Abstract
Myocardial infarction (MI) often develops when thrombosis occurs at lesions which have not previously been flow-limiting. However, the development of cardiogenic shock complicating acute myocardial infarction in such circumstances has received little attention. We studied the characteristics of 15 patients with cardiogenic shock who had no flow-limiting angiographic stenosis compared to 767 patients with at least one stenosis, who were enrolled in the SHOCK Trial and Registry. Compared to patients with at least one flow-limiting stenosis, patients with no flow-limiting stenosis were less likely to have pulmonary edema on chest x-ray (29% v 62%, P=0.008), and to have white ethnicity (53% v 82%, P = 0.011), and had lower median highest creatine kinase levels (702 v 2731 u/l; P = 0.018). For SHOCK Trial patients 1-year survival was 49% for patients with at least one flow-limiting stenosis and 71% for those with no flow-limiting stenosis (P= 0.268).
Keywords: no flow-limiting stenosis, myocardial infarction, cardiogenic shock
Introduction
Of patients surviving acute myocardial infarction (MI) up to 15% have angiographically normal or near normal coronary arteries. In patients who have undergone prior angiography, in the majority there was no flow-limiting stenosis at the site of the culprit plaque thrombus causing the index MI.1-4 Patients without flow-limiting stenosis following MI, are usually younger and more likely to be current smokers, but less likely to be diabetic. Also, such patients may be more likely to have certain inherited thrombophilias than the general population of patients hospitalized for acute MI. 3
The increasing use of invasive treatments including revascularization procedures and intra-aortic balloon counter-pulsation (IABP) has been associated reduced mortality after cardiogenic shock complicating MI.5-7 The SHould We Emergently Revascularize Occluded Coronaries for Cardiogenic ShocK (SHOCK) Trial reported 30-day and 12-month mortality rates of 47% and 57% respectively in patients randomized to emergency revascularization (ERV), which was better than initial medical stabilization (IMS).8-10 However, the subgroup of patients without angiographic flow-limiting stenosis after the development of cardiogenic shock complicating acute MI, has not been previously reported.
Methods
The SHOCK trial was a multicentre clinical trial that randomized patients with cardiogenic shock complicating acute MI to one of two strategies ERV and IMS; inclusion and exclusion criteria have been described previously.10 Fibrinolytic therapy was recommended for all patients randomized to IMS, unless there were absolute contraindications, and for patients randomized to ERV for whom immediate angiography was not possible, and IABP was recommended for all patients. Patients were initially evaluated at both tertiary care and community hospitals. The SHOCK Trial was approved by local ethical review boards.
Coronary and left ventricular cineangiograms from the SHOCK trial were reviewed at the core laboratory using the Thrombolysis In Myocardial Infarction (TIMI) criteria, the Rentrop coronary collateral circulation classification,11 and an estimate of the amount of myocardium at risk based on the coronary artery jeopardy score,12 as previously described.13-14 Each SHOCK trial angiogram was read by two independent readers using standardised data forms with discrepancies resolved by a third reader, and all readers were blinded to treatment group and enrolling centre. For SHOCK Registry patients, cardiac catheterization reports from all investigation sites were sent to the clinical coordinating centre for abstraction and central completion of a standardised report form that included the extent of coronary artery obstructions, degree of lesion severity, culprit lesion location, Thrombolysis In Myocardial Infarction (TIMI) flow characteristics and ejection fraction. Details of the angiographic analyses from the SHOCK registry have previously been reported. 14 Non-significant coronary artery disease, herewith called non-flow-limiting, was defined as <50% diameter stenosis.
Survival data was collected for 782 patients in hospital and at 30-days in the SHOCK Trial and Registry, though where data was only available for a smaller number of patients, numbers are shown in brackets. Vital status was obtained at one year for Trial patients only. Associations between patient characteristics, therapy, and outcome variables were assessed by Fisher’s exact test for categorical variables, the Wilcoxon rank sum test for ordinal and non-normally distributed continuous variables, and the Student’s t-test for normally distributed variables.
Results
Of 782 patients with cardiogenic shock due to predominant left ventricular (LV) failure, who underwent angiography in the SHOCK Trial and Registry, 15 (2%) had no flow-limiting stenosis (study group), and 767 (98%) patients had at least one artery with at least ≥50% stenosis (figure). By design, these patient groups were different with respect to angiographic coronary disease severity (Table 1). There were differences in race, with a lower proportion of patients without flow-limiting stenosis being white and non-hispanic.
Figure 1.
