Summary
The underlying molecular action of the novel uncoupling proteins 2 and 3 (UCP2 and UCP3) is still under debate. The proteins have been implicated in many cell functions, including the regulation of insulin secretion and regulation of reactive oxygen species (ROS) generation. These effects have mainly been explained by suggesting that the proteins establish a proton leak through the inner mitochondrial membrane (IMM). However, accumulating data question this mechanism and suggest that UCP2 and UCP3 may play other roles, including carrying free fatty acids from the matrix towards the intermembrane space, or contributing to the mitochondrial Ca2+ uniport. Accordingly, in this review we reflect on these actions of UCP2/UCP3 and discuss alternative explanations for the molecular mechanisms by which UCP2/UCP3 might contribute to aspects of cell function. Based on the potential role of UCP2/UCP3 in regulating mitochondrial Ca2+ uptake, we propose a scheme whereby these proteins integrate Ca2+-dependent signal transduction and energy metabolism in order to meet the energy demand of the cell for its continuous response, adaptation, and stimulation to environmental input.
Keywords: Ca2+ signaling, Diabetes, Fatty acid transport, Glucotoxicity, Lipid metabolism, Lipotoxicity, Mitochondria, Mitochondrial Ca2+ uniporter, Insulin secretion, Uncoupling proteins, UCP2, UCP3, ROS
Introduction
The original uncoupling protein, UCP1 (Link to HPRD), also called thermogenin, was first described in 1976 and was found to be almost exclusively expressed in the brown adipose tissue of mammals, where it is responsible for the inducible thermogenic activity of this tissue (for review see ref. [1]). UCP1 accounts for a proton (H+) influx through the inner mitochondrial membrane (IMM) along the H+ gradient that is established by the mitochondrial respiratory chain and thus, uncouples mitochondrial respiration from ATP production (ATP-synthase). Noteworthy, brown adipose tissue was found to contain very low amounts of ATP-synthase, compared to the components of the respiratory chain [2], thus, UCP1, which accounts for 10% of the protein mass in the IMM [1], is mainly responsible for the dissipation of the proton gradient at the IMM in this particular tissue. 21 years later, three groups found from databases of expressed sequence tags or from cDNA libraries two mRNAs encoding for two proteins that share 72% homology with each other and approximately 58% with UCP1 [3—5]. Based on this existing sequence homology with UCP1, the proteins were named uncoupling protein 2 (UCP2) and uncoupling protein 3 (UCP3) although no function could be attributed to them at this time. Meanwhile, for UCP3 two splice variants UCP3L (for long) and UCP3S (for short) were identified of which UCP3S lacks the sixth potential transmembrane region and the purine nucleotide binding domain [5].
In contrast to UCP1, the novel uncoupling proteins UCP2 and UCP3 were found in tiny concentrations of 0.01—0.1% of the mitochondrial membrane proteins in numerous tissues (UCP2: Link to HPRD; UCP3: Link to HPRD) [6]. Since both proteins are also expressed in ectothermic fish and plants that do not require thermogenesis an exclusive thermogenic function of UCP2 and UCP3 seemed unlikely and other molecular actions of UCP2 and UCP3 were sought. Following the original concept, UCP2 and UCP3 have been extensively studied in isolated systems in terms of their uncoupling potential. However, while these studies often report considerable uncoupling potential of these proteins, at least under certain conditions, controversial findings were obtained in intact cells. In this respect, Nedergaard and Cannon suggested that while the naming of UCP2 and UCP3 is comprehensible, considering their similarity to UCP1, in view of the accumulated experimental evidence that indicate that UCP2 and UCP3 most likely do not act as uncoupling proteins, this nomenclature might be misleading and may “direct thoughts towards the implied function” [7]. Indeed, despite experimental evidence of alternative functions of UCP2 and UCP3 other than uncoupling by forming a H+ channel, many reports that deal with these proteins discuss the contribution of UCP2 and UCP3 entirely on basis of their uncoupling functions. Very recently we made some novel and totally unexpected observations that suggested alternative (or additional) functions for UCP2 and UCP3. Using overexpression, knock-down techniques and UCP2−/− animals we found that both proteins are required for mitochondrial Ca2+ sequestration through the classical mitochondrial Ca2+ uniport [8]. Most of this work was performed in intact cells following stimulation with agonists which trigger an increase of the cytosolic Ca2+ concentration. Although it remains to be proved whether or not this Ca2+ function of UCP2 and UCP3 is the main physiological task of these proteins, these findings question the physiological role of UCP2 and UCP3.
Consequently, in this review, we challenge the uncoupling function of UCP2 and UCP3 and attempt to provide alternative explanations for published phenomena that describe the role of these proteins in mitochondrial Ca2+ signaling.
Molecular functions of UCP2/UCP3
At first glance, there are at least three independent molecular actions reported for UCP2 and UCP3: (i) establishing a proton leak through the IMM, (ii) carrying free fatty acids from the matrix to the intermembrane space, and (iii) contributing to mitochondrial Ca2+ uniporter activity.
Establishing a proton leak
According to the chemiosmotic theory defined by Peter Mitchell, the oxidation of the reduced coenzymes NADH/H+ and FADH2 at complexes of the respiratory chain at the IMM is linked to the transport of protons from the matrix to the intermembrane space [9]. Thus, the electron transfer to molecular oxygen by protein complexes I—IV leads to an electrochemical gradient that establishes a negative membrane potential (Δψmito) of the IMM at around −180 mV. At this potential, protons flow back into the mitochondrial matrix via the F0-F1-ATPase and the released energy is utilized to phosphorylate ADP to ATP [10]. Notably, Δψmito is of utmost significance for most mitochondrial (bioenergetic) functions and constitutes the driving force for the influx of cations such as H+ and Ca2+ into these organelles (for reviews see refs. [11,12]). Since a total and continuing loss of Δψmito results inescapably in cell death, Δψmito is maintained by multiple processes (including the respiratory chain) that compensate any kind of disturbances.
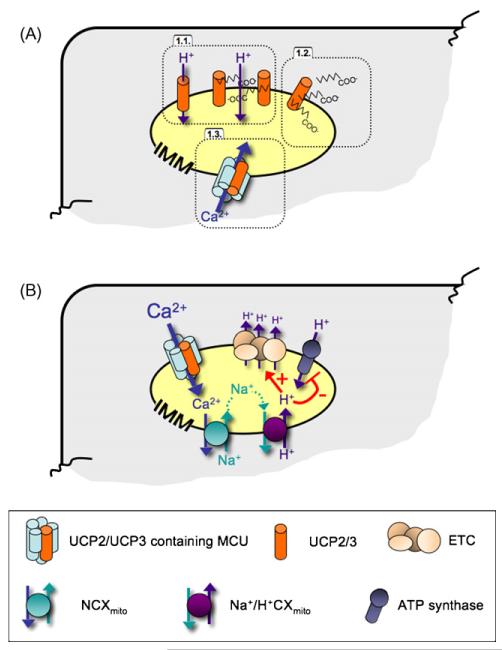
Once a high Δψmito is achieved, an additional increase of substrate supply cannot further activate respiration due to the high H+ gradient across the IMM [13]. Basically, proton influx through the ATP-synthase-complex counteracts the established high H+ gradient and thus, maintains activity of the respiratory chain. Accordingly, inhibition of ATP synthesis should cause a total loss of respiration. However, it has been demonstrated that isolated mitochondria still consume O2 even if the ATP-synthase is entirely inhibited [14], thus, indicating that the H+ gradient must be lowered via routes others than the ATP-synthase. To date, it is not entirely clear whether such a H+-leak across the IMM, which displays an energy dissipating process by “uncoupling” phosphorylation of ADP from respiration, is actively controlled by proteins or if the IMM is more or less passively permeable for H+ or small molecule H+-carriers such as free fatty acids. In this context Skulachev proposed that several proteins located at the IMM such as UCP1, the ATP/ADP translocator, the glutamate/aspartate antiporter and the dicarboxylate carrier facilitate the translocation of (fatty acid) anions (see Section ‘Free fatty acid carrier’) from the matrix to the intermembrane space [15]. Although the molecular mechanism by which the original uncoupling protein UCP1 induces heat generation by dissipation of the H+-gradient in brown adipose tissue is still elusive, it seems plausible that fatty acids are essential for stimulating uncoupling activity of UCP1 [16]. Currently, two distinct hypotheses exist, where the first suggests that UCP1 directly functions as an inward H+ carrier [17], whereas the other suggests that UCP1, as well as UCP2 and UCP3, the ATP/ADP translocator and the glutamate/aspartate carrier, are involved in a free fatty acid cycling mechanism [18—20] transporting free fatty acid anions from the matrix side to the intermembrane space (Fig. 1A). The H+ conductance is facilitated by the free fatty acid themselves, which are protonated on the outer leaflet of the inner membrane and pass through the membrane by a “flip-flop” mechanism, releasing protons in the mitochondrial matrix.
Figure 1.
Postulated molecular action of UCP2 and UCP3. (Panel A) The three proposed molecular actions of UCP2 and UCP3 are schematically illustrated as 1.1., establishing a H+-leak that is most likely dependent on free fatty acids, 1.2., free fatty acid export from the mitochondria, and 1.3., contributing to mitochondrial Ca2+ uniport. (Panel B) While Ca2+ stimulates mitochondrial ATP production due to its stimulatory effect on various dehydrogenases [51—55], excessive mitochondrial Ca2+ sequestration causes matrix acidification and depolarization that is due to the mitochondrial Na+/Ca2+ exchanger and the Na+/H+ exchanger that are activated subsequently to mitochondrial Ca2+ elevation. Accordingly, excessive mitochondrial Ca2+ uptake can cause uncoupling as a consequence of the mechanisms that lower matrix Ca2+ to avoid opening of the permeability transition pore, an initial step of mitochondriatriggered cell death.
It has been calculated that the H+-leak in mammalian liver and skeletal muscle accounts for approximately 20% of basal O2 consumption [21]. If uncoupling is considered as a controlled physiological process, it can be assumed that other proteins in the IMM are involved in this process in all tissues lacking UCP1. The two UCP1 homologues, UCP2 and UCP3 are expressed in a variety of tissues [3—5]. Notwithstanding, a strong uncoupling function of these novel UCPs under physiological conditions could not be demonstrated in intact cells so far. This is partially due to the fact that an excessive, unphysiological overexpression of any (misfolded) protein located at the IMM obviously has the potency to enhance respiration by increasing the H+-leak across the IMM artificially [22,23]. Hence extensive research on the molecular function of UCP2 and UCP3 performed in recent years, including data from knock-down approaches, remain controversial in terms of the participation of UCP2 and UCP3 in uncoupling and associated thermogenesis. Indeed, several reports exclude a typical uncoupling function of UCP2 or UCP3 [24—26]. On the other hand, a reduced H+-leak and increased ATP levels at least in some tissues of UCP2 or UCP3 ablated mice have been observed, consistent with suggestions that these proteins facilitate uncoupling of respiring mitochondria [27—29]. Notably, there is some evidence that UCP2 and UCP3 accomplish mild uncoupling, which is a term that implies a small increase in H+-leak associated with a slight reduction of the H+-gradient with little or no effect on ATP production [30]. Moreover, it has been suggested that mild uncoupling is activated by ROS and reactive alkenals such as 4-hydroxynonenal and represents a negative feedback to mitigate mitochondrial ROS generation (see Section ‘Defense of mitochondrial ROS’) [31].
