Abstract
Pereskia bleo, a leafy cactus, is a medicinal plant native to West and South America and distributed in tropical and subtropical areas. It is traditionally used as a dietary vegetable, barrier hedge, water purifier, and insect repellant and for maintaining health, detoxification, prevention of cancer, and/or treatment of cancer, hypertension, diabetes, stomach ache, muscle pain, and inflammatory diseases such as dermatitis and rheumatism. The aim of this paper was to provide an up-to-date and comprehensive review of the botanical characteristics, traditional usage, phytochemistry, pharmacological activities, and safety of P. bleo. A literature search using MEDLINE (via PubMed), Science direct, Scopus and Google scholar and China Academic Journals Full-Text Database (CNKI) and available eBooks and books in the National University of Singapore libraries in English and Chinese was conducted. The following keywords were used: Pereskia bleo, Pereskia panamensis, Pereskia corrugata, Rhodocacus corrugatus, Rhodocacus bleo, Cactus panamensis, Cactus bleo, Spinach cactus, wax rose, Perescia, and Chinese rose. This review revealed the association between the traditional usage of P. bleo and reported pharmacological properties in the literature. Further investigation on the pharmacological properties and phytoconstituents of P. bleo is warranted to further exploit its potentials as a source of novel therapeutic agents or lead compounds.
1. Introduction
Pereskia bleo is a medicinal plant of the family Cactaceae. Cacti are well-known desert plants and widely recognized by their specialized growth form of the stems and leaves. This family consists of 100 genera and about 2000 species [1, 2]. The genus Pereskia consists of 17 species with regular leaf development and function. They are generally representative of the “ancestral cactus.” This genus does not look much like other types of cacti because of having substantial leaves and thin stems [3–5]. The plants in the genus Pereskia originate from the region between Brazil and Mexico and South America and Central America [6–8] and are cultivated in many tropical and subtropical countries including India, Malaysia, Singapore, and Indonesia [1]. They also generally resemble other types of plants such as roses [3, 8]. Pereskia species are divided into Clades A and B [9] (Table 1). The two clades of Pereskia differ in their geographical distribution. Clade A is found around the Gulf of Mexico and the Caribbean Sea whereas Clade B is found in the south of the Amazon Basin. The stems of the species of Pereskia within Clade A begin to form bark early in the life of the plant like most non-cacti. In contrast, Pereskia species within Clade B typically delay forming bark, thus giving the stem the potential to become a major organ for photosynthesis [4].
Table 1.
Clades of the genus Pereskia [9].
| Clade A | Clade B |
|---|---|
|
Pereskia aureiflora F.Ritter Pereskia bleo (Kunth) DC Pereskia guamacho F.A.C.Weber Pereskia lychnidiflora DC Pereskia marcanoi Areces Pereskia portulacifolia (L.) DC Pereskia quisqueyana Alain Pereskia zinniiflora DC |
Pereskia aculeata Mill. Pereskia bahiensis Gürke Pereskia diaz-romeroana Cárdenas Pereskia grandifolia Haw. Pereskia horrida DC Pereskia nemorosa Rojas Acosta Pereskia sacharosa Griseb. Pereskia stenantha F.Ritter Pereskia weberiana K.Schum. |
Among them, Pereskia aculeata Mill (P. aculeate), Pereskia grandifolia Haw (P. grandifolia), and Pereskia bleo (Kunth) DC. (P. bleo) are listed to be found in Singapore and Malaysia [7, 10, 11]. P. bleo and P. grandifolia are used for medicinal purposes in these areas [1, 11]. Hence, more information on these three species is presented below.
1.1. Pereskia aculeata Mill
Its common names are Barbados gooseberry or lemon vine [12, 13] and it is native to tropical America [14]. This plant is a scrambling vine growing to the height of 10 m to a tree. The stems reach 2-3 cm in diameter. Younger stems have hooked thorns and older stems have clusters of woody spines. The leaves are 4–11 cm long and 1.5–4 cm wide, simple, and deciduous in the dry season. The flowers are white, cream, or pinkish with 2.5–5 cm diameter and strongly scented. This plant has translucent rounded white to pink berries which turn to yellow or orange with the diameter of 2 cm upon ripening. The fruits are edible and containing numerous small seeds. They somewhat resemble the gooseberry in appearance and are of excellent flavor [15, 16]. The leaves are also edible and are a popular vegetable in parts of the Brazilian state of Minas Gerais under the name of ora-pro-nóbis [14].
1.2. Pereskia grandifolia Haw
It is also known as rose cactus or Rhodocactus grandifolia. This plant is native to the Northeastern Brazil restingas and is cultivated in tropical and subtropical areas [7]. It is a shrub or small tree, 2–5 m high, with a grayish-brown trunk up to 20 cm in diameter. The spines range from black to brown and their number at each areole gradually increases with age. The new twigs may be spineless while the trunk may have up to 90 spines in areoles, each 2–6.5 cm long. The leaves vary in size from 9 to 23 cm long and the shapes range from elliptic to ovate and obovate-lanceolate. Usually 10–15 flowers of dense inflorescence develop at the ends of stems, but sometimes there are 30 or more. The flowers are pink-purple and look like rose with 3–5 cm diameter [12]. The leaves of P. grandifolia are edible [11].
1.3. Pereskia bleo (Kunth) DC
P. bleo is also known as Cactus bleo and has been commonly used for a variety of medicinal and non-medicinal purposes in different countries [1, 2]. However, to the best of our knowledge, a comprehensive review of P. bleo is not available. The objective of this paper is to provide a comprehensive review of the botanical characteristics, traditional usage, phytoconstituents, pharmacological activities, and safety of P. bleo. Such information will serve as a useful resource for the proper usage of this plant and for future research.
2. Method
Internet sources including MEDLINE (via Pubmed), Science direct, Scopus and Google scholar, and China Journals Full-Text Database (via CNKI) were searched for publications on this plant. The following keywords were used: Pereskia bleo, Pereskia panamensis, Pereskia corrugata, Rhodocactus corrugatus, Rhodocactus bleo, Cactus panamensis, Cactus bleo, Spinach cactus, wax rose, Perescia, and Chinese rose. No restriction on the language and date of publication was implemented. In addition, available books and eBooks in the National University of Singapore (NUS) libraries were manually searched for the relevant information.