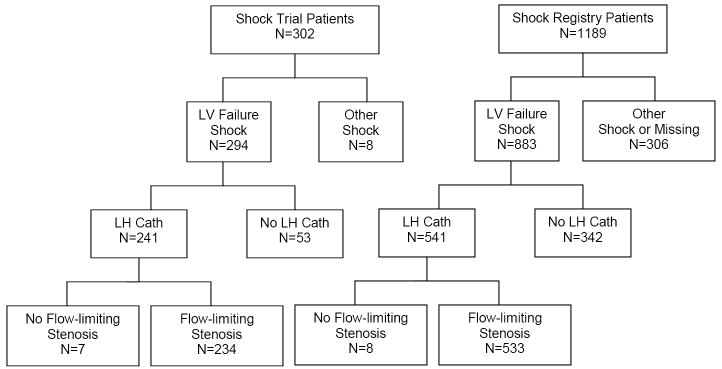
Patients with and without flow-limiting stenosis in the SHOCK trial and registry.
Table 1.
Baseline Characteristics of Patients with Predominant Left Ventricular Failure Shock
| Flow Limiting Stenosis | |||
|---|---|---|---|
|
| |||
| Variable | No (N = 15) |
Yes (N=767) |
p-value1 |
| Median Age years (IQR) | 61.1 (58.5, 72.3) | 67.1 (57.5, 74.0) | .470 |
| Randomized to ERV (N=241) | 27% | 60% | .127 |
| Men | 53% | 65% | .418 |
| White Race, Non-Hispanic | 53% | 82% | .011 |
| Transfer Admission | 53% | 55% | 1.000 |
| Cardiac Pulmonary Resuscitation Pre- | 17% | 20% | 1.000 |
| Randomization (N=185) | |||
| Previous Myocardial Infarction | 27% | 34% | .784 |
| Hypertension | 67% | 49% | .199 |
| Diabetes Mellitus | 40% | 30% | .404 |
| Smoker | 55% | 53% | 1.000 |
| Severe Elevated Lipids (N=414) | 33% | 41% | .744 |
| Prior Heart Failure | 21% | 12% | .231 |
| Renal Impairment | 0 | 7% | .615 |
| Prior Peripheral Vascular Diseases (N=541) | 17% | 16% | 1.000 |
| Prior Primary Coronary Intervention | 7% | 8% | 1.000 |
| Prior Coronary Artery Bypass Grafting | 0 | 8% | .621 |
p-values were obtained using Fisher’s Exact Test for categorical variables, Wilcoxon Rank Sum Test for non-normal variables, and Student’s t-test for normal variables.
Data is expressed as percentages except for age which is expressed as meant ± SD
The laboratory, hemodynamic, angiographic and MI characteristics of patients according to the presence or absence of flow-limiting stenosis are shown in Tables 2 - 4. Pulmonary edema on the chest x-ray occurred less frequently in patients without compared to with, flow-limiting stenosis. TIMI-3 flow was more frequently present in patients who had no flow-limiting stenosis compared to patients with at least 1 flow-limiting stenosis. Administration rates of thrombolytic therapy were similar in patients with, and without, flow-limiting stenosis (43% vs 33% respectively, P=0.601). Patients without flow-limiting stenosis were less likely to have an index inferior MI (7% vs. 35%, P=0.025). There were trends for these patients to have a higher rate of anterior index MI (86% vs 59%, P=0.053), to develop shock more quickly after an MI (median time from MI to shock 1.7 vs 6.1 hours, P=0.087), and to have higher left ventricular ejection fractions (40% vs 30%, P=0.074).
Table 2.
Laboratory and Hemodynamic Characteristics of Patients with predominant left ventricular failure shock
| Flow Limiting Stenosis | |||
|---|---|---|---|
|
| |||
| Variable | No (N =15) |
Yes (N=767) |
p-value1 |
| Median Initial Creatinine (mg/dL) (Q1, Q3) (N=818) | 1 (1, 2) | 1 (1, 2) | .557 |
| Initial Hemotocrit (N=577) | 37 ± 9 | 39 ± 8 | .403 |
| Pulmonary Edema on X-Ray (N=639) | 29 | 65 | .008 |
| Heart Rate at Time of Shock (bpm)2 | 100 ± 23 | 98 ± 24 | .770 |
| Diastolic Blood Pressure at Time of Shock (mm Hg)2 | 51 ± 23 | 55 ± 16 | .617 |
| Systolic Blood Pressure at Time of Shock (mm Hg)2 | 90 ± 22 | 90 ± 22 | .987 |
| Lowest Recorded Systolic Pressure (mm Hg)2 (N=561) | 66± 14 | 68 ± 15 | .524 |
p-values were obtained using Fisher’s Exact Test for categorical variables, Wilcoxon Rank Sum Test for non-normal variables, and Student’s t-test for normal variables
Obtained while on support measures
Table 4.
