Abstract
The incidence of obesity and type 2 diabetes mellitus (T2DM) has risen to epidemic proportions. The pathophysiology of T2DM is complex and involves insulin resistance, pancreatic β-cell dysfunction and visceral adiposity. It has been known for decades that a disruption of biological rhythms (which happens the most profoundly with shift work) increases the risk of developing obesity and T2DM. Recent evidence from basal studies has further sparked interest in the involvement of daily rhythms (and their disruption) in the development of obesity and T2DM. Most living organisms have molecular clocks in almost every tissue, which govern rhythmicity in many domains of physiology, such as rest/activity rhythms, feeding/fasting rhythms, and hormonal secretion. Here we present the latest research describing the specific role played by the molecular clock mechanism in the control of glucose metabolism and speculate on how disruption of these tissue clocks may lead to the disturbances in glucose homeostasis.
Keywords: Glucose, Diabetes, Circadian rhythm, Hypothalamus, Autonomic nervous system
1. Daily rhythms
In most organisms each day is organized into two phases: one characterized by activity and feeding and one by rest and fasting. Nutrients ingested during the active period provide substrates such as glucose, lipids and amino acids, which fuel the metabolic pathways in our cells, whereas during the resting period energy and substrates stored in our body are mobilized to sustain metabolic homeostasis [1]. The hypothalamus controls a vast array of these alternating behavioral and physiological processes, including food and water intake, but also sleep and arousal, thermoregulation, and energy expenditure. The activity/feeding and resting/fasting periods are defined by a molecular mechanism in the central clock that is located in the suprachiasmatic nuclei (SCN) of the hypothalamus and generate a rhythm of approximately 24-h (hence ‘circadian’). Lesions of the SCN cause a loss of all circadian rhythms, including those in locomotor, feeding and drinking activity [2].
The molecular mechanism governing circadian rhythmicity is based on a complex program of gene expression. A number of interlocked transcriptional and post-translational negative feedback loops are responsible for the generation and maintenance of circadian rhythms. CLOCK and BMAL1 are transcription factors that act as positive regulators of circadian gene expression and activate the expression of the negative regulators of circadian gene expression: cryptochrome (CRY1 and CRY2) and period (PER1, PER2, PER3) families. CRY and PER proteins feedback and inhibit their own expression as well as the expression of other clock-controlled genes (CCGs) [3]. REV-ERBα, a nuclear receptor that regulates lipid metabolism and adipogenesis, is regulated by the circadian clock and represses Bmal1 expression, thereby providing additional robustness to these circadian oscillations. To start a new transcriptional cycle, the CLOCK–BMAL1 complex is de-repressed through the proteolytic degradation of PER and CRY. This molecular machinery is capable of generating rhythmic patterns of gene expression with a period length of about 24 h (Figure 1).
Figure 1.
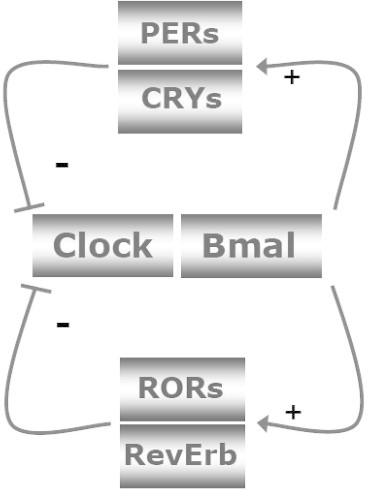
Simplified version of the molecular core clock mechanism. The core loop is formed by Clock:Bmal1 and Period 1–3 (Per1–3) and Cryptochrome 1 and 2 (Cry1–2). The Clock:Bmal1 heterodimer stimulates the transcription of Per1–3 and Cry1–2. Subsequently, Per's and Cry's heterodimerize, translocate to the nucleus, and inhibit Clock:Bmal1 activity. As a consequence, Clock:Bmal1 transcriptional activity drops, which reduces the transcription of Per and Cry genes, thereby activating Clock:Bmal1 again. Additional loops formed by RevErbs and RORs enhance the robustness of the core loop. Post-translational modifications of the clock proteins such as phosphorylation, ubiquitination and sumoylation greatly determine their stability and degradation, which plays a critical role in circadian cycle progression and setting the clock period. For clarity these posttranscriptional regulatory events have been left out of the figure. The feedback loops of the core clock can also regulate widely different phases of genes encoding regulatory components of energy metabolism, by binding to the appropriate promoter elements. On the other hand, changes in energy status have an impact on the molecular clock mechanism. AMPK phoshorylates Cry1 and thereby targets it for degradation. SIRT1 deacetylates, amongst others, Bmal1 and Per2, thereby decreasing the half-life of Per2.
Adapted from Ref. [110].
The transcriptional output time of the molecular clock in the SCN is set to exactly 24-h by retinal light input, the most important timing cue (Zeitgeber) to synchronize this central clock with the environment [4]. Until 1998 it was thought that circadian rhythms in the periphery were driven directly by SCN outputs or indirectly via SCN-driven rhythmic behavior. However, in that year Balsalobre et al. [5] showed that a serum shock could induce rhythmic gene expression in cultured mammalian cells and it emerged that (almost) all cells in the body express the molecular machinery for the circadian clock. A few years later it became clear that the feeding/fasting cycle is the main Zeitgeber in terms of the synchronization of these so-called peripheral clocks [6]. Indeed, it was shown that rhythmic feeding is both necessary and sufficient to drive the circadian expression of liver genes [7,8] and that by restricting food intake to the rest phase (i.e., the light period for nocturnal animals) it is possible to set the peripheral clocks and the central SCN clock to two different time zones, 12-h apart.
Blood glucose homeostasis can be seen as a paradigm of the circadian control of energy metabolism. Indeed, whereas during the activity/feeding period blood glucose is mainly of dietary origin, during the resting/starvation period glucose is progressively recruited from endogenous glucose production in the liver to maintain blood glucose levels within a relatively narrow range. In this process liver glycogen content undergoes large daily fluctuations to sustain blood glucose levels, as glycogen synthesis and degradation are specifically recruited during the activity/feeding and resting/starvation periods, respectively [9,10]. As will become clear, daily blood glucose homeostasis also involves control by the hypothalamic clock in the SCN as well as by peripheral clocks in, for instance, liver, pancreas, muscle and white adipose tissue.
2. Clock gene knock-outs
The awareness of a daily variation in glucose homeostasis dates back to the early seventies [9,11], but a full-blown realization of the importance of the circadian timing system for glucose homeostasis only became apparent when Turek et al. [12] described a pronounced metabolic phenotype in Clock mutant mice. Not only did these animals show severely disturbed daily feeding rhythms, they were also hyperphagic, obese, hyperleptinemic, hyperlipidemic, hyperglycemic and hypoinsulinemic. However, different results have been reported in other Clock mutant mouse models as also lack of obesity or even weight loss, despite lipid imbalance, has been reported suggesting the importance of the genetic background when analyzing the metabolic effects of the Clock gene [13–16]. In later years differential metabolic phenotypes were observed in various other core clock gene mutant mice. For instance, whole-body knockout (KO) mice of Bmal1 show fasting hypoglycemia, reduced plasma insulin levels, increased adiposity [16–18]. Both Clock mutant and Bmal1 KO mice exhibit delayed recovery from insulin-induced hypoglycemia, impaired glucose tolerance and blunted insulin sensitivity. Indeed in Clock mutant mice the circadian oscillation of both the hepatic glycogen content and the circadian mRNA and protein expression of glycogen synthase 2 (a rate-limiting enzyme of glycogenesis in the liver) was severely dampened [19]. Per2 mutant mice show reduced fasting glycemia, a loss of rhythmic glycogen accumulation in the liver, elevated plasma insulin levels and impaired gluconeogenesis [20–22], as well as a reduction in fat pad mass and plasma lipid levels [23]. Per2 null mice exhibit no glucocorticoid oscillations, indicating that PER2 may also impact glucocorticoid receptor (GR)-dependent glucose metabolism [24,25]. Glucose homeostasis is also severely disrupted in Cry-deficient mice. Cry1−/−/Cry2−/− (double KO) mice exhibit elevated blood glucose in response to acute feeding after an overnight fast and severely impaired glucose clearance in a glucose tolerance test. Even mice lacking either Cry1 (cry1−/−) or Cry2 (cry2−/−) were significantly impaired in their ability to restore normal blood glucose following a glucose injection. In contrast, Cry-deficient animals were normally responsive to insulin [26]. The impaired glucose tolerance became especially apparent when the animals were on a high-fat diet. Despite being hypophagic on the high-fat diet the dKO animals become obese rapidly, probably because of an increased insulin release and lipid storage [28]. Rev-erbα directly regulates the expression of multiple gluconeogenic enzymes in liver, including glucose-6-phosphatase and phosphoenolpyruvate [27]. Indeed, Rev-erbα−/− mice displayed increased adiposity and mild hyperglycemia, but without insulin resistance. Rev-erbα−/− mice seem to favor fatty acid oxidation at the expense of glycogen utilization [29]. An overview of the metabolic disturbances reported for the different global clock gene KOs is presented in Table 1.
