Abstract
Atrial volumetric measurement has proven clinical implications. Advances in cardiac imaging, notably the precision enabled by multidetector computed tomography (MDCT), herald the need for new criteria of what constitutes normal volumetric measurements. With use of 64-slice MDCT, we compared the atrial volumes in healthy individuals with those in individuals with coronary artery disease.
By means of manual segmentation, we measured biatrial volume in 686 participants who underwent retrospective electrocardiographic-gated MDCT angiographic evaluation. The study population included a control group of 203 persons with no cardiac abnormalities, and a study group of 483 patients with obstructive coronary artery disease. All variables were compared between men and women and between the groups.
We found a significant difference in left atrial end-systolic and end-diastolic volumes between men and women in the control group (P <0.05); however, right atrial volumes were similar. In comparison with the entire control group, the coronary artery disease group had significantly higher left atrial volume, significantly lower right atrial stroke volume, and significantly lower biatrial ejection fraction, except for left atrial ejection fraction in men. Right atrial volume and left atrial stroke volume were not significantly different. The results imply that a sex-specific reference value is necessary for left atrial volumetric evaluation, and that left atrial volume and biatrial ejection fraction (excluding left atrial ejection fraction in men) might be useful during diagnosis and prognosis in patients who have coronary artery disease.
Keywords: Atrial function; cardiac volume/physiology; coronary angiography/methods; heart atria/pathology/ultrasonography; image interpretation, computer-assisted/methods; imaging, three-dimensional; predictive value of tests; sensitivity and specificity; tomography, x-ray computed/methods/utilization
Atrial volume enlargement is an important diagnostic and prognostic indicator for patients with coronary artery disease (CAD). In various diseases, an increase in left atrial (LA) size has been associated with poor outcomes.1–5 In patients with CAD, LA enlargement is a known risk factor for an unfavorable cardiovascular prognosis.6 In addition, right atrial (RA) enlargement might indirectly contribute to this risk by affecting the systemic and coronary vasculature. Multidetector computed tomography (MDCT) enables 3-dimensional imaging of the heart and correlates well with results of echocardiography and magnetic resonance imaging (MRI).7 In this study, we used MDCT angiography to identify normal ranges of LA and RA volumes in men and women and to calculate the prevalence of atrial enlargement in individuals with CAD.
Patients and Methods
The study protocol and method of consent were approved by our institutional review board. We reviewed our clinical database of patients who had been examined for evaluation of coronary artery disease with use of computed tomographic angiography (CTA) in our department from November 2007 through December 2010. A total of 1,400 records were reviewed; any patient with a historical diagnosis of valvular disorders, atrial fibrillation, or pulmonary hypertension was excluded from consideration. The remaining 686 participants (Table I) subsequently underwent examination by means of electrocardiographic (ECG)-gated CTA. We estimated the presence and extent of CAD, and cardiac systolic function.
TABLE I.
Clinical and Demographic Characteristics of the Groups
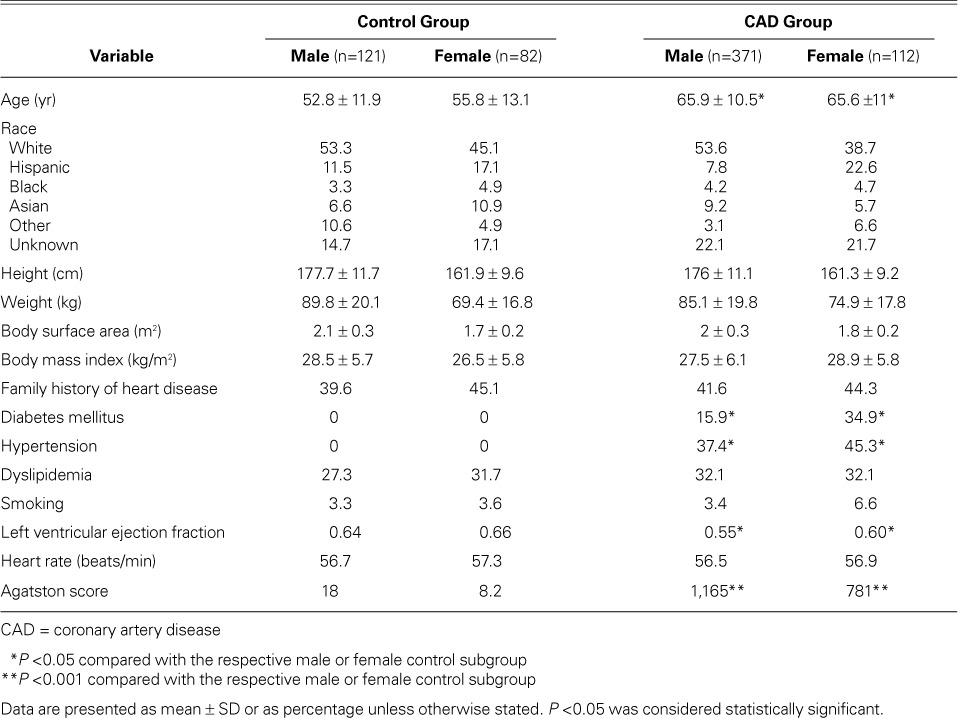
We then divided the study population into a control group (203 healthy subjects) and an obstructive-CAD group (483 patients) as diagnosed by CTA. Exclusionary criteria for the control group included a high coronary calcium burden (Agatston score, >100) and obstructive CAD as seen on CTA (>50% stenosis in any coronary artery), as well as conditions that could affect atrial diameters, including a history of hypertension (≥140 mmHg systolic pressure and ≥90 mmHg diastolic pressure), diabetes mellitus, congenital or rheumatic heart disease, lung disease, or kidney disease.
