Abstract
Basic pharmacological/transgenic studies have clearly demonstrated a cause-effect relationship between the induction and activation of matrix metalloproteinases (MMPs) and adverse changes in the structure and function of the left ventricle (LV). Thus, regulation of MMP induction and/or activation would appear to be a potential therapeutic target in the context of cardiovascular disease, such as following myocardial infarction (MI). However, pharmacological approaches to inhibit MMPs have yet to be realized for clinical applications. The endogenous inhibitors of the MMPs (TIMPs) constitute a set of 4 small molecules with unique functionality and specificity. Thus, improved understanding on the function and roles of individual TIMPs may provide important insight into the design and targets for pharmacological applications in LV remodeling processes, such as MI. Therefore, the purpose of this review will be to briefly examine biological functions and relevance of the individual TIMPs in terms of adverse LV remodeling post-MI. Second is to examine the past outcomes and issues surrounding clinical trials targeting MMPs in the post MI context and how new insights into TIMP biology may provide new pharmacological targets. This review will put forward the case that initial pharmacological attempts at MMP inhibition were over-simplistic and that future strategies must recognize the diversity of this matrix proteolytic system and that lessons from TIMP biology may lead to future therapeutic strategies.
Keywords: myocardial remodeling, tissue inhibitors of matrix metalloproteinases, fibroblasts, myocardial infarction
Introduction
Heart failure (HF) remains a major cause of morbidity, mortality, and constitutes a significant portion of medical care costs. In general clinical terms, HF is manifested by defects in cardiac pump function (ejection, filling, or a combination of both), which in turn will cause clinical signs and symptoms that are often progressive and result in emergent presentation and hospitalization. While the current standard of care for HF is appropriately focused upon the reduction in symptomatology, therapeutic strategies which specifically target the fundamental underpinnings of the HF process remains an unmet medical need and hence an important area for research and development. The term HF is not defined by a specific pathological stimulus but rather the downstream consequence of multifactorial events with the underlying causes being quite diverse, and as such, classification schemes can be problematic. Nevertheless, a generalized dual classification system has been developed that encompasses the HF presentation, and key underlying physiological manifestations have emerged. [1] Specifically, patients with a HF presentation and primarily left ventricular (LV) systolic dysfunction such as that which can occur following a myocardial infarction (MI), or that of primarily LV diastolic dysfunction, which can occur with a sustained pressure overload such as hypertension. The changes in LV geometry and myocardial structure, often referred to as LV remodeling, can also be different in these two classifications, and as such the therapeutic targets and pathways may also be distinctly different. For the purposes of this review and to maintain focus, the prototypical example of LV remodeling and progressive HF, as it applies to myocardial infarction (MI), will be utilized.
Despite significant improvements in the management of acute coronary syndromes and myocardial ischemic events, residual injury to the affected region of the myocardium (MI) can occur. This myocardial injury sets in motion a number of cellular and extracellular matrix (ECM) events. The death of cardiac myocytes in the context of ischemic injury and MI first occurs through the classical cell death pathway, necrosis. This region of necrotic myocytes then causes a cascade of biological events, which include the expression of inflammatory molecules and egress of inflammatory cells, proliferation and transdifferentiation of fibroblasts, and the induction of ECM degradation/synthetic pathways.[2,3] While this process is initially considered to be an appropriate and adaptive wound healing response, the persistence of these biological events, particularly that of continued ECM turnover, is considered to be maladaptive and contribute to the pathophysiology of LV remodeling and progression to HF.[3–5] Most specifically, the changes in ECM structure can contribute to a structural milestone in adverse post-MI remodeling - infarct expansion.[5,6] The affected region following the MI that contributes to infarct expansion, progressive LV remodeling, and systolic dysfunction not only is composed of the MI region itself but can also affect the viable myocardium surrounding the MI. Significant changes within the ECM occur during all time points post-MI and likely contribute to the overall adverse LV remodeling process. Firstly, the inflammatory response causes the release of matrix metalloproteinases (MMPs) as well as other proteases to degrade the ECM and allow for margination of inflammatory cells.[8–10] However, with a persistent inflammatory state, MMP induction will also destabilize the newly formed ECM and the nascent scar. Secondly, the transformed fibroblast population within the MI region as well as the surrounding viable myocardium causes a shift in the relative balance of MMPs and endogenous tissue inhibitors of MMPs (TIMPs),[6,8,9,11–14] favoring accelerated ECM turnover and a failure of mature scar formation. These observations led to significant initial exuberance by both industry and medical academia for the development of pharmacological reagents, which would inhibit MMP activity for the purposes of interrupting adverse LV remodeling post-MI.[15–24] However, this initial enthusiasm has been tempered by the recognition that MMPs constitute a diverse family of enzymes, all with unique functionality, and that only a subset of these MMPs may hold therapeutic relevance in terms of post-MI remodeling.[6,21–23,25,26] At the same time, there is growing awareness that the TIMPs may also be multifaceted in terms of biological roles relevant to the post-MI remodeling process well beyond that of inhibition of active MMPs.[27–36] Specifically, there are 4 known TIMPs, which appear to be differentially regulated in terms of temporal expression following tissue injury, may differentially affect MMP activity, and regulate fibroblast growth and viability.[27–32,35–43] Therefore, the purpose of this review is to place into context the functionality, expression profiles, and potential therapeutic application of these individual TIMPs in terms of post-MI remodeling.
TIMPs – small molecules with diversity of function
The TIMPs constitute 4 unique, small molecular weight proteins (~20 kDa) that are distinctly different gene products with 50% or less homology.[27–30] The first TIMP, TIMP-1, was identified in the late 1970s and TIMP-4 was first described in the late 1990s,[44,45] and while initially considered to simply bind to active MMPs in a 1:1 stoichiometric ratio, these molecules have unique and differential effects upon key aspects of ECM biology relevant to the post-MI remodeling process (Table 1). Using primary fibroblast cultures taken predominantly from either myocardial samples or from cancer related regions, [27–37] divergent effects of specific TIMPs have been identified with respect to cell growth and viability. For example, TIMP-1 induces a robust effect on fibroblast growth and proliferation, whereas TIMP-2 and TIMP-4 appear to have a more modest or negative effect.[29–37,41] On the other hand, TIMP-1 has been shown to reduce relative apoptosis rates, whereas other TIMPs can accelerate cell death.[31,32,37,41] With respect to TIMP-2, a robust effect on fibroblast transdifferentiation, as defined as a transition to a more contractile phenotype, has been reported.[37] In contradistinction to other TIMPs, TIMP-3 modulates cytokine processing through an inhibition/interruption of a disintegrin-metalloproteases (ADAMs), including both ADAM-10 and ADAM-17. [27–30,39,40]
Table 1.
