Abstract
In patients with cardiac sarcoidosis, the sarcoid granulomas usually involve the myocardium or endocardium. The disease typically presents as heart failure with ventricular arrhythmias, conduction disturbances, or both. Constrictive pericarditis has rarely been described in patients with sarcoidosis: we found only 2 reports of this association.
We report the case of a 57-year-old man who presented with clinical and hemodynamic features of constrictive pericarditis, of unclear cause. He was admitted for treatment of recurrent pleural effusion. After a complicated hospital course, he underwent pericardiectomy. His clinical and hemodynamic conditions improved substantially, and he was discharged from the hospital in good condition. The pathologic findings, the patient's clinical course, and his response to pericardiectomy led to our diagnosis of cardiac sarcoidosis presenting as constrictive pericarditis. In addition to the patient's case, we discuss the nature and diagnostic challenges of cardiac sarcoidosis. Increased awareness of this disease is necessary for its early detection, appropriate management, and potential cure.
Keywords: Cardiomyopathies/complications/diagnosis/pathology; diagnosis, differential; diagnostic imaging; myocardium/pathology; pericarditis, constrictive/complications/etiology/surgery; sarcoidosis/complications/diagnosis/pathology; treatment outcome
In the United States, one quarter of patients with systemic sarcoidosis have cardiac involvement,1 which is the usual cause of sarcoid-related deaths.2 The myocardium and endocardium are typically affected.1,3 Pure pericardial involvement, excluding the adjacent myocardium, is reported less frequently. Furthermore, sarcoidosis rarely appears to present as constrictive pericarditis.
We report the case of a man who displayed classic findings of constrictive pericarditis secondary to cardiac sarcoidosis (CS) with adjacent myocardial involvement, and we discuss the nature and diagnosis of CS.
Case Report
In December 2010, a 57-year-old man presented with progressive exertional dyspnea and lower-extremity edema. He had been healthy until approximately 6 months before and reported no chest pain, orthopnea, or paroxysmal nocturnal dyspnea. His blood pressure was 120/90 mmHg, his heart rate was 70 beats/min, and he was afebrile. His jugular venous pressure was elevated (15 cm H2O) with hepatojugular reflux. Cardiac auscultation revealed a normal S1 and S2 with no murmur, gallop, or rub. Lung auscultation revealed diminished air entry in the bases bilaterally, with no crackles. The patient had severe (4+) pitting edema of the lower extremities, extending to his thighs. A chest radiograph showed bilateral pleural effusion. A 12-lead electrocardiogram revealed sinus rhythm with low voltage. A transthoracic echocardiogram showed mild left ventricular (LV) hypertrophy with mild impairment of systolic function and interventricular septal bounce consistent with constrictive physiology. The cardiac valves appeared to be normal. A computed tomogram (CT) of the chest with contrast showed mild pericardial thickening with focal calcification, extensive mediastinal and bilateral hilar lymphadenopathy, and moderate left and large right pleural effusions. A diagnostic and therapeutic right thoracentesis yielded results consistent with transudative effusion. Right- and left-sided heart catheterization revealed nonobstructive coronary artery disease. The patient's hemodynamic data were consistent with constrictive physiology (Table I and Fig. 1). Simultaneous right ventricular (RV) and LV pressure tracings showed interventricular discordance, with diastolic equalization of the pressure in both chambers and a square root sign (Fig. 2). Pericardial calcification was noted on fluoroscopy. The diagnosis was constrictive pericarditis of unclear cause.
TABLE I.
Hemodynamic Data from Cardiac Catheterization
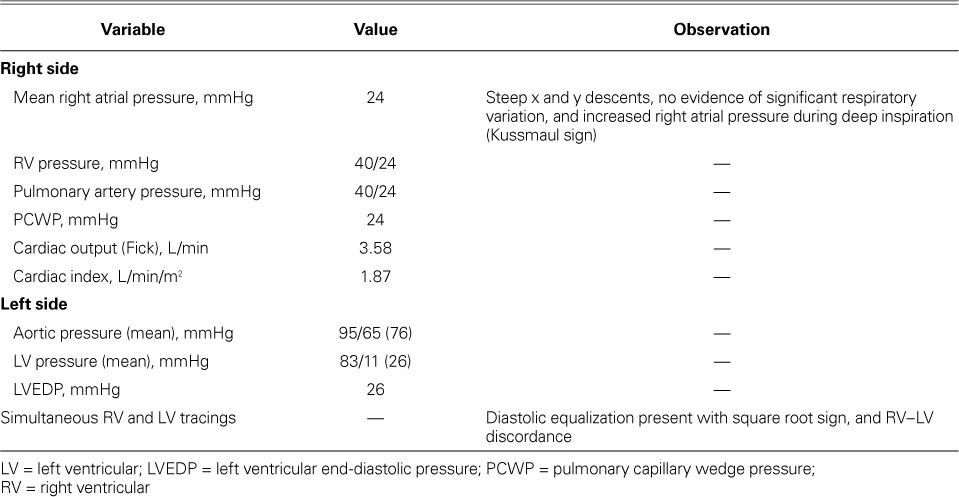
Fig. 1.
