Abstract
Better outpatient management of heart failure might improve outcomes and reduce the number of rehospitalizations. This study describes recent outpatient heart-failure management in the United States.
We analyzed data from the National Ambulatory Medical Care Survey of 2006–2008, a multistage random sampling of non-Federal physician offices and hospital outpatient departments.
Annually, 1.7% of all outpatient visits were for heart failure (51% females and 77% non-Hispanic whites; mean age, 73 ± 0.5 yr). Typical comorbidities were hypertension (62%), hyperlipidemia (36%), diabetes mellitus (35%), and ischemic heart disease (29%). Body weight and blood pressure were recorded in about 80% of visits, and health education was given in about 40%. The percentage of patients taking β-blockers was 38%; the percentage taking angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs) was 32%. Medication usage did not differ significantly by race or sex. In multivariate-adjusted logistic regression models, a visit to a cardiologist, hypertension, heart failure as a primary reason for the visit, and a visit duration longer than 15 minutes were positively associated with ACEI/ARB use; and a visit to a cardiologist, heart failure as a primary reason for the visit, the presence of ischemic heart disease, and visit duration longer than 15 minutes were positively associated with β-blocker use. Chronic obstructive pulmonary disease was negatively associated with β-blocker use. Approximately 1% of heart-failure visits resulted in hospitalization.
In outpatient heart-failure management, gaps that might warrant attention include suboptimal health education and low usage rates of medications, specifically ACEI/ARBs and β-blockers.
Keywords: Ambulatory care/standards; cardiovascular agents/therapeutic use; clinical trials as topic; comprehensive health care; drug utilization/statistics & numerical data; health care surveys; heart failure/drug therapy/economics/epidemiology/prevention & control; office visits/statistics & numerical data/trends/utilization; outcome assessment (health care)/trends; quality assurance, health care
In the United States, congestive heart failure (HF) affects 5.7 million individuals. There are 700,000 new diagnoses each year.1,2 Congestive HF is characterized by multiple relapses, with an expected one-year rate of hospital readmission of more than 50%, and one-year mortality rates of more than 30%.3,4 Accordingly, it remains the most frequent hospital-discharge diagnosis. It poses a substantial economic burden on the nation's healthcare system: more Medicare dollars are spent on its diagnosis and treatment than on any other diagnosis (total cost, $39.2 billion in 2009).1 In this context, improving the outpatient management of HF has been suggested as the cornerstone to prevent frequent hospital readmissions and thus cost.5
The outpatient treatment of HF patients is multidisciplinary and involves several steps that are listed in the American College of Cardiology/American Heart Association (ACC/AHA) management guidelines.6 These steps include initial laboratory evaluations; documented measurement of left ventricular (LV) function, body weight, and blood pressure (BP) during each visit; evaluation and management of clinical signs and symptoms of volume overload; evaluation of activity level; educating patients about disease management and health-related behavioral changes; and ensuring that patients are treated with evidence-based therapy, including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs) and β-blockers. Reducing the risk-factor burden to prevent ischemic heart disease (IHD) and diastolic dysfunction—both of which may worsen HF—is of paramount importance.
Despite the proven survival benefit of medical therapies such as ACEI/ARBs and β-blockers, mortality rates in HF patients remain high.7 Little is known about the use of these HF medications among outpatients in the U.S. In addition, most literature about HF treatment and management focuses on systolic HF, despite an equal burden of HF with preserved LV ejection fraction (LVEF).6,7 In this study, we used a national sample of outpatient visits in the U.S. to describe some important performance measures that are suggested by the ACC/AHA guidelines for the outpatient management of HF. These factors are body weight and BP measurement during each visit, evaluation of LVEF, education of patients, and appropriate use of ACEI/ARBs and β-blockers.
Patients and Methods
Study Sample
We used data from the National Ambulatory Medical Care Survey (NAMCS) 2006–2008, a multistage random sampling of non-Federal physician offices and hospital outpatient departments within the 50 United States and the District of Columbia. The sampling design and data collection procedures have been previously described.8–10 Data from the 2006, 2007, and 2008 NAMCSs were combined, and weights were divided by 3 to generate yearly national estimates of outpatient visits for HF.11
Ascertainment of Heart Failure
Data were collected with use of a standard questionnaire that was consistent for all 3 years. The questionnaire answers in regard to patient visits with HF were obtained by the following means: a census field representative who used a medical chart (46% of responses), office staff (32%), physicians (11%), and others (12%). Irrespective of the reason for the visit, the survey asked, “Does the patient now have heart failure?” This question was not included in the survey before 2006.