Differential Biological Functionality of Tissue Inhibitors of MMPs (TIMPs) Potentially Relevant to Post-MI Remodeling
| TIMP-13 | TIMP-2 | TIMP-3 | TIMP-4 | References | |
|---|---|---|---|---|---|
| Cell Growth/Proliferation1 | ++ | − | + | − | 31–34,36,37,41 |
| Cell Apoptosis | − | + | + | + | 31,36,41 |
| Cytokine Processing | ++ | + | 27,30 | ||
| Fibroblast Transdifferentiation | + | +++ | + | 36,37 | |
| Pro-MMP Complex Formation | Pro-MMP-9 | Pro-MMP-2 | Pro-MMP-2 | Pro-MMP-22 | 27,30,35,36 |
| Transgenic Deletion and Post-MI Remodeling | ↑ LV Dilation | ↑ LV Dilation | Rupture, ↑ Inflammation | LV Dilation, Accelerated HF | 17,38,40,43 |
Derived from studies previously in fibroblasts / smooth muscle cell culture
Does not yield an activation complex
Weak inhibitor of membrane bound MMPs
Other unique differences in TIMPs can be found in the relative affinity for MMP inhibition as well as activation. Specifically, TIMP-1 has been shown to have a very low affinity for the transmembrane MMPs, such as MT1-MMP.[27,28,46] Since MT1-MMP has been shown to play a significant role in ECM remodeling, including post-MI remodeling,[47,48] then the weak inhibitory capacity of TIMP-1 on MT1-MMP likely holds relevance when considering TIMPs as a therapeutic. With respect to TIMP-2, it has now been well established that an activation complex is formed between proMMP-2, TIMP-2, and MT1-MMP, which will facilitate activation of MMP-2.[27,35,47,49] Moreover, while other TIMPs, such as TIMP-4, can bind to pro-MMP-2, these pro-MMP-2/TIMP-4 complexes do not appear to facilitate MMP-2 activation.[27,28,35,36] In fact, in-vitro kinetic studies have identified TIMP-4 will inhibit the interaction and activation of pro-MMP-2 via the TIMP-2/MT1-MMP cascade and is also a potent inhibitor of MT1-MMP.[27–29,35,36,42,50] Thus, a duality of function exists for TIMP-2 whereby both MMP activation and inhibition can occur simultaneously and provide for a very precise localization of ECM turnover. With respect to TIMP-4, the direct and indirect effects on MMP activation and activity, along with the relatively restricted expression pattern to that of hollow muscular organs, such as the heart and uterus, [42,51,52] underscore the unique functionality of each TIMP and potential relevance to the post-MI remodeling process.
While TIMP binding to specific MMP sequences results in both inhibition and activation, there is growing evidence that TIMPs can directly influence cell growth and function through a ligand-receptor mediated pathway.[29–34] In cancer associated fibroblasts, it has been demonstrated that TIMP-1 binds to the membrane receptor CD63 and can cause extracellular signal-regulated kinase activation.[31] In transformed fibroblasts, TIMP-1 induced activation of protein kinase B (Akt) pathway, whereby this cellular transduction event was demonstrated to be MMP independent.[32] In cancer associated fibroblasts, TIMP-2 has been demonstrated to reduce mitogen activated signaling through a cyclic AMP mediated pathway, which in turn reduced fibroblast proliferation.[33] Moreover, TIMP-2 binds to transformed fibroblasts in a ligand-receptor mediated fashion that is saturable, whereby the binding kinetics were unaffected by co-incubation with an MMP inhibitor.[33] In other in-vitro binding studies, it has been demonstrated that the likely cognate receptor for TIMP-2 is an alpha-3/beta-1 integrin.[34] While the majority of these studies have been performed in transformed fibroblast cell lines in the context of cancer, there is likely great relevance to the post-MI remodeling process. Specifically, there are significant parallelisms in the expression profiles in the transdifferentiation process of fibroblasts associated with cancer and those which occur in fibroblasts post-MI.[13,14] Thus, the identification of specific TIMP receptors in cardiac cells, such as the myocardial fibroblast, may afford a specific mechanism by which to regulate proliferation and function of this critical ECM cell type. Indeed, differential effects of individual TIMP types on myocardial fibroblast growth and transdifferentiation have been demonstrated [37] and were likely due to specific receptor mediated interactions.
Through sequencing analysis and examination of TIMPs in invertebrate species, [27,28] it is clear that TIMPs are ancient molecules, which therefore underscore the overall biological relevance of these molecules. What is now becoming clear is past canonical thought that TIMPs simply bind and inhibit active MMPs must be revised. The diversity in biological effects of each TIMP upon cell growth, viability, and signaling as well as those that can actually facilitate MMP activation hold important considerations when developing these molecules as pharmacological agents, such as with post-MI remodeling.
TIMP profiles and expression post-MI
A number of animal and clinical studies have profiled changes in TIMP expression in the post-MI period. [see reviews 6,7,25,47] Overall, these studies uniformly demonstrated a divergence in MMP and TIMP expression and induction in the early post-MI period. Specifically, in animal and patient studies of post-MI remodeling, a robust induction of MMPs, particularly those associated with inflammation, occur early (~3–7 days post-MI) but are not necessarily accompanied by a concomitant increase in TIMPs. For example, in a rat MI model, Peterson and colleagues demonstrated temporal changes in TIMP induction over an approximate 2 week post-MI period.[53] Specifically, at early post-MI time points (<3 days) an increase in relative TIMP-1 and TIMP-2 mRNA levels occurred, which was then followed by a time-dependent decline over longer post-MI time periods. This study also reported that relative TIMP-4 concentrations actually fell from referent control values during this post-MI time period. In contrast, this past study demonstrated a robust and persistent induction of a number of MMP types, which included MMP-13, MMP-2, MMP-9, and MT1-MMP. The changes in TIMP levels post-MI also appear to be region specific within the LV, whereby TIMP levels are substantially extinguished within the MI region, and within normal limits or elevated in the remote viable regions of the myocardium.[12] Clinical studies have utilized plasma samples to profile TIMP levels,[55–56] and while arguably the sources for these TIMPs can be of concern, they can serve as a reasonable surrogate for changes in TIMP levels which are likely occurring within the myocardium of patients post-MI.[57] A summary of clinical studies that comprise over 5,000 subjects, which have measured TIMPs following MI, is shown in Table 2. Overall, these studies identified an increase in relative plasma TIMP-1 levels in patients post-MI, but relative TIMP-2 and TIMP-4 levels either remained unchanged or were reduced from referent normal values. However, these TIMP levels were also accompanied by robust increases in plasma MMP levels in the post-MI period, and the elevated MMP levels persist for weeks to months. Moreover, the relative magnitude of the increase in MMP levels is associated with the rate and extent of adverse post-MI remodeling in patients.[6,54,56] The lack of concordance between increased MMP levels and TIMP levels is further exemplified when the stoichiometric ratio of MMP/TIMP is considered. For example, a 3–4 fold increase in the MMP-9/TIMP-4 ratio has been reported in post-MI patients, which illustrates the imbalance of this ECM proteolytic system in the post-MI context.[54]
Table 2.