Mitochondrial uncoupling, the dissipation of the H+-gradient of the IMM resulting in enhanced respiration not coupled to chemical or osmotic work but associated with heat production, can be scrutinized adequately only under rather unphysiological conditions using suspended isolated mitochondria [32] or liposomes [33] and in the presence of certain substrates and toxins. Accordingly the contribution of UCP2 and UCP3 to mitochondrial uncoupling in intact cells or tissues, and the relevance of this process for physiology and pathophysiology remains unclear and requires further investigations.
Free fatty acid carrier
Uncoupling proteins are inhibited by purine nucleotides and activated by free fatty acids (for review see ref. [34]). The interaction of fatty acids with UCP1 is very well characterized in mitochondria from brown adipose tissue, but was also shown for members of the mitochondrial anion carrier protein family, e.g. ANT or the glutamate/aspartate carrier (see review ref. [15]). These proteins are considered to mediate a purine nucleotide-sensitive uniport of monovalent unipolar anions, including anionic fatty acids. The transport of protonated fatty acids from the intermembrane space to the mitochondrial matrix and the subsequent release of the H+ from the polar fatty acid headgroup leads to net H+ uniport and uncoupling [18,35,36]. Moreover, the export of excessive free fatty acids from the mitochondrial matrix by UCP2/UCP3 has been suggested to rescue mitochondria from an overload of free fatty acids (the ‘rescue hypothesis’ of Schrauwen [37—39]) (Fig. 1A).
Mitochondrial Ca2+ uptake
The contribution of mitochondria to cellular signaling cascades as well as most of the organelle’s own functions are strikingly linked to mitochondrial Ca2+ (for review see ref. [12]). Mitochondrial Ca2+ homeostasis is concertedly accomplished by multiple Ca2+ channels, pumps and exchangers which, remarkably, mostly remain genetically unidentified. We have recently demonstrated that UCP2 and UCP3 are fundamentally involved in mitochondrial Ca2+ uniport (MCU; [8]), the process that represents the main Ca2+ sequestration pathway into the mitochondrial matrix [40,41] (for review see ref. [12]). This conclusion is based on the following experimental findings (Table 1):
siRNA-triggered knock-down of UCP2/UCP3 abolishes mitochondrial Ca2+ accumulation in response to cytosolic Ca2+ elevation/Ca2+ entry in intact cells.
Overexpression of either UCP2 or UCP3 was associated with enhanced mitochondrial Ca2+ accumulation in response to cell stimulation.
Isolated liver mitochondria of UCP2−/− mice lacked ruthenium red-sensitive mitochondrial Ca2+ uptake, while it was present in the liver mitochondria of wild type littermates that contain UCP2 mRNA and protein [8] (Fig. 1A). Notably, in isolated liver mitochondria of UCP2−/− mice, a ruthenium red-insensitive mitochondrial Ca2+ uptake that exhibited approximately 50% of that found in liver mitochondria of wild type littermates was found. Whether this phenomenon represents an adaptation of the tissue in response to the lack of UCP2 or is the reason for the lack of a strong phenotype of the knock-out mice is unclear.
Site-directed mutagenesis of UCP2 and UCP3 revealed the formation of dominant negative proteins that competitively counteracted the Ca2+ function of the wild type proteins.
Table 1.
Overview on the reported evidences for an involvement of UCP2 and UCP3 in mitochondrial Ca2+ uniport [8]
| Model* | Overexpression | siRNA | UCP2−/− mouse (liver) |
|---|---|---|---|
| Basal mitochondrial Ca2+ | Unchanged | Unchanged | n.d. |
| Mitochondrial Ca2+ uptake | Increased (even under de-polarized conditions) | Strongly reduced (rescue by UCP2 in UCP3 cells treated with siRNA against UCP3) | Absent (no ruthenium red-sensitive Ca2+ entry) |
| Capacity of the MCU | Increased (even under depolarized conditions) | Strongly reduced | Reduced |
| Ca2+ sensitivity of MCU | Unchanged | n.d. | n.d. |
| Basal Δψmito | Unchanged | Unchanged | Unchanged |
| Δψmito upon stimulation/Ca2+ exposure | Unchanged | Unchanged | Unchanged |
| Basal pHmito | Unchanged | Unchanged | n.d. |
| pHmito upon stimulation | Decreased due to elevated [Ca2+]mito | Unchanged | n.d. |
| Basal ATP levels | Unchanged | n.d. | n.d. |
| ATP synthesis | Increased | n.d. | n.d. |
| Basal cytosolic Ca2+ concentration | Unchanged | Unchanged | n.d. |
| Intracellular Ca2+ mobilization | Unchanged | Unchanged | n.d. |
| Mitochondrial structure | Unchanged | Unchanged | n.d. |
| Focal contacts with the ER | Unchanged | Unchanged | n.d. |
| ER Ca2+ content | Unchanged | Unchanged | n.d. |
Most experiments were performed in human endothelial cells but were verified in HeLa, Hek293 and CHO cells.
Consistent with these findings, UCP2 and UCP3 were described to be activated by fatty acids and blocked by nucleotides [36,42,43], a phenomenon that was also recently reported for the ruthenium red-sensitive mitochondrial Ca2+ uniporter [44,45].
The findings on the contribution of UCP2/UCP3 to mitochondrial Ca2+ uptake stand against the dogma that UCP2/UCP3 depolarize mitochondria as this effect should reduce the electrical driving force for Ca2+ accumulation, and thus on would expect mitochondrial Ca2+ uptake to be decreased if UCPs are overexpressed. In contrast to UCP2 and UCP3, overexpression of UCP4 reduced store-operated mitochondrial Ca2+ influx in PC12 cells [46]. Although neither mitochondrial depolarization nor uncoupling activity of UCP4 in the intact PC12 cells was measured so far, the molecular action of UCP4 that underlies its inhibitory potential on store-operated Ca2+ influx in PC12 cells may point to an uncoupling potential of this more recently described UCP homologue [47].
The controversy on the molecular action of UCP2 and UCP3 is fueled by multiple reports that describe convincingly an uncoupling activity of these proteins in isolated membranes or bilayers while such activity could not be directly measured in intact cells [19,23,34,42]. Consequently, UCP2/UCP3 have been postulated as carriers of ions other than H+ that do not cause uncoupling, but control mitochondrial metabolic activity in CHO cells [48].
Strikingly, heterologous expression of human UCP3 in yeast did not reveal any evidence for a uncoupling activity of this protein and lead the authors to conclude “that the uncoupling of yeast mitochondria by high levels of UCP3 expression is entirely an artifact and provides no evidence for any native uncoupling activity of the protein” [23]. However, the same yeast models for heterologous expression of either UCP2 or UCP3 exhibited a strong ruthenium red-insensitive mitochondrial Ca2+ accumulation upon extramitochondrial Ca2+ while the heterologous expression of UCP2/UCP3 failed to establish ruthenium red-sensitive mitochondrial Ca2+ uniport in these yeast models [8]. Accordingly, expression of UCP2/UCP3 in yeast could not provide any support for either uncoupling or Ca2+ function of these UCPs. Whether yeast, which does not express an UCP homologue, lacks further proteins that are essential to establish either the ruthenium red-sensitive mitochondrial Ca2+ uniporter or H+ conductance, or the molecular action of UCP2/UCP3 are distinct from these postulated activities is unclear and needs further attention.
Overall, the contribution of UCP2 and UCP3 to mitochondrial Ca2+ uptake (Fig. 1A) brings some light to the understanding of the molecular action of UCP2 and UCP3 while additional studies are necessary to further approve the “Ca2+ function” of these proteins. However, in view of the recent data and the ongoing uncertainties whether or not UCP2 and UCP3 uncouple mitochondria in intact cells (see Section ‘Establishing a proton leak’), their effect on mitochondrial Ca2+ signaling needs to be considered in the attempt to explain the underlying molecular mechanisms of the physiological effects of UCP2 and UCP3.
Does (UCP2/UCP3-mediated) mitochondrial Ca2+ flux mimic uncoupling?
In view of the increasing evidence that UCP2 and UCP3 do not exhibit uncoupling function under physiological conditions, the nomenclature of these two proteins, which originally stems from their similarities with UCP1, was claimed to be inappropriate by several authors (e.g. ref. [7]). However, the recent findings on the contribution of UCP2 and UCP3 to mitochondrial Ca2+ uptake provides a mechanistic scenario in which these proteins indeed should yield (mild) uncoupling as functional consequence of mitochondrial Ca2+ accumulation. In most cell types, the extrusion of elevated mitochondrial Ca2+ is achieved by the mitochondrial Na+/Ca2+ exchanger (NCXmito; [49,50]). Subsequently, Na+ is extruded by a, so far unidentified Na+/H+ exchanger, which causes mitochondrial acidification, diminishing the H+ gradient across the IMM [12] (Figs. 1B and 2). Accordingly, Ca2+-dependent acidification of the mitochondrial matrix increases the activity of the respiratory chain without increasing ATP formation, as the reduction of the H+ gradient reduces the activity of the ATP-synthase. Since, by definition an increase in the activity of the respiratory chain that does not result in “useful work”, such as enhanced ATP formation is referred as uncoupling [15], Thus, the Ca2+ uptake function of UCP2/UCP3 may indeed yield mitochondrial uncoupling although these proteins may not exhibit a H+ conductance directly.
