Abstract
Since its first description more than 20 years ago osteopontin has emerged as an active player in many physiological and pathological processes, including biomineralization, tissue remodeling and inflammation. As an extracellular matrix protein and proinflammatory cytokine osteopontin is thought to facilitate the recruitment of monocytes/macrophages and to mediate cytokine secretion in leukocytes. Modulation of immune cell response by osteopontin has been associated with various inflammatory diseases and may play a pivotal role in the development of adipose tissue inflammation and insulin resistance. Here we summarize recent findings on the role of osteopontin in metabolic disorders, particularly focusing on diabetes and obesity.
Keywords: Osteopontin, Biomineralization, Tissue remodeling, Inflammation, Obesity, Insulin resistance, Insulin sensitivity, Non-alcoholic fatty liver disease, Diabetic nephropathy
1. Introduction
Osteopontin (OPN), also known as secreted phosphoprotein 1 (SPP 1), 44 kDa bone phosphoprotein, sialoprotein 1, 2ar, uropontin, and early T-lymphocyte activation-1 (Eta-1) is a secreted matricellular protein that was first identified in 1985 by Heingard et al. as sialoprotein derived from bovine bone matrix [1]. The most commonly used name osteopontin is derived from “osteon”, the Greek word for bone, and “pons”, the Latin word for bridge illustrating its function as a linking protein and crucial factor in bone homeostasis [2].
OPN is a negatively charged aspartic acid-rich, N-linked glycosylated phosphoprotein composed of 314 amino acid residues [3–5]. The human gene for OPN has been localized on the long arm of chromosome 4q13 directly related to four similar genes encoding for bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP) and matrix extracellular phosphoglycoprotein (MEPE) [6,7]. Due to common functional motifs and domains these five integrin-binding glycophosphoproteins are categorized as the so called SIBLING proteins (small integrin-binding ligand N-linked glycoproteins) [8]. OPN is encoded by a single copy gene, but exists in various isoforms as a result of alternative splicing, alternative translation and different posttranslational modifications (PTMs), which allow for a molecular weight ranging from 41 to 75 kDa [9–13]. To date three splice variants of the human OPN transcript have been identified: OPN a, the full-length isoform; OPN b which lacks exon 5 and OPN c which lacks exon 4 [11]. OPN has primarily been described as a secreted protein involved in several physiological as well as pathological events. However, current evidence suggests that OPN can also be found in the cytoplasm and the nucleus [14]. This form of intracellular OPN (iOPN) is the result of alternative translation and has biological functions distinct from those of secreted OPN (sOPN) [15].
OPN is expressed in various cell types and tissues including preosteoblasts, osteoblasts, osteocytes [16], chondrocytes [17], fibroblasts [18], dendritic cells, macrophages and T-cells [19], hepatocytes [20], smooth muscle cells [21], skeletal muscle [22], endothelial cells [23], inner ear [24], brain [25], placenta and mammary glands [26], deciduum and kidney [5,27]. Extracellular OPN functions through its interactions with multiple cell surface receptors, including various integrins (αvβ1, αvβ3, αvβ5, αvβ6, α4β1, α5β1, α8β1, and α9β1) and CD44 [15,16] thereby regulating cellular processes such as biomineralization, tissue remodeling and immune regulation [4,28,29]. Abundant evidence suggests that OPN plays a critical role in chronic inflammatory diseases, including multiple sclerosis [30], Crohn's disease [31] and other autoimmune disorders [32,33], several types of cancer [34–36] and cardiovascular diseases [37–40]. Furthermore, OPN may play a pivotal role in the development of adipose tissue inflammation and insulin resistance [29,41]. In this review, we will summarize the current knowledge on the role of OPN in metabolic disorders, particularly focusing on diabetes and obesity.
2. OPN in biomineralization
It is now well recognized that one major physiological function of OPN is the control of biomineralization. As a member of the SIBLING protein family with overall negative charge, OPN is able to directly bind to specific apatite crystal faces thereby governing its function as a mineralization inhibitor [4,42]. Hence, OPN−/− bones unlike those from wild type mice are hypermineralized and more fragile [4,43]. Furthermore, OPN is not only critical for bone mineralization, but is also strongly upregulated at sites of ectopic, pathologic calcification – such as vascular calcification [44], valvular calcification [45], renal crystal formation [46], and gallstone formation [47].
3. OPN in tissue remodeling processes
Although it is not required for normal bone formation and development, OPN participates as an essential component in the bone remodeling process [48,49]. Bone cells secrete OPN physiologically during the process of bone remodeling and increase OPN expression in response to mechanical stimuli [50,51]. OPN appears to stimulate adhesion, migration and bone resorption by osteoclasts [52]. OPN function in osteoclasts involves the stimulation of CD44 expression on the osteoclast surface, which was shown to be required for osteoclast motility and bone resorption. Consistently, resorption of ectopic bone is substantially impaired in the absence of OPN [53]. OPN has also been shown to regulate remodeling of soft tissues in response to pathologic stimuli. For example, in heart failure and cardiac remodeling there appears to be a sophisticated balance of OPN expression: strongly increased levels of OPN may induce deleterious fibrosis and hypertrophy while sufficient levels of OPN are needed to prevent left ventricular (LV) dilatation [37].
