Abstract
A novel four-component strategy for selective synthesis of fused azepino[5,4,3-cd]indoles and pyrazolo [3,4-b]pyridines has been established. The bond-forming efficiency, accessibility of starting materials and substrate scope provide an invaluble access to tetra-, and bis-heterocyclic scaffolds.
The functional diverse of azepino[5,4,3-cd]indole skeletons commonly exist in natural and unnatural products;1 they have been found in indole alkaloids, such as Aurantioclavine (I),2 Rucaparib (II),3 Hyrtiazepine (III)4 and Hyrtimomines F5 (Figure 1), exhibiting biological activities for serving as 5-HT1 agonists6 and PARP inhibitor.3 Aurantioclavines have served as key building blocks during biosynthesis of complex polycyclic alkaloids of the communesin family.7,8 The construction of these compounds and their structural analogues has attracted much attention in synthetic community.1-5 To the best of our knowledge, an effcient construction of tetracyclic azepino[5,4,3-cd]indole skeleton through sequential cyclizations via multicomponent domino reaction (MDR) has not been documented yet.
Figure 1.
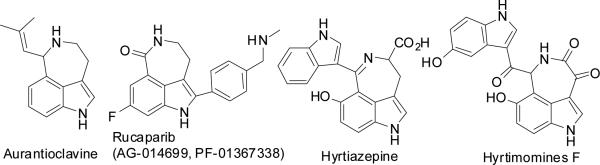
Several representative natural products.
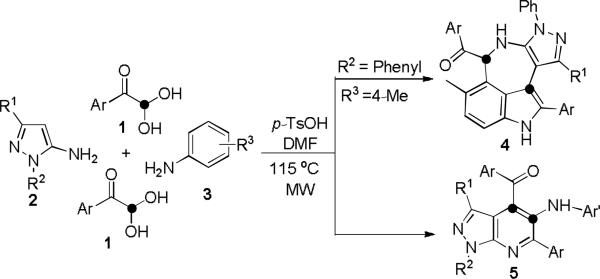
In recent years, multicomponent domino reactions have been playing an important role in synthetic methodologies, and can implement cascade reactions in total synthesis of natural products.9 Our groups10 and several others11,12 have developed a series of MDRs for the synthesis of unique heterocycles and natural mimic compounds of chemical and pharmaceutical importance. To continue our efforts on this project, we now discovered a novel ABC2 type domino reaction of arylglyoxal monohydrate 1 with electron-rich pyrazol-5-amines 2 and aromatic amines 3, leading to formation of pyrazolo[4',3':6,7]azepino[5,4,3-cd]indoles and pyrazolo[3,4-b]pyridines under microwave (MW) heating (Scheme 1). The former reaction occurred through (3+2)/(3+2+1+1) bis-cyclizations to give tetracyclic pyrazolo[4',3':6,7]azepino[5,4,3-cd]indoles in a straightforward manner, which are normally difficult to perform in a single operation. Both 2- and 3-positions of p-toluidine 3a simultaneously served as nucleophilic centers to enable the following domino cyclizations that were rarely encountered in organic reactions. Interestingly, the direct C-C formation between two electrophilic centers of arylglyoxal monohydrates can be smoothly performed through four-component [3+2+1] heteroannulation without the use of any metal catalysts.
Scheme 1.
Multicomponent synthesis of compounds 4 and 5
To optimize the reaction condition, we began our examination of the reaction of 2,2-dihydroxy-1-phenylethanone (1a), 3-methyl-1-phenyl-1H-pyrazol-5-amine (2a) and p-toluidine (3a) in DMF at 100 °C (Table 1, entry 1. See SI). When this reaction was performed in the presence of p-TsOH for 15 min, 38% yield of azepino[5,4,3-cd]indole (4a) was obtained. The structure of 4a has been determined by spectroscopic and X-ray crystallographic analysis (See SI). We next screened different Brønsted acids and Lewis acids as catalysts (entries 1-5). As shown in Table 1 (See SI), the reaction did not proceed in the presence of Brønsted acids, such as H2SO4 and CF3COOH. Lewis acids, such as FeCl3 and ZnCl2 also failed to improve the yield (entries 4-5). A variety of both polar and nonpolar solvents were also examined, and DMF was most suitable solvent for this reaction (entry 1). To verify the role of p-TsOH either as catalyst or promoter, we conducted two sets of reactions by loading p-TsOH in 1.5 equiv. (entry 10) and 30 mol% (entry 11), respectively. We found both conditions failed to give higher than 30% yield. The reaction was then performed in DMF at different temperatures in a sealed vessel under microwave irradiation for 15 min. The yield of product 4a was improved to 46% when temperature was increased to 115 °C (entry 13).