3. Results and Discussion
3.1. Botanical Characteristics
P. bleo belongs to the order of Caryophyllales Juss. ex Bercht. & J. Presl, superorder of Caryophyllanae Takht and subclass of Magnoliidae Novák ex Takht. It is in the Cactaceae family, Peresioideae subfamily, and Pereskia Mill genus [44, 45]. In the International Plant Nomenclature Index (IPNI) [46], its ID code is 273592-2 and its basionym is Cactus bleo (Kunth). Basionym name is defined as “previously published legitimate name-bringing or epithet-bringing synonym from which a new name is formed for a taxon of different rank or position taxon of different rank or position” [17]. The scientific and common names of P. bleo are listed in Table 2. This plant is also known as “Pokok Jarum Tujuh Bilah” in Malay and “Cak Sing Cam” or “Qi Xing Zhen (七星针)” in Chinese [8, 40]. Its Chinese name literally means “seven stars needle” [7].
Table 2.
Scientific and common names of P. bleo.
| Names | References |
|---|---|
| Scientific names | |
| Cactus bleo Kunth | [2, 12, 17–19] |
| Pereskia bleo (Kunth) DC | [1, 2, 12, 17, 19–21] |
| Pereskia corrugata Cutak | [17, 21] |
| Pereskia panamensis F.A.C. Weber | [2, 17] |
| Rhodocactus bleo (Kunth) F.M. Kunth | [17, 19, 21] |
| Rhodocactus corrugatus (Cutak) Backeberg | [17] |
| Common names | |
| Butarrar (Kuna Indian) | [22] |
| Cak Sing Cam, Qi xing zhen (Chinese) | [1, 8, 23] |
| Chupa, Chupa melon, Najií, Najii De Culebra, Najú de esoubas, and Bleo de chupa (Spanish) | [2, 21, 24] |
| Perescia | [7] |
| Pokok Jarum Tujuh Bilah (Malay) | [2, 25] |
| Rose cactus, Bleo, Chinese rose, Spinach cactus, wax rose, and orange rose cactus (English) | [1, 6, 7, 21, 24, 26] |
P. bleo originates from Mesoamerica (Panama), Western South America (Columbia) [1, 2, 6, 12] and is distributed in tropical and subtropical regions [1, 2]. It is a deciduous, shrubby, tree-like plant with a height of 0.6–8 m. The trunk reaches 10 cm in diameter and bears very large fascicle of spines when it is young. However, the trunk becomes naked when turning old. Young branches are red and leafy and often bear 5–7 black spines up to 1 cm in length. The spines reach 2 cm on the older stems. The leaves are thin, oblong to oblanceolate, glossy, and succulent, 6–21 cm long, and 2–7 cm wide [2]. The flowers are orange-red and grouped in 2–4 terminally and laterally. The fruits are yellow, thick walled, fleshy, and glossy and look like conical berries at maturity, up to 5 × 5 cm in size, turbinate, and containing 6–8 mm in diameter dark brown or black color seeds [1, 19, 31]. It can be propagated by stem cutting or seeds [12].
This species was collected by Bonpland during Humboldt's trip through the new world and was described and published by Kunth in 1823 [2]. In some older books and herbaria, it was confused with Pereskia grandifolia (P. grandifolia) [20] because both plants are vegetatively similar [31]. In addition, P. bleo and P. grandifolia are the only exceptions of Pereskia which grow in areas receiving considerably high annual rainfall more than 187 mm per wet month. Other Pereskia species grow in dry areas [3]. The two species can be distinguished by the leaves, flowers, and spines. P. bleo has thinner, corrugated leaves and orange-red flowers, with shorter spines compared to P. grandifolia. In contrast, P. grandifolia has thicker, uncorrugated leaves, pink to purplish-pink flowers and longer but fewer spines on the stems [11]. Figure 1 shows the photographs of different parts of P. bleo and P. grandifolia. Although they are different species, anatomical similarities in these two species support the evolution theory for cactus family [18].
Figure 1.
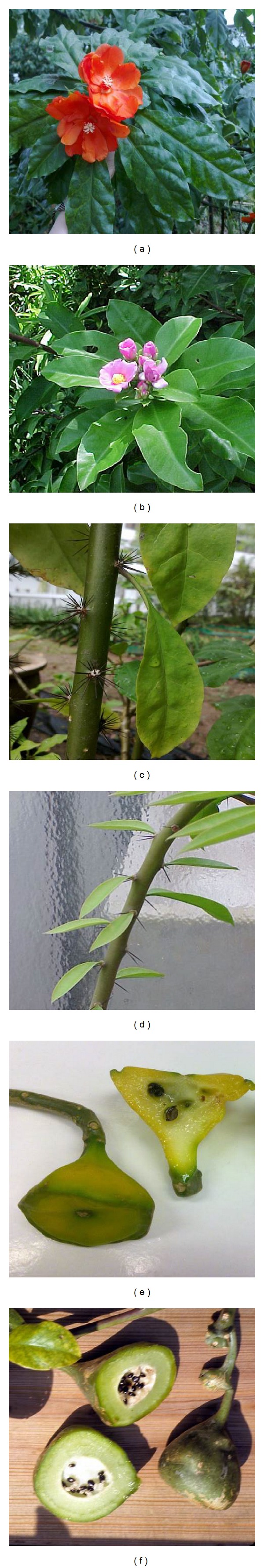
Photographs of different plant parts of P. bleo and P. grandifolia. (a) Flower of P. bleo, (b) lower of P. grandifolia [47], (c) stem and spines of P. bleo, (d) stem and spines of P. grandifolia [48], (e) ripe fruits and seeds of P. bleo, and (f) ripe fruits and seeds of P. grandifolia [49].