4. OPN in inflammation
Multiple studies have demonstrated that OPN is expressed by inflammatory cells such as macrophages and highly induced during inflammatory activation [19,54].
OPN appears to be constitutively expressed but in all cells studied it is rapidly upregulated following cellular activation by a variety of growth factors and cytokines (including LPS, NO, Ang II, IL-1β, IL-2, IL-3, IFN-γ, TNF-α, TGFβ) [55,56]. Until this date the molecular mechanisms that regulate OPN expression in macrophages during inflammation remain incompletely understood. The OPN promoter is remarkably responsive and contains various motifs including a purine-rich sequence, an ETS-like sequence, glucocorticoid and vitamin D response elements, and IFN-inducible elements [57,58]. In LPS-stimulated macrophages OPN expression was shown to be upregulated by activation of phosphoinositide 3-kinase (PI3K), extracellular signal-regulated kinase (ERK), and c-Jun NH2-terminal kinase (JNK). Furthermore, chromatin immunoprecipitation (ChIP) assays revealed that activator protein 1 (AP-1) binds to the proximal AP-1 site in the OPN promoter from LPS-stimulated macrophages [59]. We could demonstrate that cytokine-induced OPN expression in macrophages depends on AP-1 binding to a CCTCATGAC cognate (AP-1 consensus element) in the proximal OPN promotor between -80 and -71. Stimulation of macrophages with LXR ligands suppressed cytokine-induced OPN expression by inhibition of c-Jun/c-Fos DNA binding activities to the proximal OPN promotor, which impairs AP-1-dependent OPN transcription [60]. Recent work provided evidence that NF-κB also plays an important role during LPS-stimulated OPN expression through binding to a cis-regulatory element (GGAATTCCC between nt – 1817 and nt – 1808) in the distal OPN promotor in macrophages. Interestingly, LPS stimulation induced chromosomal loops in the OPN promotor between the NF-κB binding site and the AP-1 binding site involving the coactivator p300. These results identified an essential mechanism to establish higher order chromatin structure to regulate LPS-induced OPN expression [61]. Work by Oyama and co-workers demonstrated that phorbol 12-myristate 13-acetate (PMA)-induced OPN expression is significantly decreased by troglitazone and other PPARγ ligands in macrophages. Further experiments showed that PPARγ inhibits the OPN promoter activity, and the PPARγ-responsive region within the OPN promoter lies between -1000 and -970 relative to the transcription start site. Site-specific mutation analysis and electrophoretic mobility shift assays indicated that a homeobox-like A/T-rich sequence between -990 and -981, which functions as a binding site for PMA-induced nuclear factors other than PPARγ, mediates the repression of OPN expression by troglitazone. Moreover, concatenated A/T-rich sequences conferred the PPARγ responsiveness on the heterologous promoter. All in all, these data suggest that PPARγ ligand inhibits OPN gene expression through the interference with the binding of nuclear factors to A/T-rich sequence in macrophages [62].
It is now well recognized that OPN controls immune cell functions including monocyte adhesion [63], migration [64], differentiation [65], and phagocytosis [4,15,66]. The induction of monocyte and macrophage chemotaxis and cellular motility as well as migration by OPN occurs via direct interaction with several different cell surface receptors [67–69]. This interaction is mostly mediated by two different binding domains. As mentioned above, OPN interacts with αvβ1, αvβ3, αvβ5, αvβ6, α8β1 and α5β1 integrins through its RGD domain, while the SLAYGLR (SVVYGLR in human OPN) domain facilitates binding to α9β1, α4β1 and α4β7 integrins. Furthermore, OPN has been identified to interact with the CD44 hyaluronic acid receptor [54]. Additionally, OPN induces the expression of matrix metalloproteinase (MMP), in particular MMP-2 and MMP-9 [39,64,70]. Since these proteinases are important in degrading matrix for migrating cells, this represents an alternative mechanism by which OPN may profoundly enhance cellular migration. In vivo, evidence for OPN regulating monocyte/macrophage recruitment to sites of inflammation was provided by studies using either blocking antibodies or genetic approaches. Neutralizing antibodies to OPN diminished intradermal macrophage infiltration in response to a chemotactic peptide [63] and monocyte migration into joints leading to an inhibition of rheumatoid arthritis [71]. Impaired leukocyte recruitment in OPN−/− mice has further been demonstrated in a variety of different inflammatory disease processes [39,72–76]. In all these studies, OPN−/− mice consistently exhibited diminished leukocyte recruitment at sites of inflammation demonstrating the pivotal role of OPN to regulate leukocyte attraction during inflammation. OPN is not only critical for macrophage recruitment, but also regulates the secretion of cytokines during cell-mediated immunity [4]. OPN itself activates the transcription factors NF-κB and AP-1 and thereby potentially modulates the expression of a variety of inflammatory genes [77]. The engagement of CD44 and αvβ3 integrin by OPN induces PI3-kinase dependent Akt phosphorylation and enhances the interaction between phosphorylated Akt and IKKα/β. OPN also enhances NF-κB activation through phosphorylation and degradation of IκBα by inducing the IKKα/β activity [78,79]. Furthermore, SFK (Src family of tyrosine kinases) kinase activity was found to be required for integrin αvβ3-mediated NF-κB activation [80]. OPN-mediated activation of AP-1 is mediated by nuclear factor-inducing kinase (NIK)–ERK (extracellular signal-related kinase) and MEKK1 (also known as mitogen-activated protein kinase kinase kinase 1 (MAP3K1)–JNK1 (also known as MAPK8)) signaling. Upon binding to αvβ3, OPN also stimulates epidermal growth factor receptor (EGFR) transactivation and ERK phosphorylation [8]. Further studies revealed that OPN regulates crosstalk between NF-κB and AP-1 by p70S6K/mTOR phosphorylation which is unidirectional towards AP-1 that in turn regulates intercellular adhesion molecule-1 (ICAM-1) expression [77].