Myocardial Infarction Characteristics of Patients with and without Flow-limiting Stenosis
| Flow Limiting Stenosis | |||
|---|---|---|---|
|
| |||
| Variable | No (N = 15) |
Yes (N = 767) |
p-value |
| New Q waves in 2 Leads | 21% | 39% | .27 |
| New LBBB | 21% | 8% | |
| Anterior MI | 86% | 59% | .053 |
| Inferior Index MI (Non-anterior) (%) | 7% | 36% | .025 |
| Median Highest CPK (Q1, Q3) | 702 (230, 4360) | 2731 (1098, 5223) | .018 |
| Median MI onset to TT (hrs) (Q1, Q3) | 2 (1, 3) | 3 (1, 5) | .32 |
| Median MI to Shock (Q1, Q3) | 2 (1, 5) | 6 (2, 19) | .087 |
| TT administered | 33% | 43% | .601 |
| Revascularization During Hospital Stay | 0 | 73% | <.001 |
TT = thrombolytic therapy; CABG = coronary artery bypass surgery; MI = myocardial infarction; LBBB = left bundle branch block; CK = creatine kinase
We undertook further 3-way comparative analyses (0v 1v 2 or 3 vessel disease) evaluating the frequencies of various patient characteristics and outcomes in tables 1-5. In general, characteristics and outcomes of patients with one vessel disease tended to track with those multi-vessel disease rather than those with no-flow limiting stenosis, though there was a lower frequency of anterior MI with increasing numbers of diseased vessels (86% v 67% v 57%; P=0.005).
Table 5.
Outcomes in patients with predominant left ventricular failure shock, with and without flow-limiting stenosis
| Flow Limiting Stenosis | |||
|---|---|---|---|
|
| |||
| Variable | No (N = 15) |
Yes (N = 767) |
p-value1 |
| Revascularization During Hospital Stay | 0 | 73% | <.001 |
| In-hospital Survival | 60% | 55% | .797 |
| Revascularization Patients (N=560) | 0 | 59% | |
| Non-revascularization Patients (N=222) | 60% | 44% | .286 |
| 30-Day Survival (Trial Patients Only) (N=241) | 71% | 57% | .702 |
| Revascularization Patients (N=165) | 0 | 60% | |
| Non-revascularization Patients (N=76) | 71% | 51% | .435 |
| 1-Year Survival (Trial Patients Only) (N=240) | 71% | 48% | .268 |
| Revascularization Patients (N=164) | 0 | 51% | |
| Non-revascularization Patients (N=76) | 71% | 39% | .124 |
p-values were obtained using Fisher’s Exact Test for categorical variables, Wilcoxon Rank Sum Test for non-normal variables, and Student’s t-test for normal variable
In-hospital, 30-day and 1-year survival were similar for patients with and without flow-limiting stenosis (table 5). None of the 15 patients in the no flowlimiting group were revascularized. For Trial and Registry patients, in-hospital survival was 60% for no flow-limiting stenosis patients and 55% for flow-limiting stenosis patients (P=0.797). For those not undergoing revascularization, 60% of no flow-limiting stenosis patients and 44% of flow-limiting stenosis patients (P=0.286) survived. For Trial patients, 1-year survival was 71% for no flow-limiting stenosis patients and 48% for flow-limiting stenosis patients (P=0.268). For Trial patients not undergoing revascularization, 71% of no flow-limiting stenosis patients and 39% of flow-limiting stenosis patients (P=0.124) were alive at 1 year.
Discussion
Cardiogenic Shock is the major cause of mortality following hospitalization for acute MI in the reperfusion era. Patients in the SHOCK Trial and Registry surviving MI who develop cardiogenic shock and who have no flow-limiting stenosis have differences in characteristics such as less pulmonary edema. As these patients had less pulmonary edema and a tendency to better left ventricular function (p = 0.074), this implies shock may have been due to a lesser area of “myocardium risk” from the index MI.
The development of shock in patients without significant flow-limiting coronary stenosis suggests there may be a subgroup of cardiogenic shock patients with different risk factor characteristics. A minority of patients surviving an ST elevation MI, have angiographically normal arteries or have no flow-limiting stenosis in their coronary arteries. 1-3 As acute MI does not invariably develop after the formation of thrombi on atherosclerotic plaques, pro-coagulant or thrombophilic factors which may lead to complete occlusion, may have contributed to the development of acute MI causing cardiogenic shock. Also prolonged coronary spasm increasing shear stress on platelets leading to thrombus formation may have been a mechanism.