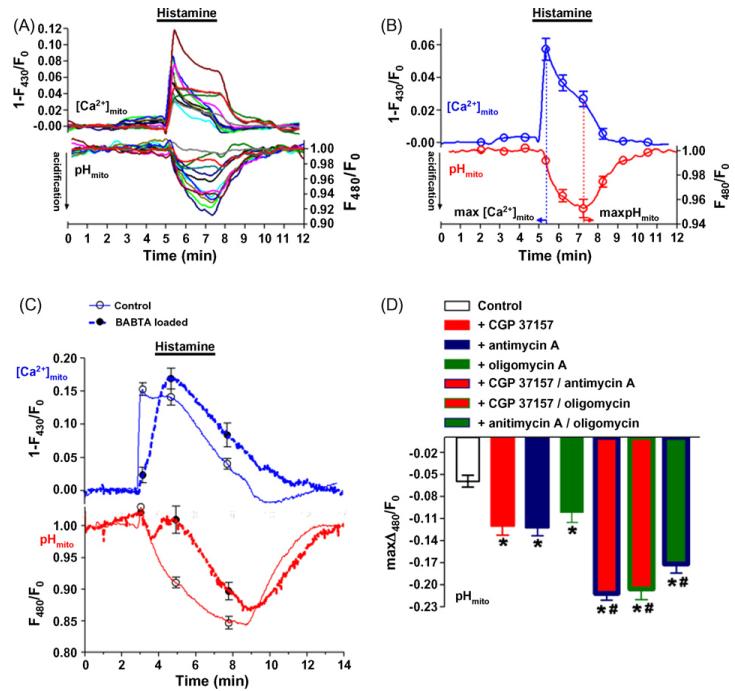
Figure 2.
Mitochondrial Ca2+ fluxes are accompanied by alterations in the pH of the mitochondrial matrix. The use of mitochondrial targeted ratiometric pericam allows simultaneous measurements of [Ca2+]mito and changes of the pH of the mitochondrial matrix at the level of the single cell. EA.hy926 cells stably expressing RPmt were alternatively illuminated at 430 and 480 nm and emission was collected at 535 nm using a wide field imaging system [49,50]. As the signals at an excitation of 430 nm are largely insensitive to changing pH, but change promptly upon variations of the mitochondrial free Ca2+ concentration, these signals can be used to measure the time course of [Ca2+]mito-fluxes. In contrast, at an excitation of 480 nm the signal mainly follows changes of the mitochondrial matrix pH, whereas alterations of [Ca2+]mito only marginally impact these values. (Panel A) Changes in [Ca2+]mito of 14 different cells from 3 independent experiments upon stimulation with 100 μM histamine in the presence of 2 mM extracellular Ca2+ are presented (upper panel). The corresponding (same color) alterations of mitochondrial luminal pH are shown in the lower panel. (Panel B) Comparison of the kinetics of changes in [Ca2+]mito (upper panel) with alterations in intramitochondrial pH (lower panel) indicate that following stimulation, mitochondrial Ca2+ elevation precedes mitochondrial acidification. (Panel C) The causative role of [Ca2+]mito in triggering mitochondrial acidification becomes further evident by a comparison of the effect of histamine on mitochondrial Ca2+ and pH in control cells (continuous line; n = 11) with BAPTA/am loaded cells (dotted lines; n = 12). Ca2+ chelation slowed the kinetics of both parameters, while the sequence of appearance remained unchanged. (Panel D) The histamine-induced mitochondrial acidification (control; n = 6) was evaluated under different conditions using pharmacological tools. Inhibition of NCXmito with 20 μM CGP 37157 strongly increased mitochondrial acidification (red column; n = 8). Similar findings were obtained using 10 μM antimycin A, a selective inhibitor of complex III (blue column, n = 9) or 5 μM oligomycin, an inhibitor of the ATP-synthase (green column, n = 9). These effects were amplified by combinations of the respecitive chemical compounds (CGP 37157/antimycin A, n = 8; CGP 37157/oligomycin, n = 8; antimycin A/oligomycin; n = 9).
Notably, such Ca2+-mediated mitochondrial uncoupling counteracts the stimulatory effect of Ca2+ on the activity of the mitochondrial dehydrogenases [51—55]. The outcome of this bidirectional regulation of mitochondrial ATP formation by Ca2+ might depend on the amount of Ca2+ sequestered by the mitochondria and may be stimulatory at lower and inhibitory at (pathologically) high intraluminal Ca2+ concentrations (see Section ‘UCP2 and islet function’).
Physiological functions of UCP2/UCP3 and their implication in pathology
Although the molecular mechanism(s) of UCP2 and UCP3 is/are still under debate there are some identified physiological functions that point to the importance of UCP2 and UCP3 in human physiology and pathology. As the physiological functions of UCP2 and UCP3 have been extensively reviewed recently [7,19,42,56—66], herein we selected the most prominent functions of these proteins and reconsider the suggested explanation in view of the Ca2+ function of UCP2 and UCP3: (i) islet function, (ii) defence of mitochondrial ROS production, and (iii) free fatty acid metabolism.
UCP2 and islet function
The oxidative metabolism of d-glucose and mitochondrial ATP production represent the key phenomenon that couples d-glucose concentration with pancreatic insulin secretion, a process referred as glucose-stimulated insulin secretion (GSIS) (for review see ref. [67]). Thus, d-glucose-induced mitochondrial ATP production that results in an elevation of cytoplasmic ATP/ADP ratio triggers insulin secretion both by blocking KATP-dependent channels and by KATP-independent mechanisms [67,68]. In view of the regulatory role of mitochondrial ATP production for GSIS, any mechanism that reduces the efficiency of mitochondrial ATP production at given activity of the respiratory chain (i.e. uncoupling) will inevitably impair insulin secretion. Since, UCP2 is normally expressed in both human and rodent pancreatic beta cells[69,70], a role of UCP2 as a negative regulator for pancreatic insulin section was proposed [70].
Evidence
An inhibitory role of UCP2 for GSIS has been described in many reports in which GSIS was reduced under conditions of upregulated UCP2 expression [69—71], while the absence of UCP2 enhances GSIS [28,72]. Moreover, an upregulation in UCP2 expression that was associated with signs of mitochondrial uncoupling was described after long term exposure of beta cells to high concentrations of fatty acids [73—75] but not by nutrition oversupply (i.e. fatty acids plus high d-glucose) or high d-glucose alone [74]). On the other hand, UCP2−/− mice have increased circulating insulin levels and isolated pancreatic islets from these mice secrete more insulin and show a higher ATP/ADP ratio in response to d-glucose [28]. Consistently, viral expression of UCP2 reversed these effects in beta cells isolated from UCP2−/− mice [76].
The controversy in explaining the molecular mechanisms of UCP2 in its contribution to GSIS
Most of these reports fuel the hypothesis that UCP2-mediated uncoupling reduces mitochondrial ATP production. As a consequence of the reduced cytosolic ATP/ADP ratio, KATP channels are insufficiently inhibited and thus, membrane depolarization is inadequate to trigger Ca2+ influx through voltage gated Ca2+ channels, which is essential for ample insulin secretion [63,68]. This concept was recently revisited as superoxide anions were found to activate UCP2 and to enhance their mitochondrial depolarizing activity[30,77,78]. Since superoxide anions are generated under overflow conditions by the respiratory chain, it was suggested that metabolism-triggered ROS production under conditions of long term high fatty acid exposure might be responsible for beta cell dysfunction and the inhibitory action of UCP2 on GSIS (for review see refs. [42,63,79,80]).
The concept was further elaborated by the introduction of free fatty acids into the model (see Section ‘Free fatty acid metabolism’). In a recent study using planar lipid bilayers, UCP1 and UCP2 were found to be activated by polyunsaturated fatty acids while saturated fatty acids had much less effect [81]. In view of the potential of fatty acids to initiate UCP2-dependent decrease in GSIS as well as upregulation of UCP2 expression [82,83], these findings further add to the idea that under metabolic overload, increased mitochondrial ROS production and fatty acid content stimulate UCP2, leading to uncoupling and reduced ATP production, eventually causing reduced GSIS[42,68,80].
While these findings are consistent with an uncoupling function of UCP2, not all studies that dealt with the effect of an overexpression of UCP2 in pancreatic beta cells revealed evidence for an UCP2-triggered uncoupling that results in a decrease in GSIS [84,85]. In transgenic mice with pancreaticspecific overexpression of UCP2 and in a respective beta cell model, d-glucose-induced ATP production and GSIS were comparable with that of control animals [84]. Moreover, no differences in resting and d-glucose-stimulated changes inΔψmito were found in beta cells of UCP2 overexpressing vs. control mice, thus, indicating that an UCP2 overexpression per se might not cause uncoupling either in vivo or in situ [84].
Thus, the findings regarding the molecular action of UCP2 in GSIS drawn from these recent reports from pancreaticspecific UCP2 overexpressing mice as well as the respective beta cell model [84] contradict the explanation derived from experiments in UCP2−/− mice [28].
It is notable that the overall ablation of UCP2−/− in mice results in slightly elevated plasma levels of inflammatory cytokines (e.g. TNFα or IFNγ) and persistent NFκB activation [86] that has been found to enhance beta cell function and insulin secretion [87]. In line with this report, attenuation of NFκB activation reduced GSIS and the expression of proteins involved in d-glucose metabolism and insulin secretion [88]. In view of these findings, Produit-Zangaffinen et al. suggested that increased GSIS in the UCP2−/− mice results from the activation of beta cells by NFκB rather than through a direct effect of the UCP2 on beta cell function [84].
However, the lack of any sign of mitochondrial uncoupling and reduction of GSIS by UCP2 overexpression per se [84] might depend on the expression level of this protein and the molecular action of moderately overexpressed UCP2 may differ from that of a supraphysiologically expressed protein [23,42]. Nevertheless, there was a striking effect of UCP2 overexpression on cytokine-induced mitochondrial ROS production in the same model [84] that is further supported by a report of the same group in which an attenuation of cytokine-induced beta cell death by increased UCP2 was described [89].
Overall, although the involvement of UCP2 in the regulation of GSIS is clear, the molecular action(s) by which UCP2 contributes to this phenomenon is still under debate and the controversy of so far reported findings may require further inputs to be finally solved.
Can the Ca2+ function of UCP2/UCP3 help resolve the inconsistencies between recent reports on the molecular action of UCP2 in GSIS?
Excessive mitochondrial Ca2+ counteracts mitochondrial ATP availability
Ca2+ plays a crucial role in GSIS. Insulin secretion depends predominantly on an elevation of cytosolic free Ca2+ [90,91] and even follows cytosolic Ca2+ oscillations [90,92]. Mechanistically, elevated cytosolic Ca2+ raises mitochondrial [Ca2+ ], which increases mitochondrial ATP production ([93] for review see refs. [12,80]) and in turn, enhances insulin release. The stimulatory effect of Ca2+ on mitochondrial ATP production is thought to be mainly mediated via stimulation of several matrix dehydrogenases (pyruvate dehydrogenase, isocitrate dehydrogenase, and 2-oxoglutarate dehydrogenase [51—55]; for review see refs.[12,80]). In line with these observations and recent findings that demonstrated an involvement of UCP2 (and UCP3) in mitochondrial Ca2+ uptake [8], a moderate elevation of mitochondrial Ca2+ uniporter activity as a result of UCP2 (and UCP3) overexpression yields elevated ATP production upon exposure to histamine [8] an agonist that raises [Ca2+ ]c via production of inositol 1,4,5-trisphosphate (Fig. 3B, upper panel).
