–24. doi: 10.1016/j.cbi.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 174.van der Meer D.L., Degenhardt T., Vaisanen S., de Groot P.J., Heinaniemi M., de Vries S.C. Profiling of promoter occupancy by PPARalpha in human hepatoma cells via ChIP-chip analysis. Nucleic Acids Research. 2010;38:2839–2850. doi: 10.1093/nar/gkq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Boergesen M., Pedersen T.A., Gross B., van Heeringen S.J., Hagenbeek D., Bindesboll C. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Molecular and Cellular Biology. 2012;32:852–867. doi: 10.1128/MCB.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Motojima K., Passilly P., Peters J.M., Gonzalez F.J., Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. Journal of Biological Chemistry. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- 177.Guo L., Fang H., Collins J., Fan X.H., Dial S., Wong A. Differential gene expression in mouse primary hepatocytes exposed to the peroxisome proliferator-activated receptor alpha agonists. BMC Bioinformatics. 2006;7(Suppl. 2):S18. doi: 10.1186/1471-2105-7-S2-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krammer J., Digel M., Ehehalt F., Stremmel W., Fullekrug J., Ehehalt R. Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells. International Journal of Medical Sciences. 2011;8:599–614. doi: 10.7150/ijms.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Aoyama T., Peters J.M., Iritani N., Nakajima T., Furihata K., Hashimoto T. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) Journal of Biological Chemistry. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 180.Hsu M.H., Savas U., Griffin K.J., Johnson E.F. Identification of peroxisome proliferator-responsive human genes by elevated expression of the peroxisome proliferator-activated receptor alpha in HepG2 cells. Journal of Biological Chemistry. 2001;276:27950–27958. doi: 10.1074/jbc.M100258200. [DOI] [PubMed] [Google Scholar]
- 181.Schoonjans K., Watanabe M., Suzuki H., Mahfoudi A., Krey G., Wahli W. Induction of the acyl-coenzyme A synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. Journal of Biological Chemistry. 1995;270:19269–19276. doi: 10.1074/jbc.270.33.19269. [DOI] [PubMed] [Google Scholar]
- 182.Dongol B., Shah Y., Kim I., Gonzalez F.J., Hunt M.C. The acyl-CoA thioesterase I is regulated by PPARalpha and HNF4alpha via a distal response element in the promoter. Journal of Lipid Research. 2007;48:1781–1791. doi: 10.1194/jlr.M700119-JLR200. [DOI] [PubMed] [Google Scholar]
- 183.Brandes R., Kaikaus R.M., Lysenko N., Ockner R.K., Bass N.M. Induction of fatty acid binding protein by peroxisome proliferators in primary hepatocyte cultures and its relationship to the induction of peroxisomal beta-oxidation. Biochimica et Biophysica Acta. 1990;1034:53–61. doi: 10.1016/0304-4165(90)90152-m. [DOI] [PubMed] [Google Scholar]
- 184.Poirier H., Niot I., Monnot M.C., Braissant O., Meunier-Durmort C., Costet P. Differential involvement of peroxisome-proliferator-activated receptors alpha and delta in fibrate and fatty-acid-mediated inductions of the gene encoding liver fatty-acid-binding protein in the liver and the small intestine. Biochemical Journal. 2001;355:481–488. doi: 10.1042/0264-6021:3550481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Simon T.C., Roth K.A., Gordon J.I. Use of transgenic mice to map cis-acting elements in the liver fatty acid-binding protein gene (Fabpl) that regulate its cell lineage-specific, differentiation-dependent, and spatial patterns of expression in the gut epithelium and in the liver acinus. Journal of Biological Chemistry. 1993;268:18345–18358. [PubMed] [Google Scholar]