Associations between the development of MI and angiographically normal or ‘near normal’ coronary arteries, and increased frequencies of inherited thrombophilic factors, including Factor V Leiden compared with other patients after MI or to age-matched controls has been reported. 15-16 The rate of inherited thrombophilias risk in patients in this cohort is unknown.
A longer time between MI and angiography in some patients without flow limiting stenosis may have allowed time for autolysis of thrombus to have occurred, as only 30% received fibrinolysis. Other factors or drugs may have been prothrombotic, including oral contraceptive agents which may have been influenced pathogenesis in premenopausal women, a group who commonly have normal arteries angiographically. 17-18 It is a limitation that we did not record the use of hormone therapies.
An intriguing potential mechanism that may explain cardiogenic shock complicating myocardial infarction, but without significant angiographic coronary stenosis is emotional stress, 19 leading to excess levels of catecholamines. It is possible that a similar mechanism and potentially indistinguishable mode of presentation when acute myocardial stunning due to sudden emotional stress.20 This latter finding has been reported predominantly in women generally without significant coronary stenosis. While our patients with shock and (near) normal coronary angiography were ~ 50% female, and most had anterior MI, the median highest creatine kinase levels of our cohort were 702 U/L, which are higher than the 133 U/L level previously reported. Also, slightly elevated troponin levels 20 may have been due to heart failure rather than myocardial infarction.21-22 We did not collect systematic data to assess the role of emotional stress in the development of cardiogenic shock post-infarction in our cohort.
Table 3.
Angiographic Characteristics of Patients with predominant left ventricular failure shock
| Flow Limiting Stenosis | |||
|---|---|---|---|
|
| |||
| Variables | No (N=15) |
Yes (N=767) |
p-value1 |
| Angiographic LVEF % (N=92) | 40 ± 13 | 30 ± 12 | .074 |
| Jeopardy Score (N=233) | 0 ± 0 | 8 ± 3 | <.001 |
| Culprit Coronary Artery (N=625) | .075 | ||
| Left Main | 33% | 6% | |
| Left Anterior Descending | 67% | 48% | |
| Right Coronary Artery | 0% | 29% | |
| Left Circumflex | 0% | 15% | |
| Saphenous Venous Graft | 0% | 1% | |
| Culprit Vessel TIMI Flow 3 (N=2, 583) | 100% | 18% | .036 |
| Culprit Vessel TIMI flow (N=2, 583) | .011 | ||
| TIMI 0-1 | 0% | 61% | |
| TIMI 2 | 0% | 20% | |
| TIMI 3 | 100% | 19% | |
| Diameter Stenosis | |||
| 50-90% (Flow-limiting Stenosis) | --- | 15% | |
| >90% (Flow-limiting Stenosis) | --- | 85% | |
p-values were obtained using Fisher’s Exact Test for categorical variables, Wilcoxon Rank Sum Test for non-normal variables, and Student’s t-test for normal variables.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Little WC, Constantinescu M, Applegate RJ, Kutcher MA, Burrows MT, Kahl FR, Santamore WP. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circ. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 2.Alpert JS. Fascination with myocardial infarction and normal coronary arteries. Eur Heart J. 2001;22:1364–1366. doi: 10.1053/euhj.2001.2590. [DOI] [PubMed] [Google Scholar]
- 3.Pinney SP, Rabbani LE. Myocardial infarction in patients with normal coronary arteries: proposed pathogenesis and predisposing risk factors. J Thromb Thrombolysis. 2001;11:11–17. doi: 10.1023/a:1008995908377. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morhpological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1267. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg RJ, Samad NA, Yarzebski J, Gurwitz J, Bigelow C, Gore JM. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med. 1999;340:1162–1168. doi: 10.1056/NEJM199904153401504. [DOI] [PubMed] [Google Scholar]
- 6.Hochman JS, Buller CE, Sleeper LA, Boland J, Dzavik V, Sanborn TA, Godfrey E, White HD, Lim J, LeJemtel T for the SHOCK Investigators. Cardiogenic shock complicating acute myocardial infarction - etiologies, management and outcome: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36:1063–1070. doi: 10.1016/s0735-1097(00)00879-2. [DOI] [PubMed] [Google Scholar]