Coronary Artery Calcium Scanning and Angiographic Protocols
Scanning for Coronary Artery Calcium. All coronary arteries and their branches were imaged with 30 to 40 contiguous 2.5-mm slices during mid-diastole with use of ECG triggering during each participant's 10-second breath-hold. Coronary artery calcium was considered to be present when a density greater than 130 Hounsfield units was detected in 3 or more contiguous pixels (>1 mm2) overlying an artery. The calcium burden was quantified in accordance with Agatston score.
Cardiac Computed Tomographic Angiography. β-Blockers were given to participants whose heart rates were faster than 65 beats/min. As a measure of contrast timing, an intravenous bolus of 15 mL of contrast agent was followed by flushing with 20 mL of normal saline solution at a rate of 4.5 mL/s, followed by one sublingual puff of nitrate (400 μg) by each participant, 5 to 10 min before contrast injection. For all coronary CTA studies, we used a dual-head Medrad® Stellant® CT Injection System (Bayer HealthCare; Indianola, Pa) to perform retrospective ECG-gated cardiac CTA in a triphasic consecutive-injection sequence. This process began with the injection of 50 mL of iopamidol 370, a nonionic intravenous contrast material (Bracco Diagnostics, Inc.; Monroe Township, NJ), at a rate of 5 mL/s, followed by 50 mL of 60% contrast agent mixed with normal saline solution, and ending with a 50-mL flush of normal saline solution. Contrast material was injected through a 18G–20G angiocatheter in an antecubital vein. The mean heart rate of participants during scanning was 56 ± 6 beats/min.
Data Acquisition. For 64-slice MDCT, we used a GE 64 LightSpeed™ VCT (General Electric Medical Systems; Wausheka, Wisc). Imaging began 1 inch above the ostium of the left main coronary artery and continued to 1 inch below the inferior aspect of the heart. The following imaging and reconstruction limits were applied: collimation, 40 × 0.625 mm; 120 kVp; 220–670 mAs; pitch, 0.18–0.24; rotation time, 0.35 s; matrix, 512 × 512 pixels; and mean effective radiation dose, 11.4 ± 1.3 mSv (8–14.5 mSv). In each instance, ECG-triggered dose modulation was applied at 400 to 600 mA in a 60% to 80% R-R interval and at 250 to 350 mA for the rest of the cardiac cycle. The R-R interval of the next cycle was 59% to 81%. The cardiac data were reconstructed from 5% to 95% of the R-R interval at 10% intervals and 1.5 mm in slice thickness. The status of the coronary vessels was reviewed with use of AW VolumeShare™ (GE Medical Systems), and volume renderings and curved multiplanar reformations were produced. Each vessel was classified as normal (no stenosis or calcium), as having nonobstructive CAD (luminal stenosis, 1%–49%), or as having obstructive CAD (luminal stenosis, ≥50%). We evaluated vessels that were at least 1.5 mm in diameter. Two experienced cardiologists (MJB and SSM), who were blinded to the clinical data, separately evaluated the arteries. The arteries were grouped as follows: 1) left main coronary artery, left anterior descending coronary artery, and diagonal branches; 2) left circumflex coronary artery and obtuse marginal branches; and 3) right coronary artery, acute marginal branches, posterior descending artery, and posterolateral branch. Left or right dominance was determined.