- 186.Storch J., McDermott L. Structural and functional analysis of fatty acid-binding proteins. Journal of Lipid Research. 2009;(Suppl. 50):S126–S131. doi: 10.1194/jlr.R800084-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fourcade S., Savary S., Albet S., Gauthe D., Gondcaille C., Pineau T. Fibrate induction of the adrenoleukodystrophy-related gene (ABCD2): promoter analysis and role of the peroxisome proliferator-activated receptor PPARalpha. European Journal of Biochemistry. 2001;268:3490–3500. doi: 10.1046/j.1432-1327.2001.02249.x. [DOI] [PubMed] [Google Scholar]
- 188.Rampler H., Weinhofer I., Netik A., Forss-Petter S., Brown P.J., Oplinger J.A. Evaluation of the therapeutic potential of PPARalpha agonists for X-linked adrenoleukodystrophy. Molecular Genetics and Metabolism. 2003;80:398–407. doi: 10.1016/j.ymgme.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 189.Leclercq S., Skrzypski J., Courvoisier A., Gondcaille C., Bonnetain F., Andre A. Effect of dietary polyunsaturated fatty acids on the expression of peroxisomal ABC transporters. Biochimie. 2008;90:1602–1607. doi: 10.1016/j.biochi.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 190.Hunt M.C., Solaas K., Kase B.F., Alexson S.E. Characterization of an acyl-coA thioesterase that functions as a major regulator of peroxisomal lipid metabolism. Journal of Biological Chemistry. 2002;277:1128–1138. doi: 10.1074/jbc.M106458200. [DOI] [PubMed] [Google Scholar]
- 191.Bardot O., Aldridge T.C., Latruffe N., Green S. PPAR-RXR heterodimer activates a peroxisome proliferator response element upstream of the bifunctional enzyme gene. Biochemical and Biophysical Research Communications. 1993;192:37–45. doi: 10.1006/bbrc.1993.1378. [DOI] [PubMed] [Google Scholar]
- 192.Marcus S.L., Miyata K.S., Zhang B., Subramani S., Rachubinski R.A., Capone J.P. Diverse peroxisome proliferator-activated receptors bind to the peroxisome proliferator-responsive elements of the rat hydratase/dehydrogenase and fatty acyl-CoA oxidase genes but differentially induce expression. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5723–5727. doi: 10.1073/pnas.90.12.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nicolas-Frances V., Dasari V.K., Abruzzi E., Osumi T., Latruffe N. The peroxisome proliferator response element (PPRE) present at positions -681/-669 in the rat liver 3-ketoacyl-CoA thiolase B gene functionally interacts differently with PPARalpha and HNF-4. Biochemical and Biophysical Research Communications. 2000;269:347–351. doi: 10.1006/bbrc.2000.2249. [DOI] [PubMed] [Google Scholar]
- 194.Shimizu M., Takeshita A., Tsukamoto T., Gonzalez F.J., Osumi T. Tissue-selective, bidirectional regulation of PEX11 alpha and perilipin genes through a common peroxisome proliferator response element. Molecular and Cellular Biology. 2004;24:1313–1323. doi: 10.1128/MCB.24.3.1313-1323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brandt J.M., Djouadi F., Kelly D.P. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. Journal of Biological Chemistry. 1998;273:23786–23792. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 196.Mascaro C., Acosta E., Ortiz J.A., Marrero P.F., Hegardt F.G., Haro D. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. Journal of Biological Chemistry. 1998;273:8560–8563. doi: 10.1074/jbc.273.15.8560. [DOI] [PubMed] [Google Scholar]
