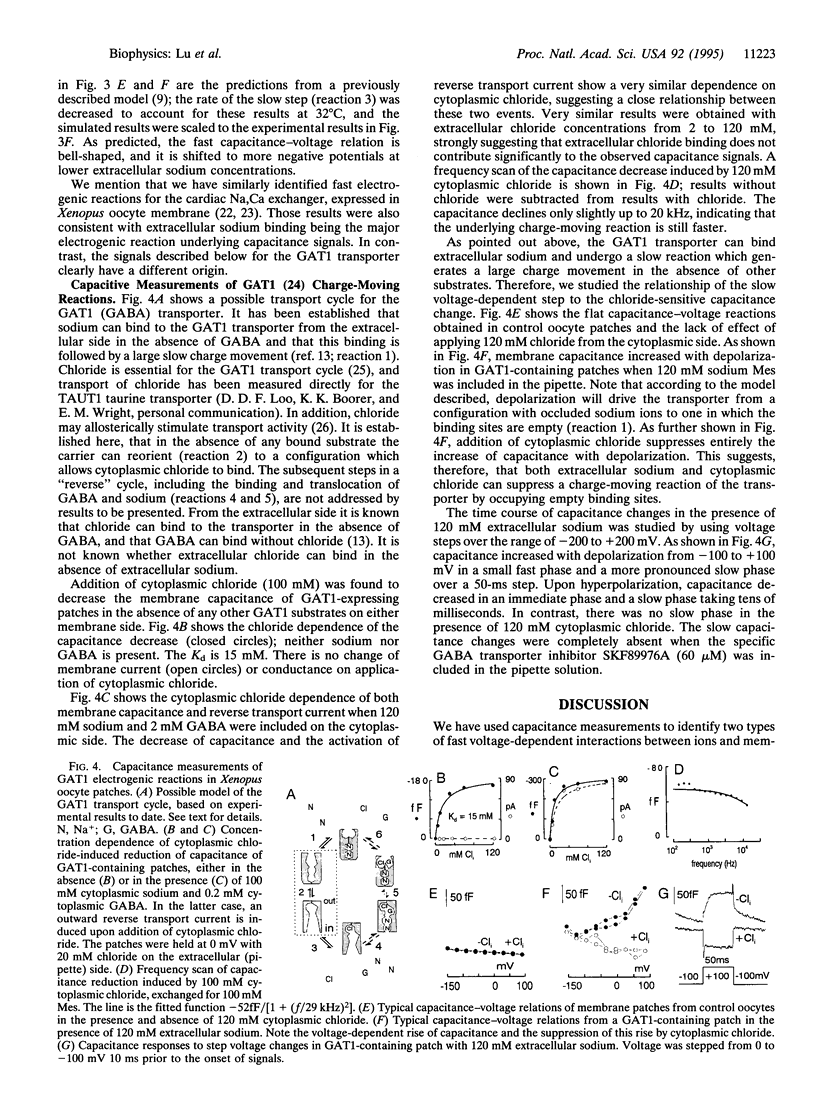
Abstract
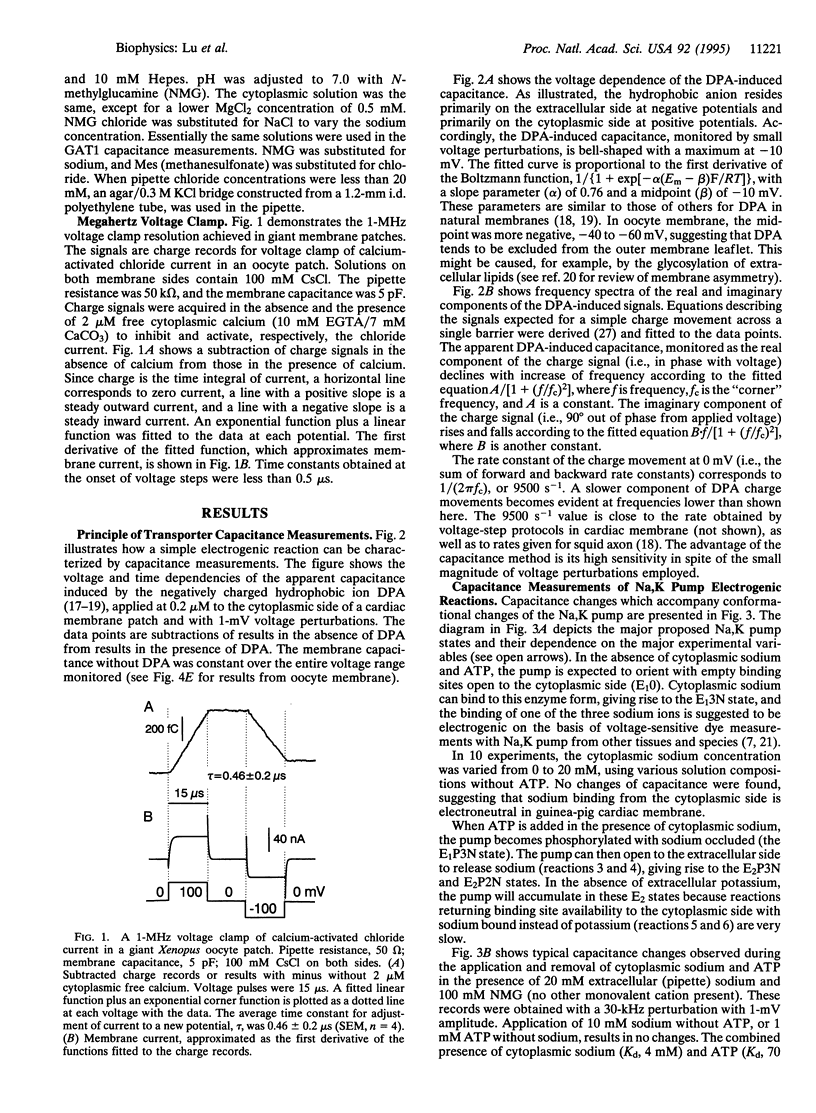
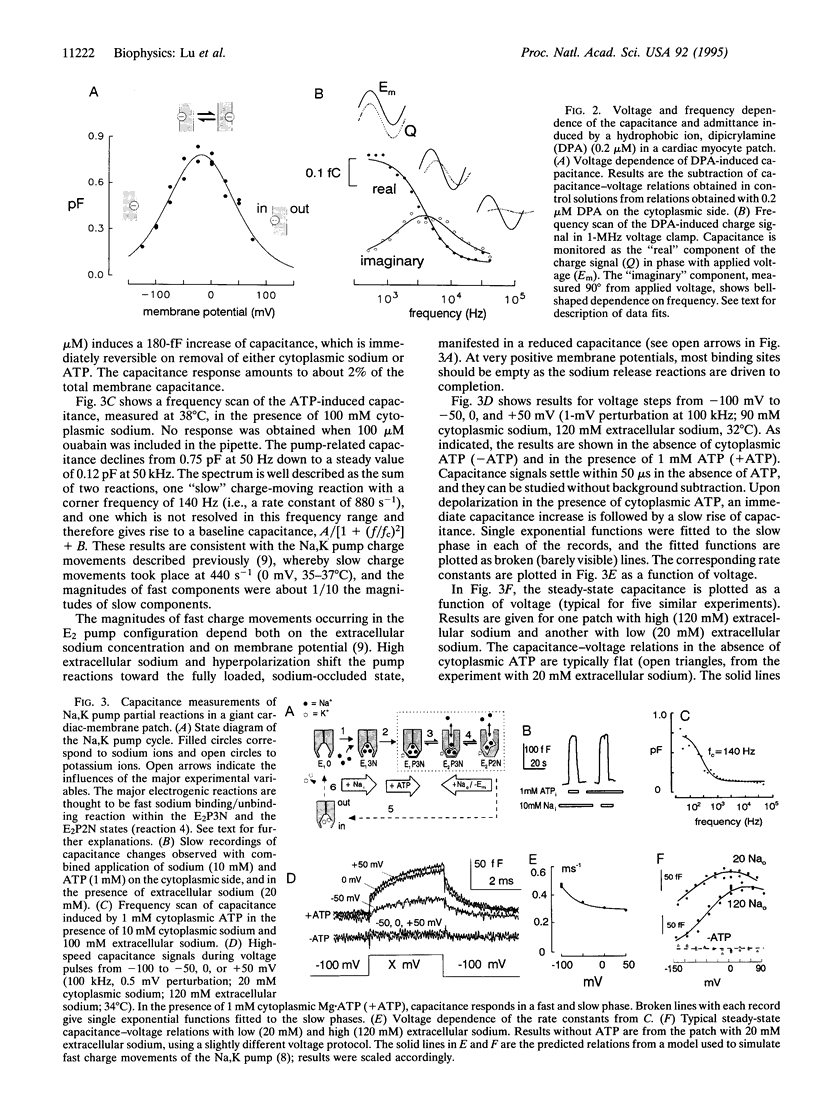
We have used capacitance measurements with a 1-microsecond voltage clamp technique to probe electrogenic ion-transporter interactions in giant excised membrane patches. The hydrophobic ion dipicrylamine was used to test model predictions for a simple charge-moving reaction. The voltage and frequency dependencies of the apparent dipicrylamine-induced capacitance, monitored by 1-mV sinusoidal perturbations, correspond to single charges moving across 76% of the membrane field at a rate of 9500 s-1 at 0 mV. For the cardiac Na,K pump, the combined presence of cytoplasmic ATP and sodium induces an increase of apparent membrane capacitance which requires the presence of extracellular sodium. The dependencies of capacitance changes on frequency, voltage, ATP, and sodium verify that phosphorylation enables a slow, 300- to 900-s-1, pump transition (the E1-E2 conformational change), which in turn enables fast, electrogenic, extracellular sodium binding reactions. For the GAT1 (gamma-aminobutyric acid,Na,Cl) cotransporter, expressed in Xenopus oocyte membrane, we find that chloride binding from the cytoplasmic side, and probably sodium binding from the extracellular side, results in a decrease of membrane capacitance monitored with 1- to 50-kHz perturbation frequencies. Evidently, ion binding by the GAT1 transporter suppresses an intrinsic fast charge movement which may originate from a mobility of charged residues of the transporter binding sites. The results demonstrate that fast capacitance measurements can provide new insight into electrogenic processes closely associated with ion binding by membrane transporters.