Left Ventricular Evaluation. Left ventricular segmentation was completed by 2 physicians (YG and SSM) who were experienced in cardiac CT study. Global wall motion was estimated, and the end-systolic (minimum) and end-diastolic (maximum) phases were chosen. The epicardial and endocardial segmentation was manually completed at end-systole and end-diastole simultaneously. The AW 4.4 Workstation (GE Medical Systems) was used. In each study, 8 to 15 slice levels with axial images were traced; the remaining slices were traced automatically by computers. Volume was calculated by means of the modified Simpson method, in which the sum of cross-sectional areas was multiplied by the sum of slice thicknesses. Left ventricular systolic function was calculated accordingly.
Left and Right Atrial Measurement. By means of segmentation and calculation methods similar to those described above, all LA and RA epicardial segmentation was completed manually at end-systole (maximal in area) and end-diastole (minimal). The atrial appendages were included in the measurements. The pericardial border at the pulmonary-vein slice levels was traced as an LA boundary (Fig. 1). All segmentation was completed by 2 skilled physicians (YG and SSM). The precision test was conducted by GC and AC.
Fig. 1.
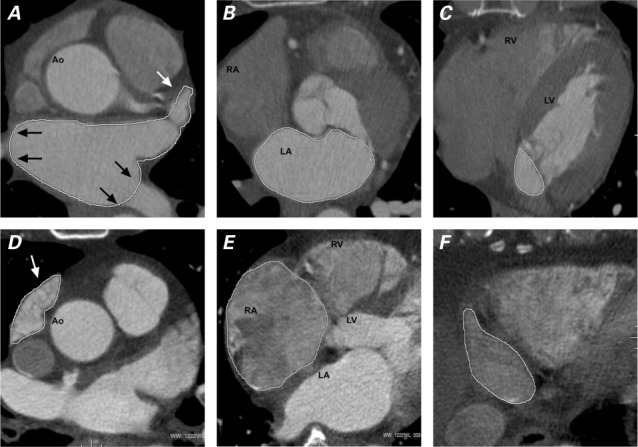
Axial computed tomographic angiograms at end-systole show manual tracings of A, B, C) the left atrium (LA) of one patient and D, E, F) the right atrium (RA) of another. These are representative slices from the top (A, D), mid (B, E), and bottom (C, F) levels of the atria. Both appendages were included for segmentation (white arrows). At the junction of the pulmonary vein and the LA body, the pericardial boundary was used as the border of the LA body (black arrows).
Ao = ascending aorta; LV = left ventricle; RV = right ventricle
Atrial Volume. In all instances, atrial volume was indexed to body surface area (BSA) as recommended by the American Society of Echocardiography.8
Atrial Stroke Volume and Atrial Ejection Fraction. Atrial stroke volume (ASV) was calculated from measurements of end-systolic volume (ESV) and end-diastolic volume (EDV) as follows:
Atrial ejection fraction (AEF) was calculated from measurements of ASV and atrial end-systolic volume (AESV) as follows:
Statistical Analysis
Continuous variables were expressed as mean ± SD among men and women separately in the 2 groups. Bonferroni-adjusted t tests were applied, and all values were adjusted for age. In the control group, means were compared between both sexes. The CAD and control groups were compared. The value of mean ± 2 SD was used as an upper or lower limit. The prevalence of LA enlargement and decrease of biatrial ejection fraction, and the relative ratio of all values compared with the control group, were computed in the CAD group. The variation of interobserver measurement for biatrial ESV was calculated as follows and represented the precision error: abs (A+B)/[0.5 × (A+B)] × 100%.
P <0.05 was considered statistically significant. All statistical analyses were performed by DL with use of SAS 9.3® software (SAS Institute Inc.; Cary, NC).
Results
The mean reference values of the left and right atrial indexed volumes, as well as the reference values for stroke volumes and ejection fractions, were derived in the control group (Table II). In that group, the women had significantly higher LA volumes than the men (P=0.01) but had similar RA volumes. In comparison with the control subgroups for each sex, LA volumes in the CAD group were significantly higher; RA stroke volumes and biatrial ejection fractions were significantly lower, except for left AEF in men; and neither RA volume nor LA stroke volume was significantly different.
TABLE II.