Polarization of Th cells to the Th1 or Th2 phenotypes, a critical aspect of cell-mediated immunity, is influenced by OPN which enhances Th1 and inhibits Th2 cytokine expression [75]. OPN induces macrophages to express IL-12 and stimulates T-cells to express INF-γ and CD40 ligand, which subsequently induces IL-12 expression from monocytes [75,81]. Thus, OPN provides an important early stimulus for IL-12 production at sites of inflammation. OPN further inhibits IL-10 expression by macrophages and thereby decreases anti-inflammatory signaling pathways [39,75]. Collectively, these in vivo studies support altered innate immune responses in OPN−/− mice, consistent with the well-described regulation of monocyte/macrophage migration and invasion by OPN, and point to an underappreciated role of OPN as a potent mediator of cellular immunity (Figure 1).
Figure 1.
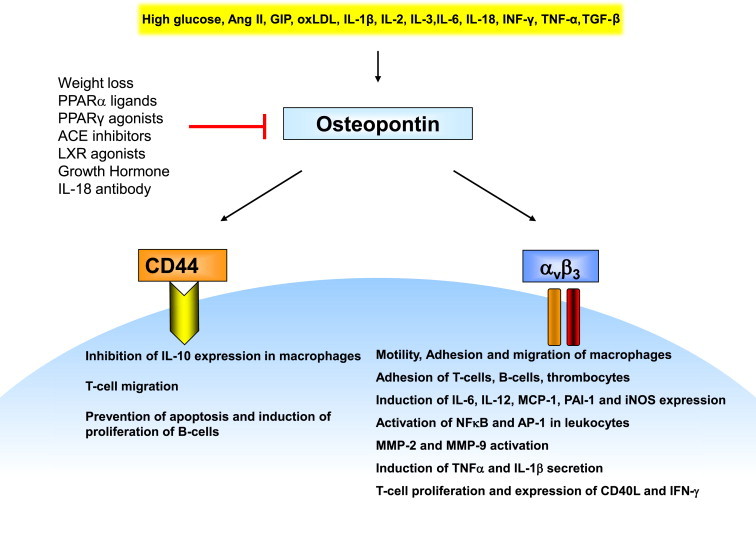
OPN is secreted by activated macrophages and T-cells and has been shown to be an important component of early cellular immune responses and inflammation. These proinflammatory effects of OPN are mediated through engagement of a number of receptors. Of particular interest are the integrin receptor αvβ3 and the CD44 receptor. Ligation to these receptors results in important proinflammatory functions allowing OPN to mediate the recruitment and activation of leukocytes at sites of inflammation.
5. OPN in obesity and diabetes
Obesity constitutes the major risk factor for the development of insulin resistance, type 2 diabetes and subsequent diabetes-related complications such as micro- and macrovascular disease [82]. Chronic low-grade inflammation has been described as a fundamental component of adipose tissue expansion in obesity. Inflamed adipose tissue is characterized by enhanced secretion of cytokines and recruitment of leukocytes, in particular macrophages [83]. Current evidence suggests that these cytokines, often referred to as adipokines, including resistin, visfatin, apelin, omentin, chemerin, IL-6, MCP-1, PAI-1, or TNF-α link obesity to the development of systemic insulin resistance [84]. Several studies have described OPN as a critical regulator of adipose tissue inflammation, insulin resistance and diabetes mellitus (Figure 2). OPN expression is drastically upregulated by 40 and 80-fold in adipose tissue from diet-induced and genetically obese mice, respectively [85]. Furthermore, mice exposed to a high-fat-diet (HFD) exhibited elevated OPN expression in macrophages recruited to the adipose tissue [29]. Using loss-of function approaches we [29] and others [41] reported that HFD-induced adipose tissue macrophage infiltration and inflammatory gene expression were markedly blunted in OPN−/− mice [29]. A similar phenotype could be observed following treatment with an OPN-neutralizing antibody [29,41]. Adipose tissue expression of IL-6, TNF-α, MCP-1 and iNOS as well as IL-6, MCP-1 and PAI-1 plasma levels were significantly reduced in mice lacking the OPN gene [29]. Importantly, OPN deficiency not only led to decreased adipose tissue inflammation, but also improved whole-body glucose tolerance and reduced insulin resistance in mice independent from body composition or energy expenditure [29,41]. This protection from metabolic deterioration by OPN depletion is already apparent after only two weeks of HFD feeding [86].