- 197.van Vlies N., Ferdinandusse S., Turkenburg M., Wanders R.J., Vaz F.M. PPAR alpha-activation results in enhanced carnitine biosynthesis and OCTN2-mediated hepatic carnitine accumulation. Biochimica et Biophysica Acta. 2007;1767:1134–1142. doi: 10.1016/j.bbabio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 198.Wen G., Ringseis R., Eder K. Mouse OCTN2 is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha) via a PPRE located in the first intron. Biochemical Pharmacology. 2010;79:768–776. doi: 10.1016/j.bcp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 199.Gulick T., Cresci S., Caira T., Moore D.D., Kelly D.P. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11012–11016. doi: 10.1073/pnas.91.23.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Frerman F.E. Acyl-CoA dehydrogenases, electron transfer flavoprotein and electron transfer flavoprotein dehydrogenase. Biochemical Society Transactions. 1988;16:416–418. doi: 10.1042/bst0160416. [DOI] [PubMed] [Google Scholar]
- 201.Rodriguez J.C., Gil-Gomez G., Hegardt F.G., Haro D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. Journal of Biological Chemistry. 1994;269:18767–18772. [PubMed] [Google Scholar]
- 202.Kroetz D.L., Yook P., Costet P., Bianchi P., Pineau T. Peroxisome proliferator-activated receptor alpha controls the hepatic CYP4A induction adaptive response to starvation and diabetes. Journal of Biological Chemistry. 1998;273:31581–31589. doi: 10.1074/jbc.273.47.31581. [DOI] [PubMed] [Google Scholar]
- 203.Muerhoff A.S., Griffin K.J., Johnson E.F. The peroxisome proliferator-activated receptor mediates the induction of CYP4A6, a cytochrome P450 fatty acid omega-hydroxylase, by clofibric acid. Journal of Biological Chemistry. 1992;267:19051–19053. [PubMed] [Google Scholar]
- 204.Wanders R.J., Komen J., Kemp S. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. FEBS Journal. 2011;278:182–194. doi: 10.1111/j.1742-4658.2010.07947.x. [DOI] [PubMed] [Google Scholar]
- 205.Miller C.W., Ntambi J.M. Peroxisome proliferators induce mouse liver stearoyl-CoA desaturase 1 gene expression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9443–9448. doi: 10.1073/pnas.93.18.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cherkaoui-Malki M., Meyer K., Cao W.Q., Latruffe N., Yeldandi A.V., Rao M.S. Identification of novel peroxisome proliferator-activated receptor alpha (PPARalpha) target genes in mouse liver using cDNA microarray analysis. Gene Expression. 2001;9:291–304. doi: 10.3727/000000001783992533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yu S., Cao W.Q., Kashireddy P., Meyer K., Jia Y., Hughes D.E. Human peroxisome proliferator-activated receptor alpha (PPARalpha) supports the induction of peroxisome proliferation in PPARalpha-deficient mouse liver. Journal of Biological Chemistry. 2001;276:42485–42491. doi: 10.1074/jbc.M106480200. [DOI] [PubMed] [Google Scholar]
- 208.Dalen K.T., Ulven S.M., Arntsen B.M., Solaas K., Nebb H.I. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. Journal of Lipid Research. 2006;47:931–943. doi: 10.1194/jlr.M500459-JLR200. [DOI] [PubMed] [Google Scholar]
- 209.Targett-Adams P., McElwee M.J., Ehrenborg E., Gustafsson M.C., Palmer C.N., McLauchlan J. A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochimica et Biophysica Acta. 2005;1728:95–104. doi: 10.1016/j.bbaexp.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 210.Dalen K.T., Schoonjans K., Ulven S.M., Weedon-Fekjaer M.S., Bentzen T.G., Koutnikova H. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes. 2004;53:1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 211.Dalen K.T., Dahl T., Holter E., Arntsen B., Londos C., Sztalryd C. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochimica et Biophysica Acta. 2007;1771:210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 212.Wolins N.E., Quaynor B.K., Skinner J.R., Tzekov A., Croce M.A., Gropler M.C. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 213.Kadereit B., Kumar P., Wang W.J., Miranda D., Snapp E.L., Severina N. Evolutionarily conserved gene family important for fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Viswakarma N., Yu S., Naik S., Kashireddy P., Matsumoto K., Sarkar J. Transcriptional regulation of Cidea, mitochondrial cell death-inducing DNA fragmentation factor alpha-like effector A, in mouse liver by peroxisome proliferator-activated receptor alpha and gamma. Journal of Biological Chemistry. 2007;282:18613–18624. doi: 10.1074/jbc.M701983200. [DOI] [PubMed] [Google Scholar]
