Spontaneous coronary artery dissection (SCAD) is an uncommon and poorly understood cause of acute coronary syndrome (ACS), myocardial infarction, and sudden cardiac death.1 Spontaneous coronary artery dissection is a sudden separation between the layers of a coronary artery wall that creates an intimal flap or intramural hematoma, obstructing blood flow (Fig. 1A) The true prevalence, causes, prognosis, recurrence rate, and optimal management of SCAD remain uncertain, but recent increases in the use of social media, in patient engagement, and in the formation of disease-specific online communities have facilitated case finding and have accelerated the progress of SCAD research.2 Advances in imaging techniques and better recognition of acute SCAD have led to several new insights and have generated interest in this underrecognized and understudied condition.
Fig. 1.
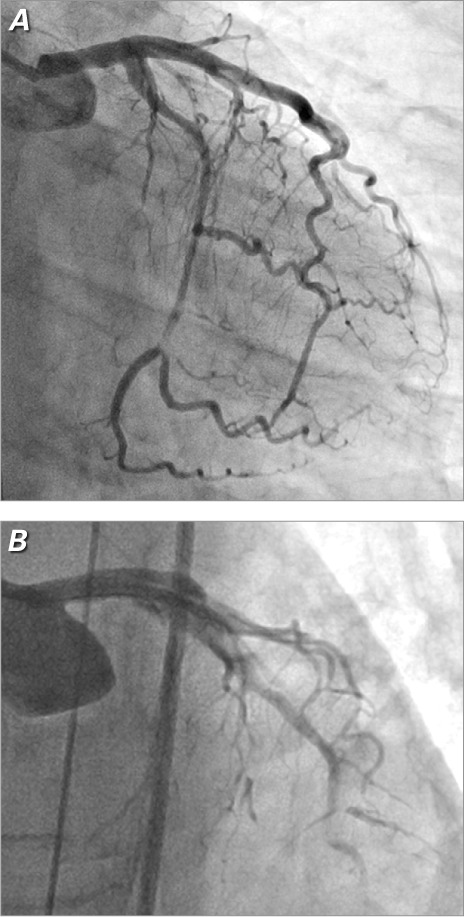
Coronary angiogram shows A) spontaneous dissection of the left circumflex coronary artery in a 40-year-old woman who presented with non-ST-elevation myocardial infarction. During an attempt at percutaneous intervention in the same patient, we saw B) extension of the left circumflex dissection into the left main and left anterior descending coronary arteries.
The demographics, causes, and natural history of ACS associated with SCAD are distinct from those of ACS caused by atherosclerosis or plaque rupture. Spontaneous coronary artery dissection patients are typically young women who do not have risk factors for atherosclerosis. The average age of incident SCAD is 42 years, with reported cases from the age of 14 years to well into the 7th decade. Approximately 80% of SCAD patients are female and, of those, 20% to 25% of cases occur in the peripartum period.
In recent accounts of series that have used careful angiographic evaluation or advanced intracoronary imaging to detect intramural hematoma and more subtle dissections, the true prevalence of SCAD (a phenomenon once believed rare) has been reported to be as high as 1% to 4% of ACS overall.3 Moreover, SCAD has been found to be a factor in up to 40% of heart attacks in women under the age of 50 years.4 About half of SCADs initially present as ST-segment-elevation myocardial infarction, and one quarter present with multivessel involvement.1
Diagnosis and Management
The diagnosis of SCAD requires careful angiographic study and a high degree of suspicion. Accurate differentiation of ACS due to SCAD from ACS due to atherosclerosis is crucial, because the approaches to both acute and long-term management are different. Intravascular ultrasonography and optical coherence tomography can be invaluable adjuncts to inadequate or nondiagnostic angiographic imaging. Advanced imaging in the acute setting should be strongly considered in individuals who do not have standard coronary heart disease risk factors, in young or postpartum women, or in patients who show an absence of plaque in noninfarct-related coronary arteries.