Figure 2.
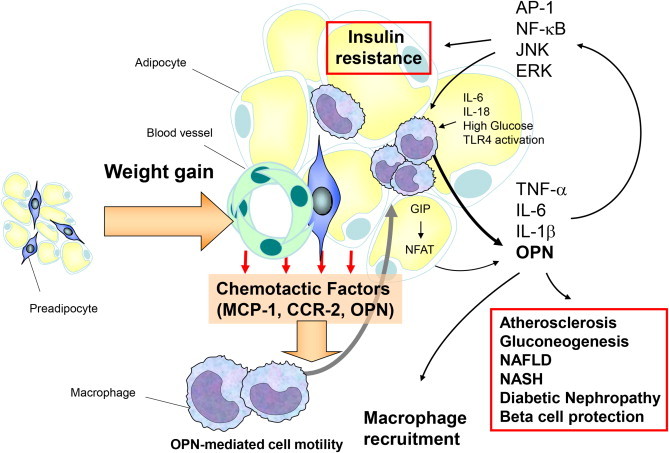
During diet-induced weight gain OPN is upregulated and mediates macrophage infiltration into adipose tissue. OPN expression in adipose tissue macrophages is enhanced by high glucose, TLR4 activation, IL-6 and IL-18. In adipocytes GIP increases OPN secretion. OPN itself activates several inflammatory signaling pathways leading to adipose tissue insulin resistance and type 2 diabetes. Furthermore, OPN was shown to directly increase atherosclerosis, NAFLD, NASH and diabetic nephropathy.
Although OPN protein expression can be induced by a variety of growth factors and cytokines [55], the mechanisms by which OPN is upregulated in adipose tissue inflammation remains incompletely understood. Analysis of adipose tissue cellular fractions revealed that the main source of OPN in human and murine genetic and diet-induced obesity are adipose tissue macrophages (ATM) [29,85]. First mechanistic insights were provided by Samuvel et al. who incubated mononuclear cells with adipocytes in a transwell system and studied how cell interaction regulated OPN expression. OPN expression in mononuclear cells was markedly increased when cocultured with adipocytes. In addition, LPS-induced TLR4 activation and high glucose further augmented coculture-stimulated OPN secretion. Neutralizing antibodies against IL-6 or siRNA mediated IL-6 knockdown in adipocytes diminished LPS mediated OPN expression in mononuclear cells in coculture with adipocytes. These results suggest, that IL-6 released from adipocytes might regulate OPN secretion from immune cells during adipose tissue inflammation [87]. Recently, Lu et al. showed that macrophage-specific growth hormone (GH) receptor-null mice (MacGHR KO) challenged with HFD exhibited impaired glucose and insulin tolerance, which was paralleled by increased adipose tissue inflammation and OPN expression. Further experiments established that GH, acting via a NF-κB site in the distal OPN promotor directly inhibits OPN promoter activity and expression [88]. Therefore administration of GH could have beneficial effects on HFD-induced adipose tissue inflammation and insulin resistance.
Although OPN expression during adipose tissue inflammation is primarily mediated by immune cells, recent work shows that the incretin hormone GIP (Glucose dependent insulinotropic peptide) increases OPN expression in primary adipocytes. Mechanistically, GIP-induced upregulation of OPN expression is mediated by a crosstalk with insulin signaling pathways, the transcriptional factor NFAT and the cAMP/PDE3B system [83,89]. Remarkably, a genetic variant with reduced GIP receptor function is correlated with lower OPN expression and improved insulin sensitivity in humans [83]. Both studies suggest a novel link between the incretin hormone GIP and OPN regulation in adipose tissue.
All these findings provide novel insights into the regulation of OPN expression in adipose tissue and support further research to elucidate the distinct molecular pathways regulating OPN secretion during HFD-induced obesity.
Until this date not much is known about the pathophysiological impact of the different OPN isoforms in terms of leukocyte recruitment and cytokine signaling in adipose tissue. Recent evidence suggests that highly phosphorylated OPN isoforms have significantly reduced capabilities to promote cell adhesion via the αvβ3-integrin receptor [90]. Further studies showed upregulation of alkaline phosphatases during adipogenesis [91] and revealed altered OPN isoform expression in adipose tissue following high-fat feeding [86]. These first clues suggest that the phosphorylation state of different OPN isoforms might regulate OPN function during adipose tissue inflammation in terms of diet-induced obesity. Future studies are greatly needed to confirm this hypothesis.