Tissue Inhibitor of Matrix Metalloproteinase Dynamics in Patients with Ischemic Heart Disease and Relation to Clinical Outcomes
| Subjects (n) |
Biomarker(s) | Primary Observation | Reference # |
|---|---|---|---|
| 233 | PINP, ICTP, PIIINP, MMP-1, TIMP-1 | Longitudinal changes in all ECM markers whereby ICTP associated with greater HF symptom progression | 33 |
| 1009 | PIIINP, MMP-1, TIMP-1, hsCRP, IL-18, IL-10 | Indices of increased ECM turnover associated with functional capacity and outcomes. | 34 |
| 39 | MMP-1, TIMP-1 and Biopsy | Shift in MMP-1/TIMP-1 balance favoring ECM degradation as evidenced by ECM biopsy histology | 26 |
| 85 | Portfolio of MMPs and TIMPs serially examined | A specific cassette of MMPs and TIMPs are increased following MI and associated with progressive LV remodeling | 37 |
| 100 | TIMP-1, -2, -4 | Early changes in plasma TIMP-4 levels associated with degree of LV dilation at 3 months post-MI | 39 |
| 404 | TIMP-1, MMP-9, NTproBNP | Changes in MMP-9 associated with adverse post-MI remodeling as defined by LV dilation and TIMP-1 levels demonstrated predictive risk for combined event endpoint (HF/death) | 40 |
| 1313 | TIMP-1,-2,-4 | Univariate and composite score relationship to major advance cardiovascular events | 58 |
| 30 | MMP-9, TIMP-1, TIMP-2 | MMp-9/TIMP-1 ratio associated with coronary plaque rupture | 59 |
| 500 | NTpro-BNP, TIMP-1 | TIMP-1 predictive of long-term outcome in ischemic heart disease | 60 |
| 389 | MMP-9, TIMP-1 | TIMP-1 predictive of mortality following MI | 61 |
| 556 | TIMP-1, TIMP-1 polymorphism | TIMP-1 associated with worsening heart failure post-MI | 62 |
| 1082 | MMP-9, TIMP-1 | TIMP-1 associated with cardiovascular risk in elderly | 63 |
Despite the relative imbalance between MMP and TIMP levels in the post-MI period, relative TIMP-1 levels increase from normal values in both animal models and patients, particularly at later post-MI time points.[6,8,53,54] This raises the question as to why elevated TIMP-1 levels in the post-MI period do not appear to favorably alter the post-MI remodeling process. Moreover, elevated plasma TIMP-1 levels have actually been demonstrated to be associated with a worsening of clinical outcomes and cardiovascular events.[57] The underlying reasons for this apparent conundrum regarding TIMP-1 may be due to several biological and structural reasons. First, the increased induction of TIMP-1 may be insufficient to effectively prevent the exuberant ECM proteolysis that persists in the post-MI period. Specifically, the relative MMP/TIMP-1 levels remain elevated throughout the post-MI period, thus favoring continued ECM degradation. Furthermore, TIMP-1 is a poor inhibitor of MT1-MMP, a likely critical MMP type in the post-MI remodeling process. Second, TIMP-1 causes fibroblast growth and transdifferentiation (Table 1), which may actually exacerbate post-MI remodeling. Third, in myocardial samples from patients with end-stage ischemic HF, TIMP-1 levels are increased, but MMP/TIMP-1 complexes are actually reduced.[70,71] This observation suggests that defects in TIMP-1 binding to active MMPs may be impaired. It has been demonstrated that post-translational modification of TIMPs, can directly affect the affinity and inhibition of active MMPs.[28–30] For example, carbamylation of the N-terminal amino group of TIMP-1 greatly reduces MMP inhibitory capacity.[66] These past binding/structural studies suggest the intriguing possibility that post-translational modification of TIMPs may occur in the context of post-MI remodeling.
TIMP modulation by transgenics and relevance to post-MI remodeling
In order to examine the potential consequence and roles of individual TIMPs in terms of post-MI remodeling, transgenic constructs through deletion of genomic fragments of the targeted TIMP gene were utilized, which in general will prevent initiation of transcription.[17,38,40,43] None of the individual TIMP transgenic knockout lines were embryonically lethal, and stable colonies could be developed. This however does not imply that the global loss of a specific TIMP yielded a phenotypically normal organism. For example, TIMP-3 deletion resulted in abnormal lung development and function as well as enhanced inflammation.[67,68] While there are a number of inherent limitations with global gene deletion in transgenic mice, MI induction in these murine constructs through coronary ligation have provided a unique insight into post-MI remodeling and TIMPs.[17,38,40,43]
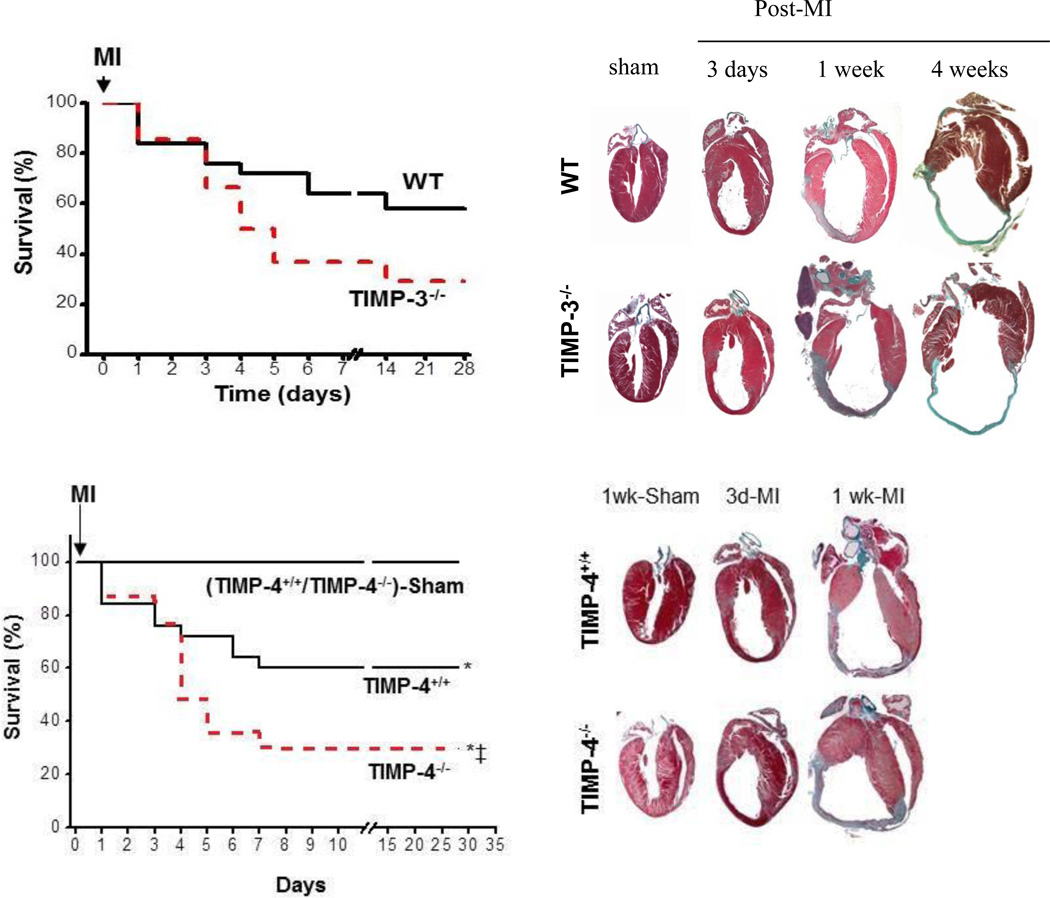
In general terms, TIMP deletion accelerated the adverse LV remodeling process post-MI, but there were differences in the changes in myocardial structure (Table 1). In the TIMP-1−/− mouse, induction of an MI caused an acceleration in global LV dilation compared to wild-type littermates, presumably due to increased infarct expansion and a loss of ECM structural integrity.[17] Notably, these effects of TIMP-1 gene deletion post-MI in terms of increased acceleration of the adverse post-MI remodeling process could be mitigated somewhat by concomitant pharmacological MMP inhibition.[17] The most significant work regarding the effects of targeted TIMP deletion and the consequences upon post-MI remodeling is from the Kassiri laboratory.[38,40,43] Using a TIMP-2−/− mouse line, these investigators demonstrated that MI induction caused increased loss of ECM structural integrity by second harmonic generation and multi-photon imaging, which was in turn associated with increased infarct expansion and LV dilation.[38] Moreover, these authors demonstrated that in these TIMP-2 null mice, which effectively abolished the pro-MMP-2/TIMP-2/MT1-MMP activation cascade, accelerated adverse LV remodeling was observed. These findings underscored several important points regarding MMP-TIMP interactions. First, this study demonstrated the requirement of TIMP-2 in an in-vivo context or MMP-2 activation. Second, these investigators identified enhanced MT1-MMP activity in the TIMP-2 null mice, underscoring the tight interaction of MMP types to specific TIMPs. Perhaps the most profound effects on post-MI remodeling was observed in the TIMP-3−/− mouse.[39] In these studies, the Kassiri laboratory demonstrated that MI induction in the TIMP-3 null mouse was associated with increased and persistent myocardial inflammation and increased MI rupture.[39] While myocardial rupture is a rather rare event post-MI in humans, this occurs with enough frequency in murine MI models to be a quantifiable response variable.[8–10,19] The increased MI rupture in the post-MI period resulted in worse survival in the TIMP-3−/− mouse when compared to strain matched wild type mice and clear evidence of LV dilation in the surviving mice (Figure 2). Finally, these investigators demonstrated differential effects of TIMP-4 gene deletion with respect to LV remodeling and survival secondary to MI or a pressure overload.[43] While the effects of TIMP-4 gene deletion in the context of a sustained pressure overload appeared modest, adverse LV remodeling and poor survival was observed post-MI (Figure 2).