Study Characteristics
The variables of interest recorded for this study were age, sex, race, body mass index, BP, the chief reason for the outpatient visit, the type of healthcare provider, time that the physician spent with the patient, and the facility's use of electronic medical records (EMR). Some relevant comorbidities recorded in the questionnaire were hypertension, hyperlipidemia, diabetes mellitus, IHD, chronic obstructive pulmonary disease (COPD), asthma, chronic renal insufficiency (CRI), cancer, and psychological depression. Heart failure as a major reason for a visit was defined when the medical billing code ICD-9CM (428.x) was the answer to any of the 3 questions about the 3 major reasons for the visit. Information was available on several quality-improvement issues, such as whether body weight and BP were measured, whether further studies were ordered (chest radiograph, electrocardiogram, or echocardiogram), and whether health education was imparted during each visit.
Medications administered in the outpatient department were recorded in accordance with the National Hospital Ambulatory Medical Care Survey List of National Drug Codes directory.12 For medications, we used variables RX1V1C1 through RX4V3C4—that is, the most detailed (level 3) classification of a maximum of 8 recorded medications. The medications included in our analysis were diuretic agents, β-blockers, ACEIs, ARBs, inotropic agents, calcium channel blockers (CCB), and antiarrhythmic agents.
Other variables of interest included healthcare system characteristics such as the clinic's location (geographically, and in a metropolitan statistical area or not), the qualifications of the healthcare provider (MD or DO), the primary payer, and the specialization of the health-care provider (cardiologist or non-cardiologist).
Statistical Analysis
The unit of analysis was the patient visit. The response rate by healthcare providers was approximately 60%. Weighted analyses were adjusted for response rates. All analyses used probability weights to generate estimates for U.S. patient-visits per year. We adhered to the standards previously identified by the National Center for Health Statistics for acceptable reliability in survey estimates.8–10
Statistical analyses were performed with use of SAS statistical software version 9.2 (SAS Institute, Inc.; Cary, NC). Descriptive statistics were estimated by means of survey routines in the SAS software to account for complex survey design. Specifically, SAS routines “Surveymeans” and “Surveylogistic” were used with adoption of clustering variables cstratm and cpsum and weight variable to account for multistage sampling during variance estimation. In addition, we divided weights for each year by 3 to derive average yearly estimates. Last, we divided the weight by number of visits within the applicable past year to derive the total number of HF patients visiting outpatient facilities in the U.S. Confidence intervals (CI) for binomial variables were calculated by using the relative standard error of the estimates (SEE). Multivariate logistic regression models were built to identify the factors associated with use of ACEI/ARBs or β-blockers during each patient-visit. All odds ratios (ORs) are presented with 95% CIs. All tests were 2-tailed, and P <0.05 was considered statistically significant.
Results
During the 3 survey years, of a total of 90,588 patient-visits, 1,555 (1.7%) involved HF. On weighted analysis, 949 million clinic visits were made each year in the U.S., and an estimated 16 million patient-visits were for HF. Based on the number of visits within the applicable past year, an estimated (± SD) 4.1 ± 0.3 million visits for HF occurred. Table I shows the characteristics of the patients with HF. The mean (± SE) age of the patients was 73 ± 0.5 years; 51% were female, and 77% were non-Hispanic whites. The mean body mass index was 30 ± 0.25 kg/m2, and the mean BP was 128/72 mmHg.
TABLE I.
Characteristics of Heart-Failure Outpatients in the United States, 2006–2008 *
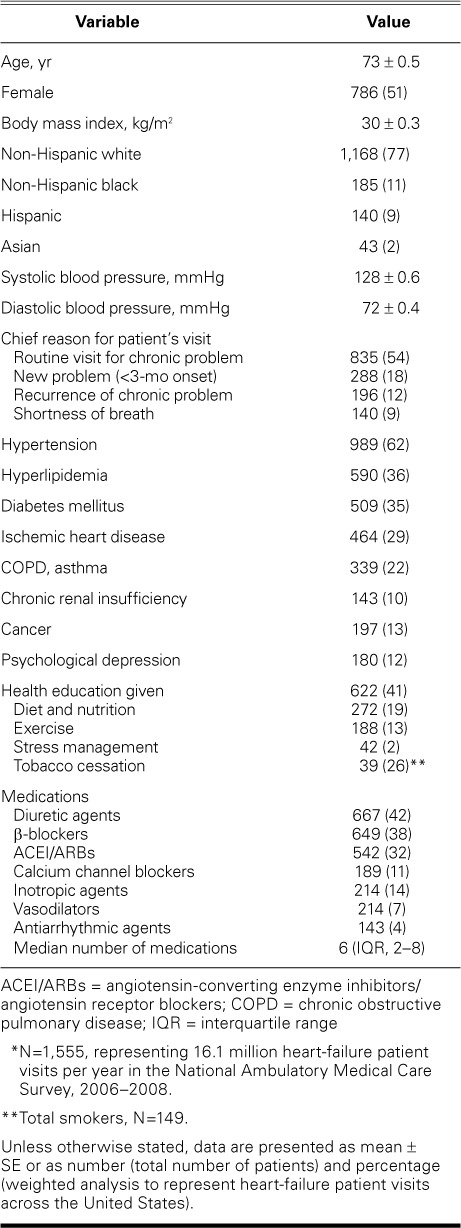
The predominant reasons for the patient-visits were as follows: a routine visit for a chronic problem (54%), a new problem within 3 months of onset (18%), recurrence of a chronic problem (12%), and shortness of breath or dyspnea (9%). According to the healthcare provider, HF was coded as the primary reason for the visit in 35% of HF-patient visits and in 0.61% of all outpatient visits.