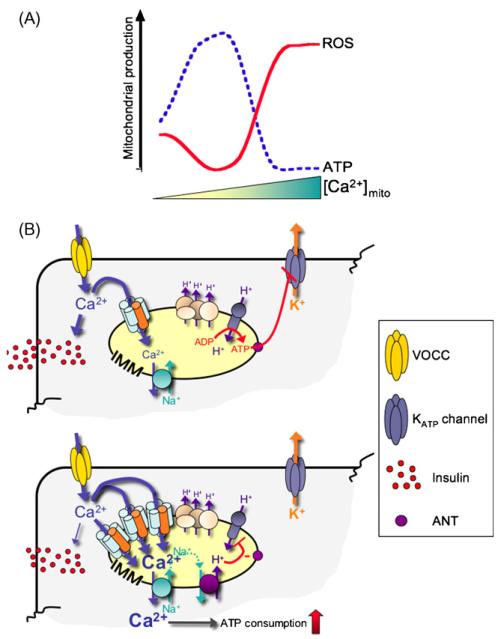
Figure 3.
Effect of mitochondrial Ca2+ on the energy balance of the cell and its subsequent effect on d-glucose-stimulated insulin secretion. (Panel A) We propose that increasing matrix [Ca2+] may cause a biphasic change in mitochondrial ATP and ROS production. At physiological Ca2+ elevations in the mitochondrial matrix, Ca2+ increases ATP formation and reduces ROS by activation of, e.g. MnSOD. However, at higher, presumably pathological Ca2+ elevations in the mitochondrial matrix, mitochondrial Ca2+ promotes ROS formation possibly via supramaximal stimulation of matrix dehydrogenases [162]. (Panel B, upper scheme) Ca2+ stimulates GSIS by two mitochondria dependent pathways. The first encompasses the stimulatory effect of matrix Ca2+ on ATP production that results in an elevated cytosolic ATP level, which, in turn, inhibits ATP-sensitive K+ channels (KATP channels). This results in a depolarization of the cell membrane and consequently, activation of voltage gated Ca2+ channels (VOCC). In addition, entering Ca2+ directly stimulates insulin secretion. (Lower scheme) Upregulation of UCP2-dependent mitochondrial Ca2+ uniporter under conditions of high nutrition overflow, could increase the Ca2+ buffering capacity of subplasmalemmal mitochondria, buffering of Ca2+ influx and consequently, reducing Ca2+-dependent insulin secretion. Moreover high [Ca2+]c increases the cell’s ATP consumption and causes mitochondrial uncoupling via excessive Ca2+ sequestration into the organelle, decreasing cytosolic ATP and so reducing insulin secretion.
The findings on the impact of UCP2 (and UCP3) on mitochondrial Ca2+ and ATP production are contradictory to an uncoupling function of these proteins (see Section ‘Establishing a proton leak’). Nevertheless, excessive mitochondrial Ca2+ sequestration via the UCP2/UCP3-containing Ca2+ uniporter might represent the turning point that converts mitochondrial Ca2+ to an inhibitory executer that prevents mitochondrial ATP production.
Since ROS promote mitochondrial Ca2+ accumulation [94] and stimulate UCP2/UCP3 activity (for review see ref. [42]), excessive mitochondrial Ca2+ accumulation might occur under physiological conditions of elevated ROS production, which increases in response to metabolic stimulation (see Section ‘Defense of mitochondrial ROS production’). Thus, as an alternative explanation of the involvement of UCP2 in GSIS, increased substrate supply that promotes electron overflow, triggering mitochondrial ROS production, might activate a UCP2-containing mitochondrial Ca2+ uniporter resulting in an robust mitochondrial Ca2+ accumulation sufficient to reduce cellular ATP levels by (i) Ca2+-stimulated ATP hydrolysis [95,96], (ii) initiation of a futile, ATP consuming mitochondrial Ca2+ cycling [97—99], (iii) a Ca2+-dependent feedback inhibition of glycolytic flux[100] and/or, (iv) Ca2+-initiated uncoupling (see Section ‘Does (UCP2/UCP3-mediated) mitochondrial Ca2+ flux mimic uncoupling?’) (Fig. 3A). Notably, such a switch from a stimulatory effect of mitochondrial Ca2+ on ATP production to an inhibitory action has been also postulated recently in a model for mitochondrial ATP production [101] (Fig. 3A). Overall, such Ca2+-dependent reduction in ATP production and/or increased ATP consumption will decrease GSIS. Thus this hypothesis may provide an explanation for the molecular action of UCP2 in GSIS that is consistent with all existing data so far,
Increased mitochondrial Ca2+ uptake suppresses cytosolic Ca2+ signaling
Besides the reversed metabolic action of pathologically elevated mitochondrial Ca2+ concentration [102] following overexpression of UCP2 to explain reduced GSIS in the respective models, mitochondrial Ca2+ uptake exhibits outstanding importance for shaping cytosolic and local Ca2+ signaling that controls multiple cell functions including insulin secretion (for review see refs. [12,103—105]). Intriguingly, mitochondria exhibit spatial Ca2+ buffering in distinct area of the cytosol. In many excitable cells including cardiac myocytes, chromaffin cells and pancreas acinar cells, subplasmalemmal mitochondria buffer Ca2+ entering the cell by local Ca2+ sequestration, a phenomenon referred to as a “mitochondrial firewall” (cited from a talk of Martin Bootman [106]) that delays, diminishes or shapes cytosolic Ca2+ signaling and its accompanied cell functions [107—109]. Accordingly, in view of the involvement of UCP2 in mitochondrial Ca2+ uptake, upregulation of UCP2 upon substrate overload in beta cells might result in an enhanced Ca2+ sequestration competence of the mitochondria. Accordingly, one might hypothesize that augmented subplasmalemmal Ca2+ buffering by superficial mitochondria, might diminish local cytosolic Ca2+-dependent processes such like vesicle transport or insulin secretion, thus, leading to a reduction of GSIS (Fig. 3B, lower panel).
Involvement of ROS in the regulatory role of UCP2 for GSIS
Recently mitochondrial ROS were found to be required for glucose/nutrition sensing of various tissues [84,110,111]. In view of these reports and the accumulating evidence that point to an involvement of UCP2 in the regulation of mitochondrial ROS production (see Section ‘Defense of mitochondrial ROS’), one might hypothesize that the regulatory role of UCP2 on insulin secretion may be associated, at least in part, with the protein’s effect on mitochondrial ROS production.
Conclusion
While there is overwhelming data that UCP2 is involved in the regulation of GSIS, the common concept on the underlying molecular mechanisms were recently challenged by increasing evidence that point to a lack of uncoupling activity of UCP2 under physiological conditions. Recent findings on the involvement of UCP2 in mitochondrial Ca2+ uptake and the Janus-faced contribution of mitochondrial Ca2+ to ATP availability might present an alternative explanation for the observed phenomena. Additional work is necessary to resolve the controversy on the molecular actions beyond the regulation of GSIS (Fig. 3B).
Defense of mitochondrial ROS production
The mitochondrial respiratory chain represents a major source of ROS [112]. It has been calculated that under basal conditions approximately 1—2% of the electrons transported by the complexes of the respiratory chain leak to produce superoxide anions [113]. The actual site of ROS generation within the complexes of the respiratory chain is not entirely clear, but complex I and complex III have attracted most attention [114,115]. ROS are potentially harmful agents that can induce severe cell damage, which has been associated with numerous diseases including diabetes, Parkinson’s disease, Alzheimer’s disease, and even ageing [58]. However, ROS are also involved in a variety of physiological signaling processes that are not linked to the development and progression of diseases [116,117]. Thus, mitochondrial generated ROS should not only be considered as toxic by-products of cellular metabolism [118,119]. Nevertheless, mitochondria are equipped with extensive ROS-defense machinery in order to balance the permanent generation of ROS and to protect against the malignant properties of ROS [120]. While mitochondrial SOD (SOD2, MnSOD) and glutathione represent the first defense line against mitochondrial ROS production [121], there is increasing evidence that UCP2 and UCP3 also significantly serve to attenuate mitochondrial ROS production [42,122]. Although the molecular mechanism behind this function of UCP2 and UCP3 is not entirely resolved, several explanations have been proposed.
Evidence
Strong evidence for an involvement of UCP2 and UCP3 in mitochondrial ROS defense comes from the phenotype of the respective knock-out mice. For instance, in UCP2−/− mice increased higher ROS markers have been described in liver[123], while pancreatic islet cells dysfunction is thought to be due to the increased ROS production in these animals [78]. In line with these findings, mice with bone morrowspecific UCP2 knock-out exhibit more oxidative stress and pronounced atherosclerosis [124]. Similar to the ablation of UCP2, UCP3 knock-out results in increased ROS generation in tissues in which it is normally expressed (e.g. skeletal muscle [29]). However, although UCPs are obviously involved in the control of mitochondrial ROS production, UCP2 and UCP3 ablated mice show neither a tendency to develop cancer nor do they have a reduced life span.
Surprisingly, only a few studies with increased levels of UCP2 or UCP3 confirm the contribution of these proteins to the suppression of mitochondrial ROS. Adenovirus-mediated overexpression of UCP2 in vascular smooth muscle cells reversed elevated ROS production in response to high d-glucose and angiotensin II [125], which led the authors to suggest that agents which increase UCP2 expression in vascular cells may prevent the development and progression of atherosclerosis. In L6 muscle cells, elevated UCP3 expression reduced mitochondrial ROS production without a measurable impact onΔψmito [126] while this effect was not obtained by others under similar conditions in the same cell type [127].
Explanation
UCPs per se do not catalyze ROS conversion to less or non-reactive compounds. Thus, the molecular function of UCP2 and UCP3 responsible for ROS mitigation seems to be accomplished by an impairment of ROS-generating processes rather than being directly involved in the decomposition of already generated ROS. It is generally believed that mitochondrial ROS generation is a function ofΔψmito [128,129]. Even under conditions of physiological pO2-levels a high Δψmito, in combination with a highly reduced electron transport chain and sufficient substrate availability, favors mitochondrial ROS formation. Hence, a reduction of Δψmito, possibly by increasing the H+-leak, was postulated as a mechanism whereby UCP2 and UCP3 may facilitate the electron flux through the respiratory chain resulting in decreased ROS formation (see Section ‘Establishing a proton leak’). Indeed, Skulachev and co-workers showed that chemical uncouplers efficiently reduce ROS production of isolated mitochondria [15,130] while in a recent study, contradictory data were described, as mild uncoupling using low doses of protonophore did not decrease mitochondrial superoxide anion levels in cultured rat cerebellar granule [131]. In line with these experiments, uncoupling does not affect mitochondrial ROS production in synaptic mitochondria[132].