3.2. Traditional Usage
P. bleo has been used for various purposes. In some areas, it is used as a food spice [1, 7]. This plant has been eaten raw as vegetables by some people in Malaysia and China or taken as a concoction brewed from fresh leaves [19, 36]. In addition, it is taken for detoxification and revitalizing the body [27, 28, 40]. Its fruit is consumed by some ethnic groups in Panama as a wild fruit [26]. The leaves of P. bleo have been traditionally used to treat cancer, hemorrhoid, hypertension, diabetes [32, 40], infections, gastric pain, headache, ulcer, and inflammatory conditions like rheumatism and asthma [28, 31]. Indigenous Colombians have used P. bleo to neutralize the effects of snakebites [33], to relax spastic muscles, and to alleviate muscle aches [29]. Apart from dietary and medicinal uses, this plant is a suitable barrier hedge because of its sharp spines, strong stem, and insect repellant properties [21]. In Central America, Kuna Indians used the crushed leaves to clarify drinking water [12]. Different methods of preparation have been reported for the plant. It is usually taken raw or as a decoction of its fresh leaves. Table 3 shows the traditional usage and different preparation methods of P. bleo. To the best of our knowledge, information on the specific preparation methods for some of the indicated traditional usages is not available.
Table 3.
Traditional usage and methods of preparation of P. bleo.
| Purpose | Method of preparation | References |
|---|---|---|
| Detoxification and prevention of cancer | Making tea by boiling the leaves and/or the fruit and then drinking it warm or cool | [27–30] |
|
| ||
| Dietary purposes and health maintenance | Eating the raw leaf, flower, and fruit | [19, 28] |
|
| ||
| Health maintenance and revitalizing the body | Making juice from the leaves and boiling in water and drinking every morning | [30] |
|
| ||
| To alleviate muscleache | Making decoction from the leaves and then using as a warm bath for muscle ache | [29] |
|
| ||
| To alleviate stomachache | Preparing “ina kuamakalet”: the inflorescence is mixed with the excrements of red ants by using a special mortar and then moistened with water. The resulted mass is moulded to oval shape objects which are dried in sun. When using the remedy, these balls are rubbed in a small container with a small amount of water. | [29] |
|
| ||
| To treat hemorrhoid, hypertension, diabetes, infections, headache, and inflammatory conditions (rheumatism and asthma) | No information is available in the literature. | [28, 31, 32] |
|
| ||
| To neutralize the effects of the snakebites | No information is available in the literature. | [33] |
3.3. Phytochemistry
The leaves are the most commonly used part of P. bleo in traditional medicine. Hence, they have been more studied compared to the other plant parts. So far, 20 phytoconstituents have been reported in the leaves and two components from the fruit as shown in Table 4. These components include alkaloids, fatty acids, glycosides, lactones, phenolic, sterol, terpenoid, and carotenoid compounds. The major isolated component from P. bleo leaves is phytol [27]. In addition, Doetsch et al. [34] reported the isolation of three alkaloids, namely, 3,4-dimethoxy-β-phenethylamine (mescaline), 3-methoxytyramine, and tyramine, from the leaves of this plant. Vitamin E (α-tocopherol) [36, 37] which is well known for its antioxidant properties; 2,4-ditert-butylphenol and dihydroactinidiolide were isolated through bioassay-guided fractionation by Malek et al. [36]. Murillo et al. [26] analyzed the fruit of P. bleo for lutein and zeaxanthin contents. The total carotenoid content of the fruit was found to be 13.3 μg/g, making P. bleo fruit a high carotenoid food source among the wild fruits in Panama.
Table 4.
Reported phytoconstituents in the leaves and fruits of P. bleo.
| Plant part | Class of the constituents | Constituents | Reference |
|---|---|---|---|
| Leaves | Alkaloids | 3,4-Dimethoxy-β-phenethylamine | [34] |
| 3-Methoxytyramine | [34] | ||
| Tyramine | [34] | ||
| Fatty acids | Methyl palmitate | [31] | |
| Methyl linoleate | |||
| Methyl α-linoleate | |||
| Flavonoid | Vitexin | [35] | |
| Phytosterol glycoside | β-Sitosterol glucoside | [35] | |
| Lactone | Dihydroactinidiolide | [28] | |
| Phenolic compounds | 2,4-Ditert-butylphenol | [36] | |
| α-Tocopherol | [36, 37] | ||
| Catechin | [37] | ||
| Epicatechin | [37] | ||
| Quercetin | [37] | ||
| Myricetin | [37] | ||
| Sterols | Campesterol | ||
| Stigmasterol | [28] | ||
| β-Sitosterol | [36] | ||
| Terpenoids | β-Carotene | [37] | |
| Phytol | [36] | ||
|
| |||
| Fruit | Carotenoids | Lutein (β,é-carotene-3,3′-diol) | [26, 37] |
| Zeaxanthin (β,β-carotene-3,3′-diol) | |||
The mineral content of the leaves was also investigated by using energy-dispersive X-ray microanalysis. Table 5 shows the weight percentage of the minerals reported by Abbdewahab et al. [38]. As can be seen, P. bleo leaves are rich in potassium (10.16%). This is more than two times of the potassium content of tomato (4.5%), a vegetable known to be high in potassium [50]. It has been shown that a high potassium diet has an important role in lowering blood pressure [51]. Therefore, it might be one of the possible reasons for the traditional usage of P. bleo as a treatment for hypertension [31].
Table 5.
Percentage (% w/w) of mineral contents in the leaves of P. bleo [38].
| Mineral elements | Percentage weight (%) |
|---|---|
| Carbon | 50.6 |
| Oxygen | 35.4 |
| Magnesium | 0.4 |
| Phosphorus | 0.4 |
| Sulfur | 1.5 |
| Chlorine | 1.2 |
| Potassium | 10.2 |
| Aluminium | ND* |
| Calcium | 0.3 |
| Silicon | ND |
| Ferrum (Iron) | ND |
*ND: not detected.
3.4. Pharmacological Properties
Pharmacological evaluation of plants is based on their traditional uses. Cancer is one of the main causes of mortality and morbidity. Since P. bleo is traditionally used to prevent and treat cancer [28, 30, 40], it has been most studied for its antiproliferative and cancer protective properties [8, 22, 28, 32, 36, 39, 40]. This is followed by investigations of its antimicrobial and antiparasitic effects in vitro [8, 38, 41–43, 52]. The snake venom neutralizing properties [33], antinociceptive effects [35], and toxicity [22, 31] of this plant have been evaluated through in vivo studies.