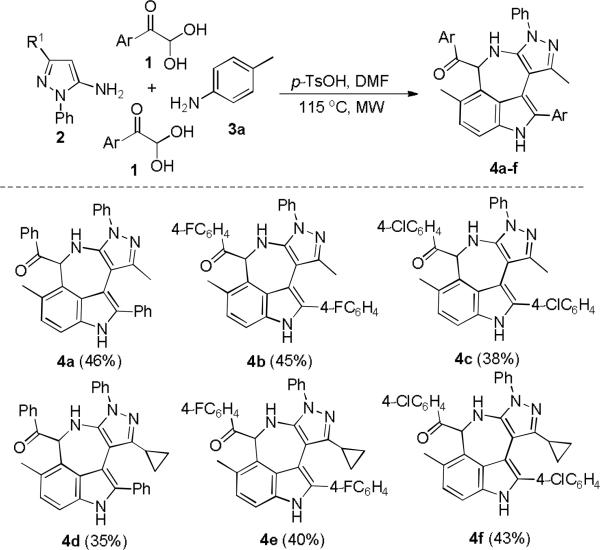
With the above condition in hand, the generality and scope of the reaction were investigated for a range of arylglyoxal monohydrates 1 and electron-rich pyrazol-5-amines 2 (Scheme 2). A variety of functional groups in substituted arylglyoxal monohydrates were found to be well tolerable under the above condition to give azepino[5,4,3-cd]indole products (4a-f); even cyclopropyl-substituted of 3-position of pyrazol-5-amines 2 can be employed for this reaction. Surprisingly, when the strong electron-donating group (MeO-) was placed at C4 of phenyl ring of 1, the reaction did not give the expected azepino[5,4,3-cd]indoles; Instead, it led to formation of multifunctionalized pyrazolo[3,4-b]pyridines 5a (Scheme 3). When electronically poor 4-chloroaniline (3b) was utilized to replace p-toluidine (3a) to react with 1 under this system, pyrazolo[3,4-b]pyridine can still be formed. This interesting observation of 1-phenyl-pyrazol-5-amine-based domino reaction indicated the electronic effect of arylglyoxals and aromatic amines may control the reaction pathways chemoselectively.
Scheme 2.
Domino formation of azepino[5,4,3-cd]indoles 4a-4f
Scheme 3.
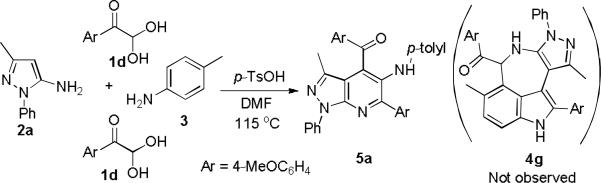
Domino formation of pyrazolo[3,4-b]pyridines 5a
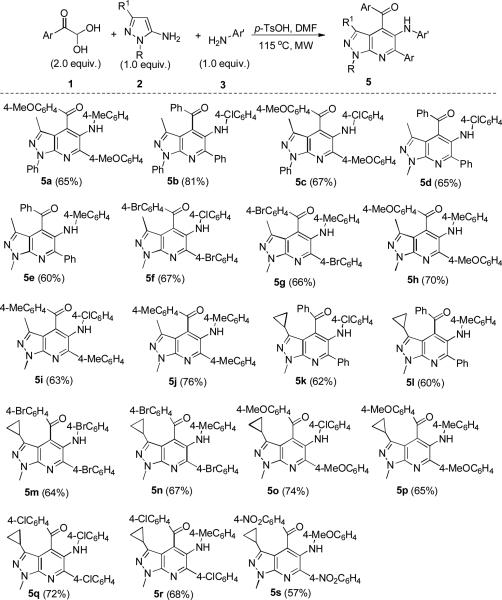
Due to the importance of pyrazolo[3,4-b]pyridines in organic synthesis and drug design in pharmaceutical sciences,13 we then focused on the feasibility of the latter reaction (Scheme 4). We found that a variety of functional groups in arylglyoxals 1 can enable the reaction to occur smoothly. Reactions involving methyl-, or chloro-substituted phenylglyoxal monohydrate 1 with 2 and 3 all worked efficiently to give the bicyclic pyrazolo[3,4-b]pyridines in moderate to good yields under microwave irradiation as shown in Scheme 4. This reaction also tolerated the substrate of 4-nitrophenylglyoxals 1 attached by of the strong electron-withdrawing group and can smoothly proceed to give pyrazolo[3,4-b]pyridines 5s in 57% yield. The substituents on N1 or C3 positions of pyrazole and on aromatic amines 3 were well tolerated to afford pyrazolo[3,4-b]pyridine 5 within short times in good yields. However, ortho-substituted aniline, such as 2-nitroaniline (3f) or o-toluidine (3g), failed to give the desired pyrazolo[3,4-b]pyridines 5. The structures of the resulting products 5 have been unambiguously confirmed by IR, NMR and HR-MS spectral analysis. In addition, one of them (5g) has been determined by X-ray diffractional analysis (see SI).