Heart-Failure Management Characteristics
In patients with HF, body weight was measured and recorded in 81% of visits and BP in 82%. Electrolyte studies were ordered in 20% of visits and a complete blood count in 10%. Imaging was ordered in 20% of clinic visits, including radiographs in 7% and echocardiograms in 8% (data available only for 2007–2008). Current tobacco use was recorded in 10% of all HF patients (149 of 1,555); however, tobacco cessation counseling was provided to only 26% of those patients (39/149). Health education was given to 41% (Table I). Specifically, appropriate education about diet and nutrition was given to 19%; about exercise, to 13%; and about stress management, to 2%.
The median number of medications used was 6 (inter-quartile range [IQR], 2–8). The use of medications specific to HF included diuretic agents, 42%; β-blockers, 38%; ACEI/ARBs, 32% (ACEI, 22%; ARB, 10%); inotropic agents, 14%; CCB, 11%; vasodilators, 7%; and antiarrhythmic agents, 4% (Table I).
In Table II, differences in performance measures from the results of the earlier NAMCS are compared with our results.13,14
TABLE II.
Differences in Some Characteristics between the 2 NAMCS Periods

Predictors of ACEI/ARB Use
In unadjusted univariate analyses, patients who had HF as the primary reason for their visit were more likely than other patients to be prescribed ACEI/ARB therapy (40% vs 27%; Fig. 1A), as were patients seen by a cardiologist versus those seen by a non-cardiologist (44% vs 28%; Fig. 1B). Hypertensive patients were more likely to be prescribed ACEI/ARB therapy than were normotensive patients (35% vs 26%; Fig. 1C). There were no significant differences in ACEI/ARB use with regard to IHD (Fig. 1D), sex (Fig. 2A), race (Fig. 2B), diabetes mellitus (Fig. 2C), CRI (Fig. 2D), or COPD/asthma (Fig. 2E). Upon multivariate logistic regression analysis, use of ACEI/ARBs among HF patients was more likely when they were seen by a cardiologist (OR=1.74; 95% CI, 1.26–2.40), when HF was the primary reason for the visit (OR=1.78; 95% CI, 1.25–2.53), in the presence of hypertension (OR=1.69; 95% CI, 1.28–2.24), and when visits lasted longer than 15 minutes (OR=1.39; 95% CI, 1.07–1.82), independent of age, race, sex, geographic region, metropolitan area, primary payer, IHD, diabetes mellitus, CRI, and COPD/asthma.
Fig. 1.
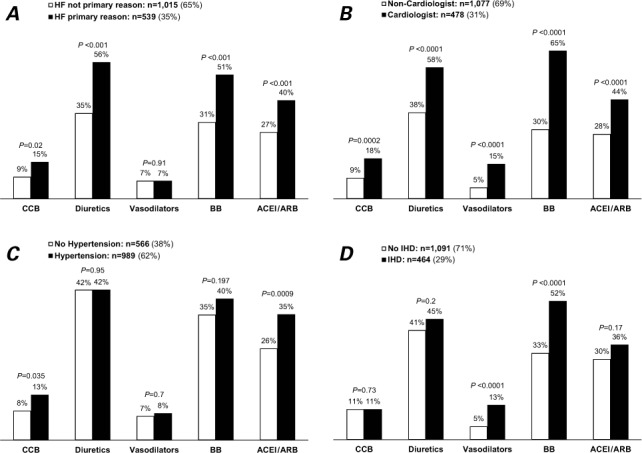
Use of some medications among heart-failure patients: National Ambulatory Medical Care Survey, 2006–2008. N=1,555; weighted n=16 million visits/yr. Graphs show visits based on whether A) heart failure was or was not the primary reason for the visit, B) examination was by a cardiologist or non-cardiologist, C) patients did or did not have hypertension, and D) patients did or did not have ischemic heart disease.