In line with UCP-triggered uncoupling that counteracts ROS production, ROS-induced formation of 4-hydroxynonenal, which induces a mild uncoupling activity by UCPs was suggested [133].
Independently of any uncoupling activity, UCP2 and UCP3 protect against ROS by facilitating the transport of superoxide and hydroperoxyl radicals across the IMM (for review see ref. [7]).
Does the Ca2+ function of UCP2/UCP3 affect mitochondrial ROS production?
Although numerous studies have demonstrated that an excessive mitochondrial Ca2+ sequestration may increase mitochondrial ROS generation and even precipitate cell death [134—137], Ca2+ uptake into mitochondria per se may dissipate Δψmito (see Section ‘Does (UCP2/UCP3-mediated) mitochondrial Ca2+ flux mimic uncoupling?’) and thus, counteracts ROS generation [138,139]. Indeed, mitochondrial Ca2+ uptake is accompanied by a long lasting luminal acidification [8] that is due to the activity of either the Na+/Ca2+ exchanger followed by Na+/H+ exchange or the Ca2+/H+ exchanger (Fig. 2). In healthy cells, changes in Δψmito, are most likely transient [140], thus, pointing to a compensatory activity of the respiratory chain, which is activated via Ca2+ under such conditions. However, independently of a change in Δψmito, the reduced proton gradient through the IMM due to the accumulation of luminal protons is by definition ‘uncoupling’ and may also counteract ROS production.
As long as mitochondrial ATP production is ensured even under conditions of Ca2+-dependent uncoupling, the cell should behave as normal, while under conditions of decreased cellular ATP availability, the system may switch to enter the apoptosis/necrosis cell death pathways (see Section ‘Excessive mitochondrial Ca2+ counteracts mitochondrial ATP availability’).
In addition to Ca2+-triggered uncoupling, mitochondrial Ca2+ can modulate the activity of various enzymes of the antioxidant defense system and thus, counteract ROS production [141,142].
Conclusion
A correlation of UCP2/UCP3-dependent enhanced mitochondrial Ca2+ influx with mitochondrial ROS production has yet to be elucidated. However, in view of existing data regarding the effect of mitochondrial Ca2+ on pH and enzyme activity, the roles of UCP2 and UCP3 in regulating mitochondrial Ca2+ may provide mechanisms for the organization and capacity of mitochondrial antioxidant defences against excessive ROS production.
Free fatty acid metabolism
The ability of UCP2 and UCP3 to transport free fatty acid anions and their participation to the mitochondrial Ca2+ uniport [8] fit very well to the hypothesis that these proteins are involved in the regulation of free fatty acid metabolism. A role of UCP2/UCP3 in the mitochondrial export of excessive free fatty acids that are formed by matrix thioesterase, converting acyl-CoA to free fatty acid and CoA-SH has been postulated [143]. Alternatively, free fatty acids may physically enter the mitochondria as neutral protonated fatty acids by a so-called “flip-flop” mechanism [37—39]. This is associated with a simultaneous H+ uniport once UCP2/UCP3 export the fatty acid anions [35] (Fig. 4A). These may re-enter the β-oxidation cycle by being converted into acylcarnitine in the cytosol and are subsequently transported into the mitochondria through the carnitine palmitoyltransferase system.
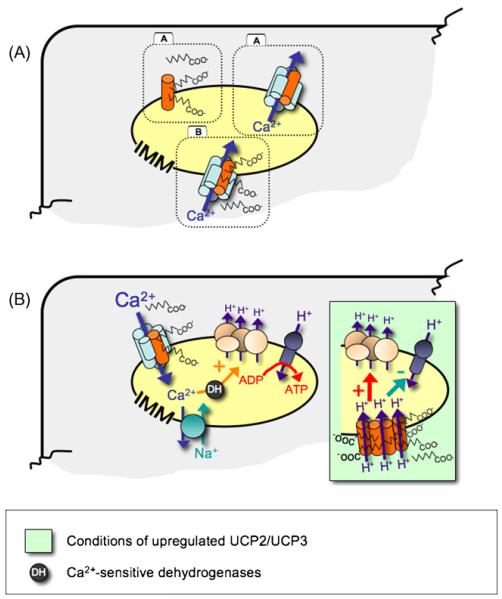
Figure 4.
Revised model of the molecular action of UCP2 and UCP3. (Panel A) A model is proposed in which UCP2 and UCP3 account for free fatty acid transport from the mitochondria and mitochondrial Ca2+ uniport. It remains to be investigated whether or not both molecular actions of UCP2/UCP3 are independent (A) or interrelated (B) functions of these proteins. (Panel B) Under physiological conditions we propose that UCP2 and UCP3 act as fatty acid carriers and as a fundamental part of the mitochondrial Ca2+ uniporter in so optimize and balance mitochondrial metabolic and signalling activity. However, upon nutrition overload, the upregulation of UCP2/UCP3 may disturb this homeostasis by, e.g. forming misfolded UCP proteins [22,23] and/or the lack of other, essential constituents of the mitochondrial Ca2+ uniporter [12], resulting in an accumulation of “free” UCP proteins in the membrane that may exhibit basal uncoupling activity, like many other members of the family of mitochondrial anion carrier protein family [15].
Evidence
Since UCP2/UCP3 gene expression is under the control of PPARs (peroxisome proliferator-activated receptors; for review see ref. [144]), and PPARs are important in the regulation of fat metabolism, it was suggested that UCP2 and UCP3 may play a role in fatty acid metabolism. Indeed, the expression of these proteins is upregulated under conditions of high fatty acid availability, such as obesity [145], response to fasting [57,145,146], high-fat diet [147], or acute exercise [148,149]. Moreover, UCP3 overexpressing mice showed increased fatty acid oxidation rates and decreased intramuscular triglyceride stores [146]. However, inconsistent results have been described in UCP3−/− mice, in which no effect on the muscular capacity to oxidize fatty acids was found[29,150].
Explanation
As outlined above evidence accumulated that point to a role of UCP2 and UCP3 in fatty acid metabolism by increasing fatty acid oxidation capacity. Notably, UCPs are more likely to transport fatty acid anions out of the mitochondrial matrix than directly translocating H+ and thus, might work as fatty acid anion transporters (for reviews see refs. [20,36]). Consistently, UCP3 was hypothesized to represent a rescue pathway for free fatty acids when mitochondria are facing excessive free fatty acid supply [143]. Under such conditions long-chain fatty acid (LCFA)-CoA accumulate in the mitochondrial matrix, decreasing availability of coenzyme A (CoA-SH), that, in turn, could prevent further oxidation of fatty acids and limit TCA cycle activity. Hence, UCP2/UCP3 may concertedly work with the mitochondrial thioesterase in the liberation of free fatty acid anions in the matrix. The free fatty acid anions are then exported by UCP2/UCP3 and reactivated by acyl-CoA synthase in the intermembrane space. Export of fatty acid anions from the matrix by UCP2/UCP3 would not only allow greater rates of fatty acid oxidation but may also reduce Δψmito and thereby diminish ROS production.
Alternatively, free fatty acids that passively cross the IMM once they get protonated might be eliminated by UCP2/UCP3 as anions and thus protect mitochondria against lipid-induced damage (lipotoxicity) [37—39].
Does the role of UCP2/UCP3 in mitochondrial Ca2+ uptake fit with their involvement in free fatty acid metabolism?
The PPAR-dependent expression of UCP2/UCP3 may point to a coherence of the molecular function of these proteins and fatty acid metabolism. Long before the discovery of UCP2 and UCP3, it was reported that mitochondria from brown adipose tissue and liver in obese ob/ob mice have enhanced Ca2+ transport capacities while Δψmito was unaffected [151]. In view of the stimulatory effect of matrix Ca2+ on the rate-limiting dehydrogenases (for review see ref. [52]), mitochondrial Ca2+ may increase the rate of fatty acid β-oxidation in the mitochondria.
Mitochondrial fatty acid anion export and Ca2+ import represent two intriguing putative molecular functions of UCP2 and UCP3. This aspect generates several additional questions and hypotheses that may deserve further investigations: is there a direct link between these two proposed functions? Does the activity of the UCP2/UCP3-dependent mitochondrial Ca2+ uniporter depend on the presence of free fatty acids? Since numerous studies have shown that free fatty acids affect cellular functions by modulating the activities of various ion channels and transporters, including Ca2+ , Cl− , K+ , and Na+ , as well as non-selective cation channels [152—156], the questions raised above seem feasible. Free fatty acids may directly bind to ion channels regulating their activity or modulate channel activities through metabolites and fatty acid-activated kinases [157]. The ability of fatty acids to affect ion channels depends on the level of unsaturation and chain length. This has also been strikingly demonstrated for the mitochondrial Ca2+ uniporter, which is activated exclusively by polyunsaturated fatty acids [44]. In line with these findings, the total membrane anion conductance of UCP2 increased in the range “palmitic < oleic < eicosatrienoic < linoleic < retinoic < arachidonic acids” [81].
Besides the proposed concerted action of free fatty acids and UCP2/UCP3 on IMM anion conductance and mitochondrial Ca2+ uniport, mitochondrial Ca2+ and free fatty acids have been shown to activate the formation of the classical [158,159] and the so called non-classical [160,161] mitochondrial transition pore.
Conclusion
Evidence has accumulated to suggest that free fatty acids and Ca2+, in a concerted action, are participating in channel/pore formation in the IMM. Nevertheless, whether the two proposed functions of UCP2/UCP3, namely the fatty acid binding/transport and the contribution to mitochondrial Ca2+ sequestration, are interrelated phenomena or occur independently of each other awaits further investigation (Fig. 4A).
Conclusions
Although convincing experimental data point to an involvement of UCP2 and UCP3 in multiple physiological processes, the molecular mechanisms of action of these two proteins are still hotly debated. In particular, the most common concept of a direct uncoupling activity by carrying H+ into the mitochondrial matrix has not been convincingly demonstrated under physiological conditions. Three striking new functions of UCPs have recently been reported and indicate involvement of UCP2 and UCP3 in mitochondrial fatty acid transport, attenuation of mitochondrial ROS production and mitochondrial Ca2+ uniport. So far no adequate mechanism has been described that integrates all these functions to explain the physiological role of UCP2 and UCP3. In the present review, we have discussed the most recently described function of UCP2 and UCP3 in mitochondrial Ca2+ uptake in the light of a range of reports that describe the involvement of UCPs in major physiological phenomena. While many questions remain unresolved, it is evident that UCP2 and UCP3 play a central role in the integration of Ca2+-sensitive signal transduction, free fatty acid and substrate metabolism that are required to meet the energy demands of the cell as it responds and adapts to continuously changing energy demands (Fig. 4B).