Scheme 4.
Domino synthesis of pyrazolo[3,4-b]pyridines 5
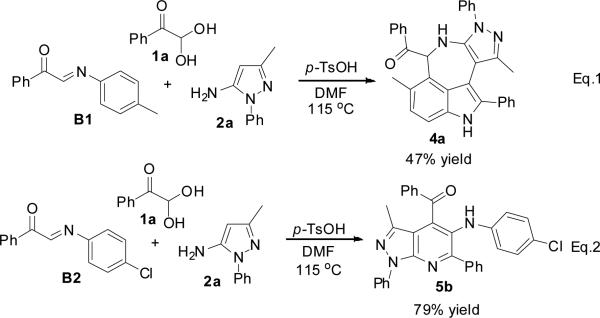
To understand the mechanism hypothesis, 1-phenyl-2-(p-tolylimino)ethanone B1 and 2-(4-chlorophenylimino)-1-phenylethanone B2 were subjected to the reaction with 1a and 2a under the standard condition. The corresponding azepino[5,4,3-cd]indoles 4a and pyrazolo[3,4-b]pyridines 5b were generated in 47% and 79% yields, respectively (Scheme 5). These observations prove that the electronic effect of aromatic amines plays a key role in controlling the reaction pathways.
Scheme 5.
Control experiments for mechanism hypothesis
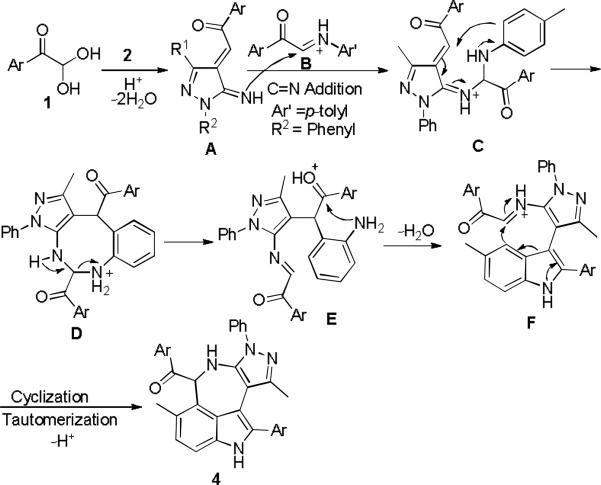
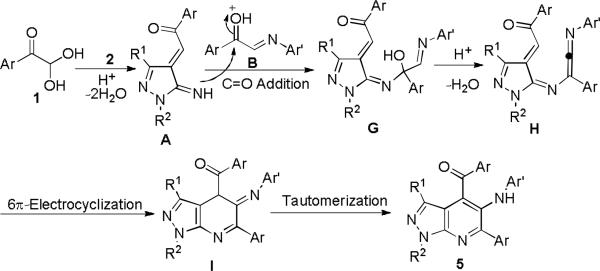
On the basis of this experiment, mechanisms for these two domino reactions are proposed as shown in Schemes 6 and 7. In the former, arylglyoxal monohydrates 1 was protonated by p-TsOH and occurred dehydration which was followed by addition reaction with electron-rich pyrazol-5-amines 2 leading to intermediate A. The intermolecular C=N addition of intermediate B and intramolecular cyclization resulted in macrocyclic intermediate D. Ring-opening of D promoted by p-TsOH afforded imine intermediate E which underwent consecutive intramolecular cyclizations and tautomerization to give azepino[5,4,3-cd]indoles 4 (Scheme 6). Similar to the former, the latter reaction occurred to give the intermediate A at the early stage. Due to the electronic effect of imines B, the carbonyl addition reaction of intermediate A with imines B proceeded to generate enone intermediate G, which was then transformed into active allene intermediate H. The following intramolecular 6π-electrocyclization and tautomerizm result in the formation of pyrazolo[3,4-b]pyridines 5 as the final product (Scheme 7).