ACEI/ARB = angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; BB = β-blockers; CCB = calcium channel blockers; HF = heart failure; IHD = ischemic heart disease
*P<0.05 was considered statistically significant.
Fig. 2.
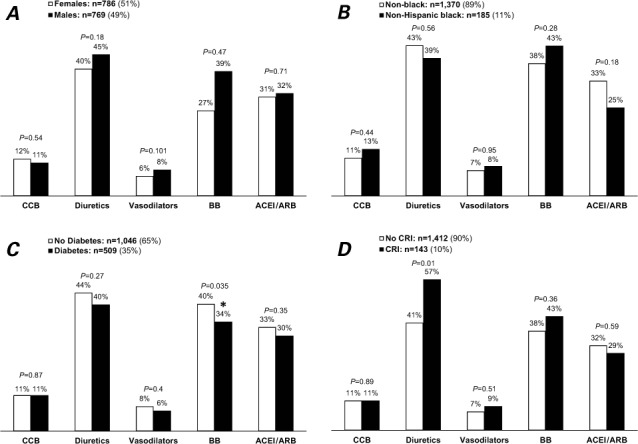
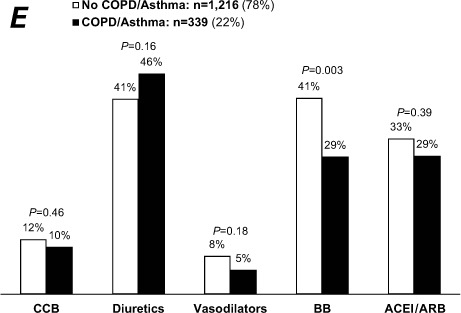
Use of some medications among heart-failure patients: National Ambulatory Medical Care Survey, 2006–2008. N=1,555; weighted n=16 million visits/yr. Graphs show visits by A) sex, B) black patients versus others, C) patients with and without diabetes mellitus, D) patients with and without chronic renal insufficiency, and E) patients with and without chronic obstructive pulmonary disease or asthma.
ACEI/ARB = angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; BB = β-blockers; CCB = calcium channel blockers; COPD = chronic obstructive pulmonary disease; CRI = chronic renal insufficiency
*P <0.05 was considered statistically significant
Fig. 2.
Continued
Predictors of β-Blocker Use
On univariate analysis, patients seen by a cardiologist were more likely to be taking β-blockers than were patients treated by other physicians (65% vs 30%; Fig. 1B), as were patients for whom HF was the primary reason for the visit (51% vs 31%; Fig. 1A) and patients with IHD (52% vs 33%; Fig. 1D). The rate of β-blocker use was higher in nondiabetic patients than in diabetic patients (40% vs 34%; Fig. 2C). There was no significant difference in β-blocker use in regard to hypertension (Fig. 1C), sex (Fig. 2A), race (Fig. 2B), or CRI (Fig. 2D). The presence of COPD/asthma was associated with lower use of β-blockers (29% vs 41%, P=0.003; Fig. 2E). Upon multivariate analysis, β-blocker use was associated with patients seen by a cardiologist (OR=3.34; 95% CI, 2.40–5.64), HF as a primary reason for the visit (OR=1.84; 95% CI, 1.40–2.53), visits lasting longer than 15 minutes (OR=1.31; 95% CI, 1.00–1.72), and the presence of IHD (OR=1.76; 95% CI, 1.23–2.52), independent of age, sex, race, geographic region, metropolitan area, primary payer, diabetes, CRI, hypertension, and COPD/asthma.
Figure 1A shows the differences in HF-related medication prescriptions between cardiologists and non-cardiologists. In addition, both body weight (90% vs 79%; P <0.001), and BP (93% vs 78%; P <0.001) were measured more often during visits to cardiologists than non-cardiologists.