Table 1.
Metabolic changes in whole body clock gene knock-outs.
| Gene | BW | WAT | Glucose | Glucose tolerance | Insulin | Insulin sensitivity | Lipids | |
|---|---|---|---|---|---|---|---|---|
| Rudic et al. [16] | Clock | Normal | Normal | Decreased | Increased | |||
| Turek et al. [12] | Clock | Increased | Increased | Normal | Increased | |||
| Oishi et al. [13] | Clock | Decreased | Normal | Normal | Decreased | |||
| Kennaway et al. [15] | Clock | Normal | Normal | Decreased | Decreased | Increased | Decreased | |
| Kudo et al. [14] | Clock | Normal | Normal | |||||
| Doi et al. [19] | Clock | Normal | ||||||
| Marcheva et al. [17] | Clock | Increased | Decreased | Decreased | Normal | |||
| Shostak et al. [105] | Clock | Increased | Increased | Decreased | ||||
| Rudic et al. [16] | Bmal | Increased | Increased | |||||
| Marcheva et al. [17] | Bmal | Decreased | ||||||
| Kennaway et al. [18] | Bmal | Decreased | Increased | Normal | Normal | Decreased | Normal | Normal |
| Grimaldi et al. [23] | Per2 | Decreased | Decreased | Decreased | ||||
| Schmutz et al. [20] | Per2 | Decreased | ||||||
| Zhao et al. [22] | Per2 | Increased | Increased | Increased | ||||
| Chappuis et al. [114] | Per2 | Normal | Normal | |||||
| Zani et al. [21] | Per2 | Normal | Decreased | Normal | Normal | Decreased | ||
| Lamia et al. [26] | Cry1/Cry2 | Increased | Decreased | Normal | Normal | |||
| Barclay et al. [28] | Cry1/Cry2 | Decreased | Normal | Normal | ||||
| Cho et al. [154] | Rev-erbα | Increased | Decreased | |||||
| Delezie et al. [29] | Rev-erbα | Normal | Increased | Increased | Normal | Normal | Decreased | |
Changes indicated represent changes in basal plasma concentrations as observed in animals on normal chow.
As noted above the metabolic disturbances observed in clock mutants much depend on their genetic background. In fact, the majority of null mutations in the circadian clock components are associated with leanness, despite normophagia or hyperphagia, indicating that global clock gene disruption affects energy metabolism in a complex manner. Moreover, in addition to clock gene mutations clock gene expression and thereby energy metabolism may be disturbed in many other ways. The p75 neurotrophin receptor (p75NTR) is a member of the tumor necrosis factor receptor superfamily with a widespread pattern of expression in tissues, including brain, liver, lung and muscle. p75NTR is an oscillating gene regulated by the CLOCK/BMAL1 heterodimer. Loss of p75NTR alters the circadian oscillation of clock genes in the SCN, liver and fibroblasts, as well as glucose and lipid homeostasis genes [30]. Indeed, p75NTR KO-mice show an increased insulin sensitivity [31]. However, it is not clear yet whether this is due to changes in the central clock or due to changes in peripheral clocks. Interestingly, the p75NTR is also highly expressed in sensory and sympathetic neurons [32].
Inhibitor of DNA binding 2 (ID2) is a helix–loop–helix transcriptional repressor that interacts with CLOCK and BMAL1 and is rhythmically expressed in many tissues. LD2 KO mice not only show increased light-induced phase-shifts [33], but also increased glucose tolerance and insulin sensitivity [34]. Fluorodeoxyglucose-positron emission tomography (FDG-PET) analysis revealed increased glucose uptake by skeletal muscle and brown adipose tissue in the LD2 KO mice [34].
Even a high-fat diet may alter the function of the circadian clock, with an altered period of the locomotor activity rhythm and changes in the rhythms of the core clock genes in adipose and liver tissue [35–40,49]. It has therefore been hypothesized that a high-fat diet may contribute to the development of obesity and insulin resistance also via alterations in circadian rhythmicity. Although the exact mechanism via which a high-fat diet affects the circadian system has not been elucidated yet, it is obvious that changes in food intake will influence the cellular energy status. Indeed, activation of adenosine monophosphate-activated protein kinase (AMPK), a sensor of low intracellular energy and nutrient state, leads to altered circadian rhythms by destabilizing the negative limb of the circadian clock [41,42]. Cyclic adenosine monophosphate (cAMP) is a further example of an acute signaling pathway that is tightly intertwined with the core clock. In a series of elegant experiments, O'Neill et al. [43] showed that cAMP is not solely an output of the SCN, but an integral component of the SCN pacemaker, regulating transcriptional cycles. The cellular energy status also influences the redox state, implying that also via this pathway food intake might be able to entrain the circadian system. Indeed, in vitro experiments have shown that the redox state of NAD can regulate DNA binding activity of the CLOCK:BMAL1 heterodimer. Interestingly, in vivo NAD levels are subjected to daily variations, thereby giving rhythmic input to the genetic clock [44–46], but there are also several indirect pathways via which the redox state can be linked to the clock [46]. For instance, the enzymes silent information regulator protein (SIRT) and poly (ADP-ribose) polymerase 1 (PARP-1) are both NAD-dependent enzymes. SIRT is expressed rhythmically and interacts with CLOCK:BMAL1 heterodimers, leading to rhythmic deacetylation of CLOCK:BMAL1, histone H3, and PER2 [47]. When subjected to daytime feeding the liver of Parp-1 KO mice showed a significantly delayed phase inversion of clock gene expression, when compared with wild type mice, suggesting that PARP-1 activity is indeed implicated in the phase entrainment of peripheral oscillators [48].
The results of all these whole-body KO studies clearly emphasized the importance of the molecular clock mechanism for glucose homeostasis. However, they provided little further understanding as to which part of the circadian timing mechanism and which aspects of the glucose regulatory system were affected and responsible for the observed metabolic phenotypes. Although SCN-lesion studies did support the observations from the KO studies [40,49], for the current subject a brain-specific KO is of little added value, contrary to other molecular mechanisms, since both SCN-lesions and a genetic elimination of the central clock will also result in a desynchronization of the peripheral clock systems. Clearly, additional studies, involving amongst others the central clock in the SCN and tissue-specific KO models, were urgently needed to elucidate further the multifaceted role of the circadian clock system in whole-body glucose metabolism.
3. The hypothalamic clock
The hypothalamus controls a vast array of physiological processes, including sleep/wake cycles, sexual behavior and reproduction, and metabolic control such as thermoregulation, energy intake/expenditure, glucose metabolism, lipid metabolism, and food and water intake. All these functions follow circadian rhythms. In addition to the SCN, the hypothalamus is made up of several other interconnected nuclei, including the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), lateral hypothalamus (LH), dorsomedial hypothalamus (DMH) and paraventricular nucleus (PVN). The hypothalamus senses nutrients such as glucose and lipids [50,51] and via a specialized area of the blood brain barrier (BBB) in the ARC it also detects circulating metabolic hormones such as leptin, insulin, thyroid hormone, adiponectin and ghrelin [52], produced in peripheral glands and tissues including the intestine, pancreas, stomach and adipose tissue. This specialized area of the BBB is called a circumventricular organ, an area in which blood to brain transport is facilitated through regulated exchanges.
The first evidence that the SCN are involved in the daily rhythm in glucose metabolism came from the work of Nagai and Nakagawa, who showed that SCN lesions abolished the daily rhythms in plasma concentrations of glucose and insulin [53] and revealed the existence of a pronounced day/night difference in the response to 2-deoxy glucose, a glucose-utilization inhibitor [54]. SCN-lesioned rats, however, do not have a rhythm in food intake either [55]. Therefore, an indirect effect of the lack of a feeding rhythm on glucose metabolism could not be excluded, as restricted feeding during the daytime will induce the glucose and insulin peak to shift to the daytime [56]. To test whether the SCN exerts a direct influence on glucose metabolism which is independent from its effect on feeding behavior, we performed a series of experiments using a 6-meals-a-day feeding schedule with an identical meal every 4 h, which effectively removes the pronounced day/night difference in food intake.