Further approaches analyzed whether OPN could be causally involved in the pathogenesis of type 2 diabetes. First clues were provided by studies of Chapman et al. revealing a reduction of HFD-induced hyperleptinemia and reduced adipocyte hypertrophy in OPN−/− mice [86]. In human adipose tissue several OPN receptor chains have been detected on adipocytes and non-adipocytes at high levels. In contrast to integrin chains αv, β1, and β5, which are expressed in adipocytes, in the adipose-derived stromal vascular fraction (dSVF) and in ATM, α9 and CD44 were expressed only in non-adipocytes (dSVC). Integrins α4 and α8 were selectively detected in ATM and dSVC, respectively. The β3 chain was only marginally found exclusively in dSVC. Interestingly, OPN was found to directly stimulate inflammatory signaling pathways and secretion of cytokines in isolated human adipose tissue macrophages and adipocytes [92]. Analysis of signaling molecules revealed a substantial phosphorylation of Akt, p38 MAPK, and ERK, as well as degradation of IκB-α following OPN stimulation in human macrophages, whereas phosphorylation of JNK was only less affected. OPN further upregulates TNF-α and MCP-1 expression in human macrophages. In human adipocytes OPN impaired differentiation and insulin sensitivity of primary adipocytes as determined by peroxisomal proliferator-activated receptor-γ (PPARγ) and adiponectin gene expression and insulin-stimulated glucose uptake. Furthermore, in adipocytes OPN induces phosphorylation of JNK and ERK while leaving the NF-κB pathway as well as phosphorylation of Akt and p38 MAPK virtually unaffected [92]. These results identified OPN as a key component in the development of HFD-induced insulin resistance.
Since OPN has been established as a crucial component in the development of experimental adipose tissue inflammation and insulin resistance, a number of human studies have focused on its role in patients with obesity and type 2 diabetes. First clinical approaches yielded results similar to rodent studies. Likewise, OPN expression in adipose tissue as well as circulating OPN levels were substantially elevated in obese patients compared with lean subjects, and were further increased in obese diabetic or insulin resistant patients [85,93–95]. Conversely, dietary weight loss significantly decreased OPN concentrations [93,96]. Surprising results were found in four studies analyzing OPN levels in morbidly obese patients before and after bariatric surgery. Subjects who previously underwent gastric banding or Roux-en Y gastric bypass exhibited decreased body weight, body mass index, inflammation markers as well as reduced insulin resistance. However, all studies consistently reported a gradual increase of OPN blood concentrations after bariatric surgery [97–100]. Further investigations are needed to differentiate whether these changes are secondary to alterations of bone metabolism or an adaption to weight loss. We previously demonstrated that OPN expression in human macrophages is upregulated by a variety of proinflammatory mediators known to be elevated in type 2 diabetes and cardiovascular disease, including TNF-α, IL-6, and oxidized LDL [101]. This induction of OPN expression in macrophages was suppressed by the PPARα ligands bezafibrate and WY14643 through a mechanism involving an inhibition of AP-1-dependent transactivation of the proximal OPN promoter. Finally, using a translational approach we observed decreased OPN plasma levels in type 2 diabetic patients after short-term treatment with bezafibrate [101]. More recently, Ahmad et al. reported simultaneous upregulation of IL-18 and OPN in PBMCs (peripheral blood mononuclear cells) from obese individuals compared to lean group. Intriguingly, treatment with a neutralizing IL-18 antibody diminished OPN secretion from PBMCs, indicating that IL-18 regulates OPN expression [94]. These findings point toward a specific pathophysiological role of OPN also in human inflammatory processes linked to obesity-induced adipose inflammation and insulin resistance. Therefore, pharmacological inhibition of OPN expression might be a novel approach for the treatment of type 2 diabetes and its complications.
As mentioned above it has been widely accepted that local OPN expression is strongly elevated in adipose tissue during the development of HFD-induced obesity [29,85,93]. However, several approaches analyzing systemic circulating OPN levels yielded inconclusive results. We and others found a slight but significant increase of systemic OPN levels in HFD-induced obese mice [29,97], whereas another group reported no difference in OPN plasma levels in a murine model of diet-induced obesity [85]. Nevertheless, a number of human studies consistently reported elevated circulating OPN levels in obese individuals compared with lean subjects [85,93,94,97]. Surprisingly, although weight loss was paralleled by a decrease of circulating OPN levels, there was no correlation between serum OPN and body fat percentage [96]. Kiefer and co-workers further showed that OPN expression in adipose tissue is not correlated with plasma OPN levels in obese patients [85]. This was strengthened by work from Bertola et al. who reported a substantial increase of circulating OPN levels despite downregulation of local OPN expression in white adipose tissue in obese patients who underwent bariatric surgery [97]. Consistently, elegant studies analyzing arteriovenous differences across adipose tissue from obese subjects revealed that adipose tissue does not secret OPN [97], indicating that local concentrations of OPN in adipose tissue do not appear to affect its systemic levels. Future studies are greatly needed to reveal the organ origin of circulating OPN levels in diet-induced obesity.