The most important reasons for accurately diagnosing SCAD are that patients undergoing percutaneous coronary intervention (PCI) for acute SCAD have technical success rates that are markedly reduced (Fig. 1B) compared with PCI success rates for atherosclerotic ACS (62% vs 92%)1,5; and the substantial rate of spontaneous vascular healing1,6 suggests a role for conservative management in stable SCAD patients who have preserved coronary flow.5 Although conservative management has generally been associated with favorable outcomes,6 this approach is associated with a small early hazard of dissection progression and the consequent need for intervention. Therefore, our practice is to provide, for as long as 4 to 5 days, careful inpatient monitoring of those treated conservatively for a SCAD event. Because the coronary arteries of patients who have experienced SCAD can be more friable and prone to new or worsening dissection, repeat coronary angiography risks iatrogenic injury. We strongly advise against performing coronary angiography solely for monitoring purposes, without a strong clinical indication. Functional testing or coronary computed tomographic angiography can be helpful in the initial evaluation of chest pain or other symptoms in SCAD patients.
Although long-term survival after SCAD is improved in comparison with survival after atherosclerotic ACS, 10-year recurrence rates of up to 20%, predominantly in women,1 underscore the need for close and long-term follow-up, and for more research. Unlike effective secondary prevention measures for atherosclerotic ACS, no such measures have been identified for SCAD. In a retrospective case series, statins were associated with recurrent SCAD.1 Medication recommendations after SCAD should target a patient's non-SCAD conditions and indications (for example, angiotensin-converting enzyme inhibitors for left ventricular dysfunction, or dual antiplatelet agents after stent placement), rather than SCAD itself. Although evidence of benefit is lacking, we recommend the administration of low-dose aspirin and the prescription of statins only when hyperlipidemia is present (goal: low-density-lipoprotein cholesterol level, <100 mg/dL). Nitrate-responsive chest pain is common after SCAD. Functional testing should be considered in order to exclude demand-induced ischemia due to coronary artery or stent obstruction. If that testing is negative, a trial of long-acting nitrates is often effective for potential coronary vasospasm and is less likely to cause hypotension than are calcium channel blockers.
Cardiac rehabilitation should be recommended to all SCAD patients. Many SCAD patients are not initially referred for cardiac rehabilitation, perhaps because of such factors as female gender, youth, overall good health, or fear of recurrent SCAD. This lack of rehabilitation has the effect of delaying patients' return to normal activity and mental health. Cardiac rehabilitation is particularly important in SCAD patients, who often have substantial anxiety, depression, and ongoing physical symptoms.
The association of recent extreme physical activity with SCAD has led some healthcare providers to prohibit even minimal physical exertion, such as lifting more than 5 pounds. This approach has no evidence to support it and, if applied over a lifetime, has the potential to substantially increase the risk of such health conditions as osteoporosis, injuries from falls, obesity, and atherosclerosis. Until there is evidence that moderate exercise is harmful, it seems prudent to provide activity guidelines that include 30 to 40 minutes of moderate aerobic activity daily. We advise the avoidance of weightlifting and bodybuilding but generally encourage resistance training, with light weights and high repetitions. We advise against competitive racing or athletic pursuits at high levels. Individuals with recurrent SCAD or extensive fibromuscular dysplasia (FMD) might warrant more conservative activity recommendations.
Causes
In the vast majority of patients, the underlying cause of SCAD is uncertain, although numerous associated conditions have been identified in case series and in isolated case reports (Table I). Some patients report extreme physical exertion or high levels of emotional stress shortly before the occurrence of SCAD—stress that often is considerably out of the ordinary for them. The relationship between pregnancy and SCAD has been known for several decades, and the extreme hormonal fluxes and the stress of labor are often cited as precipitants; however, the pathophysiology is not fully understood. Pregnancy after SCAD is not recommended, even in patients who did not experience peripartum SCAD. An effective contraceptive method, preferably without the use of systemic hormones, is advised.
TABLE I.
Conditions Associated with Spontaneous Coronary Artery Dissection

Fibromuscular dysplasia is a more recently identified associated condition.1,7 Spontaneous coronary artery dissection patients might present with no symptoms or signs of FMD and with only the most subtle evidence of visceral, carotid, vertebral, or renal artery FMD, detectable with sensitive imaging techniques (Fig. 2). Other patients have more overt findings, such as bruits or symptomatic extracoronary aneurysms or dissections. The extent and severity of FMD has not been correlated with SCAD risk. Although neither the role nor the optimal method of screening for extracoronary vasculopathy has been well established, we recommend that patients who have symptoms or signs suggestive of extracoronary vascular disease—or a family history of aneurysms, dissections, or premature or unexplained sudden death—undergo computed tomographic angiography from the base of the skull to the pelvis in a single procedure, to exclude clinically important pathologic conditions. If such conditions are detected, additional screening for intracranial cerebrovascular abnormalities is suggested.