Healthcare System Characteristics
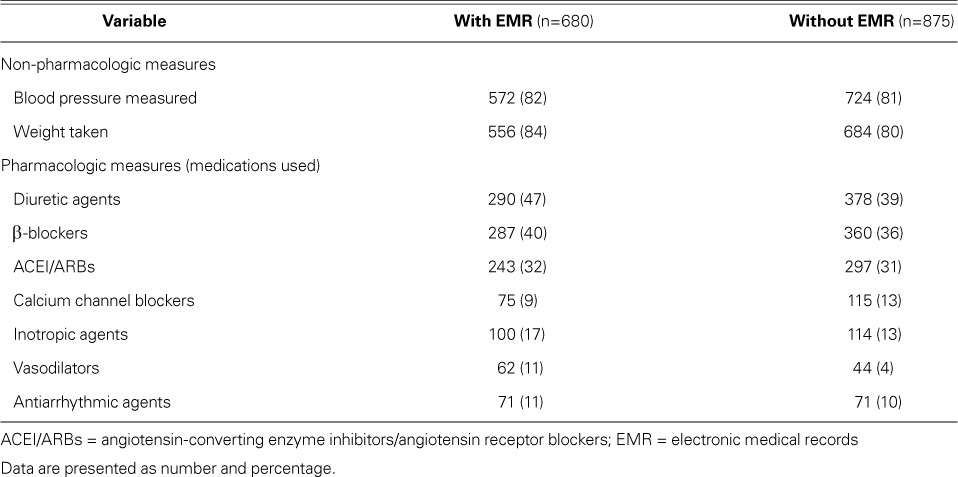
Healthcare system characteristics are summarized in Table III. A plurality of HF patient visits took place in the southern region of the U.S. (43%). More than three quarters of all HF patients (77%) had Medicare, Medicaid, or both as their primary payer. Most patients were seen by an MD (93%). Approximately half of HF patients were seen by a general or family medicine practitioner or an internist, and 22% were seen by a cardiologist. The median duration of the appointment was 15 minutes (IQR, 15–25 min). Electronic medical records were fully used in 27% of HF visits and partially used in 14%; however, in 58%, EMRs were not used at all. When facilities using and not using EMRs were compared, there was no appreciable difference in either the measurement of BP and body weight or in pharmacologic prescriptions (Table IV). Approximately 1.04% of HF patient-visits resulted in hospitalization.
TABLE III.
Healthcare System Characteristics in Out-patient Settings in the United States, 2006–2008 *
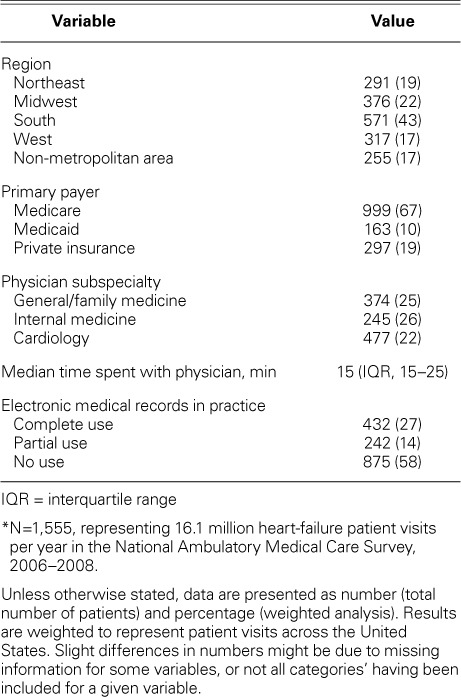
TABLE IV.
Comparison of Quality-of-Care Variables in Patient Visits with and without the Use of Electronic Medical Records
Discussion
This study provides a comprehensive account of the recent management of HF patients in U.S. outpatient settings. Our important findings were that, in comparison with non-cardiologists, cardiologists had higher rates of prescribing anti-HF medications (notably ACEI/ARBs and β-blockers) and more frequently measured patients' body weight and BP during clinical visits. By providing data on general outpatient settings for all HF patients during recent years, we extend the results of the Pinnacle15 and Improve HF16 studies, which were limited to cardiology practices.
In addition, even though there might be differences in HF definition (that is, dependence in previous surveys on ICD codes only, as compared with our questionnaire query specifically about the presence of HF), the number of outpatient HF visits increased from 6 million in 199414 (1.1% of all outpatient visits) to 16 million visits per year in our study (1.7% of all visits).