Scheme 6.
Proposed mechanism for forming azepinoindoles 4
Scheme 7.
Proposed mechanism for forming pyrazolopyridines 5
Conclusions
In conclusion, we have established novel chemoselective four-component domino reactions for rapid accesses to azepino[5,4,3-cd]indoles 4 and pyrazolo[3,4-b]pyridines 5. The reactions are easy to perform under consice conditions under microwave irradiation. The mechanisms for these two new reactions were proposed and partially confirmed by control experiments. The reactions show good substrate scope, particularly, the simultaneous formations of two C-N and two C-C bonds through a key 6π-electrocyclization in the latter reaction. Further study of these two new reactions and their applications will be conducted in our laboratories in due course.
Supplementary Material
Acknowledgment
We are grateful for financial support from the NSFC (No. 21232004, 21272095 and 21102124), Jiangsu Science and Technology Support Program (No. SBE2011045), the Qing Lan Project (12QLG006), Robert A. Welch Foundation (D-1361, USA) and NIH (R33DA031860, USA).
Footnotes
Electronic supplementary information (ESI) available. CCDC 977865 (4a) and 977866 (5g)
References
- 1.a Qu S-J, Liu Q-W, Tan C-H, Jiang S-H, Zhu D-Y. Planta Medica. 2006;72:264. doi: 10.1055/s-2005-873195. [DOI] [PubMed] [Google Scholar]; b Yamada K, Teranishi S, Miyashita A, Ishikura M, Somei M. Heterocycles. 2011;83:2547. [Google Scholar]; c Strandtmann MV, Cohen MP, Shavel J., Jr. J. Med. Chem. 1965;8:200. doi: 10.1021/jm00326a012. [DOI] [PubMed] [Google Scholar]; d Ishikura M, Abe T, Choshi T, Hibino S. Nat. Prod. Rep. 2013;30:694. doi: 10.1039/c3np20118j. [DOI] [PubMed] [Google Scholar]
- 2.a Kozlovskii AG, Soloveva TF, Sakharobskii VG, Adanin VM. Dokl. Akad. Nauk SSSR. 1981;260:230. [PubMed] [Google Scholar]; b Yamada F, Makita Y, Suzuki T, Somei M. Chem. Pharm. Bull. 1985;33:2162. [Google Scholar]; c Hegedus LS, Toro JL, Miles WH, Harrington PJ. J. Org. Chem. 1987;52:3319. [Google Scholar]; d Yamada K, Namerikawa Y, Haruyama T, Miwa Y, Yanada R, Ishikura M. Eur. J. Org. Chem. 2009:5752. [Google Scholar]
- 3.a Porcelli L, Quatrale A, Mantuano P, Leo MG, Silvestris N, Rolland JF, Carioggia E, Lioce M, Paradiso A, Azzariti A. Mol. Oncol. 2013;7:308. doi: 10.1016/j.molonc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Plummer R, Lorigan P, Steven N, Scott L, Middleton MR, Wilson RH, Mulligan E, Curtin N, Wang D, Dewji R, Abbattista A, Gallo J, Calvert H. Cancer Chemother. Pharmacol. 2013;71:1191. doi: 10.1007/s00280-013-2113-1. [DOI] [PubMed] [Google Scholar]
- 4.a Ito F, Shudo K-I, Yamaguchi K. Tetrahedron. 2011;67:1805. [Google Scholar]; b Sauleau P, Martin M-T, Dau M-ETH, Youssef DTA, Bourguet-Kondracki M-L. J. Nat. Prod. 2006;69:1676. doi: 10.1021/np060132r. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka N, Momose R, Takahashi-Nakaguchi A, Gonoi T, Fromont J, Kobayashi J. Tetrahedron. 2014;70:832. [Google Scholar]
- 6.Benson W, K Van C, C Gregory P, Wolf KU, Preuschoff U, Tulp M, Hulkenberg T, Van IW. Eur. Pat. EP 525584 A1. 19930203:1993. [Google Scholar]
- 7.a May JA, Stoltz BM. Tetrahedron. 2006;62:5262. [Google Scholar]; b May JA, Zeidan RK, Stoltz BM. Tetrahedron Lett. 2003;44:1203. [Google Scholar]