Fig. 2.
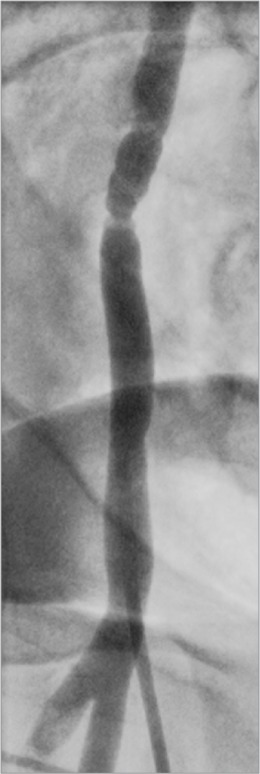
Fibromuscular dysplasia was incidentally noted in the right iliac artery during coronary angiography in a patient with myocardial infarction caused by spontaneous coronary artery dissection.
Although several monogenic mutations associated with vascular fragility, aneurysms, or dissections have been reported in SCAD patients (Table I), few such patients, in our experience, have manifested any of these mutations—indeed, there have been no reported mutations specific to either SCAD or FMD. Medical genetics consultation can be helpful in identifying individuals who warrant additional genetic testing.
In summary, SCAD is a markedly underrecognized and important cause of ACS, particularly in young women. The pathophysiology, causes, and responses to standard treatment differ from those of atherosclerotic ACS, so proper management demands accurate diagnosis. Novel intracoronary imaging can be valuable both in confirming the diagnosis and in guiding treatment decisions, because percutaneous coronary procedures carry high rates of procedural complications in SCAD patients, and conservative management is more appropriate in some cases. Although the underlying cause of SCAD is still largely unknown, FMD has emerged as an associated and possibly causal factor. Patient engagement and disease-specific online communities can promote research and a better understanding of SCAD and other rare conditions.
Acknowledgments
The author wishes to thank the many SCAD patients and family members who sustain and encourage our work, and to acknowledge the contributions of the entire Mayo Clinic SCAD Research Program team.
Footnotes
★ CME Credit
Presented at the 4th Annual Symposium on Risk, Diagnosis and Treatment of Cardiovascular Disease in Women; Texas Heart Institute, Houston; 19 October 2013.
Section Editor: Stephanie A. Coulter, MD
References
- 1.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Len-non RJ et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126(5):579–88. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 2.Tweet MS, Gulati R, Aase LA, Hayes SN. Spontaneous coronary artery dissection: a disease-specific, social networking community-initiated study. Mayo Clin Proc. 2011;86(9):845–50. doi: 10.4065/mcp.2011.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2013 Sep 11 doi: 10.1177/2048872613504310. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Grosseto D, Santarelli A, Carigi S, Baldazzi F, Franco N, Santoro D et al. Incidence of spontaneous coronary artery dissection in all comers patients referred for acute coronary syndrome [abstract 194] Eur Heart J: Acute Cardiovasc Care. 2012;1(1 Suppl):61. Available from: http://acc.sagepub.com/content/1/1_suppl/7.full.pdf+html [cited 2014 Apr 2] [Google Scholar]
- 5.Tweet M, Hayes S, Lerman A, Rihal C, Gulati R. Percutaneous coronary intervention for acute spontaneous coronary artery dissection is associated with reduced rates of technical success [abstract] Circulation. 2012;126(21 Suppl):A17969. Available from: http://circ.ahajournals.org/cgi/content/meeting_abstract/126/21_MeetingAbstracts/A17969?sid=35b9d318-dea2-4b3d-819b-f82a26a103d3 [cited 2014 Apr 2] [Google Scholar]
- 6.Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jimenez-Quevedo P et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. 2012;5(10):1062–70. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013;6(1):44–52. doi: 10.1016/j.jcin.2012.08.017. [DOI] [PubMed] [Google Scholar]