The first experiments with this scheduled feeding regimen in rats revealed a clear daily rhythm in meal-induced responses in glucose and insulin, i.e., similar meals resulted in larger glucose and insulin responses when consumed during the dark period than when consumed during the light period (Figure 2; [57]). In addition, the daily rhythm in basal plasma glucose concentrations of the 6-meals-a-day fed animals nicely resembled the daily rhythm – a daily rise at the time of awakening – observed in animals fed ad libitum, which clearly suggests a direct influence of the SCN on plasma glucose concentrations, independent of the feeding rhythm [58]. Another argument in favor of a direct influence of the SCN on glucose metabolism, independent of its effect on feeding behavior, was the fact that the rhythmicity was maintained in plasma glucose concentrations during fasting. On the other hand, we did not find an obvious and direct influence of the SCN on basal plasma insulin or glucagon concentrations [58,59]. Clearly, feeding behavior and/or glucose concentrations are more important to set basal insulin and glucagon secretion than a direct SCN input.
Figure 2.
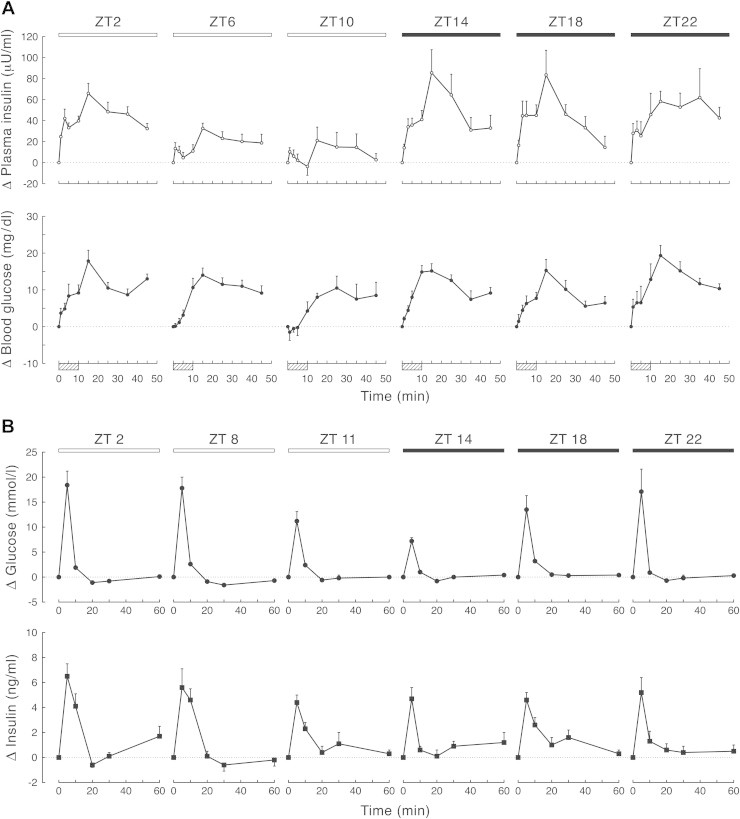
(upper part): Plasma insulin and blood glucose responses after meal ingestion during the light period and dark period in animals on a regular feeding regimen of 6-meals-a-day. Before sampling animals had been on this regimen for 2–3 weeks. Boxes indicate meals. Despite similar meals (3.1 ± 03 g) and comparable glucose increments insulin responses significantly differ depending on the time of day. (lower part): Plasma glucose and insulin responses after the intravenous injection of a glucose bolus (500 mg/kg BW) at different times of the light/dark cycle. The maximal glucose increment at ZT14 was significantly lower than the ones at the other 5-time points. On the other hand, the total amount of insulin released did not differ between the different time points. Responses are expressed as the difference from the respective t = 0 values. Black bars indicates meals in the dark period. ZT = Zeitgeber Time; ZT12 being defined as the onset of the dark period. Adapted from Ref. [57] (upper figure) and Ref. [60] (lower figure).
The daily rise in plasma glucose concentrations at awakening could either result from a decrease in glucose uptake or from an increase in glucose output. Glucose uptake did indeed show a clear 24-h rhythm, but, surprisingly, with the highest uptake at the end of the light period (Figure 2; [60]). A similar situation occurs in humans; before waking, glucose production and glucose concentrations are increased while at the same time glucose utilization is high [61]. Consequently, the increase in plasma glucose concentrations before the onset of activity is due to increased glucose production and not the result of a decreased glucose utilization. It is obvious that the simultaneous rise in plasma glucose concentrations and glucose uptake can only occur when the glucose output to the general circulation is such that it compensates for the increased glucose uptake.
The liver is the main source of endogenous glucose. Interestingly, our anatomical tracing experiments revealed synaptic connections between the SCN and the liver, via the autonomic nervous system (ANS) [62,63]. The involvement of the ANS in SCN-driven changes in glucose metabolism was first suggested by Nagai and colleagues. They showed that electrical stimulation of the SCN resulted in hyperglycaemia, an effect that could be prevented by blocking ANS activity by means of (i.p.) administration of α and β-adrenergic blockers [64,65]. Administering an adrenergic blocker i.p., however, has differential effects in many organs and more recent experiments therefore used hepatic sympathetic denervations to determine the role of autonomic innervation of the liver for the generation of the daily rhythm in plasma glucose concentrations. From these studies it became clear that the SCN does indeed need an intact sympathetic innervation of the liver to generate a daily rhythm in plasma glucose concentrations [63,66].
The SCN does not directly innervate autonomic motor neurons, but transmits its signal to other areas within the hypothalamus. The PVN is the most important target area for the SCN to affect autonomic signaling to peripheral organs [67] as it has extensive projections to sympathetic and parasympathetic motor neurons in the spinal cord and in the brainstem, respectively [68–71]. The functional importance of this SCN–PVN connection in controlling plasma glucose concentrations was revealed by introducing different SCN transmitter agonists and antagonists into the vicinity of the PVN [63]. The most pronounced effects on plasma glucose concentrations were observed after the administration of either bicuculline (BIC; a GABA-A antagonist) or NMDA (an agonist of glutamatergic receptors) into the PVN, both resulting in a prolonged and significant increase in plasma glucose concentrations. These drugs also increased plasma glucagon concentrations (which may stimulate glucose output) but did not affect plasma insulin concentrations in any significant way. Blockade of GABA-ergic receptors also resulted in increased plasma concentrations of corticosterone, which – like glucagon – is known to increase gluconeogenesis. Stimulating the glutamate receptors, however, did not. These data indicate that it is unlikely that the hyperglycemia induced by stimulating PVN neurons is a result of changes in either insulin or corticosterone release, although increased glucagon release could be a causative factor. Later experiments showed that prior selective denervation of the sympathetic, but not the parasympathetic, autonomic input to the liver completely prevented the hyperglycemic effects of both BIC and NMDA [63]. The hyperglycemic effects disappeared in the sympathetic denervated animals, notwithstanding pronounced increases of plasma concentrations of glucagon and corticosterone. Together, these functional studies demonstrate that stimulating neuronal activity in the PVN results in hyperglycemia through activating sympathetic input to the liver. Repeating the above experiments at different times of the day and in SCN-lesioned animals confirmed the SCN as the major site of origin for the GABA and glutamatergic inputs to the PVN with respect to the control of glucose homeostasis [72].
Another target area of the SCN is the perifornical area (PF) [73,74]. Follow-up studies using a stable glucose isotope to quantify hepatic glucose production showed that this was the most effective area for increasing hepatic glucose production [75]. The PF contains a major part of the hypothalamic population of orexin-containing neurons. Nowadays, the neuropeptide orexin (also known as hypocretin) is best known for its involvement in sleep and arousal (i.e., a lack of orexin causes narcolepsy), but as can be inferred from its name it is also involved in food intake and energy metabolism. The hypothalamic orexin system shows a pronounced day/night rhythm, with peak activity during the waking period in both nocturnal and diurnal species [76,77]. The daily rhythm in the activity of orexin neurons seems to be controlled primarily via an inhibitory GABAergic input [78]. Thus we hypothesized – and were able to show – that an increased activity of the orexin neurons at the end of the sleep period, due to a withdrawal of the GABAergic SCN inhibition, will not only result in arousal but also in an increased hepatic glucose production (Figure 3; [75]). In fact, by virtue of its stimulatory effect on the sympathetic branch of the ANS the increased activity of the orexin system at arousal may also adapt other physiological parameters, such as heart rate, body temperature and glucose uptake, to the waking state [79].
Figure 3.