Left and Right Atrial Values in the Groups, Indexed to Body Surface Area
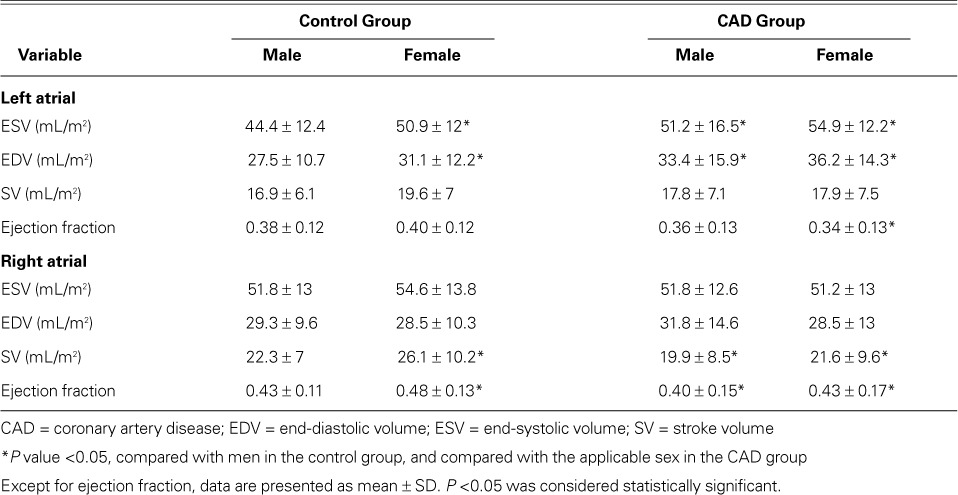
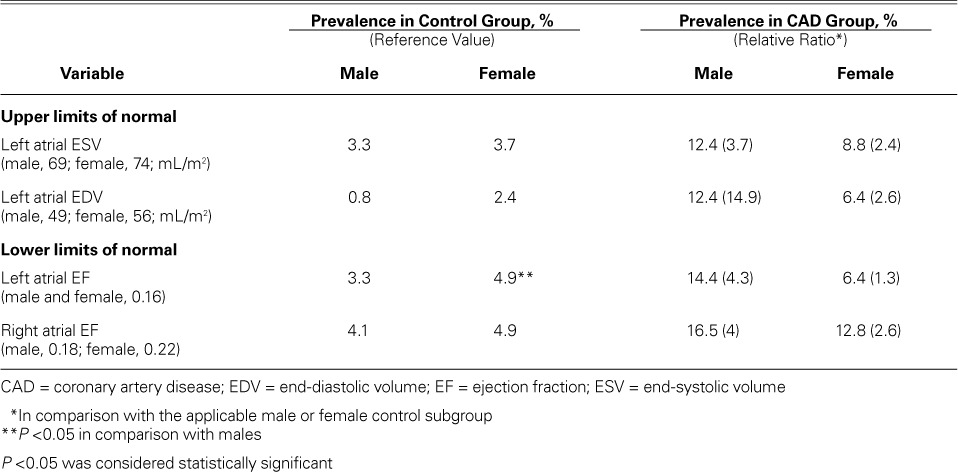
Table III shows the upper limits of LA enlargement and lower limits of biatrial ejection fraction. According to those limits, men in the CAD group had a prevalence of abnormal left atrial ESV of 12.4%; a prevalence of abnormal left atrial EDV of 12.4%; of left AEF, 14.4%; and of right AEF, 16.5%. In women, the corresponding values for each of these variables were 8.8%, 6.4%, 6.4%, and 12.8%.
TABLE III.
Prevalence of Left Atrial Enlargement and Atrial Ejection Fraction Decrease in the Groups
The precision error for left atrial ESV was 4.8% ± 3.5% (r =0.97, P <0.001); the error for right atrial ESV was 5.8% ± 4.1% (r =0.96, P <0.001).
Discussion
Atrial volumes have substantial clinical significance. Echocardiography is most often used for measurement. Modern MDCT systems are capable of obtaining high-quality data for the volumetric evaluation of heart chambers, including the atria. Our study revealed a significant difference in left atrial ESV and EDV between men and women in the control group. In comparison, the CAD group had significantly higher LA volume and significantly lower biatrial ejection fraction, except for left AEF in men. This result implies that a sex-specific reference value is necessary for LA volumetric evaluation, and that LA volume and biatrial ejection fraction (excluding left AEF in men) might be used for diagnosis and prognosis in patients with CAD.