Non-Pharmacologic Performance Measures
Body weight was not measured in 19% of patient-visits, BP was not measured in 18% of patient-visits, and health education was not given in 59% of visits. Dietary and nutritional counseling was supplied in 19% of all visits. The rates of measuring BP and of supplying dietary and nutritional counseling did not change greatly from the NAMCS of 1989–1994 (Table II).13 No references could be found on body-weight measurements in the NAMCS to check for differences. Factors such as the busy nature of outpatient practice, lack of complete EMR, and lack of economic incentives for providing complete records might contribute to the low prevalence of reporting these non-pharmacologic measures.15
Pharmacologic Performance Measures
Certain CCBs (nondihydropyridines such as diltiazem and verapamil) can worsen HF and increase mortality rates; conversely, other CCBs are considered to be safe.17–22 The use of CCBs declined from 15% in 1994 to 11% in our analysis (Table II).14 Patients seen by a cardiologist and those with hypertension were more likely to be prescribed CCB therapy (Figs. 1B–C). When HF was the primary reason for a visit, the usage rate of diuretic agents decreased from 62% in 1994 to 55% in our study (Table II).14
Evidence-based guidelines support the use of ACEI/ARBs in HF.7 The combined usage rate of ACEI/ARBs in 2006–2008 among patients for whom HF was coded as the primary reason for their visit increased from 31% to 40% (1989–1994); however, the overall usage rate among patients with HF was 32% (Table II).14 The use of ACEI/ARBs was twice as likely when the patient visited a cardiologist rather than a non-cardiologist, which perhaps points to one of two conclusions: either HF can be optimally managed by specialists, or the profile of patients who visit cardiologists is different from that of patients who visit other doctors.23 No significant differences in ACEI use were found when HF was the primary reason for the visit (39% vs 38%; Table II). Upon comparison of current study data with those published for 1989–1994 for patient-visits primarily for HF, the prescription rate for ACEIs increased among non-cardiologists (22% formerly to 38% now), and they decreased among cardiologists (46% formerly to 39% now) (Table II).14 No significant differences in ACEI use were found when HF was the primary reason for the visit (39% vs 38%, Table II). The prescription rate for ACEI/ARBs by cardiologists in our study was lower (44%) than in previous reports (80%–86%, restricted to patients with reduced LVEF).15,16 This can be explained by our study's inclusion of HF patients with preserved LVEF, whereas previous reports included patients with reduced LVEF (≤0.40),15 a mean LVEF of 0.25,16 and disproportionate numbers of males.16 Angiotensin-converting enzyme inhibitors and ARBs have not shown effectiveness in reducing HF-related deaths in patients with HF and preserved LVEF (approximately half the entire HF population).24,25 A comparative analysis (2005–2007) of inpatient admissions of HF patients with LVEFs <0.35 yielded an 87% rate of ACEI/ARB use upon their discharge from the hospital.26 However, in our study, the use of ACEI/ARBs was not statistically significant in HF patients with IHD (36% vs 30%; Fig. 1D).
Contrary to our expectations, we found no significant differences in ACEI/ARB use among patients with and without CRI (Fig. 2D) or with and without diabetes (Fig. 2C). We did not study whether the lower use was motivated by the expected presence of HF with preserved LVEF in about half of HF patients, or by side effects such as hyperkalemia. Several barriers might hinder physicians' prescribing ACEI/ARBs, such as unfamiliarity with the drugs, concerns about safety profiles (hypotension, worsening renal failure, hyperkalemia, and cough), and lack of reimbursement incentives to provide preventive care.27–30
Despite the importance of β-blockers in HF therapy,31–34 their usage rate increased only slightly, from 32% in 200035 to 38% in our study. These rates are low when compared with the β-blocker usage rates of 86% to 93% in cardiology outpatients with reduced LVEFs.15,16 Of note, HF patients with IHD were 1.6 times more likely to have been prescribed this therapy.
It is widely accepted that HF education and management by mid-level providers is essential for the optimization and maintenance of recommended medical therapies and lifestyle interventions that might save tremendous amounts of resources.7,36–40 Because so many HF patients are treated by non-cardiovascular experts, educating non-cardiologists to maximize the treatment of HF patients might improve patients' adherence to interventions and therapeutic regimens. Wider dissemination of the current HF-management guidelines in formats to suit the busy schedule of healthcare providers might be useful.
Strengths and Limitations of the Study
Whereas previous studies could comment only on patients whose chief reason for a doctor visit was HF, our inclusion of a mandatory survey question about congestive HF as a comorbidity enabled more generalization. No HF diagnosis was verified independently, although previous reports indicate very high positive predictive value for that diagnosis in hospital administrative databases (~95%).41,42
Several limitations warrant mention. The unit of analysis was a patient-visit rather than a patient. It is plausible that a single patient made more than one visit. We were able to estimate numbers of patients by accounting for the number of visits that each patient made during the last year. In addition, it was noteworthy that data were collected from visits during one-week periods and were given very low sampling probability for each visit; the chances of re-sampling a patient during and across survey years were minimal. Data were insufficient to characterize HF type (systolic vs diastolic), HF symptoms and their duration, New York Heart Association functional status, physical examination findings, laboratory results such as basic metabolic panel, biomarkers such as pro-brain natriuretic peptide, and diagnostic testing such as echocardiography. Data on the optimal doses, contraindications, and adverse effects of ACEI, ARB, and β-blockers were also sparse. Data on medication compliance were unavailable. Of importance, medication use might have been underreported, because the permissible number of medications to record was 8. Generalization to care by physicians who practice in publicly funded clinics cannot be made, because of their exclusion from the NAMCS database.