Right atrial pressure tracing shows rapid x and y descent.
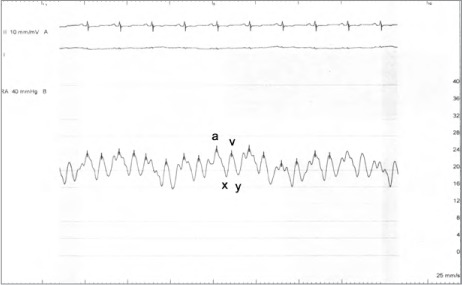
Fig. 2.
Simultaneous recordings of left ventricular (LV) and right ventricular (RV) pressures show ventricular discordance. Note the decrease in RV systolic pressure during expiration (4th beat) and the marked rise at peak inspiration.
The patient was admitted to the hospital for treatment of recurrent pleural effusion, and he underwent right pleurodesis with biopsy of the mediastinal lymph node. His hospital course was prolonged and complicated by cardiogenic shock, severe anasarca, and respiratory and renal failure. After 6 weeks, he underwent extensive resection of the pericardium, which resulted in remarkable symptomatic and hemodynamic improvement. The excised pericardium showed pink-tan to purple-gray thickened fibrous tissue adhering to yellow, lobulated adipose tissue (Fig. 3). Histopathologic analysis of the excised specimens confirmed thickened pericardial tissue with dystrophic calcification, chronic inflammation, and no granulomas. The lymph node biopsy yielded nonnecrotizing and granulomatous inflammation and sclerosis (Fig. 4). Polymerase chain reaction analysis yielded no evidence of Mycobacterium tuberculosis complex DNA. Other diagnostic evaluations for infectious processes such as acid-fast bacilli and fungi produced no unusual results. Results of fluid cytology were negative for malignant cells. A cardiac magnetic resonance (CMR) image with gadolinium showed delayed enhancement involving the LV myocardium at the mid anteroseptal and basal inferolateral walls, consistent with infiltrative disease (Fig. 5). The final diagnosis was constrictive pericarditis caused by CS.
Fig. 3.
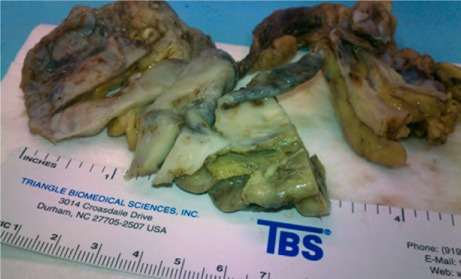
Photograph of the excised pericardium shows pink-tan to purple-gray thickened fibrous tissue adherent to yellow, lobulated adipose tissue.
Fig. 4.
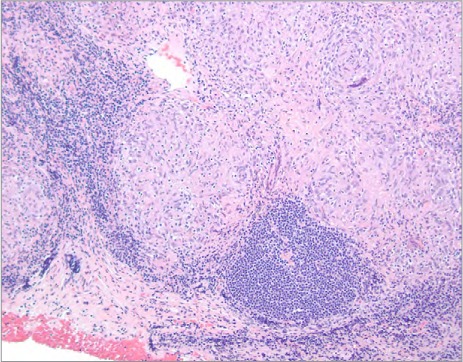
Photomicrograph of the lymph node biopsy specimen shows nonnecrotizing and granulomatous inflammation and sclerosis (H & E, orig. ×100).
Fig. 5.
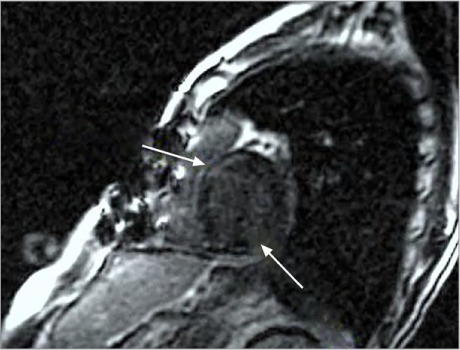
Cardiovascular magnetic resonance image shows late enhancement in the anteroseptal and basal inferolateral segments of the left ventricular myocardium (arrows).