3.4.1. Antiproliferative Properties
The effects of different P. bleo extracts have been reported on various cell lines in vitro. The crude methanol extract and its ethyl acetate fraction had significant cytotoxic effects against human nasopharyngeal epidermoid carcinoma cell line (KB) [36]. In addition, the ethyl acetate fraction was more active than the methanol extract against human colon carcinoma (HCT116) and hormone dependent breast carcinoma cell lines (MCF7) [36]. Table 6 shows the reported IC50 values (μg/mL) for the antiproliferative effects of P. bleo extracts and fractions.
Table 6.
IC50 values (μg/mL) of P. bleo leaf extracts and fractions on different cell lines.
| Cell line | Extracts and fractions (IC50: μg/mL) | Positive control (IC50: μg/mL) | Negative control | References | ||||
|---|---|---|---|---|---|---|---|---|
| Methanol | Water | Hexane | Dichloromethane | Ethylacetate | ||||
| 4T1 | >50 | >50 | NA | NA | NA | Cisplatin (NA) | NA | [32] |
| CasKi | 40.5 | — | 89.5 | NA | 58 | Doxorubicin (6 × 10−3) | NA | [36] |
| CEM-ss | — | NA | — | — | — | NA | VC | [8] |
| HT29 and HCT116 | >30 | NA | >30 | >30 | >30 | NA | VC | [8] |
| 41.6 | — | 67.5 | NA | 22 | Doxorubicin (3.6 × 10−1) | NA | [36] | |
| KB | 6.5 | — | 28 | NA | 4.5 | Doxorubicin (1.2 × 10−2) | NA | [36] |
| MCF-7 | >30 | NA | >30 | >30 | >30 | NA | VC | [8] |
| 39 | — | 25 | NA | 28 | Doxorubicin (7.5 × 10−2) | NA | [36] | |
| MRC-5 | — | — | — | NA | — | Doxorubicin (5.5 × 10−1) | NA | [36] |
| NIH/3T3 | ≥200 | ≥200 | NA | NA | NA | Cisplatin | NA | [32] |
| Saos-2 | — | NA | NA | NA | NA | Cisplatin | NA | [39] |
| T-47D | 2 | NA | NA | NA | NA | DNase I | VC | [40] |
| V79 | — | NA | NA | NA | NA | Nitracrine | VC | [22] |
IC50: 50% of maximum cell inhibition. IC50 < 20 μg/mL is considered active, 100 > IC50 > 20 μg/mL is relatively active, and IC50 > 100 is not active [8].
(—): no activity.
4T1: mouse mammary cancer cell line; CasKi: human cervical carcinoma cell line; CEM-ss: human T-4 lymphoblastoid cell line; HT29 and HCT116: human colon carcinoma cell line; KB: human nasopharyngeal epidermoid carcinoma cell line; MCF-7: hormone dependent breast carcinoma cell line; MRC-5: normal human fibroblast cell lines; NIH/3T3: normal mouse fibroblast cell line; Saos-2: human osteosarcoma cell line; T-47D: human breast carcinoma cell line; V79: Chinese hamster lung fibroblasts.
NA: not available.
VC: vehicle control.
Gupta et al. [22] reported high tumor inhibition activity in “potato disc inhibition assay” using crown gall tumors (LC50 77 ppm). Their result was accompanied by a significant DNA peak reduction in the DNA intercalation test for the methanol extract of the whole plant.
To date, no report is available on the in vivo antiproliferative activities of P. bleo.
(1) Cytotoxic Components. Some of the cytotoxic components in P. bleo have been reported. Table 7 shows the reported IC50 (μg/mL) values of these components in the different human cell lines. The effects of these compounds and the mixture of the isolated sterols were not as high as doxorubicin, that is, a chemotherapy drug [28]. In another study, phytol isolated from P. bleo leaves was found to have a significant antitumor activity against some mouse cancer cell lines [36].
Table 7.
Reported IC50 values (μg/mL) of selected P. bleo phytoconstituents on human cell lines [28].
| Compound | IC50 (μg/mL) of different cell lines | |||||
|---|---|---|---|---|---|---|
| KB | MCF7 | CasKi | HCT 116 | A549 | MRC-5 | |
| Dihydroactinidiolide | 6.7 | 30 | 40 | 5 | 97 | 91.3 |
| β-sitosterol | >100 | 72 | 62 | >100 | 78 | >100 |
| 2,4-ditertbutylphenol | 0.81 | 5.75 | 4.5 | 29 | 6 | 20 |
| α-tocopherol | 8 | 7.5 | 6 | 31 | 6 | 30.5 |
| Phytol | 7.1 | 34 | 18 | 100 | 31 | 74.1 |
| Mixture of sterols | >100 | >100 | >100 | >100 | >100 | >100 |
| Doxorubicin | 1.3 × 10−2 | 7.6 × 10−2 | 6.0 × 10−3 | 3.6 × 10−1 | 2.2 × 10−1 | 5.5 × 10−1 |
A549: human lung carcinoma cell line, CasKi: human cervical carcinoma cell Line, HCT116: human colon carcinoma cell Line, KB: human nasopharyngeal epidermoid carcinoma cell Line, MCF-7: hormone dependent breast carcinoma cell Line, MRC-5: normal human fibroblast cell Lines.
(2) Proposed Antiproliferative Mechanism. The antiproliferative activity of the methanol extract of P. bleo against human breast carcinoma cell line (T-47D) was found to be apoptotic in nature through the activation of caspase-3 and c-myc pathways [40]. Caspase-3 and c-myc are frequently activated death proteases which catalyze the specific cleavage of many key cellular proteins. They are also essential for normal development of the tissues as well as apoptosis in the tissues and cell types [53]. Komiya et al. [54] reported the induction of apoptosis as a mechanism of action for cytotoxic activity of phytol. DNA intercalation is another proposed mechanism of antiproliferative activity for P. bleo [22]. However, in some studies, P. bleo did not show appreciable cytotoxic effect [32]. Differences in the sources of plants, extraction methods, assay methods, and cell lines can be the possible reasons for these discrepancies. On the other hand, P. bleo may contain some prodrugs which are metabolized to the active metabolites. Therefore, further studies are needed to better understand its antiproliferative activity.