Figure 1.
The effects of transgenic deletion of specific TIMPs with respect to post-MI remodeling have been the subject of several studies by the Kassiri laboratory,[38,40,43] and representative findings from TIMP-3 gene deletion and that of TIMP-4 gene deletion are shown here. Left panels demonstrated a worsening survival in the TIMP-3 and TIMP-4 gene knockout mice following MI, but the pathophysiology for this reduced survival may be distinctly different. In the TIMP-3 null mice, increased inflammation and incidence of MI rupture were observed, whereas accelerated LV dilation and progression to HF was the observation in the TIMP-4 null mice. In both cases, adverse LV remodeling characterized by increased LV volumes, MI thinning, and expansion was observed in both the TIMP-3 and TIMP-4 null mice (right panels). Reproduced from references #40 and 43.
Clinical studies of pharmacological MMP inhibition post-MI: Lessons learned
The initial pharmacological compounds advanced to clinical application for MMP inhibition contained structures which would bind to the common sequence of the catalytic domain of active MMPs. These initial MMP inhibitors were non-peptides and contained a hydroxymate structure.[6,21–23] Continued improvements in the structural design yielded MMP inhibitors with nanomolar potency and were successfully used in several animal models.[15–17,19,24] One of the MMP inhibitors that was successfully advanced to clinical trials for the indication of post-MI remodeling was the pharmacological MMP inhibitor (PG116800).[16,20,69] This specific MMP inhibitor had been utilized in pre-clinical large animal models in order to develop pharmacokinetic data and initial proof of concept.[16,70] Following which, a clinical study was undertaken entitled Selective Matrix Metalloproteinase Inhibitor to Prevent Ventricular Remodeling After Myocardial Infarction (Prevention of Myocardial Infarction Early Remodeling-PREMIER).[20,69] The PREMIER study recruited 253 patients, primarily from international study centers that were with an identified MI, and then randomized to an active treatment arm consisting MMP inhibition or placebo. Treatment began within 48 hours of presentation with an MI, and initially the study utilized a 200 mg dose to be given orally twice daily for the entire study interval of 180 days.[69] However, due to historical concerns regarding the potential risk of systemic side effects, the dosing regimen was reduced to 200 mg dose once/day.[20,69] Changes in LV end-diastolic volume from baseline compared to that obtained at 90 days post-MI was the primary response variable. Surprisingly and contrary to other major clinical studies,[1–7] LV volumes only increased slightly (~10%), suggesting that infarct expansion had not occurred in these post-MI patients, irrespective of treatment. As a function of baseline values, the change in LV volumes was 8.4% in the PG11680 group and 10.3% in the placebo group (p=0.31). The neutral findings from the PREMIER trial were likely multifactorial and included an inadequate dosing regimen and a minimal change in the primary response variable. For example, based upon pharmacokinetic computations as well as the known volume of distribution for humans of PG11680,[16,69] it is unlikely that this study achieved significant therapeutic efficacy, that is MMP inhibition, in a large number of patients [for further computations and discussion, see reference 6]. Inexplicably, plasma profiles for PG11680 were not reported in the PREMIER trial, and thus this issue remains of concern. In the PREMIER study, the relative magnitude of post-MI remodeling as a function of change in LV end-diastolic volume were unusually modest (5 mL/m2). Based upon the initial power estimates,[69] it would be necessary for PG11680 to reduce the change in LV end-diastolic volume during the post-MI period by 80% when compared to placebo values. These dramatic reductions in LV end-diastolic volumes have never been achieved in pre-clinical animal studies of MMP inhibition despite optimal dosing conditions and experimental designs. Despite the significant shortcomings in design and limitations in the primary response variable, the PREMIER trial did have one effective outcome: the closure of a number of research and development programs focusing upon MMP inhibition for adverse LV remodeling.
An entirely different and initially rather surprising MMP inhibitor that was advanced to clinical trials was that of doxycycline.[18,71] In a preclinical large animal study, Villarreal and colleagues demonstrated that specific doxycycline doses would reduce adverse LV remodeling post-MI and that this effect was independent of any anti-microbial action.[18] Tetracyclines were developed as a result of the screening of soil samples for antibiotic organisms (an extensive review on the pleiotropy of tetracyclines can be found in [72]). This family of antibiotics was found to be highly effective against various pathogens including rickettsiae, Gram-positive, and Gram-negative bacteria, thus becoming a class of broad-spectrum antibiotics. The antibiotic mechanism of action of tetracyclines is thought to be related to the inhibition of protein synthesis. Over time, many other “protective” actions have been reported for tetracyclines. Minocycline, which can readily cross cell membranes, is known to be a potent anti-apoptotic agent. Its mechanism of action appears to relate to specific effects exerted on apoptosis signaling pathways. Another tetracycline, doxycycline, is known to exert antiprotease activities. Doxycycline can inhibit MMPs, which can contribute to tissue destruction activities as seen with gingivitis, thus their FDA approval for this use under the category of MMP inhibition. A large body of literature has provided evidence for additional “beneficial” actions of tetracyclines, including their ability to act as oxygen radical scavengers and anti-inflammatory agents. Their unique capacity to accumulate in injured tissues (such as infarcted myocardium) also makes them appear to act as smart drugs. The recognition by scientists and clinicians of these collections of properties and of the safety profile of this class of drugs led to the implementation of pre-clinical and clinical trials to explore their possible beneficial effects in the setting of a wide variety of diseases including cardiovascular pathologies.