Acknowledgements
The authors would like to express their highest gratitude and thanks to Prof. Michael Duchen for his efforts and time and his insightful guidance and comments. Work conducted in the authors’ laboratory was founded by the Austrian Science Funds FWF (P16860-B09, P-20181-B05 and SFB F3010-B05).
References
- [1].Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- [2].Kramarova TV, Shabalina IG, Andersson U, Westerberg R, Carlberg I, Houstek J, Nedergaard J, Cannon B. Mitochondrial ATP synthase levels in brown adipose tissue are governed by the c-Fo subunit P1 isoform. FASEB J. 2008;22:55–63. doi: 10.1096/fj.07-8581com. [DOI] [PubMed] [Google Scholar]
- [3].Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- [4].Vidal-Puig A, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem. Biophys. Res. Commun. 1997;235:79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- [5].Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- [6].Esteves TC, Brand MD. The reactions catalysed by the mitochondrial uncoupling proteins UCP2 and UCP3. Biochim. Biophys. Acta. 2005;1709:35–44. doi: 10.1016/j.bbabio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- [7].Nedergaard J, Cannon B. The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp. Physiol. 2003;88:65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- [8].Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling-proteins 2 and 3 are elementary for mitochondrial Ca2+ uniport. Nat. Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- [10].Stock D, Leslie AG, Walker JE. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- [11].Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [12].Graier WF, Frieden M, Malli R. Mitochondria and Ca2+ signaling: old guests, new functions. Pflugers Arch. 2007;455:375–396. doi: 10.1007/s00424-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nicholls DG. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur. J. Biochem. 1974;50:305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- [14].Gnaiger E, Steinlechner-Maran R, Mendez G, Eberl T, Margreiter R. Control of mitochondrial and cellular respiration by oxygen. J. Bioenerg. Biomembr. 1995;27:583–596. doi: 10.1007/BF02111656. [DOI] [PubMed] [Google Scholar]
- [15].Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- [16].Garlid KD, Jaburek M, Jezek P. The mechanism of proton transport mediated by mitochondrial uncoupling proteins. FEBS Lett. 1998;438:10–14. doi: 10.1016/s0014-5793(98)01246-0. [DOI] [PubMed] [Google Scholar]
- [17].Klingenberg M, Winkler E, Echtay K. Uncoupling protein, H+ transport and regulation. Biochem. Soc. Trans. 2001;29:806–811. doi: 10.1042/0300-5127:0290806. [DOI] [PubMed] [Google Scholar]
- [18].Skulachev VP. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991;294:158–162. doi: 10.1016/0014-5793(91)80658-p. [DOI] [PubMed] [Google Scholar]
- [19].Jezek P, Zackova M, Ruzicka M, Skobisova E, Jaburek M. Mitochondrial uncoupling proteins—-facts and fantasies. Physiol. Res. 2004;53:S199–S211. [PubMed] [Google Scholar]
- [20].Garlid KD, Jaburek M, Jezek P. Mechanism of uncoupling protein action. Biochem. Soc. Trans. 2001;29:803–806. doi: 10.1042/0300-5127:0290803. [DOI] [PubMed] [Google Scholar]
- [21].Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- [22].Stuart JA, Harper JA, Brindle KM, Jekabsons MB, Brand MD. A mitochondrial uncoupling artifact can be caused by expression of uncoupling protein 1 in yeast. Biochem. J. 2001;356:779–789. doi: 10.1042/0264-6021:3560779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harper JA, Stuart JA, Jekabsons MB, Roussel D, Brindle KM, Dickinson K, Jones RB, Brand MD. Artifactual uncoupling by uncoupling protein 3 in yeast mitochondria at the concentrations found in mouse and rat skeletal-muscle mitochondria. Biochem. J. 2002;361:49–56. doi: 10.1042/0264-6021:3610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- [25].Couplan E, del Mar Gonzalez-Barroso M, Alves-Guerra MC, Ricquier D, Goubern M, Bouillaud F. No evidence for a basal, retinoic, or superoxide-induced uncoupling activity of the uncoupling protein 2 present in spleen or lung mitochondria. J. Biol. Chem. 2002;277:26268–26275. doi: 10.1074/jbc.M202535200. [DOI] [PubMed] [Google Scholar]
- [26].Gong DW, Monemdjou S, Gavrilova O, Leon LR, Marcus-Samuels B, Chou CJ, Everett C, Kozak LP, Li C, Deng C, Harper ME, Reitman ML. Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J. Biol. Chem. 2000;275:16251–16257. doi: 10.1074/jbc.M910177199. [DOI] [PubMed] [Google Scholar]
- [27].Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI. In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J. Biol. Chem. 2001;276:20240–20244. doi: 10.1074/jbc.M102540200. [DOI] [PubMed] [Google Scholar]
- [28].Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- [29].Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM, Lowell BB. Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- [30].Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- [31].Echtay KS, Brand MD. 4-Hydroxy-2-nonenal and uncoupling proteins: an approach for regulation of mitochondrial ROS production. Redox. Rep. 2007;12:26–29. doi: 10.1179/135100007X162158. [DOI] [PubMed] [Google Scholar]
- [32].Gnaiger E. Oxygen conformance of cellular respiration. A perspective of mitochondrial physiology. Adv. Exp. Med. Biol. 2003;543:39–55. doi: 10.1007/978-1-4419-8997-0_4. [DOI] [PubMed] [Google Scholar]
- [33].Jaburek M, Garlid KD. Reconstitution of recombinant uncoupling proteins: UCP1, −2, and −3 have similar affinities for ATP and are unaffected by coenzyme Q10. J. Biol. Chem. 2003;278:25825–25831. doi: 10.1074/jbc.M302126200. [DOI] [PubMed] [Google Scholar]
- [34].Nicholls DG. The physiological regulation of uncoupling proteins. Biochim. Biophys. Acta. 2006;1757:459–466. doi: 10.1016/j.bbabio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- [35].Jaburek M, Miyamoto S, Di Mascio P, Garlid KD, Jezek P. Hydroperoxy fatty acid cycling mediated by mitochondrial uncoupling protein UCP2. J. Biol. Chem. 2004;279:53097–53102. doi: 10.1074/jbc.M405339200. [DOI] [PubMed] [Google Scholar]
- [36].Jezek P. Fatty acid interaction with mitochondrial uncoupling proteins. J. Bioenerg. Biomembr. 1999;31:457–466. doi: 10.1023/a:1005496306893. [DOI] [PubMed] [Google Scholar]
- [37].Schrauwen P, Hesselink MK. The role of uncoupling protein 3 in fatty acid metabolism: protection against lipotoxicity? Proc. Nutr. Soc. 2004;63:287–292. doi: 10.1079/PNS2003336. [DOI] [PubMed] [Google Scholar]
- [38].Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes. 2004;53:1412–1417. doi: 10.2337/diabetes.53.6.1412. [DOI] [PubMed] [Google Scholar]
- [39].Schrauwen P, Hoeks J, Schaart G, Kornips E, Binas B, Van De Vusse GJ, Van Bilsen M, Luiken JJ, Coort SL, Glatz JF, Saris WH, Hesselink MK. Uncoupling protein 3 as a mitochondrial fatty acid anion exporter. FASEB J. 2003;17:2272–2274. doi: 10.1096/fj.03-0515fje. [DOI] [PubMed] [Google Scholar]
- [40].Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- [41].Saris NE, Allshire A. Calcium ion transport in mitochondria. Methods Enzymol. 1989;174:68–85. doi: 10.1016/0076-6879(89)74011-8. [DOI] [PubMed] [Google Scholar]
- [42].Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- [43].Jezek P. Possible physiological roles of mitochondrial uncoupling proteins--UCPn. Int. J. Biochem. Cell Biol. 2002;34:1190–1206. doi: 10.1016/s1357-2725(02)00061-4. [DOI] [PubMed] [Google Scholar]
- [44].Zhang BX, Ma X, Zhang W, Yeh CK, Lin A, Luo J, Sprague EA, Swerdlow RH, Katz MS. Polyunsaturated fatty acids mobilize intracellular Ca2+ in NT2 human teratocarcinoma cells by causing release of Ca2+ from mitochondria. Am. J. Physiol. Cell. Physiol. 2006;290:C1321–C1333. doi: 10.1152/ajpcell.00335.2005. [DOI] [PubMed] [Google Scholar]
- [45].Litsky ML, Pfeiffer DR. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: the uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- [46].Chan SL, Liu D, Kyriazis GA, Bagsiyao P, Ouyang X, Mattson MP. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store depletion-induced apoptosis in neural cells. J. Biol. Chem. 2006;281:37391–37403. doi: 10.1074/jbc.M605552200. [DOI] [PubMed] [Google Scholar]
- [47].Erlanson-Albertsson C. Uncoupling proteins—-a new family of proteins with unknown function. Nutr. Neurosci. 2002;5:1–11. doi: 10.1080/10284150290007038. [DOI] [PubMed] [Google Scholar]
- [48].Mozo J, Ferry G, Studeny A, Pecqueur C, Rodriguez M, Boutin JA, Bouillaud F. Expression of UCP3 in CHO cells does not cause uncoupling, but controls mitochondrial activity in the presence of glucose. Biochem. J. 2006;393:431–439. doi: 10.1042/BJ20050494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Malli R, Frieden M, Osibow K, Zoratti C, Mayer M, Demaurex N, Graier WF. Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J. Biol. Chem. 2003;278:44769–44779. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- [50].Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the ER. J. Biol. Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- [51].Hansford RG, Chappell JB. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem. Biophys. Res. Commun. 1967;27:686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- [52].Hansford RG, Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol. Cell Biochem. 1998;184:359–369. [PubMed] [Google Scholar]
- [53].McCormack JG, Denton RM. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem. J. 1979;180:533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McCormack JG, Denton RM. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+ Biochem. J. 1984;218:235–247. doi: 10.1042/bj2180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- [56].Bezaire V, Seifert EL, Harper ME. Uncoupling protein-3: clues in an ongoing mitochondrial mystery. FASEB J. 2007;21:312–324. doi: 10.1096/fj.06-6966rev. [DOI] [PubMed] [Google Scholar]
- [57].Boss O, Hagen T, Lowell BB. Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes. 2000;49:143–156. doi: 10.2337/diabetes.49.2.143. [DOI] [PubMed] [Google Scholar]