Apart from the cytotoxic activities against cancer cell lines, crude methanol extract and its fractions (hexane, water, and ethyl acetate) did not show any cytotoxicity to the normal human fibroblast cell lines, MRC-5 [36].
3.4.2. Antioxidant Activity
The adverse effects of oxidative stress on human health have become a serious issue. Oxidative stress causes production of free radicals in the body that facilitate the development of degenerative diseases such as cardiovascular diseases, cancers, neurodegenerative disorders [55], Alzheimer's, and inflammatory diseases [56]. One solution to this problem is to supplement the diet with antioxidant compounds found in natural plant sources [57]. Hence, in the literature, the antioxidant effects of P. bleo were evaluated using different assays as follows.
2,2-Diphenyl-1-picrylhydrazyl Hydrate (DPPH) Assay. The methanol, dichloromethane, ethyl acetate, and hexane extracts of P. bleo leaves were tested [8, 25]. The hexane extract exhibited the most effective radical scavenging activity (EC50 210 μg/mL) followed by the ethyl acetate extract (EC50 225 μg/mL). This spectrophotometric assay uses a stable radical 2,2′-diphenylpicrylhydrazyl (DPPH) as a reagent [8, 25].
Ferric Reducing Antioxidant Potential Assay (FRAP). The hexane, water, and methanol extracts of P. bleo leaves were found to reduce Fe3+/ferric cyanide complex to the ferrous form. Although the reduction was statistically significant, it was not more than ascorbic acid (vitamin C) and butylated hydroxyanisole (BHA) as positive controls [25]. Hassanbaglou et al. [37] compared the antioxidant activity of the ethyl acetate extract with that of hexane, ethanol, and methanol extracts. They showed that the ethyl acetate extract had significantly higher antioxidant properties compared to the rest of the tested extracts. FRAP measures the ability of test samples to reduce ferric ion to the ferrous form of TPTZ (2,4,6-tripyridyl-s-triazine).
β-Carotene-Linoleic Bleaching Assay. The ethyl acetate extract of P. bleo demonstrated the strongest antioxidant activity followed by the methanol extract reported by Sim et al. [25]. In this assay, the linoleate free radicals formed during the reaction are neutralized by antioxidants.
In general, although different studies used plant materials from different sources and nonsimilar extraction methods, ethyl acetate and hexane extracts appear to be the strongest antioxidant extracts from the P. bleo leaves [8, 25, 37]. Moreover, this antioxidant capacity is strongly associated with the total phenolic compounds and flavonoid content of the plant leaves [25, 37, 58]. The above studies suggest that P. bleo has antioxidant properties which can be one of the possible reasons for its traditional usage for detoxification and prevention of cancer.
3.4.3. Antimicrobial Properties
P. bleo has been shown to possess antibacterial, antiviral, and antifungal properties in vitro. Table 8 shows the effect of P. bleo extracts on selected bacteria and fungi. As can be seen, the methanol and hexane extracts demonstrated great antibacterial activities against Salmonella choleraesuis and Pseudomonas aeruginosa. In addition, its dichloromethane extract showed promising antibacterial effect against Methicillin resistant Staphylococcus aureus [8, 38]. All of the mentioned bacteria are among the main causes of nosocomial infections and they have been developing antibiotic resistance [59–61]. Therefore, the potential antibacterial activity of P. bleo needs to be further investigated to identify the lead(s) antibacterial component(s).
Table 8.
Reported effects of P. bleo extracts on the growth of selected bacteria and fungi.
| Organism | Antibacterial and antifungal effect of the extracts | Positive control | References | |||||
|---|---|---|---|---|---|---|---|---|
| Methanol | Water | Hexane | Dichloroethane | Ethyl acetate | Chloroform | |||
| Bacillus subtilis a | − | NA | − | − | − | NA | Streptomycin* | [8] |
| − | − | − | NA | − | NA | Gentamicin, ampicillin | [41] | |
| NA | NA | − | − | NA | NA | Streptomycin | [38] | |
| Escherichia coli b | − | NA | NA | − | NA | NA | Streptomycin | [42] |
| Escherichia coli a | − | − | − | NA | − | NA | Gentamicin, ampicillin | [41] |
| Helicobacter pylori b | − | NA | NA | − | NA | NA | Streptomycin | [42] |
| Klebsiella pneumoniae b | − | NA | NA | − | NA | NA | Streptomycin | [42] |
| Methicillin resistant Staphylococcus aureus a | − | NA | − | +++ | − | NA | Streptomycin* | [8] |
| NA | NA | − | ++ | NA | NA | Streptomycin | [38] | |
| Mycobacterium smegmatis b | − | NA | NA | − | NA | NA | Streptomycin | [42] |
| Pseudomonas aeruginosa a | ++ | NA | +++ | + | + | NA | Streptomycin | [8] |
| + | − | − | NA | + | NA | Gentamicin, ampicillin | [41] | |
| NA | NA | +++ | + | NA | NA | Streptomycin* | [38] | |
| Pseudomonas aeruginosa b | − | NA | NA | − | NA | NA | Streptomycin | [42] |
| Salmonella choleraesuis a | ++ | NA | +++ | − | − | NA | Streptomycin* | [8] |
| NA | NA | +++ | − | NA | NA | Streptomycin | [38] | |
| Staphylococcus aureus b | − | NA | NA | − | NA | NA | Streptomycin | [42] |
| Staphylococcus aureus a | − | − | − | NA | − | NA | Gentamicin, ampicillin | [41] |
| Candida albicans c | − | − | NA | NA | NA | − | Propiconazole, miconazole | [43] |
| Candida albicans b | − | NA | NA | − | NA | NA | Amphotericin B | [42] |
| Cladosporium cucumerinum c | + | + | NA | NA | NA | + | Propiconazole, miconazole | [43] |
aThe screening for antibacterial effect was carried out by determining the zone of inhibition using paper disc, + stands for activity between 6–9 mm, ++ stands for activity between 9–14 mm, +++ stands for activity more than 14 mm [38].
b(+) stands for activity at 100 μg/mL for E. coli, S. aureus, K. pneumoniae, M. smegmatis, C. albicance, P. aeruginosa and at 12.5 μg/mL for H. pylori, (−) stands for inactive samples.
cagar overlay assay and (+) stands for active extracts at 50 μg/mL, (−) stands for inactive extract.