Villarreal and colleagues demonstrated that early, short term treatment of rats subjected to MI with doxycycline significantly decreased cardiac hypertrophy, myocyte cross-sectional area, and internal LV diameter while preserving infarcted wall thickness.[18] Doxycycline yielded parallel left shifts in LV pressure-volume relationships and epicardial scar area strain patterns similar to normal myocardium. The assessment of LV global MMP and MMP-2 and -9 activities 1 hour after MI also evidenced significant differences with doxycycline. Subsequent studies from the same research group further substantiated these observations in large animals [73] and also expanded on the anti-remodeling properties of doxycycline when given later post-MI (from 2–7 days post-infarction). [74] Interestingly, the anti-protease properties of doxycycline also appear to be exerted on other enzymes known to potentially play adverse roles in the setting of ongoing remodeling, such as plasmin. [75] As a result of these efforts, a clinical trial was recently implemented.[76] The “TIPTOP“ trial evaluated the efficacy of submicrobial doses of doxycycline (100 mg for 7 days BID started immediately post-percutaneous intervention, n=110) on post-MI remodeling. Results yielded significant decreases in LV end-diastolic volumes, infarct size, and severity in the doxycycline treated group. Thus, emerging evidence support a possible role for tetracyclines in ameliorating adverse cardiac remodeling. However, larger clinical studies will be implemented to validate the use of this class of compounds as a means to limit adverse cardiac remodeling and may include non-antimicrobial variants (known as chemically modified tetracyclines or CMTs), which have demonstrated an apparent safe clinical profile. [77]
TIMP delivery to the myocardium as a therapeutic - Proof of concept
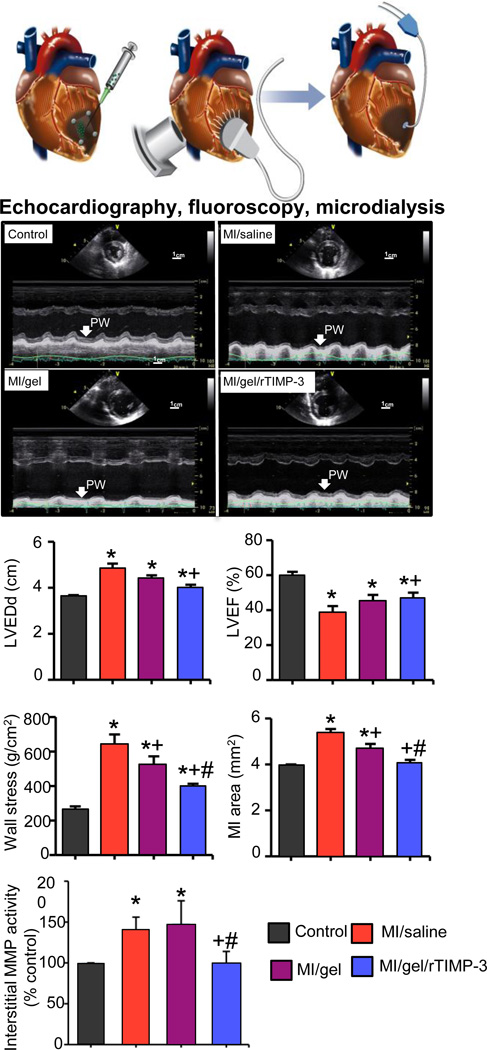
In light of the fact that systemic delivery of broad spectrum MMP inhibitors (with the possible exception of doxycycline or CMTs) is unlikely to gain a clinical foothold in terms of post-MI remodeling, then more specifically targeted approaches must be developed. One possible therapeutic direction is to augment local TIMP levels on a regional basis following MI. As briefly outlined in a previous section and detailed previously,[27–30,39,40] TIMP-3 demonstrates unique biological functions which include a high affinity to bind to the ECM through interactions with glycosaminoglycans, an influence on cytokine processing, and the ability to alter fibroblast phenotype in-vitro. Thus, localized augmentation of TIMP-3 in the context of post-MI remodeling constitutes a novel and translationally relevant therapeutic approach. Accordingly, a study was recently completed whereby the effects of regional delivery of exogenous TIMP-3 within the MI region upon infarct expansion and the course of post-MI remodeling was examined.[78] Specifically, a recombinant, full length human TIMP-3 (rTIMP-3) was synthesized and delivered to the MI region in adult pigs. In order to maintain a continuously high level of this low molecular weight construct, and to avoid systemic, off target delivery, the rTIMP-3 was encapsulated in a hyaluronic acid (HA) based hydrogel. As such, targeted injections of a HA hydrogel formulation that achieved sustained release of rTIMP-3 into the MI region was attained (Figure 3). Using this localized approach for rTIMP-3 delivery and post-MI large animal model, significant changes in the post-MI remodeling process were observed. Specifically, the rate of infarct expansion was reduced as was the degree of LV dilation - both key indices of post-MI remodeling. This translational study provides the proof of concept that regional, sustained delivery of a recombinant TIMP effectively interrupts the adverse post-MI remodeling process. This puts forth a new therapeutic paradigm in terms of modulating local biology of the MI and the infarct expansion process. Finally, this proof of concept study demonstrates that systemic or for that matter global interference of matrix proteolytic pathways is not necessary to favorably affect post-MI remodeling.
Figure 2.
(TOP PANELS) Intra-myocardial injections of a hydrogel (HA-gel) containing a recombinant full length TIMP-3 (r-TIMP-3), HA gel alone (gel), or saline, using a 9-point array pattern were performed in adult pigs at the time of MI induction. The goal of this study was to demonstrate that continuous release of a recombinant TIMP within the MI region would alter the natural history of adverse LV remodeling. Age matched, non-MI pigs were included in the study design and were considered referent controls. Serial studies of LV geometry by echocardiography and MI expansion by fluoroscopic marker localization were performed up to 14 days post-MI, which were then followed by microdialysis in order to measure MMP interstitial activity. (MIDDLE PANELS) Representative LV echocardiograms revealed LV dilation and poor posterior wall motion (PW) at 14 days post-MI, which were improved in the MI/gel/r-TIMP-3 group when compared to either MI and saline injections or MT and gel only injections. (LOWER PANELS) Quantitation of LV echocardiograms revealed a reduction in the degree of LV dilation as measured by LV end-diastolic dimension (LVEDd), LV peak wall stress, and MI area (reflective of infarct expansion) in the MI/gel/r-TIMP-3 group, which was associated with an improved LV pump function as measured by LV ejection fraction (LVEF). Notably, LV wall stress and MI area were also reduced, albeit to a more modest degree, in the MI/gel group underscoring that there are effects of these biomaterials alone. While interstitial MMP activity was increased in the MI region in the MI/saline and MI/gel groups, this was normalized in the MI/gel/r-TIMP-3 group. Reproduced from reference #78.