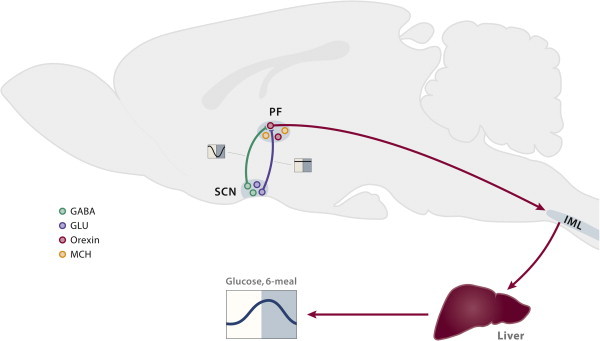
Midsagittal view of the rat brain presenting the proposed involvement of orexin neurons in the control of the daily plasma glucose rhythm. (i) Orexin-containing neurons in the perifornical area (PF) are innervated by both glutamatergic and GABAergic projections from the biological clock (SCN). During the main part of the light period, activation of the orexin neurons by the excitatory glutamatergic inputs is prevented by the simultaneous release of the inhibitory neurotransmitter GABA (the daily activity pattern of these inputs is indicated by the lines in the yellow/blue boxes aside the projections). The circadian withdrawal of the GABAergic input at the end of the light period allows the orexin neurons to become active at the onset of darkness. (ii) Subsequently, the excitatory effect of orexin on the preganglionic neurons in the intermediolateral column (IML) of the spinal cord will (iii) activate the sympathetic input to the liver and result in increased hepatic glucose production. Orexin also stimulates glucose uptake in skeletal muscle via an action in the VMH and mediated through the sympathetic nervous system [80]; but, as it is not clear yet how this message is propagated from the VMH to the autonomic nervous system, this action has not been incorporated in this schema. Possibly the effect of orexin in the VMH on glucose uptake is mediated via VMH projections to the pre-autonomic neurons in the PVN. Moreover, also the effect of orexin on hepatic glucose production might in fact in involve a projection of the PF orexin neurons to the pre-autonomic neurons in the PVN, instead of, or in addition to, the direct projection of the PF orexin neurons to the spinal cord as drawn in the figure.
Adapted from Ref. [152].
In conclusion, the central biological clock seems to affect all aspects of glucose homeostasis, including glucose production, glucose uptake, and insulin release and insulin sensitivity. However, from the above studies it has not become clear yet if, and if so, how these central timing mechanisms might be involved in the current increased propensity of type 2 diabetes and obesity.
4. The liver clock
The liver plays a pivotal role in maintaining optimum glucose levels by balancing glucose entry into and out of the circulation. From a hypothalamic and chronobiological point of view, glucose production by the liver is especially interesting because of the clear involvement of both the sympathetic and parasympathetic input to the liver in glucose metabolism [81–83] and the strong circadian control of (glucose) metabolism in the liver [84–86]. As mentioned before, it has been shown that restricted feeding shifts rhythmic patterns of clock genes in the liver and thus uncouples them from the central clock – where no changes in rhythmic patterns of clock genes are observed [6]. Over 350 circadian transcripts have been identified in the liver, 10% of which, including the core gene Per2, maintain rhythmicity in the absence of a functional hepatocyte clock. This leaves room for a role for behavioral, hormonal and autonomic rhythms, in the regulation of liver gene expression rhythms [8]. However, although insulin has long been a suspect, concrete evidence is still lacking. Acute insulin injections do cause a phase-advance of the PER2 and REV-ERBα rhythms in the liver [87], and also other studies demonstrated a phase advance of the hepatic clock when insulin signaling may be affected [88,89], but liver clock gene oscillations are maintained in streptozotocin-induced diabetic mice [90]. The same holds for glucocorticoids. A major part of the liver transcriptome is dependent on the adrenal hormones [91], and acute injections of glucocorticoids cause a shift in the expression pattern of hepatic clock genes. This shift is prevented by deletion of the GR in the liver [92]. In addition, glucocorticoid signaling is critical for maintaining fasting glucose by stimulating hepatic gluconeogenesis [93], and abnormal activation of the GR has been shown to contribute to diabetic hyperglycemia [94]. However, the exact role of GR in mediating the circadian regulation of hepatic metabolism remains to be determined, as a GR-KO does not prevent the shift of hepatic clock genes upon restricted feeding [95]. Also, the role of the autonomic innervation is not clear yet; although both a sympathetic and a parasympathetic hepatic denervation result in an abolition of the daily glucose rhythm, clock gene rhythms are only affected to a minor extent. The autonomic innervation is thus not a prerequisite for the maintenance of clock gene rhythms in the liver, and clock gene rhythms are not sufficient to drive the plasma glucose rhythm [66,96].
More precise roles for the core clock genes in hepatic glucose metabolism have been clearly shown using tissue specific clock KOs. Liver-specific Bmal1 disruption in mice increases glucose tolerance, with normal insulin production and normal body fat content [97], whereas a liver-specific Cry KO inhibits glucagon-induced gluconeogenesis and overexpression of Cry protein in the liver of diabetic db/db mice improves glucose tolerance [98]. CRY1 has been found to form a complex with GR in hepatocytes and to subsequently repress transcription of phosphoenolpyruvate kinase (PEPCK; [26]).
Thus, a liver-specific KO of the molecular clock mechanism seems to result in impaired glycogenesis and therefore, probably, in reduced hepatic glucose production and increased glucose tolerance.
5. The muscle clock
Daily rhythms have been well documented, also in skeletal muscle, where over 200 genes exhibit a rhythmic pattern of expression [99,100]. Moreover, clock gene disruption may seriously affect muscle function [101]. Rev-Erbα is highly expressed in oxidative skeletal muscle and its elimination leads to profound mitochondrial dysfunction in muscle tissue [102]. A deficiency in Rev-Erbα might therefore have an impact on glucose metabolism because skeletal muscle is the major site for glucose uptake in the organism, i.e., after a meal >80% of the glucose is taken up by muscle tissue. Although the effects of a muscle-specific clock gene knock-out on glucose homeostasis have not been studied, two distinct mouse models have been generated in which the circadian clock mechanism has been disrupted in a cardiomyocyte-specific manner. These models each targeted a different critical clock component: CLOCK and BMAL1 [103,104]. In contrast to the whole animal CLOCK and BMAL1 KO models the cardiac-specific animals display normal behavioral and neurohumoral rhythms. Note that systemic glucose metabolism appears to be normal despite clear changes in glycolysis, glycogen synthesis and glucose oxidation rhythms in the KO hearts [104].
In conclusion, although muscle tissue is an important player in glucose metabolism, skeletal muscle-specific clock KOs have not yet been produced and the potential implication of disturbed muscle clocks for the effects of circadian desynchronization on glucose metabolism remains to be determined.
6. The adipose tissue clock
Although originally considered to be an inert tissue serving as a fat depot, adipose tissue is now widely recognized as an important endocrine organ. The adipokines (cytokines secreted by the adipose tissues) play crucial roles in controlling various physiological events, including glucose and energy metabolism. Adipose tissue has a functional clock and expresses many genes in a circadian manner in a number of species, including humans [105–107]. These clocks are present in both the white adipose tissues (WAT, the predominant form of adipose tissue) and the brown adipose tissues (BAT, the major center for heat production) [108,109]. Several adipokines, such as leptin, adiponectin and visfatin, are also secreted in a circadian manner [110]. Leptin not only regulates satiety, but also stimulates energy expenditure and increases insulin sensitivity. Plasma levels of leptin and adiponectin show opposite patterns in their daily rhythms [111,112]. In rodents the diurnal rhythm in leptin levels is dependent on an intact SCN [113]. Although it is likely that disorders in the circadian rhythmicity of adipokines may promote whole-body insulin resistance, solid evidence is lacking so far. Per2 null mice show a lean phenotype with strong reductions in adipose tissue mass and plasma lipid levels [23]. Although these mice represent a whole-body KO, the phenotype seems to be mainly dependent on a tissue specific effect of Per2 deletion on PPARgamma activity in the white adipose tissue, as these animals show normal food intake and no obvious disturbances in locomotor or SCN activity rhythms. However, the pronounced effects of the Per2 KO on white [23] and brown [114] adipose tissue metabolism do not seem to be responsible for the changes in glucose metabolism, but more so its effects on pancreas and liver [21,22]. On the other hand, adipocyte-specific KO of Bmal1 leads to obesity, which confirms the crucial role of the adipose tissue clock in metabolic homeostasis [115]. The Bmal1 KO animals display increased food consumption during the daytime, suggesting an effect on feeding behavior controlled by the central nervous system. Increased daytime food intake is a behavior that has been linked to an increase in body weight. Thus, disruption of the circadian clock in the adipose tissue can alter the hypothalamic control of feeding behavior and cause obesity; although some caution is necessary since adipocyte protein 2 (aP2) (the promoter used to make an adipocyte-specific deletion of Bmal1) is also expressed in the brain, including the hypothalamus. On the other hand, when measuring clock gene rhythms in human white adipose tissue biopsies no significant effects of increased body weight or type 2 diabetes on rhythmic gene expression were found [106].