NA: not applicable as there is no report in the literature.
*Streptomycin showed 20 to 23 mm inhibition zone. The rest of the studies did not report the exact value of the inhibition for their positive controls.
The antifungal activity of the water and methanol extract of P. bleo leaves against Cladosporium cucumerinum, a plant pathogenic fungus, has been reported [43], but they were not active against Candida albicans, a common human pathogen [42, 43].
The antiviral properties of the water and methanol extracts of P. bleo leaves were evaluated against Herpes Simplex Virus-I (HSV-1) and Human Immunodeficiency Virus (HIV) by Matsuse et al. [62]. Both of the extracts demonstrated anti-HIV activity. However, the result of this study was not promising because of the low selectivity index of 0.94. Besides, in another study by Hattori et al. [63], the same extracts did not demonstrate any antiviral activity against HSV-1. In general, the available data on the antiviral activity of P. bleo is neither sufficient nor conclusive. Therefore, further research needs to be carried out.
3.4.4. Antiparasitic Properties
The only antiparasitic investigation on P. bleo was reported by Marston et al. [52]. In their study, the chloroform, methanol, and water extracts of this plant did not exert any antiparasitic activity against schistosomiasis.
3.4.5. Neutralizing Snake Venom
Otero et al. [33] evaluated the neutralizing effect of the ethanol extract of P. bleo on hemorrhagic activity of “Bothrops atrox venom” in mice. This extract did not show any neutralizing effect against the tested venom.
3.4.6. Antinociceptive Properties
Wahab et al. [35] evaluated the antinociceptive activity of the ethanol extract and its fractions using two in vivo analgesic models: peripheral formalin-induced licking and acetic acid-induced abdominal writhing. They showed that the ethanol extract, hexane fraction, dichloromethane fraction, and ethyl acetate fraction of P. bleo had moderate antinociceptive effects. However, no compound was identified in their study.
3.5. Toxicity Studies
Acute toxicity effect of the leave's extracts of P. bleo was evaluated by in vitro and in vivo studies. Er et al. [32] showed that the water extract may form mutagenic compound(s) upon metabolization by the liver enzymes in vitro. In another study by Gupta et al. [22], the methanol extract of the whole plant had moderate toxicity in brine shrimp toxicity assay (LD50 77 ppm). In the only in vivo study by Sim et al. [31], the methanol extract did not have any toxicity effect on ICR mice (LD50 > 2500 mg/kg). Although animal models have around 70–80% predictability for human toxicities [64, 65], the long term toxicity and the mutagenicity of metabolites of P. bleo should be further investigated.
4. Conclusion
A comprehensive review on Pereskia bleo has been presented. It provides an overview of the botanical characteristics, traditional usage, phytoconstituents, pharmacological activities, and safety of P. bleo. The current review highlights the association between the traditional usage of the plant and the reported anticancer, antibacterial, and antinociceptive effects tested in different studies. Although P. bleo has been traditionally used for a variety of therapeutic and prophylactic purposes, only a few of them has been investigated. Hence, more research is warranted to further study its biological activities and chemical properties to understand its traditional usage and to develop novel therapeutics. Understanding the traditional uses, knowing the available scientific evidences, and identifying the gaps in research will allow the proper translation of promising research results into a safe and efficacious usage of herbal medicine and discovery of new therapeutics. It will also assist in setting appropriate policy and guidelines in the usage of herbal medicine.
Acknowledgments
Funding from the National University of Singapore (NUS) research Grant (R-148-000-137-112 to KHL) and Leeward Pacific Pte Ltd. (R-148-000-140-592 to KHL) and research scholarship from the Singapore International Graduate Award (SINGA, SZ) are acknowledged.
Conflict of Interests
All authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Wiart C. Medicinal Plants of Asia and the Pacific: Drugs of the Future. Singapore: World Scientific; 2006. [Google Scholar]
- 2.Britton NL, Rose JN. The Cactaceae, Descriptions and Illustrations of Plants of the Cactus Family. Washington, DC, USA: Dover Publications; 2009. [Google Scholar]
- 3.Edwards EJ, Donoghue MJ. Pereskia and the origin of the cactus life-form. American Naturalist. 2006;167(6):777–793. doi: 10.1086/504605. [DOI] [PubMed] [Google Scholar]
- 4.Edwards EJ, Nyffeler R, Donoghue MJ. Basal cactus phylogeny: implications of Pereskia (Cactaceae) paraphyly for the transition to the cactus life form. American Journal of Botany. 2005;92(7):1177–1188. doi: 10.3732/ajb.92.7.1177. [DOI] [PubMed] [Google Scholar]
- 5.Ogburn RM, Edwards EJ. Anatomical variation in Cactaceae and relatives: trait lability and evolutionary innovation. American Journal of Botany. 2009;96(2):391–408. doi: 10.3732/ajb.0800142. [DOI] [PubMed] [Google Scholar]
- 6.Boo CM, Ou-Yang CL, Omar-Hor K. 1001 Garden Plants in Singapore. 2nd edition. Singapore: National Parks Board; 2007. [Google Scholar]
- 7.Singapore Npark Board. NParks Flora&Founa web, 2010, https://florafaunaweb.nparks.gov.sg/Special-Pages/plant-detail.aspx?id=2324#.