Developing TIMPs for therapeutic interventions on post-MI remodeling - Future directions
Since there are unique peptide sequences of each TIMP, then identifying the specific structure-function relationship of the regions within each TIMP with respect to MMP binding and other biological effects would allow for improved specificity in drug design. Indeed, past studies have already demonstrated that the N-terminus sequence and that of the C-terminus sequence of each TIMP may hold unique functionality in terms of MMP inhibition and non-MMP inhibitory roles.[28–30,79,80] For example, it has been shown that the C-domain of TIMP-2 is essential for formation of the proMMP-2 activation cascade but is not required for MMP inhibition.[70] On the other hand, a single point mutation in the N-terminal domain of TIMP-3 did not alter ADAM-17 inhibition but significantly impaired MMP inhibition.[80] Thus, truncated forms of TIMPs, which contain either the C or N-terminus regions, may provide enhanced specificity relevant to the post-MI remodeling process. In addition, engineering recombinant TIMPs with additional functional side chains may also provide for more localized specificity. For example, Diafarzadeh et al. added a glycosylphosphatidylinositol anchor to a recombinant form of human TIMP-1 which resulted in increased binding to the cell surface, and more localized effects on MMP activity.[81] Moreover, these investigators demonstrated that this membrane anchored form of TIMP-1 increased the rate of experimental wound closure. It has been demonstrated that specific point mutations, such as within the TIMP-1 sequence, can result in specific discrimination between MMP types.[26–29] For example, double and triple mutations at position Val4 and Ser68 within the TIMP-1 molecule altered inhibitory profiles for MMP-1, -2, and -3 when compared to the native form.[82]. Higashi et al reported that a fusion protein, whereby a 10 amino acid sequence was added to the N terminus of TIMP-2, resulted in significant inhibitory selectivity for active MMP-2.[83] Thus, future directions would include the design of recombinant TIMPs with specific amino acid substitutions that may confer MMP selectivity and hence target MMP types that are considered to play pathological roles in post-MI remodeling. It may also be possible to engineer a TIMP structure to prevent rapid proteolytic degradation as well as improve tissue penetration and specificity. Indeed, regions of the nascent TIMPs have been identified to be glycosylated and form cross-linked regions, which in turn affect 3-dimensional structure and biological activity.[28] Moreover, localized delivery of recombinant TIMPs, such as through the use of eluting biomaterials as exemplified in the previous paragraph or through induction of TIMP designs through gene transfer techniques, would also enhance the specificity as well as local concentrations of these molecules.
Acknowledgements
This work was supported by the National Institute of Health grants HL11090, HL089944, HL43617, HL67922, and a Merit Award from the Veterans’ Affairs Health Administration. Drs. Francis Spinale and Francisco Villarreal are supported by the Research Service of the Department of Veterans Affairs and University of California San Diego, respectively. The authors wish to express their sincere appreciation to Ashley Sapp for her editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography and References Cited
- 1.Hunt SA American College of Cardiology. American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005 Sep 20;46(6):e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000 Jun 27;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995 Jun;27(6):1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 4.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011 Jan;4(1):98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Weir RA, McMurray JJ, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol. 2006 May 22;97(10A):13F–25F. doi: 10.1016/j.amjcard.2006.03.005. Epub 2006 Apr 21. [DOI] [PubMed] [Google Scholar]
- 6.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007 Oct;87(4):1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012 Jul;32(7):1552–1562. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther. 2012 Feb;30(1):31–41. doi: 10.1111/j.1755-5922.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012 Jan 6;110(1):159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005 Apr 29;96(8):881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 11.Dixon JA, Gaillard WF, 2nd, Rivers WT, Koval CN, Stroud RE, Mukherjee R, Spinale FG. Heterogeneity in MT1-MMP activity with ischemia-reperfusion and previous myocardial infarction: relation to regional myocardial function. Am J Physiol Heart Circ Physiol. 2010 Dec;299(6):H1947–H1958. doi: 10.1152/ajpheart.00314.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003 Jun 10;107(22):2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. Epub 2003 May 27. [DOI] [PubMed] [Google Scholar]
- 13.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010 Nov;225(3):631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldsmith EC, Bradshaw AD, Spinale FG. Cellular Mechanisms of Tissue Fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol. 2013 Mar;304(5):C393–C402. doi: 10.1152/ajpcell.00347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003 Feb 4;107(4):618–625. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 16.King MK, Coker ML, Goldberg A, McElmurray JH, 3rd, Gunasinghe HR, Mukherjee R, Zile MR, O'Neill TP, Spinale FG. Selective matrix metalloproteinase inhibition with developing heart failure: effects on left ventricular function and structure. Circ Res. 2003 Feb 7;92(2):177–185. doi: 10.1161/01.res.0000052312.41419.55. [DOI] [PubMed] [Google Scholar]
- 17.Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005 Jan;288(1):H149–H158. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 18.Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003 Sep 23;108(12):1487–1492. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005 Mar;115(3):599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006 Jul 4;48(1):15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 21.Matter H, Schudok M. Recent advances in the design of matrix metalloprotease inhibitors. Curr Opin Drug Discov Devel. 2004 Jul;7(4):513–535. [PubMed] [Google Scholar]
- 22.Dormán G, Kocsis-Szommer K, Spadoni C, Ferdinandy P. MMP inhibitors in cardiac diseases: an update. Recent Pat Cardiovasc Drug Discov. 2007 Nov;2(3):186–194. doi: 10.2174/157489007782418964. [DOI] [PubMed] [Google Scholar]