- 7.Jeger RV, Radovanovic D, Hunziker PR, Pfisterer ME, Stauffer JC, Erne P, Urban P for the AMIS Plus Registry Investigators. Ten-Year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008;149:618–626. doi: 10.7326/0003-4819-149-9-200811040-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH, Picard MH, Menegus MA, Boland J, Dzavik V, Thompson CR, Wong SC, Steingart R, Forman R, Aylward PE, Godfrey E, Desvigne-Nickens P for the SHOCK investigators. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 9.Hochman JS, Sleeper LA, White HD, Dzavik V, Wong SC, Menon V, Webb JG, Steingart R, Picard MH, Menegus MA, Boland J, Sanborn T, Buller CE, Modur S, Forman R, Desvigne-Nickens P, Jacobs AK, Slater JN, LeJemtel TH SHOCK Investigators. One-year survival following early revascularization for cardiogenic shock. JAMA. 2001;285:190–202. doi: 10.1001/jama.285.2.190. [DOI] [PubMed] [Google Scholar]
- 10.Hochman JS, Sleeper LA, Godfrey E, Sleeper LA, McKinlay SM, Sanborn T, Col J, LeJemtel T for the SHOCK Trial Study Group. Should we emergently revascularize occluded coronaries for cardiogenic shock: an international randomized trial of emergency PTCA/CABG -- trial design. Am Heart J. 1999;137:313–321. doi: 10.1053/hj.1999.v137.95352. [DOI] [PubMed] [Google Scholar]
- 11.Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circ. 1986;74:469–476. doi: 10.1161/01.cir.74.3.469. [DOI] [PubMed] [Google Scholar]
- 12.Califf RM, Harrell FE, Jr, Lee KL, Rankin JS, Mark DB, Hlatky MA, Muhlbaier LH, Wechsler AS, Jones RH, Oldham HN., Jr Changing efficacy of coronary revascularization. Implications for patient selection Circ. 1988;78:1185–1191. [PubMed] [Google Scholar]
- 13.Sanborn TA, Sleeper LA, Webb JG, French JK, Bergman G, Parikh M, Wong SC, Boland J, Pfisterer M, Slater JN, Sharma S, Hochman JS SHOCK Investigators. Correlates of one-year survival in patients with cardiogenic shock complicating acute myocardial infarction: angiographic findings from the SHOCK trial. J Am Coll Cardiol. 2003;42:1373–1379. doi: 10.1016/s0735-1097(03)01051-9. [DOI] [PubMed] [Google Scholar]
- 14.Wong SC, Sanborn T, Sleeper LA, Webb JG, Pilchik R, Hart D, Mejnartowicz S, Antonelli TA, Lange R, French JK, Bergman G, LeJemtel T, Hochman JS for the SHOCK Investigators. Angiographic findings and clinical correlates in patients with cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK trial registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36:1077–1083. doi: 10.1016/s0735-1097(00)00873-1. [DOI] [PubMed] [Google Scholar]
- 15.Van de Water NS, French JK, Lund M, Hyde TA, White HD, Browett Prevalence of factor v leiden and prothrombin variant g20210a in patients age <50 years with no significant stenosis at angiography three to four weeks after myocardial infarction. J Am Coll Cardiol. 2000;36:717–722. doi: 10.1016/s0735-1097(00)00772-5. [DOI] [PubMed] [Google Scholar]
- 16.Da Costa A, Isaaz K, Faure E, Mourot S, Cerisier A, Lamaud M. Clinical characteristics, aetiological factors and long-term prognosis of myocardial infarction with an absolutely normal coronary angiogram: a 3-year follow-up study of 91 patients. Eur Heart J. 2001;22:1459–1465. doi: 10.1053/euhj.2000.2553. [DOI] [PubMed] [Google Scholar]
- 17.Rosendaal FR, Siscovick DS, Schwartz SM. Factor V Leiden (resistance to activated protein C) increases the risk of myocardial infarction in young women. Blood. 1997;89:2817–2821. [PubMed] [Google Scholar]
- 18.Ardissino D, Mannucci PM, Merlini PA, Duca F, Fetiveau R, Tagliabue R, Tubaro M, Galvani M, Ottani F, Ferrario M, Corral J, Margaglione M. Prothrombotic genetic risk factors in young survivors of myocardial infarction. Blood. 1999;94:46–51. [PubMed] [Google Scholar]
- 19.Geleini M, Hochman JS. Acute Myocardial Infarction Triggered by Emotional Stress. AM J Cardiol. 1992;69:1512–1513. doi: 10.1016/0002-9149(92)90918-o. [DOI] [PubMed] [Google Scholar]
- 20.Wittstein IS, Thiemann IJR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of Myocardial Stunning Due to sudden Emotional Stress. N Eng Med J. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 21.French JK, White HD. Clinical implications of the new definition of myocardial infarction. Heart. 2004;90:99–106. doi: 10.1136/heart.90.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristian Thygesen, Alpert JosephS, White HarveyD on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal Definition of Myocardial Infarction. Circ. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]