Coiled-coil domain containing 80 (Ccdc80) is a secreted protein highly enriched in mouse and human WAT and plays an important role during adipocyte differentiation in vitro. Mice lacking Ccdc80 show increased sensitivity to diet-induced hyperglycemia and glucose intolerance while displaying reduced glucose-stimulated insulin secretion in vivo. Gene expression analysis by microarray revealed that Ccdc80 might play a role in fine-tuning the expression of some circadian clock components in peripheral tissues. In all tissues examined expression of the core clock member Arntl/Bmal1 was reduced whereas that of the oscillating transcription factors Dbp and Tef was increased. Furthermore, knockdown of Ccdc80 in 3T3-L1 cells led to an increase of Dbp mRNA levels during adipocyte differentiation, suggesting that Ccdc80 might be involved in the regulation of this gene in a cell-autonomous manner [116]. However, despite its high expression in WAT and its clear effects on clock gene expression it is still very well possible that the metabolic phenotype of the KO is driven by the absence of Ccdc80 from other tissues and independent from its effect on clock gene expression.
In conclusion, although the changes in feeding behavior also resulted in changes in the glucose rhythm, no profound changes in hepatic glucose production, glucose tolerance or insulin sensitivity were found. Thus whole body Bmal1 KO and liver-specific Bmal1 KO, but not adipocyte-specific Bmal1 KO, causes increased insulin sensitivity [16,97].
7. The pancreatic clock
Insulin and glucagon secretion by the pancreatic islet cells are endocrine signals vital for glucose homeostasis. Plasma glucose concentration displays circadian variation, with the highest levels during the beginning of the active phase. Since feeding induces insulin secretion, plasma insulin levels follow the daily rhythm in food intake and may show a daily rhythm as well. On the other hand, autonomous circadian rhythms have been observed in mouse and human pancreatic islet cells [117–119]. This notion underscores the presence of a circadian control over pancreatic function. Indeed, glucose-stimulated insulin release appears to be dependent on a functional clock. Islets from Clock mutant mice or Bmal1−/− mice display drastic reduction in glucose-induced insulin secretion. In addition, Clock mutants, as well as Bmal1 mutants, show impaired glucose tolerance, reduced insulin secretion and defects in size and proliferation of pancreatic islets, symptoms which worsen with age [17]. It was shown that Clock disruption alters expression of islet genes involved in growth, survival and synaptic vesicle assembly. Therefore, additional studies sought to understand the specific role of a pancreatic clock in vivo. To do so, three research groups went on to target Bmal1 specifically in the pancreas [17,120] or in the beta-cells [121] and indeed showed that conditional ablation of the pancreatic clock causes diabetes mellitus type 2 due to defective β-cell function. These mice display elevated glucose levels, impaired glucose tolerance and decreased insulin secretion. Interestingly, the insulin content in the islets from the KO mice was similar to that of the islets from the wild type mice, suggesting that insulin secretion, but not synthesis, was defective in the mutant animals. In addition, it was shown that also REV-ERBα down-regulation by moderately interfering RNA treatment in islet cells and MIN-6 cells impaired glucose-induced insulin secretion [122]. In contrast to the hypoinsulinemic phenotype observed when knocking down Bmal or Clock, down-regulation of Per or Cry expression results in the opposite phenotype. Per2 KOs show an enhanced glucose-stimulated insulin secretion and reduced insulin clearance [22] and Cry double KO mice are hyperinsulinemic [28].
In conclusion, the major effect of disturbed clock mechanisms in the endocrine beta-cells seems to be an impaired insulin release, which, of course, ultimately will result in hyperglycemia [123,124]. On the other hand, recently it was shown that disturbing the clock in the glucagon producing alpha-cells resulted in a decreased glucagon release [125]. Thus in a desynchronized pancreas the hyperglycemic effect of reduced insulin release might be partly compensated for by the reduced release of glucagon.
8. Clock genes in humans
Interestingly, genetic variation of circadian genes in humans is correlated with glucose homeostasis, in line with the aforementioned animal experimental data. A study of polymorphisms in the Clock and Bmal1 gene in humans revealed that the molecular clock may play a role in the susceptibility for obesity and type 2 diabetes, whereas polymorphisms in the Npas2 and Per2 gene have been linked to high fasting glucose levels [126–129]. Moreover, a genomic-association study in non-diabetic participants indicated that CRY2 gene variants are associated with glucose levels [130,131]. Furthermore, carriers of specific CLOCK SNPs display lower glucose levels and improved insulin sensitivity when on a particular diet [132,133]. On the other hand, Rev-erbα gene polymorphisms seem to modulate adiposity, but not plasma glucose or insulin levels [134]. Interestingly, also mutations in the melatonin receptor, highly expressed in the SCN, but also in many peripheral tissues, have been linked to an increased risk of type 2 diabetes [135–137].
However, the strongest evidence linking circadian disruption and type 2 diabetes, comes from epidemiological studies consistently showing an increased risk for type 2 diabetes in shift workers [138–140]. These findings are supported by additional epidemiological studies showing that also individuals with deficient or disrupted sleep exhibit an increased risk for type 2 diabetes [141–143]. Moreover, a number of laboratory studies examining the impact of circadian and/or sleep disruption on glucose homeostasis under controlled conditions, support the above mentioned epidemiological findings [144–147].
9. Conclusion
Endocrine rhythms play a key role in almost every aspect of our life. Disruption of the circadian clock, because of shift-work or bad sleeping habits, can cause severe disturbances in these rhythms [146]. The resulting hormonal dysfunctions may lead to metabolic diseases such as diabetes and obesity, partly via effects on glucose homeostasis. The observations in whole body circadian gene KO animals were key in raising this awareness, but were unable to distinguish the contribution of separate tissues. Therefore, studies in tissue-specific KO models for the different clock genes were urgently needed to elucidate further the diverse roles of the circadian clock in whole body glucose metabolism. The first evidence until now supports a role for pancreatic clock genes in the development of type 2 diabetes via their role in insulin release, and for liver clock genes in the control of glucose tolerance through their effect on hepatic gluconeogenesis. Adipocyte clock genes, on the other hand, do not seem to affect glucose homeostasis directly, but may have a more indirect effect by affecting the appetite regulatory centers in the hypothalamus, probably via changes in the daily rhythms of adipokine release or plasma free fatty acid concentrations. For the muscle clock genes no clear effects on glucose metabolism have been shown so far.
These first results from the tissue-specific KO animals indicate that the complete picture may be even more complex than initially thought, as in the whole body KO models some effects of the loss of clock gene function may have been masked by opposing effects in different tissues. For instance, the hyperglycemia observed in the whole body KO animals may be due primarily to a reduced insulin release and not so much to insulin resistance. In fact, many KO animals are insulin sensitive at a young age and the insulin resistance is probably secondary to the increased obesity instead of a direct effect of clock genes on insulin sensitivity.
Moreover, shift-work is generally seen as a mismatch between the light-sensitive central pacemaker in the SCN and the energy-sensitive peripheral oscillators, but also here things may be more complex than initially thought. The major part of the metabolic work in chronobiology has focussed on the liver and, indeed, unlike the SCN many rhythms in the liver show a rapid phase-shift when the timing of food availability is shifted. However, not all organs and tissues adapt to a new feeding time with the same speed and magnitude as the liver [148–150]. Clearly a differential shift of metabolically important organs such as liver and muscle might also have profound effects on glucose metabolism [151].
In conclusion, a better insight in the tissue specific effects of clock gene disturbances is of utmost importance, but in the end only integrative physiological studies in the whole animal/human will be able to provide a complete understanding of the desynchronizing effects of our today's society on glucose and energy metabolism.
Note added in proof
After submission of our manuscript we became aware of a recent study in which glucose metabolism was studied in a muscle-specific Bmal1 KO [153]. These animals showed reduced protein levels of GLUT4 and an impaired insulin-stimulated glucose uptake in their muscles.
Acknowledgements
Parts of the research presented were financially supported by NWO-ZonMw (TOP91207036).
Conflict of interest
None declared.