In conclusion, we found an underuse of both pharmacologic and non-pharmacologic measures. Patients' having been seen by cardiologists, and longer time spent during visits, were strong predictors of higher usage rates of β-blockers and ACEI/ARBs.
Footnotes
Dr. Yaemsiri is now at the National Center of Health Statistics, Bethesda, Maryland. Dr. Alvarez is now at the Department of Cardiology, Temple University Hospital, Philadelphia, Pennsylvania.
A poster of this study was presented at the Joint Conference on Nutrition, Physical Activity and Metabolism and Cardiovascular Epidemiology and Prevention 2011 Scientific Sessions. Available from: http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_323597.pdf
References
- 1.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133(6):703–12. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association [published errata appear in Circulation 2011;123(6):e240 and Circulation 2011;124(16):e246] Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosiborod M, Lichtman JH, Heidenreich PA, Normand SL, Wang Y, Brass LM, Krumholz HM. National trends in outcomes among elderly patients with heart failure. Am J Med. 2006;119(7):616.e1–7. doi: 10.1016/j.amjmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Rathore SS, Masoudi FA, Wang Y, Curtis JP, Foody JM, Havranek EP, Krumholz HM. Socioeconomic status, treatment, and outcomes among elderly patients hospitalized with heart failure: findings from the National Heart Failure Project. Am Heart J. 2006;152(2):371–8. doi: 10.1016/j.ahj.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oddone EZ, Weinberger M, Horner M, Mengel C, Goldstein F, Ginier P et al. Classifying general medicine readmissions. Are they preventable? Veterans Affairs Cooperative Studies in Health Services Group on Primary Care and Hospital Readmissions. J Gen Intern Med. 1996;11(10):597–607. doi: 10.1007/BF02599027. [DOI] [PubMed] [Google Scholar]
- 6.Bonow RO, Bennett S, Casey DE, Jr, Ganiats TG, Hlatky MA, Konstam MA et al. ACC/AHA Clinical Performance Measures for Adults with Chronic Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures): endorsed by the Heart Failure Society of America. Circulation. 2005;112(12):1853–87. doi: 10.1161/CIRCULATIONAHA.105.170072. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation [published erratum appears in J Am Coll Cardiol 2009;54(25):2464] J Am Coll Cardiol. 2009;53(15):e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Report. 2008;(3):1–39. [PubMed] [Google Scholar]
- 9.Cherry DK, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2005 summary. Adv Data. 2007;(387):1–39. [PubMed] [Google Scholar]
- 10.Middleton K, Hing E, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 outpatient department summary. Adv Data. 2007;(389):1–34. [PubMed] [Google Scholar]
- 11.Hugli O, Braun JE, Kim S, Pelletier AJ, Camargo CA., Jr. United States emergency department visits for acute decompensated heart failure, 1992 to 2001. Am J Cardiol. 2005;96(11):1537–42. doi: 10.1016/j.amjcard.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. National drug code directory. Silver Spring (MD): 1995. [Google Scholar]
- 13.Stafford RS, Saglam D, Causino N, Starfield B, Culpepper L, Marder WD, Blumenthal D. Trends in adult visits to primary care physicians in the United States. Arch Fam Med. 1999;8(1):26–32. doi: 10.1001/archfami.8.1.26. [DOI] [PubMed] [Google Scholar]
- 14.Stafford RS, Saglam D, Blumenthal D. National patterns of angiotensin-converting enzyme inhibitor use in congestive heart failure. Arch Intern Med. 1997;157(21):2460–4. [PubMed] [Google Scholar]
- 15.Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56(1):8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M et al. Heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Circ Heart Fail. 2008;1(2):98–106. doi: 10.1161/CIRCHEARTFAILURE.108.772228. [DOI] [PubMed] [Google Scholar]
- 17.Cohn JN. The management of chronic heart failure. N Engl J Med. 1996;335(7):490–8. doi: 10.1056/NEJM199608153350707. [DOI] [PubMed] [Google Scholar]
- 18.Deedwania PC, Carbajal EV. Secondary prevention after myocardial infarction. Too many choices, which ones work? Arch Intern Med. 1993;153(3):285–8. [PubMed] [Google Scholar]
- 19.Deedwania PC, Amsterdam EA, Vagelos RH. Evidence-based, cost-effective risk stratification and management after myocardial infarction. California Cardiology Working Group on Post-MI Management. Arch Intern Med. 1997;157(3):273–80. [PubMed] [Google Scholar]