- 8.Wahab SIA, Abdul AB, Mohan SM, Al-Zubairi AS, Elhassan MM, Ibrahim MY. Biological activities of Pereskia bleo extracts. International Journal of Pharmacology. 2009;5(1):71–75. [Google Scholar]
- 9.Bárcenas RT, Yesson C, Hawkins JA. Molecular systematics of the Cactaceae. Cladistics. 2011;27(5):470–489. doi: 10.1111/j.1096-0031.2011.00350.x. [DOI] [PubMed] [Google Scholar]
- 10.Chong KY, Tan HT, Corlett RT. A Checklist of the Total Vascular Plant Flora of Singapore: Native, Naturalised and Cultivated Species. Singapore: Raffles Museum of Biodiversity Research, National University of Singapore; 2009. http://rmbr.nus.edu.sg/raffles_museum_pub/flora_of_singapore_tc.pdf. [Google Scholar]
- 11.Sri Nurestri AM, Sim KS, Norhanom AW. Phytochemical and cytotoxic investigations of Pereskia grandifolia Haw. (Cactaceae) leaves. Journal of Biological Sciences. 2009;9(5):488–493. [Google Scholar]
- 12.Anderson E. The Cactus Family. 1st edition. Puritana, Ore, USA: Timber Press; 2001. (pp. 566–568). [Google Scholar]
- 13.Wunderlin RP, Hansen BF. Atlas of Florida Vascular Plants. 2008, http://www.plantatlas.usf.edu/
- 14.Taylor NP, Zappi D, Braun P, Machado M. Pereskia aculeata. Iucn Red List of Threatened Species. Version 2013. 2. 2013, http://www.iucnredlist.org.
- 15.Pooley E. A Field Guide to Wild Flowers KwaZulu Natal and the Eastern Region. Durban, South Africa: Natal Flora Publications Trust; 1999. [Google Scholar]
- 16.Natural Heritage Trust AGI. Weed Management Guide, Leaf cactus, Pereskia aculeata . 2003, http://www.environment.gov.au/biodiversity/invasive/weeds/publications/guidelines/alert/pubs/p-aculeata.pdf.
- 17.Tropicos. Missouri Botanical Garden (MBG) 2012, http://www.tropicos.org/Name/5100482.
- 18.Leuenberger BE. Pereskia, Maihuenia, and Blossfeldia-taxonomic history, updates, and notes. Haseltonia. 2008;(14):54–93. [Google Scholar]
- 19.Nugent J. Permaculture Plants, agaves and cacti. 2007, http://books.google.com.sg/books?id=YVwMM2OdO34C&q=Pereskia+bleo#v=snippet&q=Pereskia%20bleo&f=false.
- 20.Leuenberger BE. Humboldt & Bonpland's Cactaceae in the herbaria at Paris and Berlin. Willdenowia. 2002;32:137–153. [Google Scholar]
- 21.Liamas KA. Tropical Flowering Plants: A Guide to Identification and Cultivation. Portland, Ore, USA: Timber Press; 2003. [Google Scholar]
- 22.Gupta MP, Monge A, Karikas GA, et al. Screening of Panamanian medicinal plants for brine shrimp toxicity, crown gall tumor inhibition, cytotoxicity and DNA intercalation. Pharmaceutical Biology. 1996;34(1):19–27. [Google Scholar]
- 23.Kazama CC, Uchida DT, Canzi KN, et al. Involvement of arginine-vasopressin in the diuretic and hypotensive effects of Pereskia grandifolia Haw. (Cactaceae) Journal of Ethnopharmacology. 2012;144(1):86–93. doi: 10.1016/j.jep.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 24.USDA. National Genetic Resources Program, Germplasm Resources Information Network—(GRIN) [Online Database], 2012, http://www.ars-grin.gov/cgi-bin/npgs/html/tax_search.pl?Pereskia+bleo%.
- 25.Sim KS, Sri Nurestri AM, Norhanom AW. Phenolic content and antioxidant activity of crude and fractionated extracts of Pereskia bleo (Kunth) DC. (Cactaceae) African Journal of Pharmacy and Pharmacology. 2010;4(5):193–201. [Google Scholar]
- 26.Murillo E, Meléndez-Martínez AJ, Portugal F. Screening of vegetables and fruits from Panama for rich sources of lutein and zeaxanthin. Food Chemistry. 2010;122(1):167–172. [Google Scholar]
- 27.Hostettmann K, Marston A, Maillard M, Hamburger M. Phytochemistry of Plants Used in Traditional Medicine. Oxford, UK: Oxford University Press; 1995. (pp. 373–376). [Google Scholar]
- 28.Malek SNA, Shin SK, Wahab NA, Yaacob H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules. 2009;14(5):1713–1724. doi: 10.3390/molecules14051713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta MP, Correa A, Mireya D, et al. Medicinal plant inventory of Kuna Indians: part 1. Journal of Ethnopharmacology. 1993;40(2):77–109. doi: 10.1016/0378-8741(93)90054-9. [DOI] [PubMed] [Google Scholar]
- 30.Rahmat A, Saib FP, Buslima NA. Comparing the effect of ficus benjamina extract and Pereskia saecnarosa extract on tthe level of micro and macro minerals in normal and induced liver cancer rats. Proceedings of the 4th International Conference on Biomedical Engineering in Vietnam; 1980; pp. 208–212. [Google Scholar]
- 31.Sim KS, Sri Nurestri AM, Sinniah SK, Kim KH, Norhanom AW. Acute oral toxicity of Pereskia bleo and Pereskia grandifolia in mice. Pharmacognosy Magazine. 2010;6(21):67–70. doi: 10.4103/0973-1296.59969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Er HM, Cheng E, Radhakrishnan AK. Anti-proliferative and mutagenic activities of aqueous and methanol extracts of leaves from Pereskia bleo (Kunth) DC (Cactaceae) Journal of Ethnopharmacology. 2007;113(3):448–456. doi: 10.1016/j.jep.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Otero R, Núñez V, Barona J, et al. Snakebites and ethnobotany in the northwest region of Colombia, part III: neutralization of the haemorrhagic effect of Bothrops atrox venom. Journal of Ethnopharmacology. 2000;73(1-2):233–241. doi: 10.1016/s0378-8741(00)00321-4. [DOI] [PubMed] [Google Scholar]
- 34.Doetsch PW, Cassady JM, McLaughlin JL. Cactus alkaloids: XL. Identification of mescaline and other β-phenethylamines in Pereskia, Pereskiopsis and Islaya by use of fluorescamine conjugates. Journal of Chromatography A. 1980;189(1):79–85. [Google Scholar]
- 35.Wahab IRA, Guilhon CC, Fernandes PD, Boylan F. Anti-nociceptive activity of Pereskia bleo Kunth. (Cactaceae) leaves extracts. Journal of Ethnopharmacology. 2012;144(3):741–746. doi: 10.1016/j.jep.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Malek SNA, Wahab NA, Yaacob H, et al. Cytotoxic activity of Pereskia bleo (Cactaceae) against selected human cell lines. International Journal of Cancer Research. 2008;4(1):20–27. [Google Scholar]