- 215.Matsusue K., Kusakabe T., Noguchi T., Takiguchi S., Suzuki T., Yamano S. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metabolism. 2008;7:302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Costet P., Legendre C., More J., Edgar A., Galtier P., Pineau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. Journal of Biological Chemistry. 1998;273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- 217.Stienstra R., Mandard S., Patsouris D., Maass C., Kersten S., Muller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148:2753–2763. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]
- 218.Liu A., Krausz K.W., Fang Z.Z., Brocker C., Qu A., Gonzalez F.J. Gemfibrozil disrupts lysophosphatidylcholine and bile acid homeostasis via PPARalpha and its relevance to hepatotoxicity. Archives of Toxicology. 2014;88:983–996. doi: 10.1007/s00204-013-1188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yamazaki T., Wakabayashi M., Ikeda E., Tanaka S., Sakamoto T., Mitsumoto A. Induction of 1-acylglycerophosphocholine acyltransferase genes by fibrates in the liver of rats. Biological and Pharmaceutical Bulletin. 2012;35:1509–1515. doi: 10.1248/bpb.b12-00243. [DOI] [PubMed] [Google Scholar]
- 220.Kok T., Wolters H., Bloks V.W., Havinga R., Jansen P.L., Staels B. Induction of hepatic ABC transporter expression is part of the PPARalpha-mediated fasting response in the mouse. Gastroenterology. 2003;124:160–171. doi: 10.1053/gast.2003.50007. [DOI] [PubMed] [Google Scholar]
- 221.Ghonem N.S., Ananthanarayanan M., Soroka C.J., Boyer J.L. Peroxisome proliferator-activated receptor alpha activates human multidrug resistance transporter 3/ATP-binding cassette protein subfamily B4 transcription and increases rat biliary phosphatidylcholine secretion. Hepatology. 2013;59:1030–1042. doi: 10.1002/hep.26894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hunt M.C., Yang Y.Z., Eggertsen G., Carneheim C.M., Gafvels M., Einarsson C. The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. Journal of Biological Chemistry. 2000;275:28947–28953. doi: 10.1074/jbc.M002782200. [DOI] [PubMed] [Google Scholar]
- 223.Chinetti G., Lestavel S., Bocher V., Remaley A.T., Neve B., Torra I.P. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nature Medicine. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 224.Chinetti G., Gbaguidi F.G., Griglio S., Mallat Z., Antonucci M., Poulain P. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation. 2000;101:2411–2417. doi: 10.1161/01.cir.101.20.2411. [DOI] [PubMed] [Google Scholar]
- 225.Lopez D., McLean M.P. Activation of the rat scavenger receptor class B type I gene by PPARalpha. Molecular and Cellular Endocrinology. 2006;251:67–77. doi: 10.1016/j.mce.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 226.Mardones P., Pilon A., Bouly M., Duran D., Nishimoto T., Arai H. Fibrates down-regulate hepatic scavenger receptor class B type I protein expression in mice. Journal of Biological Chemistry. 2003;278:7884–7890. doi: 10.1074/jbc.M211627200. [DOI] [PubMed] [Google Scholar]
- 227.Bertolotti M., Concari M., Loria P., Abate N., Pinetti A., Guicciardi M.E. Effects of different phenotypes of hyperlipoproteinemia and of treatment with fibric acid derivatives on the rates of cholesterol 7 alpha-hydroxylation in humans. Arteriosclerosis Thrombosis and Vascular Biology. 1995;15:1064–1069. doi: 10.1161/01.atv.15.8.1064. [DOI] [PubMed] [Google Scholar]