References
- 1.Peek C.B., Ramsey K.M., Marcheva B., Bass J. Nutrient sensing and the circadian clock. Trends in Endocrinology and Metabolism. 2012;23:312–318. doi: 10.1016/j.tem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephan F.K., Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reppert S.M., Weaver D.R. Molecular analysis of mammalian circadian rhythms. Annual Review of Physiology. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 4.Lucas R.J., Lall G.S., Allen A.E., Brown T.M. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Progress in Brain Research. 2012;199:1–18. doi: 10.1016/B978-0-444-59427-3.00001-0. [DOI] [PubMed] [Google Scholar]
- 5.Balsalobre A., Damiola F., Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 6.Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes & Development. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara R., Wan K.K., Wakamatsu H., Aida R., Moriya T., Akiyama M. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes to Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 8.Kornmann B., Schaad O., Bujard H., Takahashi J.S., Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biology. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peret J., Macaire I., Chanez M. Schedule of protein ingestion, nitrogen and energy utilization and circadian rhythm of hepatic glycogen, plasma corticosterone and insulin in rats. Journal of Nutrition. 1973;103:866–874. doi: 10.1093/jn/103.6.866. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong S. A chronometric approach to the study of feeding behavior. Neuroscience and Biobehavioural Reviews. 1980;4:27–53. doi: 10.1016/0149-7634(80)90024-x. [DOI] [PubMed] [Google Scholar]
- 11.Jarrett R.J., Baker I.A., Keen H., Oakley N.W. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon and evening. British Medical Journal. 1972;1:199–201. doi: 10.1136/bmj.1.5794.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turek F.W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi K., Atsumi G., Sugiyama S., Kodomari I., Kasamatsu M., Machida K. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Letters. 2006;580:127–130. doi: 10.1016/j.febslet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 14.Kudo T., Tamagawa T., Kawashima M., Mito N., Shibata S. Attenuating effect of clock mutation on triglyceride contents in the ICR mouse liver under a high-fat diet. Journal of Biological Rhythms. 2007;22:312–323. doi: 10.1177/0748730407302625. [DOI] [PubMed] [Google Scholar]
- 15.Kennaway D.J., Owens J.A., Voultsios A., Boden M.J., Varcoe T.J. Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. American Journal of Physiology. 2007;293:R1528–R1537. doi: 10.1152/ajpregu.00018.2007. [DOI] [PubMed] [Google Scholar]
- 16.Rudic R.D., McNamara P., Curtis A.M., Boston R., Panda S., Hogenesch J.B. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biology. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcheva B., Ramsey K.M., Buhr E.D., Kobayashi Y., Su H., Ko C.H. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennaway D.J., Varcoe T.J., Voultsios A., Boden M.J. Global loss of bmal1 expression alters adipose tissue hormones, gene expression and glucose metabolism. PLoS One. 2013;8:e65255. doi: 10.1371/journal.pone.0065255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doi R., Oishi K., Ishida N. CLOCK regulates circadian rhythms of hepatic glycogen synthesis through transcriptional activation of Gys2. Journal of Biological Chemistry. 2010;285:22114–22121. doi: 10.1074/jbc.M110.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmutz I., Ripperger J.A., Baeriswyl-Aebischer S., Albrecht U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes & Development. 2010;24:345–357. doi: 10.1101/gad.564110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zani F., Breasson L., Becattini B., Vukolic A., Montani J.P., Albrecht U. PER2 promotes glucose storage to liver glycogen during feeding and acute fasting by inducing Gys2 PTG and G L expression. Molecular Metabolism. 2013;2:292–305. doi: 10.1016/j.molmet.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y., Zhang Y., Zhou M., Wang S., Hua Z., Zhang J. Loss of mPer2 increases plasma insulin levels by enhanced glucose-stimulated insulin secretion and impaired insulin clearance in mice. FEBS Letters. 2012;586:1306–1311. doi: 10.1016/j.febslet.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Grimaldi B., Bellet M.M., Katada S., Astarita G., Hirayama J., Amin R.H. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metabolism. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So A.Y., Bernal T.U., Pillsbury M.L., Yamamoto K.R., Feldman B.J. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S., Liu A., Weidenhammer A., Cooksey R.C., McClain D., Kim M.K. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamia K.A., Papp S.J., Yu R.T., Barish G.D., Uhlenhaut N.H., Jonker J.W. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin L., Wu N., Curtin J.C., Qatanani M., Szwergold N.R., Reid R.A. Rev-erba, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 28.Barclay J.L., Shostak A., Leliavski A., Tsang A.H., Jöhren O., Müller-Fielitz H. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. American Journal of Physiology – Endocrinology and Metabolism. 2013;304:E1053–E1063. doi: 10.1152/ajpendo.00512.2012. [DOI] [PubMed] [Google Scholar]
- 29.Delezie J., Dumont S., Dardente H., Oudart H., Gréchez-Cassiau A., Klosen P. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB Journal. 2012;26:3321–3335. doi: 10.1096/fj.12-208751. [DOI] [PubMed] [Google Scholar]
- 30.Baeza-Raja B., Eckel-Mahan K., Zhang L., Vagena E., Tsigelny I.F., Sassone-Corsi P. p75 neurotrophin receptor is a clock gene that regulates oscillatory components of circadian and metabolic networks. Journal of Neuroscience. 2013;33:10221–10234. doi: 10.1523/JNEUROSCI.2757-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baeza-Raja B., Li P., Le Moan N., Sachs B.D., Schachtrup C., Davalos D. p75 neurotrophin receptor regulates glucose homeostasis and insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5838–5843. doi: 10.1073/pnas.1103638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philosophical Transactions of the Royal Society of London Series B Biological Sciences. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffield G.E., Watson N.P., Mantani A., Peirson S.N., Robles-Murguia M., Loros J.J. A role for Id2 in regulating photic entrainment of the mammalian circadian system. Current Biology. 2009;19:297–304. doi: 10.1016/j.cub.2008.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew D., Zhou P., Pywell C.M., Van Der Veen D.R., Shao J., Xi Y. Ablation of the ID2 gene results in altered circadian feeding behavior, and sex-specific enhancement of insulin sensitivity and elevated glucose uptake in skeletal muscle and brown adipose tissue. PLoS One. 2013;8:e73064. doi: 10.1371/journal.pone.0073064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohsaka A., Laposky A.D., Ramsey K.M., Estrada C., Joshu C., Kobayashi Y. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metabolism. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Barnea M., Madar Z., Froy O. High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology. 2009;150:161–168. doi: 10.1210/en.2008-0944. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh M.C., Yang S.C., Tseng H.L., Hwang L.L., Chen C.T., Shieh K.R. Abnormal expressions of circadian-clock and circadian clock-controlled genes in the livers and kidneys of long-term, high-fat-diet-treated mice. International Journal of Obesity (London) 2010;34:227–239. doi: 10.1038/ijo.2009.228. [DOI] [PubMed] [Google Scholar]
- 38.Mendoza J., Pevet P., Challet E. High-fat feeding alters the clock synchronization to light. Journal of Physiology. 2008;586:5901–5910. doi: 10.1113/jphysiol.2008.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pendergast J.S., Branecky K.L., Yang W., Ellacott K.L., Niswender K.D., Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. European Journal of Neuroscience. 2013;37:1350–1356. doi: 10.1111/ejn.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coomans C.P., van den Berg S.A., Houben T., Van Klinken J.B., Van Den Berg R., Pronk A.C. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB Journal. 2013;27:1721–1732. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 41.Um J.H., Yang S., Yamazaki S., Kang H., Viollet B., Foretz M. Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. Journal of Biological Chemistry. 2007;282:20794–20798. doi: 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- 42.Lamia K.A., Sachdeva U.M., DiTacchio L., Williams E.C., Alvarez J.G., Egan D.F. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Neill J.S., Maywood E.S., Chesham J.E., Takahashi J.S., Hastings M.H. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merrow M., Roenneberg T. Circadian clocks: running on redox. Cell. 2001;106:141–143. doi: 10.1016/s0092-8674(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 45.Rutter J., Reick M., Wu L.C., McKnight S.L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 46.Asher G., Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metabolism. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Asher G., Gatfield D., Stratmann M., Reinke H., Dibner C., Kreppel F. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 48.Asher G., Reinke H., Altmeyer M., Gutierrez-Arcelus M., Hottiger M.O., Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142:943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Coomans C.P., van den Berg S.A., Lucassen E.A., Houben T., Pronk A.C., van der Spek R.D. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62:1102–1108. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karnani M., Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2011;300:R47–R55. doi: 10.1152/ajpregu.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moulle V.S., Picard A., Le Foll C., Levin B.E., Magnan C. Lipid sensing in the brain and regulation of energy balance. Diabetes & Metabolism. 2014;40:29–33. doi: 10.1016/j.diabet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Cone R.D., Cowley M.A., Butler A.A., Fan W., Marks D.L., Low M.J. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. International Journal of Obesity and Related Metabolic Disorders. 2001;25(Suppl. 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto H., Nagai K., Nakagawa H. Role of the SCN in daily rhythms of plasma glucose, FFA, insulin and glucagon. Chronobiology International. 1987;4:483–491. doi: 10.3109/07420528709078539. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto H., Nagai K., Nakagawa H. Bilateral lesions of the SCN abolish lipolytic and hyperphagic responses to 2DG. Physiology and Behaviour. 1984;32:1017–1020. doi: 10.1016/0031-9384(84)90295-6. [DOI] [PubMed] [Google Scholar]