- [58].Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- [59].Criscuolo F, Mozo J, Hurtaud C, Nubel T, Bouillaud F. UCP2, UCP3, avUCP, what do they do when proton transport is not stimulated? Possible relevance to pyruvate and glutamine metabolism. Biochim. Biophys. Acta. 2006;1757:1284–1291. doi: 10.1016/j.bbabio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [60].Dulloo AG, Samec S. Uncoupling proteins: their roles in adaptive thermogenesis and substrate metabolism reconsidered. Br. J. Nutr. 2001;86:123–139. doi: 10.1079/bjn2001412. [DOI] [PubMed] [Google Scholar]
- [61].Harper ME, Himms-Hagen J. Mitochondrial efficiency: lessons learned from transgenic mice. Biochim. Biophys. Acta. 2001;1504:159–172. doi: 10.1016/s0005-2728(00)00244-9. [DOI] [PubMed] [Google Scholar]
- [62].Harper JA, Dickinson K, Brand MD. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes. Rev. 2001;2:255–265. doi: 10.1046/j.1467-789x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- [63].Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- [64].Sluse FE, Jarmuszkiewicz W, Navet R, Douette P, Mathy G, Sluse-Goffart CM. Mitochondrial UCPs: new insights into regulation and impact. Biochim. Biophys. Acta. 2006;1757:480–485. doi: 10.1016/j.bbabio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [65].Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem. J. 2000;345:161–179. [PMC free article] [PubMed] [Google Scholar]
- [66].Ricquier D, Bouillaud F. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J. Physiol. 2000;529:3–10. doi: 10.1111/j.1469-7793.2000.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- [68].Chan CB, Saleh MC, Koshkin V, Wheeler MB. Uncoupling protein 2 and islet function. Diabetes. 2004;53:S136–S142. doi: 10.2337/diabetes.53.2007.s136. [DOI] [PubMed] [Google Scholar]
- [69].Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302–1310. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- [70].Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–1486. doi: 10.2337/diabetes.48.7.1482. [DOI] [PubMed] [Google Scholar]
- [71].Hong Y, Fink BD, Dillon JS, Sivitz WI. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142:249–256. doi: 10.1210/endo.142.1.7889. [DOI] [PubMed] [Google Scholar]
- [72].Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51:3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- [73].Lameloise N, Muzzin P, Prentki M, Assimacopoulos-Jeannet F. Uncoupling protein 2: a possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes. 2001;50:803–809. doi: 10.2337/diabetes.50.4.803. [DOI] [PubMed] [Google Scholar]
- [74].Li LX, Skorpen F, Egeberg K, Jorgensen IH, Grill V. Induction of uncoupling protein 2 mRNA in beta-cells is stimulated by oxidation of fatty acids but not by nutrient oversupply. Endocrinology. 2002;143:1371–1377. doi: 10.1210/endo.143.4.8717. [DOI] [PubMed] [Google Scholar]
- [75].Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, Daniel KW, Collins S. Regulation of the uncoupling protein-2 gene in INS-1 beta-cells by oleic acid. J. Biol. Chem. 2002;277:42639–42644. doi: 10.1074/jbc.M208645200. [DOI] [PubMed] [Google Scholar]
- [76].Joseph JW, Koshkin V, Saleh MC, Sivitz WI, Zhang CY, Lowell BB, Chan CB, Wheeler MB. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J. Biol. Chem. 2004;279:51049–51056. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- [77].Echtay KS, Murphy MP, Smith RA, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J. Biol. Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- [78].Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic {beta} cell dysfunction. J. Clin. Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- [80].Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147:2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- [81].Beck V, Jaburek M, Demina T, Rupprecht A, Porter RK, Jezek P, Pohl EE. Polyunsaturated fatty acids activate human uncoupling proteins 1 and 2 in planar lipid bilayers. FASEB J. 2007;21:1137–1144. doi: 10.1096/fj.06-7489com. [DOI] [PubMed] [Google Scholar]
- [82].Ito E, Ozawa S, Takahashi K, Tanaka T, Katsuta H, Yamaguchi S, Maruyama M, Takizawa M, Katahira H, Yoshimoto K, Nagamatsu S, Ishida H. PPAR-gamma overexpression selectively suppresses insulin secretory capacity in isolated pancreatic islets through induction of UCP-2 protein. Biochem. Biophys. Res. Commun. 2004;324:810–814. doi: 10.1016/j.bbrc.2004.08.238. [DOI] [PubMed] [Google Scholar]
- [83].Tordjman K, Standley KN, Bernal-Mizrachi C, Leone TC, Coleman T, Kelly DP, Semenkovich CF. PPARalpha suppresses insulin secretion and induces UCP2 in insulinoma cells. J. Lipid Res. 2002;43:936–943. [PubMed] [Google Scholar]
- [84].Produit-Zengaffinen N, Davis-Lameloise N, Perreten H, Becard D, Gjinovci A, Keller PA, Wollheim CB, Herrera P, Muzzin P, Assimacopoulos-Jeannet F. Increasing uncoupling protein-2 in pancreatic beta cells does not alter glucose-induced insulin secretion but decreases production of reactive oxygen species. Diabetologia. 2007;50:84–93. doi: 10.1007/s00125-006-0499-6. [DOI] [PubMed] [Google Scholar]
- [85].Ravnskjaer K, Boergesen M, Rubi B, Larsen JK, Nielsen T, Fridriksson J, Maechler P, Mandrup S. Peroxisome proliferator-activated receptor alpha (PPARalpha) potentiates, whereas PPARgamma attenuates, glucose-stimulated insulin secretion in pancreatic beta-cells. Endocrinology. 2005;146:3266–3276. doi: 10.1210/en.2004-1430. [DOI] [PubMed] [Google Scholar]
- [86].Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW, Collins S. Persistent nuclear factor-kappa B activation in UCP2−/− mice leads to enhanced nitric oxide and inflammatory cytokine production. J. Biol. Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hammar EB, Irminger JC, Rickenbach K, Parnaud G, Ribaux P, Bosco D, Rouiller DG, Halban PA. Activation of NF-kappaB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic beta cells. J. Biol. Chem. 2005;280:30630–30637. doi: 10.1074/jbc.M502493200. [DOI] [PubMed] [Google Scholar]
- [88].Norlin S, Ahlgren U, Edlund H. Nuclear factor-{kappa} B activity in {beta}-cells is required for glucose-stimulated insulin secretion. Diabetes. 2005;54:125–132. doi: 10.2337/diabetes.54.1.125. [DOI] [PubMed] [Google Scholar]
- [89].Zengaffinen N, Perreten H, Lameloise N, Keller P, Muzzin P, Assimacopoulos-Jeannet F. Uncoupling protein 2 overexpression prevents cytokine-induced reactive oxygene species production and apoptosis in pancreatic beta cells. Diabetologia. 2005;48(Suppl. 1):A38. [Google Scholar]
- [90].Jonas JC, Gilon P, Henquin JC. Temporal and quantitative correlations between insulin secretion and stably elevated or oscillatory cytoplasmic Ca2+ in mouse pancreatic beta-cells. Diabetes. 1998;47:1266–1273. doi: 10.2337/diab.47.8.1266. [DOI] [PubMed] [Google Scholar]
- [91].Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- [92].Quesada I, Villalobos C, Nunez L, Chamero P, Alonso MT, Nadal A, Garcia-Sancho J. Glucose induces synchronous mitochondrial calcium oscillations in intact pancreatic islets. Cell Calcium. 2008;43:39–47. doi: 10.1016/j.ceca.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [93].Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jornot L, Maechler P, Wollheim CB, Junod AF. Reactive oxygen metabolites increase mitochondrial calcium in endothelial cells: implication of the Ca2+/Na+ exchanger. J. Cell Sci. 1999;112:1013–1022. doi: 10.1242/jcs.112.7.1013. [DOI] [PubMed] [Google Scholar]
- [95].Detimary P, Gilon P, Henquin JC. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem. J. 1998;333:269–274. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ainscow EK, Rutter GA. Glucose-stimulated oscillations in free cytosolic ATP concentration imaged in single islet betacells: evidence for a Ca2+-dependent mechanism. Diabetes. 2002;51:S162–S170. doi: 10.2337/diabetes.51.2007.s162. [DOI] [PubMed] [Google Scholar]
- [97].Magnus G, Keizer J. Model of beta-cell mitochondrial calcium handling and electrical activity. I. Cytoplasmic variables. Am. J. Physiol. 1998;274:C1158–C1173. doi: 10.1152/ajpcell.1998.274.4.C1158. [DOI] [PubMed] [Google Scholar]
- [98].Krippeit-Drews P, Dufer M, Drews G. Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic B-cells. Biochem. Biophys. Res. Commun. 2000;267:179–183. doi: 10.1006/bbrc.1999.1921. [DOI] [PubMed] [Google Scholar]
- [99].Kindmark H, Kohler M, Brown G, Branstrom R, Larsson O, Berggren PO. Glucose-induced oscillations in cytoplasmic free Ca2+ concentration precede oscillations in mitochondrial membrane potential in the pancreatic beta-cell. J. Biol. Chem. 2001;276:34530–34536. doi: 10.1074/jbc.M102492200. [DOI] [PubMed] [Google Scholar]
- [100].Jung SK, Kauri LM, Qian WJ, Kennedy RT. Correlated oscillations in glucose consumption, oxygen consumption, and intracellular free Ca2+ in single islets of Langerhans. J. Biol. Chem. 2000;275:6642–6650. doi: 10.1074/jbc.275.9.6642. [DOI] [PubMed] [Google Scholar]
- [101].Bertram R, Gram Pedersen M, Luciani DS, Sherman A. A simplified model for mitochondrial ATP production. J. Theor. Biol. 2006;243:575–586. doi: 10.1016/j.jtbi.2006.07.019. [DOI] [PubMed] [Google Scholar]
- [102].Luciani DS, Misler S, Polonsky KS. Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J. Physiol. 2006;572:379–392. doi: 10.1113/jphysiol.2005.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca2+ signals. J. Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- [104].Petersen OH, Sutton R, Criddle DN. Failure of calcium microdomain generation and pathological consequences. Cell Calcium. 2006;40:593–600. doi: 10.1016/j.ceca.2006.08.020. [DOI] [PubMed] [Google Scholar]
- [105].Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium. 2005;38:161–169. doi: 10.1016/j.ceca.2005.06.023. [DOI] [PubMed] [Google Scholar]
- [106].Bootman MD, Higazi DR, Coombes S, Roderick HL. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J. Cell Sci. 2006;119:3915–3925. doi: 10.1242/jcs.03223. [DOI] [PubMed] [Google Scholar]