- 228.Post S.M., Duez H., Gervois P.P., Staels B., Kuipers F., Princen H.M. Fibrates suppress bile acid synthesis via peroxisome proliferator-activated receptor-alpha-mediated downregulation of cholesterol 7alpha-hydroxylase and sterol 27-hydroxylase expression. Arteriosclerosis Thrombosis and Vascular Biology. 2001;21:1840–1845. doi: 10.1161/hq1101.098228. [DOI] [PubMed] [Google Scholar]
- 229.Stahlberg D., Angelin B., Einarsson K. Effects of treatment with clofibrate, bezafibrate, and ciprofibrate on the metabolism of cholesterol in rat liver microsomes. Journal of Lipid Research. 1989;30:953–958. [PubMed] [Google Scholar]
- 230.Stahlberg D., Reihner E., Rudling M., Berglund L., Einarsson K., Angelin B. Influence of bezafibrate on hepatic cholesterol metabolism in gallstone patients: reduced activity of cholesterol 7 alpha-hydroxylase. Hepatology. 1995;21:1025–1030. doi: 10.1002/hep.1840210421. [DOI] [PubMed] [Google Scholar]
- 231.Gbaguidi G.F., Agellon L.B. The inhibition of the human cholesterol 7alpha-hydroxylase gene (CYP7A1) promoter by fibrates in cultured cells is mediated via the liver X receptor alpha and peroxisome proliferator-activated receptor alpha heterodimer. Nucleic Acids Research. 2004;32:1113–1121. doi: 10.1093/nar/gkh260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Marrapodi M., Chiang J.Y. Peroxisome proliferator-activated receptor alpha (PPARalpha) and agonist inhibit cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. Journal of Lipid Research. 2000;41:514–520. [PubMed] [Google Scholar]
- 233.Li F., Patterson A.D., Krausz K.W., Tanaka N., Gonzalez F.J. Metabolomics reveals an essential role for peroxisome proliferator-activated receptor alpha in bile acid homeostasis. Journal of Lipid Research. 2012;53:1625–1635. doi: 10.1194/jlr.M027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li T., Chiang J.Y. Regulation of bile acid and cholesterol metabolism by PPARs. PPAR Research. 2009;2009:501739. doi: 10.1155/2009/501739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Szatmari I., Pap A., Ruhl R., Ma J.X., Illarionov P.A., Besra G.S. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. Journal of Experimental Medicine. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sun Y., Ng L., Lam W., Lo C.K., Chan P.T., Yuen Y.L. Identification and characterization of a novel mouse peroxisome proliferator-activated receptor alpha-regulated and starvation-induced gene, Ppsig. International Journal of Biochemistry and Cell Biology. 2008;40:1775–1791. doi: 10.1016/j.biocel.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 237.Jitrapakdee S., Slawik M., Medina-Gomez G., Campbell M., Wallace J.C., Sethi J.K. The peroxisome proliferator-activated receptor-gamma regulates murine pyruvate carboxylase gene expression in vivo and in vitro. Journal of Biological Chemistry. 2005;280:27466–27476. doi: 10.1074/jbc.M503836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.White H.M., Koser S.L., Donkin S.S. Differential regulation of bovine pyruvate carboxylase promoters by fatty acids and peroxisome proliferator-activated receptor-alpha agonist. Journal of Dairy Science. 2011;94:3428–3436. doi: 10.3168/jds.2010-3960. [DOI] [PubMed] [Google Scholar]
- 239.Wu P., Peters J.M., Harris R.A. Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor alpha. Biochemical and Biophysical Research Communications. 2001;287:391–396. doi: 10.1006/bbrc.2001.5608. [DOI] [PubMed] [Google Scholar]