6. OPN in NASH
Obesity and type 2 diabetes are strongly associated with a spectrum of hepatic disorders collectively referred to as non-alcoholic fatty liver disease (NAFLD). NAFLD spans a spectrum from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH) and steatofibrosis ultimately leading to liver cirrhosis and hepatocellular carcinoma [97]. Notably, OPN expression is also markedly upregulated in the liver in obesity, and hepatic OPN levels correlate with liver triacylglycerol levels [97,102]. Within the liver, increased OPN expression was mainly found in hepatocytes and inflammatory cells [103]. In addition, studies from Sahai et al. suggest a role for OPN in the development of NAFLD in mice fed a methionine- and choline-deficient diet [104,105]. This was further supported by three experimental studies demonstrating that antibody-mediated OPN neutralization and OPN deficiency protect against HFD-induced hepatic macrophage infiltration [41,102] and d-galactosamine-induced inflammatory liver injury [103]. Consistently, OPN−/− mice were protected from obesity-induced hepatic steatosis which was mediated, at least in part, through diminished hepatic triacylglycerol synthesis. Euglycemic–hyperinsulinemic clamp studies exhibited that insulin resistance and excess hepatic gluconeogenesis in obesity were significantly attenuated in OPN−/− mice. OPN deficiency markedly improved hepatic insulin signaling as shown by enhanced Akt and IRS-2 (insulin receptor substrate-2) phosphorylation and prevented upregulation of the major hepatic transcription factor FOXO1 (Forkhead box O1) and its gluconeogenic target genes [102]. Further experiments showed that OPN reduces activation of hepatic signal transducer and activator of transcription 3 (STAT3), which is essential for glucose homeostasis and insulin sensitivity. Additionally, OPN neutralization diminished expression of hepatic gluconeogenic markers including phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6 phosphatase (G6P) [41]. Collectively, these results identified OPN as an important mediator of obesity-associated hepatic inflammation, steatosis and insulin resistance.
7. OPN in diabetic nephropathy
During the last decade a number of studies analyzed the role of OPN in the pathogenesis of diabetic nephropathy. At first, OPN has been reported to be highly expressed in the tubular epithelium of the renal cortex and in glomeruli in rat and mouse models of diabetic nephropathy [106–108]. This was associated with extensive macrophage accumulation in the kidney interstitium indicating that OPN upregulation and macrophage recruitment may play a role in the tubulo-interstitial injury in diabetic nephropathy [107]. Consistently, OPN−/− mice are protected from diabetes-induced albuminuria and renal damage, possibly by modulating podocyte signaling and motility [109]. In humans, plasma OPN levels are independently associated with the presence and severity of diabetic nephropathy [110]. Compelling evidence in the literature provides interesting clues about a link between the renin–angiotensin system (RAS) and OPN in diabetic kidney disease. Diabetes-induced OPN expression and macrophage accumulation in the kidney interstitium of diabetic rats are significantly ameliorated after treatment with the long acting ACE inhibitor perindopril [107]. This was further supported by work from Li et al. showing that treatment with ramipril for nine month improves creatinine clearance rate and decreases urinary protein excretion, systolic blood pressure, development of glomerulosclerosis, tubulo-interstitial fibrosis and inflammatory cell infiltration in a diabetic rat model. Of note, all these effects of ACE inhibition were associated with markedly suppressed OPN expression, suggesting that blockade of the RAS by ramipril may confer renoprotection by decreasing OPN secretion in diabetic nephropathy [111]. Liver X receptors (LXRs) have been identified as important lipid-dependent regulators of glucose metabolism and immune functions in leukocytes [112]. Synthetic LXR ligands can inhibit cytokine-induced OPN expression in macrophages [60]. Tachibana and colleges recently observed decreased urinary albumin excretion and substantially attenuated macrophage infiltration, mesangial matrix accumulation and interstitial fibrosis in streptozotocin-induced diabetic mice following administration of the LXR agonist T0901317. Notably, this was paralleled by diminished OPN expression in the kidney cortex indicating that inhibition of renal OPN by LXR activation may provide a potential therapeutical approach for diabetic nephropathy [113].
8. OPN in type 1 diabetes
Apart from its role in obesity-induced adipose tissue inflammation and insulin resistance, OPN has also been described as a novel protective islet protein in type 1 diabetes, which is upregulated in rat pancreatic islets and ducts during beta cell destruction following administration of streptozotocin (STZ). OPN blunted STZ-induced cytotoxicity, partly via an RGD-dependent NO regulatory mechanism [114]. Furthermore, OPN prevented apoptosis and stimulated cell proliferation in human insulin-secreting cells. Interestingly, the incretin hormone GIP showed similar effects in beta cells and additionally stimulated OPN expression, suggesting a link between GIP and OPN in preservation of functional beta cell mass in humans [115]. On the other hand genetic studies of single nucleotide polymorphisms in humans suggest that the OPN encoding gene might be associated with an increased susceptibility to the development of type 1 diabetes [116,117]. These observations emphasize the pleiotropic effects and the versatile character of OPN and support further research to understand the specific role of OPN during the pathogenesis of type 1 diabetes.