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams J. P., Leslie A. G., Lutter R., Walker J. E. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994 Aug 25;370(6491):621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- Benz R., Läuger P. Transport kinetics of dipicrylamine through lipid bilayer membranes. Effects of membrane structure. Biochim Biophys Acta. 1977 Jul 14;468(2):245–258. doi: 10.1016/0005-2736(77)90118-3. [DOI] [PubMed] [Google Scholar]
- Benz R., Nonner W. Structure of the axolemma of frog myelinated nerve: relaxation experiments with a lipophilic probe ion. J Membr Biol. 1981 Apr 15;59(2):127–134. doi: 10.1007/BF01875710. [DOI] [PubMed] [Google Scholar]
- Bühler R., Stürmer W., Apell H. J., Läuger P. Charge translocation by the Na,K-pump: I. Kinetics of local field changes studied by time-resolved fluorescence measurements. J Membr Biol. 1991 Apr;121(2):141–161. doi: 10.1007/BF01870529. [DOI] [PubMed] [Google Scholar]
- Cammack J. N., Rakhilin S. V., Schwartz E. A. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994 Oct;13(4):949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Collins A., Somlyo A. V., Hilgemann D. W. The giant cardiac membrane patch method: stimulation of outward Na(+)-Ca2+ exchange current by MgATP. J Physiol. 1992 Aug;454:27–57. doi: 10.1113/jphysiol.1992.sp019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Fernández J. M., Taylor R. E., Bezanilla F. Induced capacitance in the squid giant axon. Lipophilic ion displacement currents. J Gen Physiol. 1983 Sep;82(3):331–346. doi: 10.1085/jgp.82.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Rakowski R. F., De Weer P. Extracellular access to the Na,K pump: pathway similar to ion channel. Science. 1993 Apr 2;260(5104):100–103. doi: 10.1126/science.7682009. [DOI] [PubMed] [Google Scholar]
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M. C., Davidson N., Lester H. A., Kanner B. I. Cloning and expression of a rat brain GABA transporter. Science. 1990 Sep 14;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Heyse S., Wuddel I., Apell H. J., Stürmer W. Partial reactions of the Na,K-ATPase: determination of rate constants. J Gen Physiol. 1994 Aug;104(2):197–240. doi: 10.1085/jgp.104.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches. Science. 1994 Mar 11;263(5152):1429–1432. doi: 10.1126/science.8128223. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W., Nicoll D. A., Philipson K. D. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature. 1991 Aug 22;352(6337):715–718. doi: 10.1038/352715a0. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22(1):1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4548–4552. doi: 10.1073/pnas.85.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo D. D., Hazama A., Supplisson S., Turk E., Wright E. M. Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5767–5771. doi: 10.1073/pnas.90.12.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager S., Naeve J., Quick M., Labarca C., Davidson N., Lester H. A. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993 Feb;10(2):177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Schwappach B., Stürmer W., Apell H. J., Karlish S. J. Binding of sodium ions and cardiotonic steroids to native and selectively trypsinized Na,K pump, detected by charge movements. J Biol Chem. 1994 Aug 26;269(34):21620–21626. [PubMed] [Google Scholar]