After 9 weeks total, the patient was discharged from the hospital, in good condition, on β-blocker and angiotensin-converting enzyme inhibitor therapy. He was prescribed oral prednisone, 30 mg/d for 6 weeks, with gradual tapering of the dosage over the following 8 weeks. As of June 2013, he had reported no cardiovascular symptoms during outpatient clinical visits.
Discussion
The clinical diagnosis of CS can be challenging because of the wide variety of clinical presentations, especially when the cardiac involvement precedes other manifestations of systemic sarcoidosis.4 Most often, patients present with conduction abnormalities and congestive heart failure. Other manifestations include valvular insufficiencies, myocardial infarction, recurrent pericardial effusion, and sudden death.1,5,6 Cardiac sarcoidosis should be part of the differential diagnosis in patients who present with refractory heart failure, conduction abnormalities, or ventricular arrhythmias.7 Pericardial involvement—especially constrictive pericarditis, as in our patient—appears to be an extremely rare clinical manifestation of this disease.
Initially, we thought that our patient's clinical presentation might be due to systolic heart failure caused by dilated cardiomyopathy, so we performed coronary angiography to exclude ischemic causes.8 The patient's mild coronary artery disease did not explain his LV dysfunction. On the other hand, the additional diagnostic results were consistent with constrictive physiologic conditions: pericardial calcification (on fluoroscopy and chest CT), interventricular septal bounce (echocardiography), and elevated right-sided filling pressure (catheterization). On tissue biopsy, the presence of bilateral hilar lymphadenopathy and noncaseating granulomas helped to confirm the diagnosis of systemic sarcoidosis in the absence of tuberculosis or fungal infection.3
Most available diagnostic algorithms for CS arise from histologic or clinical criteria.9,10 In our patient, the presence of systemic sarcoidosis with LV asynergy, the intracardiac pressure abnormalities found during catheterization, and the CMR findings fulfilled the clinical criteria for the diagnosis of CS.11 In addition, the diagnosis of sarcoid constrictive pericarditis was on the basis of the clinical presentation, the echocardiogram, and right-sided catheterization findings.
Congestive heart failure in patients with systemic sarcoidosis usually occurs after the sarcoid granuloma invades the myocardium or endocardium and causes dilated or restrictive cardiomyopathy.1,3 Conversely, pericardial involvement is thought to be infrequent without myocardial involvement. Small pericardial effusions have been found echocardiographically in up 19% of patients with sarcoidosis; however, only a few cases of clinically significant pericardial effusion together with biopsy-proven CS have been reported. The effusion and CS can manifest themselves as pericarditis or pericardial tamponade.12,13
Cardiac sarcoidosis usually presents as restrictive cardiomyopathy, and differentiating it from constrictive pericarditis requires careful attention to the hemodynamic effects and echocardiographic features of both entities.9 During cardiac catheterization, hemodynamic data that support the diagnosis of constrictive pericarditis include prominent X and Y descent, absent variation of right atrial pressure during respiration, dip and plateau of the LV pressure waveform, and RV–LV discordance (Figs. 1 and 2).
Sarcoidosis is apparently an extremely rare cause of constrictive pericarditis. In the largest 2 series of constrictive pericarditis (from the Mayo and Cleveland Clinics), the most frequent causes were cardiac surgery, idiopathic pericarditis, and prior irradiation.14,15 We screened our patient for the rarer cause of tuberculosis.16
We found only 2 reports of constrictive pericarditis associated with CS. The first patient was a young man whose presentation was similar to that of our patient.17 The 2nd case of constrictive pericarditis was confirmed by means of RV and LV biopsy.18 Endomyocardial biopsy can be considered in patients whose clinical features suggest CS6,7; however, the sensitivity of the test is low because of heterogeneous involvement.19 Cardiac imaging, including scintigraphy with technetium-99m, thallium-201, and gallium-67, had limited sensitivity and specificity in the diagnosis of CS.20 Conversely, CMR, by showing enhanced signal intensity in the mid portion of the myocardium and epicardium, reportedly had a sensitivity of 100% and a specificity of 78%.20
Evaluation of the pericardial tissue in our patient, as well as that in the first reported case of sarcoid constrictive pericarditis,17 yielded thickened pericardial tissue with dystrophic calcification, chronic inflammation, and no granulomas. Similarly, the most frequent histopathologic findings after pericardiectomy in patients with connective-tissue disease are nonspecific chronic inflammation, fibrosis, and calcification.18,21
Management of Cardiac Sarcoidosis
Corticosteroids, the mainstay of therapy for CS, play a major role in preventing LV remodeling22 and they improve survival rates.5,23 However, pericardiectomy is considered to be the treatment of choice for most patients with constrictive pericarditis. This surgery serves as a potential cure with substantial improvement in symptoms (as in our patient), and it is associated with improved long-term survival rates.14,15
Despite some controversy, we think that most clinicians would agree with implantable cardioverter-defibrillator (ICD) therapy for primary prevention of sudden cardiac death in CS. Cardiac sarcoidosis can be associated with life-threatening ventricular arrhythmias when the myocardium is involved. Placement of an ICD should be considered in high-risk patients, including those with a history of syncope, LV dysfunction, and spontaneous or induced cardiac arrhythmia during electrophysiologic studies.6,10,19,24 Increased awareness of CS and its challenges is necessary for early detection, selection of the appropriate management strategy, and potential cure.