- [107].Hernandez-Guijo JM, Maneu-Flores VE, Ruiz-Nuno A, Villarroya M, Garcia AG, Gandia L. Calcium-dependent inhibition of L, N, and P/Q Ca2+ channels in chromaffin cells: role of mitochondria. J. Neurosci. 2001;21:2553–2560. doi: 10.1523/JNEUROSCI.21-08-02553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tanaami T, Ishida H, Seguchi H, Hirota Y, Kadono T, Genka C, Nakazawa H, Barry WH. Difference in propagation of Ca2+ release in atrial and ventricular myocytes. Jpn. J. Physiol. 2005;55:81–91. doi: 10.2170/jjphysiol.R2077. [DOI] [PubMed] [Google Scholar]
- [109].Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Benani A, Troy S, Carmona MC, Fioramonti X, Lorsignol A, Leloup C, Casteilla L, Penicaud L. Role for mitochondrial reactive oxygen species in brain lipid sensing: redox regulation of food intake. Diabetes. 2007;56:152–160. doi: 10.2337/db06-0440. [DOI] [PubMed] [Google Scholar]
- [111].Leloup C, Magnan C, Benani A, Bonnet E, Alquier T, Offer G, Carriere A, Periquet A, Fernandez Y, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes. 2006;55:2084–2090. doi: 10.2337/db06-0086. [DOI] [PubMed] [Google Scholar]
- [112].Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J. Bioenerg. Biomembr. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- [115].Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–164. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- [116].Graier WF, Posch K, Fleischhacker E, Wascher TC, Kostner GM. Increased superoxide anion formation in endothelial cells during hyperglycemia: an adaptive response or initial step of vascular dysfunction? Diabetes Res. Clin. Pract. 1999;45:153–160. doi: 10.1016/s0168-8227(99)00045-5. [DOI] [PubMed] [Google Scholar]
- [117].Spitaler MM, Graier WF. Vascular targets of redox signalling in diabetes mellitus. Diabetologia. 2002;45:476–494. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- [118].Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- [119].Schaeffer G, Levak-Frank S, Spitaler MM, Fleischhacker E, Esenabhalu VE, Wagner AH, Hecker M, Graier WF. Intercellular signalling within vascular cells under high d-glucose involves free radical-triggered tyrosine kinase activation. Diabetologia. 2003;46:773–783. doi: 10.1007/s00125-003-1091-y. [DOI] [PubMed] [Google Scholar]
- [120].Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- [121].Melov S, Coskun P, Patel M, Tuinstra R, Cottrell B, Jun AS, Zastawny TH, Dizdaroglu M, Goodman SI, Huang TT, Miziorko H, Epstein CJ, Wallace DC. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- [123].Horimoto M, Fulop P, Derdak Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386–392. doi: 10.1002/hep.20047. [DOI] [PubMed] [Google Scholar]
- [124].Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- [125].Park JY, Park KG, Kim HJ, Kang HG, Ahn JD, Kim HS, Kim YM, Son SM, Kim IJ, Kim YK, Kim CD, Lee KU, Lee IK. The effects of the overexpression of recombinant uncoupling protein 2 on proliferation, migration and plasminogen activator inhibitor 1 expression in human vascular smooth muscle cells. Diabetologia. 2005;48:1022–1028. doi: 10.1007/s00125-005-1712-8. [DOI] [PubMed] [Google Scholar]
- [126].MacLellan JD, Gerrits MF, Gowing A, Smith PJ, Wheeler MB, Harper ME. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes. 2005;54:2343–2350. doi: 10.2337/diabetes.54.8.2343. [DOI] [PubMed] [Google Scholar]
- [127].Duval C, Camara Y, Hondares E, Sibille B, Villarroya F. Overexpression of mitochondrial uncoupling protein-3 does not decrease production of the reactive oxygen species, elevated by palmitate in skeletal muscle cells. FEBS Lett. 2007;581:955–961. doi: 10.1016/j.febslet.2007.01.085. [DOI] [PubMed] [Google Scholar]
- [128].Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci. Rep. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- [129].Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- [130].Korshunov SS, Korkina OV, Ruuge EK, Skulachev VP, Starkov AA. Fatty acids as natural uncouplers preventing generation of O2−• and H2O2 by mitochondria in the resting state. FEBS Lett. 1998;435:215–218. doi: 10.1016/s0014-5793(98)01073-4. [DOI] [PubMed] [Google Scholar]
- [131].Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild Uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J. Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- [132].Tretter L, Adam-Vizi V. Uncoupling is without an effect on the production of reactive oxygen species by in situ synaptic mitochondria. J. Neurochem. 2007;103:1864–1871. doi: 10.1111/j.1471-4159.2007.04891.x. [DOI] [PubMed] [Google Scholar]
- [133].Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Dykens JA. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated Ca2+ and Na+ : implications for neurodegeneration. J. Neurochem. 1994;63:584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- [135].Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2003;28:1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- [137].Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and - independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- [139].Starkov AA, Polster BM, Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J. Neurochem. 2002;83:220–228. doi: 10.1046/j.1471-4159.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- [140].Duchen MR. Ca2+-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem. J. 1992;283:41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006;27:821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- [142].Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Himms-Hagen J, Harper ME. Physiological role of UCP3 may be export of fatty acids from mitochondria when fatty acid oxidation predominates: an hypothesis. Exp. Biol. Med. 2001;226:78–84. doi: 10.1177/153537020122600204. [DOI] [PubMed] [Google Scholar]
- [144].Villarroya F, Iglesias R, Giralt M. PPARs in the control of uncoupling proteins gene expression. PPAR Res. 2006;2007:74364. doi: 10.1155/2007/74364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Millet L, Vidal H, Andreelli F, Larrouy D, Riou JP, Ricquier D, Laville M, Langin D. Increased uncoupling protein-2 and -3 mRNA expression during fasting in obese and lean humans. J. Clin. Invest. 1997;100:2665–2670. doi: 10.1172/JCI119811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Bezaire V, Spriet LL, Campbell S, Sabet N, Gerrits M, Bonen A, Harper ME. Constitutive UCP3 overexpression at physiological levels increases mouse skeletal muscle capacity for fatty acid transport and oxidation. FASEB J. 2005;19:977–979. doi: 10.1096/fj.04-2765fje. [DOI] [PubMed] [Google Scholar]
- [147].Schrauwen P, Hoppeler H, Billeter R, Bakker AH, Pendergast DR. Fiber type dependent upregulation of human skeletal muscle UCP2 and UCP3 mRNA expression by high-fat diet. Int. J. Obes. Relat. Metab. Disord. 2001;25:449–456. doi: 10.1038/sj.ijo.0801566. [DOI] [PubMed] [Google Scholar]
- [148].Schrauwen P, Hesselink MK, Vaartjes I, Kornips E, Saris WH, Giacobino JP, Russell A. Effect of acute exercise on uncoupling protein 3 is a fat metabolism-mediated effect. Am. J. Physiol. Endocrinol. Metab. 2002;282:E11–E17. doi: 10.1152/ajpendo.2002.282.1.E11. [DOI] [PubMed] [Google Scholar]
- [149].Tsuboyama-Kasaoka N, Tsunoda N, Maruyama K, Takahashi M, Kim H, Ikemoto S, Ezaki O. Up-regulation of uncoupling protein 3 (UCP3) mRNA by exercise training and down-regulation of UCP3 by denervation in skeletal muscles. Biochem. Biophys. Res. Commun. 1998;247:498–503. doi: 10.1006/bbrc.1998.8818. [DOI] [PubMed] [Google Scholar]
- [150].Hoeks J, Hesselink MK, Sluiter W, Schaart G, Willems J, Morrisson A, Clapham JC, Saris WH, Schrauwen P. The effect of high-fat feeding on intramuscular lipid and lipid peroxidation levels in UCP3-ablated mice. FEBS Lett. 2006;580:1371–1375. doi: 10.1016/j.febslet.2006.01.059. [DOI] [PubMed] [Google Scholar]
- [151].Fraser DR, Trayhurn P. Mitochondrial Ca2+ transport in lean and genetically obese (ob/ob) mice. Biochem. J. 1983;214:163–170. doi: 10.1042/bj2140163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Liu L, Barrett CF, Rittenhouse AR. Arachidonic acid both inhibits and enhances whole cell calcium currents in rat sympathetic neurons. Am. J. Physiol. Cell. Physiol. 2001;280:C1293–C1305. doi: 10.1152/ajpcell.2001.280.5.C1293. [DOI] [PubMed] [Google Scholar]
- [153].Ferrier GR, Redondo I, Zhu J, Murphy MG. Differential effects of docosahexaenoic acid on contractions and L-type Ca2+ current in adult cardiac myocytes. Cardiovasc. Res. 2002;54:601–610. doi: 10.1016/s0008-6363(02)00275-4. [DOI] [PubMed] [Google Scholar]
- [154].Schonfeld P, Sayeed I, Bohnensack R, Siemen D. Fatty acids induce chloride permeation in rat liver mitochondria by activation of the inner membrane anion channel (IMAC) J. Bioenerg. Biomembr. 2004;36:241–248. doi: 10.1023/b:jobb.0000031975.72350.c6. [DOI] [PubMed] [Google Scholar]
- [155].Hong MP, Kim HI, Shin YK, Lee CS, Park M, Song JH. Effects of free fatty acids on sodium currents in rat dorsal root ganglion neurons. Brain Res. 2004;1008:81–91. doi: 10.1016/j.brainres.2004.02.033. [DOI] [PubMed] [Google Scholar]
- [156].Mies F, Shlyonsky V, Goolaerts A, Sariban-Sohraby S. Modulation of epithelial Na+ channel activity by long-chain n-3 fatty acids. Am. J. Physiol. Renal Physiol. 2004;287:F850–F855. doi: 10.1152/ajprenal.00078.2004. [DOI] [PubMed] [Google Scholar]
- [157].Rychkov GY, Litjens T, Roberts ML, Barritt GJ. Arachidonic acid inhibits the store-operated Ca2+ current in rat liver cells. Biochem. J. 2005;385:551–556. doi: 10.1042/BJ20041604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- [159].Di Paola M, Lorusso M. Interaction of free fatty acids with mitochondria: coupling, uncoupling and permeability transition. Biochim. Biophys. Acta. 2006;1757:1330–1337. doi: 10.1016/j.bbabio.2006.03.024. [DOI] [PubMed] [Google Scholar]
- [160].Sultan A, Sokolove PM. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 2001;386:37–51. doi: 10.1006/abbi.2000.2194. [DOI] [PubMed] [Google Scholar]
- [161].Sultan A, Sokolove PM. Free fatty acid effects on mitochondrial permeability: an overview. Arch. Biochem. Biophys. 2001;386:52–61. doi: 10.1006/abbi.2000.2195. [DOI] [PubMed] [Google Scholar]
- [162].Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]