- 55.Van Den Pol A., Powley T. A fine-grained anatomical analysis of the role of the rat suprachiasmatic nucleus in circadian rhythms of feeding and drinking. Brain Research. 1979;160:307–326. doi: 10.1016/0006-8993(79)90427-x. [DOI] [PubMed] [Google Scholar]
- 56.Escobar C., Diaz-Munoz M., Encinas F., Aguilar-Roblero R. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. American Journal of Physiology. 1998;274:R1309–R1316. doi: 10.1152/ajpregu.1998.274.5.R1309. [DOI] [PubMed] [Google Scholar]
- 57.Kalsbeek A., Strubbe J.H. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiology and Behaviour. 1998;63:553–560. doi: 10.1016/s0031-9384(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 58.La Fleur S.E., Kalsbeek A., Wortel J., Buijs R.M. An SCN generated rhythm in basal glucose levels. Journal of Neuroendocrinology. 1999;11:643–652. doi: 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 59.Ruiter M., La Fleur S.E., Van Heijningen C., Van Der Vliet J., Kalsbeek A., Buijs R.M. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52:1709–1715. doi: 10.2337/diabetes.52.7.1709. [DOI] [PubMed] [Google Scholar]
- 60.La Fleur S.E., Kalsbeek A., Wortel J., Fekkes M.L., Buijs R.M. A daily rhythm in glucose tolerance. A role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 61.Bolli G.B., Gerich J.E. The “Dawn-Phenomenon” – a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. New England Journal of Medicine. 1984;310:746–750. doi: 10.1056/NEJM198403223101203. [DOI] [PubMed] [Google Scholar]
- 62.La Fleur S.E., Kalsbeek A., Wortel J., Buijs R.M. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Research. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- 63.Kalsbeek A., La Fleur S.E., Van Heijningen C., Buijs R.M. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. Journal of Neuroscience. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujii T., Inoue S., Nagai K., Nakagawa H. Involvement of adrenergic mechanism in hyperglycemia due to SCN stimulation. Hormone and Metabolic Research. 1989;21:643–645. doi: 10.1055/s-2007-1009309. [DOI] [PubMed] [Google Scholar]
- 65.Nagai K., Fujii T., Inoue S., Takamura Y., Nakagawa H. Electrical stimulation of the suprachiasmatic nucleus of the hypothalamus causes hyperglycemia. Hormone and Metabolic Research. 1988;20:37–39. doi: 10.1055/s-2007-1010743. [DOI] [PubMed] [Google Scholar]
- 66.Cailotto C., La Fleur S.E., Van Heijningen C., Wortel J., Kalsbeek A., Feestra M. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? European Journal of Neuroscience. 2005;22:2531–2540. doi: 10.1111/j.1460-9568.2005.04439.x. [DOI] [PubMed] [Google Scholar]
- 67.Buijs R.M., Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nature Neuroscience Reviews. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 68.Buijs R.M. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell and Tissue Research. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- 69.Swanson L.W., McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. Journal of Comparative Neurology. 1979;188:87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- 70.Luiten P.G.M., Ter Horst G.J., Steffens A.B. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Progress in Neurobiology. 1987;28:1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 71.Jansen A.S.P., Van Nguyen X., Karpitskiy V., Mettenleiter T.C., Loewy A.D. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 72.Kalsbeek A., Foppen E., Schalij I., Van Heijningen C., Van Der Vliet J., Fliers E. Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS One. 2008;3:e3194. doi: 10.1371/journal.pone.0003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abrahamson E.E., Leak R.K., Moore R.Y. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- 74.Deurveilher S., Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 75.Yi C.X., Serlie M.J., Ackermans M.T., Foppen E., Buijs R.M., Sauerwein H.P. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes. 2009;58:1998–2005. doi: 10.2337/db09-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S., Zeitzer J.M., Yoshida Y., Wisor J.P., Nishino S., Edgar D.M. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep. 2004;27:619–627. doi: 10.1093/sleep/27.4.619. [DOI] [PubMed] [Google Scholar]
- 77.Zeitzer J.M., Buckmaster C.L., Parker K.J., Hauck C.M., Lyons D.M., Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: Implications for the consolidation of wakefulness. Journal of Neuroscience. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alam M.N., Kumar S., Bashir T., Suntsova N., Methippara M.M., Szymusiak R. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. Journal of Physiology. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuwaki T., Zhang W. Orexin neurons as arousal-associated modulators of central cardiorespiratory regulation. Respiratory Physiology & Neurobiology. 2010;174:43–54. doi: 10.1016/j.resp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 80.Shiuchi T., Haque M.S., Okamoto S., Inoue T., Kageyama H., Lee S. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metabolism. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 81.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metabolism Reviews. 1987;3:185–206. doi: 10.1002/dmr.5610030109. [DOI] [PubMed] [Google Scholar]
- 82.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 83.Puschel G.P. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anatomical Record. 2004;280A:854. doi: 10.1002/ar.a.20091. [DOI] [PubMed] [Google Scholar]
- 84.Akhtar R.A., Reddy A.B., Maywood E.S., Clayton J.D., King V.M., Smith A.G. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Current Biology. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 85.Oishi K., Miyazaki K., Kadota K., Kikuno R., Nagase T., Atsumi G. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. Journal of Biological Chemistry. 2003;278:41519–41527. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- 86.Panda S., Antoch M.P., Miller B.H., Su A.I., Schook A.B., Straume M. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 87.Kuriyama K., Sasahara K., Kudo T., Shibata S. Daily injection of insulin attenuated impairment of liver circadian clock oscillation in the streptozotocin-treated diabetic mouse. FEBS Letters. 2004;572:206–210. doi: 10.1016/j.febslet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 88.Eckel-Mahan K.L., Patel V.R., de Mateo S., Orozco-Solis R., Ceglia N.J., Sahar S. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirota T., Lewis W.G., Liu A.C., Lee J.W., Schultz P.G., Kay S.A. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20746–20751. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oishi K., Kasamatsu M., Ishida N. Gene- and tissue-specific alterations of circadian clock gene expression in streptozotocin-induced diabetic mice under restricted feeding. Biochemical and Biophysical Research Communications. 2004;317:330–334. doi: 10.1016/j.bbrc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 91.Oishi K., Amagai N., Shirai H., Kadota K., Ohkura N., Ishida N. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Research. 2005;12:191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- 92.Balsalobre A., Brown S.A., Marcacci L., Tronche F., Kellendonk C., Reichardt H.M. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 93.Lin H.V., Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metabolism. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Raalte D.H., Ouwens D.M., Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? European Journal of Clinical Investigation. 2009;39:81–93. doi: 10.1111/j.1365-2362.2008.02067.x. [DOI] [PubMed] [Google Scholar]
- 95.LeMinh N., Damiola F., Tronche F., Schutz G., Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO Journal. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cailotto C., van Heijningen C., van der Vliet J., van der Plasse G., Habold C., Kalsbeek A. Daily rhythms in metabolic liver enzymes and plasma glucose require a balance in the autonomic output to the liver. Endocrinology. 2008;149:1914–1925. doi: 10.1210/en.2007-0816. [DOI] [PubMed] [Google Scholar]
- 97.Lamia K.A., Storch K.F., Weitz C.J. Physiological significance of a peripheral tissue circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang E.E., Liu Y., Dentin R., Pongsawakul P.Y., Liu A.C., Hirota T. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nature Medicine. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller B.H., McDearmon E.L., Panda S., Hayes K.R., Zhang J., Andrews J.L. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCarthy J.J., Andrews J.L., McDearmon E.L., Campbell K.S., Barber B.K., Miller B.H. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiological Genomics. 2007;31:86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pastore S., Hood D.A. 2013 Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. Journal of Applied Physiology. 1985;114:1076–1084. doi: 10.1152/japplphysiol.01505.2012. [DOI] [PubMed] [Google Scholar]