- 240.Fisher F.M., Estall J.L., Adams A.C., Antonellis P.J., Bina H.A., Flier J.S. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jonker J.W., Suh J.M., Atkins A.R., Ahmadian M., Li P., Whyte J. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kersten S., Mandard S., Tan N.S., Escher P., Metzger D., Chambon P. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. Journal of Biological Chemistry. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 244.Rakhshandehroo M., Stienstra R., de Wit N.J., Bragt M.C., Haluzik M., Mensink R.P. Plasma mannose-binding lectin is stimulated by PPARalpha in humans. American Journal of Physiology – Endocrinology and Metabolism. 2012;302:E595–E602. doi: 10.1152/ajpendo.00299.2011. [DOI] [PubMed] [Google Scholar]
- 245.Mattijssen F., Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochimica et Biophysica Acta. 2012;1821:782–789. doi: 10.1016/j.bbalip.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 246.Tachibana K., Takeuchi K., Inada H., Sugimoto K., Ishimoto K., Yamashita M. Human mannose-binding lectin 2 is directly regulated by peroxisome proliferator-activated receptors via a peroxisome proliferator responsive element. Journal of Biochemistry. 2013;154:265–273. doi: 10.1093/jb/mvt050. [DOI] [PubMed] [Google Scholar]
- 247.Rommelaere S., Millet V., Gensollen T., Bourges C., Eeckhoute J., Hennuyer N. PPARalpha regulates the production of serum vanin-1 by liver. FEBS Letters. 2013;587:3742–3748. doi: 10.1016/j.febslet.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 248.van Diepen, J.A., Jansen, P.A., Ballak, D.B., Hijmans, A., Hooiveld, G.J., Mensink, R.P., et al. PPAR-alpha dependent regulation of Vanin-1 mediates hepatic lipid metabolism. Journal of Hepatology. In press. [DOI] [PubMed]
- 249.Blaauboer B.J., van Holsteijn C.W., Bleumink R., Mennes W.C., van Pelt F.N., Yap S.H. The effect of beclobric acid and clofibric acid on peroxisomal beta-oxidation and peroxisome proliferation in primary cultures of rat, monkey and human hepatocytes. Biochemical Pharmacology. 1990;40:521–528. doi: 10.1016/0006-2952(90)90551-u. [DOI] [PubMed] [Google Scholar]
- 250.Cattley R.C., DeLuca J., Elcombe C., Fenner-Crisp P., Lake B.G., Marsman D.S. Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans? Regulatory Toxicology and Pharmacology: RTP. 1998;27:47–60. doi: 10.1006/rtph.1997.1163. [DOI] [PubMed] [Google Scholar]
- 251.Lambe K.G., Woodyatt N.J., Macdonald N., Chevalier S., Roberts R.A. Species differences in sequence and activity of the peroxisome proliferator response element (PPRE) within the acyl CoA oxidase gene promoter. Toxicology Letters. 1999;110:119–127. doi: 10.1016/s0378-4274(99)00151-4. [DOI] [PubMed] [Google Scholar]
- 252.Vu-Dac N., Schoonjans K., Kosykh V., Dallongeville J., Fruchart J.C., Staels B. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. Journal of Clinical Investigation. 1995;96:741–750. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Prieur X., Coste H., Rodriguez J.C. The human apolipoprotein AV gene is regulated by peroxisome proliferator-activated receptor-alpha and contains a novel farnesoid X-activated receptor response element. Journal of Biological Chemistry. 2003;278:25468–25480. doi: 10.1074/jbc.M301302200. [DOI] [PubMed] [Google Scholar]
- 254.Schultze A.E., Alborn W.E., Newton R.K., Konrad R.J. Administration of a PPARalpha agonist increases serum apolipoprotein A-V levels and the apolipoprotein A-V/apolipoprotein C-III ratio. Journal of Lipid Research. 2005;46:1591–1595. doi: 10.1194/jlr.C500010-JLR200. [DOI] [PubMed] [Google Scholar]
- 255.Prieur X., Lesnik P., Moreau M., Rodriguez J.C., Doucet C., Chapman M.J. Differential regulation of the human versus the mouse apolipoprotein AV gene by PPARalpha. Implications for the study of pharmaceutical modifiers of hypertriglyceridemia in mice. Biochimica et Biophysica Acta. 2009;1791:764–771. doi: 10.1016/j.bbalip.2009.03.015. [DOI] [PubMed] [Google Scholar]