- 20.O'Connor CM, Carson PE, Miller AB, Pressler ML, Belkin RN, Neuberg GW et al. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective Randomized Amlodipine Survival Evaluation. Am J Cardiol. 1998;82(7):881–7. doi: 10.1016/s0002-9149(98)00496-2. [DOI] [PubMed] [Google Scholar]
- 21.Cohn JN, Ziesche S, Smith R, Anand I, Dunkman WB, Loeb H et al. Effect of the calcium antagonist felodipine as supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril: V-HeFT III. Vasodilator-Heart Failure Trial (V-HeFT) Study Group. Circulation. 1997;96(3):856–63. doi: 10.1161/01.cir.96.3.856. [DOI] [PubMed] [Google Scholar]
- 22.Packer M, O'Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996;335(15):1107–14. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 23.Edep ME, Shah NB, Tateo IM, Massie BM. Differences between primary care physicians and cardiologists in management of congestive heart failure: relation to practice guidelines. J Am Coll Cardiol. 1997;30(2):518–26. doi: 10.1016/s0735-1097(97)00176-9. [DOI] [PubMed] [Google Scholar]
- 24.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 25.Fu M, Zhou J, Sun A, Zhang S, Zhang C, Zou Y et al. Efficacy of ACE inhibitors in chronic heart failure with preserved ejection fraction–a meta analysis of 7 prospective clinical studies. Int J Cardiol. 2012;155(1):33–8. doi: 10.1016/j.ijcard.2011.01.081. [DOI] [PubMed] [Google Scholar]
- 26.Piccini JP, Hernandez AF, Dai D, Thomas KL, Lewis WR, Yancy CW et al. Use of cardiac resynchronization therapy in patients hospitalized with heart failure. Circulation. 2008;118(9):926–33. doi: 10.1161/CIRCULATIONAHA.108.773838. [DOI] [PubMed] [Google Scholar]
- 27.Bart BA, Gattis WA, Diem SJ, O'Connor CM. Reasons for underuse of angiotensin-converting enzyme inhibitors in patients with heart failure and left ventricular dysfunction. Am J Cardiol. 1997;79(8):1118–20. doi: 10.1016/s0002-9149(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 28.Houghton AR, Cowley AJ. Why are angiotensin converting enzyme inhibitors underutilised in the treatment of heart failure by general practitioners? Int J Cardiol. 1997;59(1):7–10. doi: 10.1016/s0167-5273(96)02904-x. [DOI] [PubMed] [Google Scholar]
- 29.Philbin EF, Andreaou C, Rocco TA, Lynch LJ, Baker SL. Patterns of angiotensin-converting enzyme inhibitor use in congestive heart failure in two community hospitals. Am J Cardiol. 1996;77(10):832–8. doi: 10.1016/s0002-9149(97)89177-1. [DOI] [PubMed] [Google Scholar]
- 30.Pearson TA, McBride PE, Miller NH, Smith SC. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for coronary disease events. Task Force 8. Organization of preventive cardiology service. J Am Coll Cardiol. 1996;27(5):1039–47. doi: 10.1016/0735-1097(96)87736-9. [DOI] [PubMed] [Google Scholar]
- 31.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–7. [PubMed] [Google Scholar]
- 32.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13. [PubMed] [Google Scholar]
- 33.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 34.Sin DD, McAlister FA. The effects of beta-blockers on morbidity and mortality in a population-based cohort of 11,942 elderly patients with heart failure. Am J Med. 2002;113(8):650–6. doi: 10.1016/s0002-9343(02)01346-3. [DOI] [PubMed] [Google Scholar]
- 35.Stafford RS, Radley DC. The underutilization of cardiac medications of proven benefit, 1990 to 2002. J Am Coll Cardiol. 2003;41(1):56–61. doi: 10.1016/s0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson LW. Projecting heart failure into bankruptcy in 2012? Am Heart J. 2011;161(6):1007–11. doi: 10.1016/j.ahj.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333(18):1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 38.Philbin EF. Comprehensive multidisciplinary programs for the management of patients with congestive heart failure. J Gen Intern Med. 1999;14(2):130–5. doi: 10.1046/j.1525-1497.1999.00291.x. [DOI] [PubMed] [Google Scholar]
- 39.Rich MW, Nease RF. Cost-effectiveness analysis in clinical practice: the case of heart failure. Arch Intern Med. 1999;159(15):1690–700. doi: 10.1001/archinte.159.15.1690. [DOI] [PubMed] [Google Scholar]
- 40.Gustafsson F, Arnold JM. Heart failure clinics and outpatient management: review of the evidence and call for quality assurance. Eur Heart J. 2004;25(18):1596–604. doi: 10.1016/j.ehj.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43(2):182–8. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Goff DC, Jr, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160(2):197–202. doi: 10.1001/archinte.160.2.197. [DOI] [PubMed] [Google Scholar]