- 8.a Yang J, Wu H, Shen L, Qin Y. J. Am. Chem. Soc. 2007;129:13794. doi: 10.1021/ja075705g. (4) For synthesis of communesin see. [DOI] [PubMed] [Google Scholar]; b iu PL, Seo JH, Weinreb SM. Angew. Chem., Int. Ed. 2010;49:2000. doi: 10.1002/anie.200906818. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Siengalewicz P, Gaich T, Mulzer J. Angew. Chem., Int. Ed. 2008;80:47, 8170. doi: 10.1002/anie.200801735. For a review on synthetic efforts toward communesins, see. [DOI] [PubMed] [Google Scholar]
- 9.a Brauch S, van Berkel SS, Westermann B. Chem. Soc. Rev. 2013;42:4948. doi: 10.1039/c3cs35505e. For selected reviews and books on domino reactions, see. [DOI] [PubMed] [Google Scholar]; b Gawande MB, Bonifacio VDB, Luque R, Branco PS, Varma RS. Chem. Soc. Rev. 2013;42:5522. doi: 10.1039/c3cs60025d. [DOI] [PubMed] [Google Scholar]; c Tietze LF, Brasche G, Gericke K. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. [Google Scholar]; d Domling A, Wang W, Wang K. Chem. Rev. 2012;112:3083. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Toure BB, Hall DG. Chem. Rev. 2009;109:4439. doi: 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]; f Zhu JP, Bienayme H. Multicomponent Reactions. Wiley-VCH; Weinheim: 2004. [Google Scholar]; g Jiang B, Rajale T, Wever W, u S-JT, Li G. Chem.–Asian J. 2010;5:2318. doi: 10.1002/asia.201000310. [DOI] [PubMed] [Google Scholar]
- 10.a Jiang B, Yi M-S, Shi F, Tu S-J, Pindi S, McDowell P, Li G. Chem. Commun. 2012:808. doi: 10.1039/c1cc15913e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jiang B, Feng B-M, Wang S-L, Tu S-J, Li G. Chem.-Eur. J. 2012;18:9823. doi: 10.1002/chem.201201109. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jiang B, Wang X, Xu H-W, Tu M-S, u S-JT, Li G. Org. Lett. 2013;15:1540. doi: 10.1021/ol400322v. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Fan W, e QY, Xu H-W, iang BJ, Wang S-L, Tu S-J. Org. Lett. 2013;15:2258. doi: 10.1021/ol4008266. [DOI] [PubMed] [Google Scholar]
- 11.a Wang J, Wang J, Zhu Y, Lu P, Wang Y. Chem. Commun. 2011:3275. doi: 10.1039/c0cc04922k. [DOI] [PubMed] [Google Scholar]; b Zheng L, Ju J, Bin Y, Hua R. J. Org. Chem. 2012;77:5794. doi: 10.1021/jo3010414. [DOI] [PubMed] [Google Scholar]; c Umland K-D, Palisse A, Haug TT, Kirsch SF. Angew. Chem., Int. Ed. 2011;50:9965. doi: 10.1002/anie.201103961. [DOI] [PubMed] [Google Scholar]; d Kaschel J, Schneider TF, Kratzert D, Stalke D, Werz DB. Angew. Chem., Int. Ed. 2012;51:11153. doi: 10.1002/anie.201205880. [DOI] [PubMed] [Google Scholar]; e Grossman A, Enders D. Angew. Chem., Int. Ed. 2012;51:314. doi: 10.1002/anie.201105415. [DOI] [PubMed] [Google Scholar]
- 12.a Ruijter E, Scheffelaar R, Orru VAR. Angew. Chem., Int. Ed. 2011;50:6234. doi: 10.1002/anie.201006515. [DOI] [PubMed] [Google Scholar]; b Stearman CJ, Wilson M, Padwa A. J. Org. Chem. 2009;74:349. doi: 10.1021/jo9003579. [DOI] [PubMed] [Google Scholar]; c France S, Boonsombat J, Leverett CA, Padwa A. J. Org. Chem. 2008;73:8120. doi: 10.1021/jo8016956. [DOI] [PubMed] [Google Scholar]
- 13.a Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schroder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M. Nature. 2001;410:212. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]; b Witherington J, Bordas V, Gaiba A, Garton NS, Naylor A, Rawlings AD, Slingsby BP, Smith DG, Takle AK, Ward RW. Bioorg. Med. Chem. Lett. 2003;13:3055. doi: 10.1016/s0960-894x(03)00645-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.