A small MDCT study of 103 healthy individuals9 yielded volume-indexed measurements of 54.4 ± 11.9 mL/m2 for left atrial ESV and 59.7 ± 15 mL/m2 for right atrial ESV, slightly larger than the normal mean values in our control group (left atrial ESV, men 44.4 ± 12.4 mL/m2 and women 50.9 ± 12 mL/m2; right atrial ESV, men 51.8 ± 13 mL/m2 and women 54.6 ± 13.8 mL/m2). This might be because of different populations or measurement techniques.
Studies have shown that LA volumetric measurements are more accurate than linear-dimension measurements in evaluating asymmetric enlargement of the LA, a condition usually caused by restriction of the anteroposterior dimension of the thoracic cavity.8 Left atrial volume is also a better predictor of cardiovascular disease than is LA linear dimension.10 Multidetector computed tomography has proved to be a good tool that correlates with results of MRI and echocardiography in ventricular volumetric measurement.6 As echocardiographically measured, LA volume was compared with cine computed tomography, biplane contrast ventriculography, and MRI, and echocardiography was proved to have good correlation or underestimation.11,12
Left atrial volume was echocardiographically measured in the Framingham Heart Study cohort. In those 1,099 nonobese subjects (20–45 years of age, of average height, and with no cardiovascular disease), LA volume was 22 to 52 mL in women versus 18 to 58 mL in men, whereas the corresponding volume indexed to BSA was 22 ± 6 mL in both sexes.8 In another echocardiographic study of 767 healthy individuals,10 LA volumes in men ranged from 29 to 69 mL and in women from 23 to 54 mL. Echocardiography underestimates true atrial volumes, because it cannot procure images of the LA to the origins of the pulmonary veins. Accordingly, MDCT normal values are much more consistent with those yielded by MRI than with those of echocardiography. Contrast or 3-dimensional echocardiography might enable better viewing of the entire atria and more accurate determination of volumes.
Body size and age have a relation to LA size. Sex variations in LA size are also attributed to body size,10,13 and indeed we found a significant difference in the LA volumes indexed to BSA among men versus women. Individuals with an enlarged LA have an increased risk of adverse cardiovascular events.14,15
Comparatively few studies have focused on measuring RA size. Right atrial size can vary by sex; however, the American Society of Echocardiography has not recommended separate reference values. In a study of 54 healthy individuals,16 indexed RA volumes appeared to be slightly smaller in women. No significant difference in RA volume between men and women was found in the control group in that study, implying that the co-reference value can be used for RA volume evaluation with use of CTA. The right ASV and right AEF were significantly lower in the CAD groups. The investigators suggested that right ASV and right AEF might enable diagnosis and prognosis in patients with CAD.16
Multidetector computed tomography can be used in volumetric quantification of the atrium on contrast and noncontrast scans.17 Left atrial measurements should be reported with the MDCT angiographic results, to better evaluate prognosis in high-risk patients. The data can be used as a baseline for future studies into the indexed atrial measurements obtained with use of 64-slice MDCT. Large, randomized clinical trials will be necessary to establish the normal range and importance of biatrial volume measurements in cardiac CTA.17–19 In view of the increasing use of MDCT imaging in preventive and diagnostic cardiology,20,21 atrial volumetric measurement can be performed at no additional participant burden.22 We used the retrospective triggered helical scan to evaluate coronary arterial and cardiac function. The radiation doses in our study were much higher (8–14.5 mSv) than those in contemporary studies, because at the time of acquisition we lacked current dose-reduction algorithms, including prospective triggering, low kVp, and iterative reconstruction.23 The International Commission on Radiological Protection has documented that CT radiation doses approaching or exceeding 50 mSv can result in an increase in cancer, and CT doses are always well below that level.24 Although acquiring atrial volumes requires no additional scanning or radiation, measurement does add time to the conventional analysis of coronary arteries on coronary CTA. Furthermore, automated software can detect and measure the volumes, in most cases. Nonetheless, from a practical standpoint, atrial volumetric evaluation can be a valuable additional measurement with no increased radiation, participant burden, or extra scanning.
Limitations of the Study
An important limitation of our study—one true in all CTA studies—was our inability to measure Doppler values of LA enlargement along with measurements of LA pressure. The tricuspid valve, the junction of the RA with the superior or inferior vena cava, and the junction of the pulmonary vein with the LA could not be delineated clearly, and this affected accurate, precise segmentation despite high spatial and contrast resolution. Regardless, we think that MDCT evaluation of biatrial volumetric values affords meaningful diagnostic and prognostic benefits in patients who have obstructive CAD.
Footnotes
Dr. Budoff is on the speakers bureau for General Electric Medical Systems.