- 23.Dormán G, Cseh S, Hajdú I, Barna L, Kónya D, Kupai K, Kovács L, Ferdinandy P. Matrix metalloproteinase inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs. 2010 May 28;70(8):949–964. doi: 10.2165/11318390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Peterson JT, Hallak H, Johnson L, Li H, O'Brien PM, Sliskovic DR, Bocan TM, Coker ML, Etoh T, Spinale FG. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001 May 8;103(18):2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 25.Iyer RP, Patterson NL, Fields GB, Lindsey ML. The history of matrix metalloproteinases: milestones, myths, and misperceptions. Am J Physiol Heart Circ Physiol. 2012 Oct 15;303(8):H919–H930. doi: 10.1152/ajpheart.00577.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010 Jun;20(3):161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010 Jan;1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas DA, Shi YE, Sang QA. Computational sequence analysis of the tissue inhibitor of metalloproteinase family. J Protein Chem. 1997 May;16(4):237–255. doi: 10.1023/a:1026348808069. [DOI] [PubMed] [Google Scholar]
- 29.Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol. 2012 Jan;180(1):12–16. doi: 10.1016/j.ajpath.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006 Feb 15;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Gong Y, Scott E, Lu R, Xu Y, Oh WK, Yu Q. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS One. 2013 Oct 15;8(10):e77366. doi: 10.1371/journal.pone.0077366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, Liu S, Zhang S, Cai G, Jiang H, Su H, Li X, Hong Q, Zhang X, Chen X. Tissue inhibitor of metalloproteinase-1 promotes NIH3T3 fibroblast proliferation by activating p-Akt and cell cycle progression. Mol Cells. 2011 Mar;31(3):225–230. doi: 10.1007/s10059-011-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoegy SE, Oh HR, Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J Biol Chem. 2001 Feb 2;276(5):3203–3214. doi: 10.1074/jbc.M008157200. [DOI] [PubMed] [Google Scholar]
- 34.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003 Jul 25;114(2):171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 35.Bigg HF, Morrison CJ, Butler GS, Bogoyevitch MA, Wang Z, Soloway PD, Overall CM. Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res. 2001 May 1;61(9):3610–3618. [PubMed] [Google Scholar]
- 36.Hernandez-Barrantes S1, Shimura Y, Soloway PD, Sang QA, Fridman R. Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem Biophys Res Commun. 2001 Feb 16;281(1):126–130. doi: 10.1006/bbrc.2001.4323. [DOI] [PubMed] [Google Scholar]
- 37.Lovelock JD1, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005 Feb;288(2):H461–H468. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 38.Kandalam V, Basu R, Abraham T, Wang X, Soloway PD, Jaworski DM, Oudit GY, Kassiri Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ Res. 2010 Mar 5;106(4):796–808. doi: 10.1161/CIRCRESAHA.109.209189. [DOI] [PubMed] [Google Scholar]
- 39.Troeberg L, Fushimi K, Scilabra SD, Nakamura H, Dive V, Thøgersen IB, Enghild JJ, Nagase H. The C-terminal domains of ADAMTS-4 and ADAMTS-5 promote association with N-TIMP-3. Matrix Biol. 2009 Oct;28(8):463–469. doi: 10.1016/j.matbio.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol. 2010 Oct;299(4):H1012–H1023. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo YH, Gao W, Li Q, Li PF, Yao PY, Chen K. Tissue inhibitor of metalloproteinases-4 suppresses vascular smooth muscle cell migration and induces cell apoptosis. Life Sci. 2004 Oct 1;75(20):2483–2493. doi: 10.1016/j.lfs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol Cancer. 2008 Nov 21;7:85. doi: 10.1186/1476-4598-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kytö V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem. 2010 Aug 6;285(32):24487–24493. doi: 10.1074/jbc.M110.136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer EA, Stricklin GP, Jeffrey JJ, Eisen AZ. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975 May 5;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- 45.Greene J1, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996 Nov 29;271(48):30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 46.Nagase H, Murphy G. Tailoring TIMPs for selective metalloproteinase inhibition. In: Edwards D, Hoyer-Hansen G, Blasi F, Sloane BF, editors. The Cancer Degradome. 2008. pp. 787–810. [Google Scholar]
- 47.Spinale FG, Janicki JS, Zile MR. Membrane associated matrix proteolysis and heart failure. Circ Res. 2013 Jan 4;112(1):195–208. doi: 10.1161/CIRCRESAHA.112.266882. PMID: 23287455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spinale FG, Mukherjee R, Zavadzkas JA, Koval CN, Bouges S, Stroud RE, Dobrucki LW, Sinusas AJ. Cardiac restricted over-expression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J Biol Chem. 2010 Sep 24;285(39):30316–30327. doi: 10.1074/jbc.M110.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strongin AY, Marmer BL, Grant GA, Goldberg GI. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J Biol Chem. 1993 Jul 5;268(19):14033–14039. [PubMed] [Google Scholar]
- 50.Bigg HF, Shi YE, Liu YE, Steffensen B, Overall CM. Specific, high affinity binding of tissue inhibitor of metalloproteinases-4 (TIMP-4) to the COOH-terminal hemopexin-like domain of human gelatinase A. TIMP-4 binds progelatinase A and the COOH-terminal domain in a similar manner to TIMP-2. J Biol Chem. 1997 Jun 13;272(24):15496–15500. doi: 10.1074/jbc.272.24.15496. [DOI] [PubMed] [Google Scholar]
- 51.Liu YE, Wang M, Greene J, Su J, Ullrich S, Li H, Sheng S, Alexander P, Sang QA, Shi YE. Preparation and characterization of recombinant tissue inhibitor of metalloproteinase 4 (TIMP-4) J Biol Chem. 1997 Aug 15;272(33):20479–20483. doi: 10.1074/jbc.272.33.20479. [DOI] [PubMed] [Google Scholar]
- 52.Olson TM, Hirohata S, Ye J, Leco K, Seldin MF, Apte SS. Cloning of the human tissue inhibitor of metalloproteinase-4 gene (TIMP4) and localization of the TIMP4 and Timp4 genes to human chromosome 3p25 and mouse chromosome 6, respectively. Genomics. 1998 Jul 1;51(1):148–151. doi: 10.1006/geno.1998.5362. [DOI] [PubMed] [Google Scholar]
- 53.Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res. 2000 May;46(2):307–315. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 54.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006 Sep 5;114(10):1020–1027. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 55.Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998 Aug;32(2):368–372. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 56.Wagner DR, Delagardelle C, Ernens I, Rouy D, Vaillant M, Beissel J. Matrix metalloproteinase-9 is a marker of heart failure after acute myocardial infarction. J Card Fail. 2006 Feb;12(1):66–72. doi: 10.1016/j.cardfail.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Velagaleti RS, Gona P, Sundström J, Larson MG, Siwik D, Colucci WS, Benjamin EJ, Vasan RS. Relations of biomarkers of extracellular matrix remodeling to incident cardiovascular events and mortality. Arterioscler Thromb Vasc Biol. 2010 Nov;30(11):2283–2288. doi: 10.1161/ATVBAHA.110.208462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly D, Squire IB, Khan SQ, Dhillon O, Narayan H, Ng KH, Quinn P, Davies JE, Ng LL. Usefulness of plasma tissue inhibitors of metalloproteinases as markers of prognosis after acute myocardial infarction. Am J Cardiol. 2010 Aug 15;106(4):477–482. doi: 10.1016/j.amjcard.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 59.Furenes EB, Arnesen H, Solheim S, Grøgaard HK, Hoffmann P, Seljeflot I. The profile of circulating metalloproteinases after PCI in patients with acute myocardial infarction or stable angina. Thromb Res. 2009 Nov;124(5):560–564. doi: 10.1016/j.thromres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 60.West MJ, Nestel PJ, Kirby AC, Schnabel R, Sullivan D, Simes RJ, Pollicino C, Lubos E, Münzel TF, White HD, Tonkin AM, Bickel C, Tiret L, Blankenberg S LIPID Study Investigators. The value of N-terminal fragment of brain natriuretic peptide and tissue inhibitor of metalloproteinase-1 levels as predictors of cardiovascular outcome in the LIPID study. Eur Heart J. 2008 Apr;29(7):923–931. doi: 10.1093/eurheartj/ehn007. [DOI] [PubMed] [Google Scholar]