References
- 1.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58(6):1204–11. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 2.Iwai K, Tachibana T, Takemura T, Matsui Y, Kitaichi M, Kawabata Y. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn. 1993;43(7–8):372–6. doi: 10.1111/j.1440-1827.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 3.Roberts WC, McAllister HA, Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11) Am J Med. 1977;63(1):86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 4.Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physicians Lond. 1981;15(4):245–6. 249–53. [PMC free article] [PubMed] [Google Scholar]
- 5.Chapelon-Abric C, de Zuttere D, Duhaut P, Veyssier P, Wechsler B, Huong DL et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004;83(6):315–34. doi: 10.1097/01.md.0000145367.17934.75. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Judson MA, Donnino R, Gold M, Cooper LT, Jr, Prystowsky EN, Prystowsky S. Cardiac sarcoidosis. Am Heart J. 2009;157(1):9–21. doi: 10.1016/j.ahj.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50(19):1914–31. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation [published erratum appears in Circulation 2010;121(12):e258] Circulation. 2009;119(14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 9.Chinnaiyan KM, Leff CB, Marsalese DL. Constrictive pericarditis versus restrictive cardiomyopathy: challenges in diagnosis and management. Cardiol Rev. 2004;12(6):314–20. doi: 10.1097/01.crd.0000144368.59679.8c. [DOI] [PubMed] [Google Scholar]
- 10.Mantini N, Williams B, Jr, Stewart J, Rubinsztain L, Kacharava A. Cardiac sarcoid: a clinician's review on how to approach the patient with cardiac sarcoid. Clin Cardiol. 2012;35(7):410–5. doi: 10.1002/clc.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol. 2005;184(1):249–54. doi: 10.2214/ajr.184.1.01840249. [DOI] [PubMed] [Google Scholar]
- 12.Zelcer AA, LeJemtel TH, Jones J, Stahl J. Pericardial tamponade in sarcoidosis. Can J Cardiol. 1987;3(1):12–3. [PubMed] [Google Scholar]
- 13.Kinney E, Murthy R, Ascunce G, Donohoe R, Zelis R. Pericardial effusions in sarcoidosis. Chest. 1979;76(4):476–8. doi: 10.1378/chest.76.4.476. [DOI] [PubMed] [Google Scholar]
- 14.Ling LH, Oh JK, Schaff HV, Danielson GK, Mahoney DW, Seward JB, Tajik AJ. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100(13):1380–6. doi: 10.1161/01.cir.100.13.1380. [DOI] [PubMed] [Google Scholar]
- 15.Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P, Ozduran V, Houghtaling PL et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43(8):1445–52. doi: 10.1016/j.jacc.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 16.Blake S, Bonar S, O'Neill H, Hanly P, Drury I, Flanagan M, Garrett J. Aetiology of chronic constrictive pericarditis. Br Heart J. 1983;50(3):273–6. doi: 10.1136/hrt.50.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrett J, O'Neill H, Blake S. Constrictive pericarditis associated with sarcoidosis. Am Heart J. 1984;107(2):394. doi: 10.1016/0002-8703(84)90394-6. [DOI] [PubMed] [Google Scholar]
- 18.Cameron J, Oesterle SN, Baldwin JC, Hancock EW. The etiologic spectrum of constrictive pericarditis. Am Heart J. 1987;113(2 Pt 1):354–60. doi: 10.1016/0002-8703(87)90278-x. [DOI] [PubMed] [Google Scholar]
- 19.Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282–8. doi: 10.1136/hrt.2005.080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45(10):1683–90. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 21.Thould AK. Constrictive pericarditis in rheumatoid arthritis. Ann Rheum Dis. 1986;45(2):89–94. doi: 10.1136/ard.45.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95(1):143–6. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 23.Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006–10. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 24.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons [published erratum appears in Circulation 2009;120(5):e34–5] Circulation. 2008;117(21):e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]