References
- 1.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47(12):2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 2.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90(12):1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 3.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151(2):412–8. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Kim BS, Lee HJ, Kim JH, Jang HS, Bae BS, Kang HJ et al. Relationship between left atrial size and stroke in patients with sinus rhythm and preserved systolic function. Korean J Intern Med. 2009;24(1):24–32. doi: 10.3904/kjim.2009.24.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas PS. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;42(7):1206–7. doi: 10.1016/s0735-1097(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsui JM, Dourado PM, Elhendy A, Falcao SN, Goes RM, Chagas AC et al. Prognostic value of left atrial volume in patients who underwent dobutamine stress echocardiography for known or suspected coronary artery disease. Am Heart J. 2008;156(6):1110–6. doi: 10.1016/j.ahj.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 7.van der Vleuten PA, Willems TP, Gotte MJ, Tio RA, Greuter MJ, Zijlstra F, Oudkerk M. Quantification of global left ventricular function: comparison of multidetector computed tomography and magnetic resonance imaging. A meta-analysis and review of the current literature. Acta Radiol. 2006;47(10):1049–57. doi: 10.1080/02841850600977760. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008;1(6):782–6. doi: 10.1016/j.jcmg.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41(6):1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 11.Kircher B, Abbott JA, Pau S, Gould RG, Himelman RB, Higgins CB et al. Left atrial volume determination by biplane two-dimensional echocardiography: validation by cine computed tomography. Am Heart J. 1991;121(3 Pt 1):864–71. doi: 10.1016/0002-8703(91)90200-2. [DOI] [PubMed] [Google Scholar]
- 12.Vandenberg BF, Weiss RM, Kinzey J, Acker M, Stark CA, Stanford W et al. Comparison of left atrial volume by two-dimensional echocardiography and cine-computed tomography. Am J Cardiol. 1995;75(10):754–7. doi: 10.1016/s0002-9149(99)80676-6. [DOI] [PubMed] [Google Scholar]
- 13.Spencer KT, Mor-Avi V, Gorcsan J, 3rd, DeMaria AN, Kimball TR, Monaghan MJ et al. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi-institution acoustic quantification study. Heart. 2001;85(3):272–7. doi: 10.1136/heart.85.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beinart R, Boyko V, Schwammenthal E, Kuperstein R, Sagie A, Hod H et al. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol. 2004;44(2):327–34. doi: 10.1016/j.jacc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79(8):1008–14. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Gutman JM, Heilbron D, Wahr D, Schiller NB. Atrial volume in a normal adult population by two-dimensional echocardiography. Chest. 1984;86(4):595–601. doi: 10.1378/chest.86.4.595. [DOI] [PubMed] [Google Scholar]
- 17.Budoff MJ, Mao S, Wang S, Bakhsheshi H, Brundage BH. Simple single-section method for measurement of left and right atrial volumes with electron-beam CT. Acad Radiol. 1999;6(8):481–6. doi: 10.1016/s1076-6332(99)80167-6. [DOI] [PubMed] [Google Scholar]
- 18.Shinbane JS, Girsky MJ, Chau A, Mao S, Budoff MJ. Three-dimensional computed tomography imaging of left atrial anatomy for atrial fibrillation ablation. Clin Cardiol. 2005;28(2):100. doi: 10.1002/clc.4960280211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budoff MJ, Zhiroff K, Young E, Wong C, Liu ST, Gopal A et al. The measurement of left atrial volume by cardiac computed tomography [abstract] J Nuc Cardiol. 2007;14(2):S91. [Google Scholar]
- 20.Budoff MJ. Computed tomography: new horizons. Tex Heart Inst J. 2006;33(2):197–200. [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi RT, Sarraf G, Budoff M. Isolated noncompaction of the left ventricular myocardium diagnosed upon cardiovascular multidetector computed tomography. Tex Heart Inst J. 2010;37(3):374–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Budoff MJ, Shittu A, Hacioglu Y, Gang E, Li D, Bhatia H et al. Comparison of transesophageal echocardiography versus computed tomography for detection of left atrial appendage filling defect (thrombus) Am J Cardiol. 2014;113(1):173–7. doi: 10.1016/j.amjcard.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000;154(2):178–86. doi: 10.1667/0033-7587(2000)154[0178:rrcral]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Managing patient dose in computed tomography. A report of the International Commission on Radiological Protection. Ann ICRP. 2000;30(4):7–45. doi: 10.1016/s0146-6453(01)00049-5. [DOI] [PubMed] [Google Scholar]