- 61.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J. 2006 May;151(5):1101.e1–1101.e8. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 62.Goldbergova MP, Parenica J, Jarkovsky J, Kala P, Poloczek M, Manousek J, Kluz K, Kubkova L, Littnerova S, Tesak M, Toman O, Pavek N, Cermakova Z, Tomandl J, Vasku A, Spinar J. The association between levels of tissue inhibitor of metalloproteinase-1 with acute heart failure and left ventricular dysfunction in patients with ST elevation myocardial infarction treated by primary percutaneous coronary intervention. Genet Test Mol Biomarkers. 2012 Oct;16(10):1172–1178. doi: 10.1089/gtmb.2012.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansson J, Vasan RS, Ärnlöv J, Ingelsson E, Lind L, Larsson A, Michaëlsson K, Sundström J. Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: cohort study. PLoS One. 2011 Jan 19;6(1):e16185. doi: 10.1371/journal.pone.0016185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000 Oct 17;102(16):1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 65.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998 May 5;97(17):1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 66.Wingfield PT, Sax JK, Stahl SJ, Kaufman J, Palmer I, Chung V, Corcoran ML, Kleiner DE, Stetler-Stevenson WG. Biophysical and functional characterization of full-length, recombinant human tissue inhibitor of metalloproteinases-2 (TIMP-2) produced in Escherichia coli. Comparison of wild type and amino-terminal alanine appended variant with implications for the mechanism of TIMP functions. J Biol Chem. 1999 Jul 23;274(30):21362–21368. doi: 10.1074/jbc.274.30.21362. [DOI] [PubMed] [Google Scholar]
- 67.Gill SE, Gharib SA, Bench EM, Sussman SW, Wang RT, Rims C, Birkland TP, Wang Y, Manicone AM, McGuire JK, Parks WC. Tissue inhibitor of metalloproteinases-3 moderates the proinflammatory status of macrophages. Am J Respir Cell Mol Biol. 2013 Nov;49(5):768–777. doi: 10.1165/rcmb.2012-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gill SE, Pape MC, Leco KJ. Absence of tissue inhibitor of metalloproteinases 3 disrupts alveologenesis in the mouse. Dev Growth Differ. 2009 Jan;51(1):17–24. doi: 10.1111/j.1440-169X.2008.01075.x. [DOI] [PubMed] [Google Scholar]
- 69.A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of 200mg oral dose of PG-116800 given as the sodium salt (PG-530742) twice daily for 90 days to patients following acute myocardial infarction, with post-treatment follow-up. Feb 12. Investigator’s Regulatory Brochure #2002135. 2004:8–51. [Google Scholar]
- 70.Yarbrough WM, Mukherjee R, Escobar GP, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Lowry AS, O'Neill TP, Spinale FG. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation. 2003 Oct 7;108(14):1753–1759. doi: 10.1161/01.CIR.0000091087.78630.79. [DOI] [PubMed] [Google Scholar]
- 71.Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004 Apr;24(4):733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 72.Griffin MO, Fricovsky E, Ceballos G, Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010 Sep;299(3):C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villarreal F, Omens J, Dillmann W, Risteli J, Nguyen J, Covell J. Early degradation and serum appearance of type I collagen fragments after myocardial infarction. J Mol Cell Cardiol. 2004 Apr;36(4):597–601. doi: 10.1016/j.yjmcc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Garcia RA, Go KV, Villarreal FJ. Effects of timed administration of doxycycline or methylprednisolone on post-myocardial infarction inflammation and left ventricular remodeling in the rat heart. Mol Cell Biochem. 2007 Jun;300(1–2):159–169. doi: 10.1007/s11010-006-9379-0. [DOI] [PubMed] [Google Scholar]
- 75.Griffin MO, Jinno M, Miles LA, Villarreal FJ. Reduction of myocardial infarct size by doxycycline: a role for plasmin inhibition. Mol Cell Biochem. 2005 Feb;270(1–2):1–11. doi: 10.1007/s11010-005-2540-3. [DOI] [PubMed] [Google Scholar]
- 76.Cerisano G, Buonamici P, Valenti R, Sciagrà R, Raspanti S, Santini A, Carrabba N, Dovellini EV, Romito R, Pupi A, Colonna P, Antoniucci D. Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: the TIPTOP trial. Eur Heart J. 2014 Jan;35(3):184–191. doi: 10.1093/eurheartj/eht420. [DOI] [PubMed] [Google Scholar]
- 77.Gu Y, Walker C, Ryan ME, Payne JB, Golub LM. Non-antibacterial tetracycline formulations: clinical applications in dentistry and medicine. J Oral Microbiol. 2012;4 doi: 10.3402/jom.v4i0.19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eckhouse SR, Purcell BP, Oelsen JM, Logdon CB, Rawls WF, Patel RK, Zellars KN, Stroud RE, Jones JA, Mukherjee R, Gorman JH, III, Gorman RC, Black RA, Burdick JA, Spinale FG. Targeted injection and release of recombinant tissue inhibitor of metalloproteinase attenuates left ventricular remodeling after myocardial infarction. Sci Transl Med. 2014 Feb 12;6(223):223ra21. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Worley JR, Thompkins PB, Lee MH, Hutton M, Soloway P, Edwards DR, Murphy G, Knäuper V. Sequence motifs of tissue inhibitor of metalloproteinases 2 (TIMP-2) determining progelatinase A (proMMP-2) binding and activation by membrane-type metalloproteinase 1 (MT1-MMP) Biochem J. 2003 Jun 15;372(Pt 3):799–809. doi: 10.1042/BJ20021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei S, Kashiwagi M, Kota S, Xie Z, Nagase H, Brew K. Reactive site mutations in tissue inhibitor of metalloproteinase-3 disrupt inhibition of matrix metalloproteinases but not tumor necrosis factor-alpha-converting enzyme. J Biol Chem. 2005 Sep 23;280(38):32877–32882. doi: 10.1074/jbc.C500220200. [DOI] [PubMed] [Google Scholar]
- 81.Djafarzadeh R, Sauter M, Notohamiprodjo S, Noessner E, Goyal P, Siess W, Wörnle M, Ribeiro A, Himmelein S, Sitter T, Nelson PJ. Recombinant GPI-anchored TIMP-1 stimulates growth and migration of peritoneal mesothelial cells. PLoS One. 2012;7(4):e33963. doi: 10.1371/journal.pone.0033963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei S, Chen Y, Chung L, Nagase H, Brew K. Protein engineering of the tissue inhibitor of metalloproteinase 1 (TIMP-1) inhibitory domain. In search of selective matrix metalloproteinase inhibitors. J Biol Chem. 2003 Mar 14;278(11):9831–9834. doi: 10.1074/jbc.M211793200. [DOI] [PubMed] [Google Scholar]
- 83.Higashi S, Hirose T, Takeuchi T, Miyazaki K. Molecular design of a highly selective and strong protein inhibitor against matrix metalloproteinase-2 (MMP-2) J Biol Chem. 2013 Mar 29;288(13):9066–9076. doi: 10.1074/jbc.M112.441758. [DOI] [PMC free article] [PubMed] [Google Scholar]