- 37.Hassanbaglou B, Hamid AA, Roheeyati A, et al. Antioxidant activity of different extracts from leaves of Pereskia bleo (Cactaceae) Journal of Meidinal Plants Research. 2012;6(15):2932–2937. [Google Scholar]
- 38.Abbdewahab SI, Ain NM, Abdul AB, Taha MME, Ibrahim TAT. Energy-dispersive X-ray microanalysis of elements’ content and antimicrobial properties of Pereskia bleo and Goniothalamus umbrosus . African Journal of Biotechnology. 2009;8(10):2375–2378. [Google Scholar]
- 39.Liew SY, Stanbridge EJ, Yusoff K, Shafee N. Hypoxia affects cellular responses to plant extracts. Journal of Ethnopharmacology. 2012;144(2):453–456. doi: 10.1016/j.jep.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Tan ML, Sulaiman SF, Najimuddin N, Samian MR, Muhammad TST. Methanolic extract of Pereskia bleo (Kunth) DC. (Cactaceae) induces apoptosis in breast carcinoma, T47-D cell line. Journal of Ethnopharmacology. 2005;96(1-2):287–294. doi: 10.1016/j.jep.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Philip K, Malek SNA, Sani W, et al. Antimicrobial activity of some medicinal plants from Malaysia. American Journal of Applied Sciences. 2009;6(8):1613–1617. [Google Scholar]
- 42.Rüegg T, Calderón AI, Queiroz EF, et al. 3-farnesyl-2-hydroxybenzoic acid is a new anti-Helicobacter pylori compound from Piper multiplinervium . Journal of Ethnopharmacology. 2006;103(3):461–467. doi: 10.1016/j.jep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Rahalison L, Hamburger M, Hostettmann K, et al. Screening for antifungal activity of Panamanian plants. International Journal of Pharmacognosy. 1993;31(1):68–76. [Google Scholar]
- 44.Candolle APD. Pereskia bleo (Kunth) DC. 2011, http://www.tropicos.org/Name/5100482.
- 45.USDA. Taxon: Pereskia bleo . 2013, http://www.ars-grin.gov/cgi-bin/npgs/html/tax_search.pl?Pereskia+bleo%.
- 46.IPNI. International Plant Names Index. 2005, http://www.ipni.org/ipni/plantNameByVersion.do?id=273592-2&version=1.3.
- 47.Gardener M. Tropical plants library online. 2014, http://mgonline.com/articles/exotics.aspx.
- 48.Kurt S. Stem of P. grandifolia . 2014, http://www.biolib.de/
- 49.NRCS. Natural Resources and Conservation Service of USDA, Plant profile of P. grandifolia Haw. 2013, http://plants.usda.gov/core/profile?symbol=PEGR14#.
- 50.Sanders DC, Grayson AS, Monaco TJ. Mineral content of tomato (Lycopersicon esculentum) and four competing weed species. Weed Science. 1981;29(5):590–593. [Google Scholar]
- 51.Geleijnse JM, Witteman JC, Bak AA, den Breeijen JH, Grobbee DE. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension. British Medical Journal. 1994;309(6952):436–440. doi: 10.1136/bmj.309.6952.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marston A, Dudan G, Gupta MP, Solis PN, Correa MD, Hostettmann K. Screening of Panamanian plants for molluscicidal activity. Pharmaceutical Biology. 1996;34(1):15–18. [Google Scholar]
- 53.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death and Differentiation. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 54.Komiya T, Kyohkon M, Ohwaki S, et al. Phytol induces programmed cell death in human lymphoid leukemia Molt 4B cells. International Journal of Molecular Medicine. 1999;4(4):377–380. doi: 10.3892/ijmm.4.4.377. [DOI] [PubMed] [Google Scholar]
- 55.Gerber M, Boutron-Ruault MC, Hercberg S, Riboli E, Scalbert A, Siess MH. Food and cancer: state of the art about the protective effect of fruits and vegetables. Bulletin du Cancer. 2002;89(3):293–312. [PubMed] [Google Scholar]
- 56.di Matteo V, Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Current Drug Targets-CNS & Neurological Disorders. 2003;2(2):95–107. doi: 10.2174/1568007033482959. [DOI] [PubMed] [Google Scholar]
- 57.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. British Medical Journal. 1996;312(7029):478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mustafa RA, Abdul Hamid A, Mohamed S, Bakar FA. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. Journal of Food Science. 2010;75(1):C28–C35. doi: 10.1111/j.1750-3841.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- 59.Breidenstein EB, de la Fuente-Núñez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends in Microbiology. 2011;19(8):419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Chu C, Chiu C-H, Wu W-Y, Chu C-H, Liu T-P, Ou JT. Large drug resistance virulence plasmids of clinical isolates of Salmonella enterica serovar Choleraesuis . Antimicrobial Agents and Chemotherapy. 2001;45(8):2299–2303. doi: 10.1128/AAC.45.8.2299-2303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. Journal of Antimicrobial Chemotherapy. 1997;40(1):135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 62.Matsuse IT, Lim YA, Hattori M, Correa M, Gupta MP. A search for anti-viral properties in Panamanian medicinal plants: the effects on HIV and its essential enzymes. Journal of Ethnopharmacology. 1998;64(1):15–22. doi: 10.1016/s0378-8741(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 63.Hattori M, Nakabayashi T, Lim YA, et al. Inhibitory effects of various Ayurvedic and Panamanian medicinal plants on the infection of herpes simplex virus-1 in vitro and in vivo . Phytotherapy Research. 1995;9(4):270–276. [Google Scholar]
- 64.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nature Reviews Drug Discovery. 2004;3(8):711–716. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 65.Olson H, Betton G, Robinson D, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regulatory Toxicology and Pharmacology. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]