- 102.Woldt E., Sebti Y., Solt L.A., Duhem C., Lancel S., Eeckhoute J. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nature Medicine. 2013;19:1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bray M.S., Shaw C.A., Moore M.W., Garcia R.A., Zanquetta M.M., Durgan D.J. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. American Journal of Physiology – Heart and Circulatory Physiology. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 104.Durgan D.J., Pat B.M., Laczy B., Bradley J.A., Tsai J.Y., Grenett M.H. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. Journal of Biological Chemistry. 2011;286:44606–44619. doi: 10.1074/jbc.M111.278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shostak A., Husse J., Oster H. Circadian regulation of adipose function. Adipocyte. 2013;2:201–206. doi: 10.4161/adip.26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Otway D.T., Mantele S., Bretschneider S., Wright J., Trayhurn P., Skene D.J. Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes. 2011;60:1577–1581. doi: 10.2337/db10-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gomez-Santos C., Gomez-Abellan P., Madrid J.A., Hernandez-Morante J.J., Lujan J.A., Ordovas J.M. Circadian rhythm of clock genes in human adipose explants. Obesity (Silver Spring) 2009;17:1481–1485. doi: 10.1038/oby.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zvonic S., Ptitsyn A.A., Conrad S.A., Scott L.K., Floyd Z.E., Kilroy G. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 109.van der Veen D.R., Shao J., Chapman S., Leevy W.M., Duffield G.E. A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity (Silver Spring) 2012;20:1527–1529. doi: 10.1038/oby.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Spek R., Kreier F., Fliers E., Kalsbeek A. Circadian rhythms in white adipose tissue. Progress in Brain Research. 2012;199:183–201. doi: 10.1016/B978-0-444-59427-3.00011-3. [DOI] [PubMed] [Google Scholar]
- 111.Gavrila A., Peng C.K., Chan J.L., Mietus J.E., Goldberger A.L., Mantzoros C.S. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. Journal of Clinical Endocrinology & Metabolism. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 112.Scheer F.A., Chan J.L., Fargnoli J., Chamberland J., Arampatzi K., Shea S.A. Day/night variations of high-molecular-weight adiponectin and lipocalin-2 in healthy men studied under fed and fasted conditions. Diabetologia. 2010;53:2401–2405. doi: 10.1007/s00125-010-1869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalsbeek A., Fliers E., Romijn J.A., La Fleur S.E., Wortel J., Bakker O. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- 114.Chappuis S., Ripperger J.A., Schnell A., Rando G., Jud C., Wahli W. Role of the circadian clock gene Per2 in adaptation to cold temperature. Molecular Metabolism. 2013;2:184–193. doi: 10.1016/j.molmet.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paschos G.K., Ibrahim S., Song W.L., Kunieda T., Grant G., Reyes T.M. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nature Medicine. 2012;18:1768–1777. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tremblay F., Huard C., Dow J., Gareski T., Will S., Richard A.M. Loss of coiled-coil domain containing 80 negatively modulates glucose homeostasis in diet-induced obese mice. Endocrinology. 2012;153:4290–4303. doi: 10.1210/en.2012-1242. [DOI] [PubMed] [Google Scholar]
- 117.Peschke E., Peschke D. Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia. 1998;41:1085–1092. doi: 10.1007/s001250051034. [DOI] [PubMed] [Google Scholar]
- 118.Delattre E., Cipolla-Neto J., Boschero A.C. Diurnal variations in insulin secretion and K+ permeability in isolated rat islets. Clinical and Experimental Pharmacology and Physiology. 1999;26:505–510. doi: 10.1046/j.1440-1681.1999.03073.x. [DOI] [PubMed] [Google Scholar]
- 119.Allaman-Pillet N., Roduit R., Oberson A., Abdelli S., Ruiz J., Beckmann J.S. Circadian regulation of islet genes involved in insulin production and secretion. Molecular and Cellular Endocrinology. 2004;226:59–66. doi: 10.1016/j.mce.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 120.Sadacca L.A., Lamia K.A., deLemos A.S., Blum B., Weitz C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee J., Moulik M., Fang Z., Saha P., Zou F., Xu Y. Bmal1 and beta-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced beta-cell failure in mice. Molecular and Cellular Biology. 2013;33:2327–2338. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vieira E., Marroqui L., Batista T.M., Caballero-Garrido E., Carneiro E.M., Boschero A.C. The clock gene Rev-erbalpha regulates pancreatic beta-cell function: modulation by leptin and high-fat diet. Endocrinology. 2012;153:592–601. doi: 10.1210/en.2011-1595. [DOI] [PubMed] [Google Scholar]
- 123.Marcheva B., Ramsey K.M., Bass J. Circadian genes and insulin exocytosis. Cellular Logistics. 2011;1:32–36. doi: 10.4161/cl.1.1.14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lamia K.A., Evans R.M. Metabolism: tick, tock, a beta-cell clock. Nature. 2010;466(7306):571–572. doi: 10.1038/466571a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vieira E., Marroquí L., Figueroa A.L., Merino B., Fernandez-Ruiz R., Nadal A. Involvement of the clock gene Rev-erb alpha in the regulation of glucagon secretion in pancreatic alpha-cells. PLoS One. 2013;8(7):e69939. doi: 10.1371/journal.pone.0069939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scott E.M., Carter A.M., Grant P.J. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. International Journal of Obesity (London) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 127.Sookoian S., Gemma C., Gianotti T.F., Burgueno A., Castano G., Pirola C.J. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. American Journal of Clinical Nutrition. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 128.Englund A., Kovanen L., Saarikoski S.T., Haukka J., Reunanen A., Aromaa A. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. Journal of Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Woon P.Y., Kaisaki P.J., Braganca J., Bihoreau M.T., Levy J.C., Farrall M. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dupuis J., Langenberg C., Prokopenko I., Saxena R., Soranzo N., Jackson A.U. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nature Genetics. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu C., Li H., Qi L., Loos R.J., Qi Q., Lu L. Variants in GLIS3 and CRY2 are associated with type 2 diabetes and impaired fasting glucose in Chinese Hans. PLoS One. 2011;6:e21464. doi: 10.1371/journal.pone.0021464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garaulet M., Lee Y.C., Shen J., Parnell L.D., Arnett D.K., Tsai M.K. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. American Journal of Clinical Nutrition. 2009;90:1466–1475. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garcia-Rios A., Gomez-Delgado F.J., Garaulet M., Alcala-Diaz J.F., Delgado-Lista F.J., Marin C. Beneficial effect of CLOCK gene polymorphism rs1801260 in combination with low-fat diet on insulin metabolism in the patients with metabolic syndrome. Chronobiology International. 2014;31:401–408. doi: 10.3109/07420528.2013.864300. [DOI] [PubMed] [Google Scholar]
- 134.Goumidi L., Grechez A., Dumont J., Cottel D., Kafatos A., Moreno L.A. Impact of REV-ERB alpha gene polymorphisms on obesity phenotypes in adult and adolescent samples. International Journal of Obesity (London) 2013;37:666–672. doi: 10.1038/ijo.2012.117. [DOI] [PubMed] [Google Scholar]
- 135.Prokopenko I., Langenberg C., Florez J.C., Saxena R., Soranzo N., Thorleifsson G. Variants in MTNR1B influence fasting glucose levels. Nature Genetics. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lyssenko V., Nagorny C.L., Erdos M.R., Wierup N., Jonsson A., Spegel P. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nature Genetics. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bouatia-Naji N., Bonnefond A., Cavalcanti-Proenca C., Sparso T., Holmkvist J., Marchand M. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nature Genetics. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 138.Kroenke C.H., Spiegelman D., Manson J., Schernhammer E.S., Colditz G.A., Kawachi I. Work characteristics and incidence of type 2 diabetes in women. American Journal of Epidemiology. 2007;165:175–183. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 139.Suwazono Y., Dochi M., Oishi M., Tanaka K., Kobayashi E., Sakata K. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiology International. 2009;26:926–941. doi: 10.1080/07420520903044422. [DOI] [PubMed] [Google Scholar]
- 140.Pan A., Schernhammer E.S., Sun Q., Hu F.B. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Medicine. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meisinger C., Heier M., Loewel H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 142.Gangwisch J.E., Heymsfield S.B., Boden-Albala B., Buijs R.M., Kreier F., Pickering T.G. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Beihl D.A., Liese A.D., Haffner S.M. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Annals of Epidemiology. 2009;19:351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 144.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 145.Buxton O.M., Pavlova M., Reid E.W., Wang W., Simonson D.C., Adler G.K. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Donga E., van Dijk M., van Dijk J.G., Biermasz N.R., Lammers G.J., Van Kralingen K.W. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. Journal of Clinical Endocrinology & Metabolism. 2010;95:2963–2968. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 148.Davidson A.J., Castanon-Cervantes O., Leise T.L., Molyneux P.C., Harrington M.E. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. European Journal of Neuroscience. 2009;29:171–180. doi: 10.1111/j.1460-9568.2008.06534.x. [DOI] [PubMed] [Google Scholar]
- 149.Salgado-Delgado R., Angeles-Castellanos M., Buijs M.R., Escobar C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience. 2008;154:922–931. doi: 10.1016/j.neuroscience.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 150.Stokkan K.A., Yamazaki S., Tei H., Sakaki Y., Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 151.Reznick J., Preston E., Wilks D.L., Beale S.M., Turner N., Cooney G.J. Altered feeding differentially regulates circadian rhythms and energy metabolism in liver and muscle of rats. Biochimica et Biophysica Acta. 2013;1832:228–238. doi: 10.1016/j.bbadis.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 152.Kalsbeek A., Yi C.-X., La Fleur S.E., Fliers E. The hypothalamic clock and its control of glucose homeostasis. Trends in Endocrinology and Metabolism. 2010;21:402–410. doi: 10.1016/j.tem.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 153.Dyar K.A., Ciciliot S., Wright L.E., Biensø R.S., Tagliazucchi G.M., Patel V.R. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Molecular Metabolism. 2014;3:29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cho H., Zhao X., Hatori M., Yu R.T., Barish G.D., Lam M.T. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]