9. Pharmacological interventions
Since many studies emphasized OPN as a crucial mediator of diet-induced adipose tissue inflammation, insulin resistance, type 2 diabetes, NASH/NAFLD and diabetic nephropathy, targeting OPN could provide a novel approach for the treatment of obesity-related metabolic disorders. Several studies using loss-of-function approaches already demonstrated the value of OPN as a therapeutic target in preclinical animal models [29,41]. Thus, silencing OPN using siRNA and short hairpin RNA (shRNA) technology, or specific antibodies neutralizing OPN might provide potential targets. Furthermore, inhibition of OPN expression by PPARα ligands, PPARγ agonists, ACE inhibitors, LXR agonists, GH and IL-18 neutralizing antibodies might be potential pharmacological interventions. Since OPN is upregulated by the incretin hormone GIP, antagonism of the GIP receptor with (Pro3)GIP could also be a therapeutic target for the treatment of metabolic abnormalities [118]. Because OPN acts through several receptor mechanisms including both integrins and CD44, targeting these receptor ligand interactions as already under investigation in cancer therapy might be of interest also for the treatment of type 2 diabetes [8]. Current data justify speculation that OPN could be a viable target for new antidiabetic therapies.
10. Conclusions
Since its first description in the 1980's OPN has been identified as a key regulator of many metabolic and inflammatory diseases including obesity, diabetes, diabetic nephropathy, NAFLD and cardiovascular disease. Particularly, the use of OPN-deficient murine models significantly enhanced our understanding of OPN and its role in various metabolic pathologies. However, several key questions remain. What are the exact molecular mechanisms of OPN-mediated adipose tissue inflammation and insulin resistance? What is the impact of different OPN splice variants and isoforms in terms of leukocyte recruitment and cytokine signaling in adipose tissue? Furthermore, while OPN has been widely accepted as being causally involved in the pathogenesis of insulin resistance and type 2 diabetes, several studies identified OPN as a protective islet protein preserving insulin secretion. Therefore, these seemingly conflicting data demand further research to define the specific role and function of OPN in the development of diabetes. Especially the recent identification of a previously unrecognized crosstalk between OPN and the incretin system in terms of insulin resistance and beta cell function might open new avenues for future therapeutic strategies.
Conflict of interest
None declared.
References
- 1.Franzen A., Heinegard D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochemical Journal. 1985;232:715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinholt F.P., Hultenby K., Oldberg A., Heinegard D. Osteopontin – a possible anchor of osteoclasts to bone. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadkol S.S., Lin A.Y., Barak V., Kalickman I., Leach L., Valyi-Nagy K. Osteopontin expression and serum levels in metastatic uveal melanoma: a pilot study. Investigative Ophthalmology & Visual Science. 2006;47:802–806. doi: 10.1167/iovs.05-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scatena M., Liaw L., Giachelli C.M. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 5.Reza S., Shaukat A., Arain T.M., Riaz Q.S., Mahmud M. Expression of osteopontin in patients with thyroid dysfunction. PloS One. 2013;8:e56533. doi: 10.1371/journal.pone.0056533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosby A.H., Edwards S.J., Murray J.C., Dixon M.J. Genomic organization of the human osteopontin gene: exclusion of the locus from a causative role in the pathogenesis of dentinogenesis imperfecta type II. Genomics. 1995;27:155–160. doi: 10.1006/geno.1995.1018. [DOI] [PubMed] [Google Scholar]
- 7.Fedarko N.S., Jain A., Karadag A., Fisher L.W. Three small integrin binding ligand N-linked glycoproteins (siblings) bind and activate specific matrix metalloproteinases. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2004;18:734–736. doi: 10.1096/fj.03-0966fje. [DOI] [PubMed] [Google Scholar]
- 8.Bellahcene A., Castronovo V., Ogbureke K.U., Fisher L.W., Fedarko N.S. Small integrin-binding ligand N-linked glycoproteins (siblings): multifunctional proteins in cancer. Nature Reviews. Cancer. 2008;8:212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anborgh P.H., Mutrie J.C., Tuck A.B., Chambers A.F. Pre- and post-translational regulation of osteopontin in cancer. Journal of Cell Communication and Signaling. 2011;5:111–122. doi: 10.1007/s12079-011-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sainger R., Grau J.B., Poggio P., Branchetti E., Bavaria J.E., Gorman J.H., 3rd Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers: Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals. 2012;17:111–118. doi: 10.3109/1354750X.2011.642407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimba E.R., Tilli T.M. Human osteopontin splicing isoforms: known roles, potential clinical applications and activated signaling pathways. Cancer Letters. 2013;331:11–17. doi: 10.1016/j.canlet.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Mirza M., Shaughnessy E., Hurley J.K., Vanpatten K.A., Pestano G.A., He B. Osteopontin-c is a selective marker of breast cancer. International Journal of Cancer. Journal International du Cancer. 2008;122:889–897. doi: 10.1002/ijc.23204. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara M.L., Kim H.J., Kim J.H., Garcia V.A., Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junaid A., Moon M.C., Harding G.E., Zahradka P. Osteopontin localizes to the nucleus of 293 cells and associates with polo-like kinase-1. American Journal of Physiology. Cell physiology. 2007;292:C919–C926. doi: 10.1152/ajpcell.00477.2006. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M., Shinohara M.L. Intracellular osteopontin (iOPN) and immunity. Immunologic Research. 2011;49:160–172. doi: 10.1007/s12026-010-8179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q., Shou P., Zhang L., Xu C., Zheng C., Han Y. An osteopontin-integrin interaction plays a critical role in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem Cells. 2014 Feb;32(2):327–337. doi: 10.1002/stem.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pullig O., Weseloh G., Gauer S., Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biology: Journal of the International Society for Matrix Biology. 2000;19:245–255. doi: 10.1016/s0945-053x(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z., Singh M., Siwik D.A., Joyner W.L., Singh K. Osteopontin inhibits interleukin-1beta-stimulated increases in matrix metalloproteinase activity in adult rat cardiac fibroblasts: role of protein kinase C-zeta. Journal of Biological Chemistry. 2003;278:48546–48552. doi: 10.1074/jbc.M302727200. [DOI] [PubMed] [Google Scholar]
- 19.Lund S.A., Giachelli C.M., Scatena M. The role of osteopontin in inflammatory processes. Journal of Cell Communication and Signaling. 2009;3:311–322. doi: 10.1007/s12079-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokairin T., Nishikawa Y., Watanabe H., Doi Y., Omori Y., Yoshioka T. Osteopontin expression in the liver with severe perisinusoidal fibrosis: autopsy case of down syndrome with transient myeloproliferative disorder. Pathology International. 2008;58:64–68. doi: 10.1111/j.1440-1827.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 21.Sodhi C.P., Phadke S.A., Batlle D., Sahai A. Hypoxia stimulates osteopontin expression and proliferation of cultured vascular smooth muscle cells: potentiation by high glucose. Diabetes. 2001;50:1482–1490. doi: 10.2337/diabetes.50.6.1482. [DOI] [PubMed] [Google Scholar]
- 22.Zanotti S., Gibertini S., Di Blasi C., Cappelletti C., Bernasconi P., Mantegazza R. Osteopontin is highly expressed in severely dystrophic muscle and seems to play a role in muscle regeneration and fibrosis. Histopathology. 2011;59:1215–1228. doi: 10.1111/j.1365-2559.2011.04051.x. [DOI] [PubMed] [Google Scholar]
- 23.Singh K., Balligand J.L., Fischer T.A., Smith T.W., Kelly R.A. Glucocorticoids increase osteopontin expression in cardiac myocytes and microvascular endothelial cells. Role in regulation of inducible nitric oxide synthase. Journal of Biological Chemistry. 1995;270:28471–28478. doi: 10.1074/jbc.270.47.28471. [DOI] [PubMed] [Google Scholar]
- 24.Davis R.L., Lopez C.A., Mou K. Expression of osteopontin in the inner ear. Annals of the New York Academy of Sciences. 1995;760:279–295. doi: 10.1111/j.1749-6632.1995.tb44638.x. [DOI] [PubMed] [Google Scholar]
- 25.Shin S.L., Cha J.H., Chun M.H., Chung J.W., Lee M.Y. Expression of osteopontin mRNA in the adult rat brain. Neuroscience Letters. 1999;273:73–76. doi: 10.1016/s0304-3940(99)00516-9. [DOI] [PubMed] [Google Scholar]
- 26.Nemir M., Bhattacharyya D., Li X., Singh K., Mukherjee A.B., Mukherjee B.B. Targeted inhibition of osteopontin expression in the mammary gland causes abnormal morphogenesis and lactation deficiency. Journal of Biological Chemistry. 2000;275:969–976. doi: 10.1074/jbc.275.2.969. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z.X., Shek K., Wang S., Huang X., Lau A., Yin Z. Osteopontin expressed in tubular epithelial cells regulates NK cell-mediated kidney ischemia reperfusion injury. Journal of Immunology. 2010;185:967–973. doi: 10.4049/jimmunol.0903245. [DOI] [PubMed] [Google Scholar]
- 28.Collins A.R., Schnee J., Wang W., Kim S., Fishbein M.C., Bruemmer D. Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. Journal of the American College of Cardiology. 2004;43:1698–1705. doi: 10.1016/j.jacc.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 29.Nomiyama T., Perez-Tilve D., Ogawa D., Gizard F., Zhao Y., Heywood E.B. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. Journal of Clinical Investigation. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato W., Tomita A., Ichikawa D., Lin Y., Kishida H., Miyake S. CCR2(+)CCR5(+) t cells produce matrix metalloproteinase-9 and osteopontin in the pathogenesis of multiple sclerosis. Journal of Immunology. 2012;189:5057–5065. doi: 10.4049/jimmunol.1202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agnholt J., Kelsen J., Schack L., Hvas C.L., Dahlerup J.F., Sorensen E.S. Osteopontin, a protein with cytokine-like properties, is associated with inflammation in Crohn's disease. Scandinavian Journal of Immunology. 2007;65:453–460. doi: 10.1111/j.1365-3083.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 32.Wong C.K., Lit L.C., Tam L.S., Li E.K., Lam C.W. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:602–606. doi: 10.1093/rheumatology/keh558. [DOI] [PubMed] [Google Scholar]
- 33.Xu L., Ma X., Wang Y., Li X., Qi Y., Cui B. The expression and pathophysiological role of osteopontin in Graves' disease. Journal of Clinical Endocrinology and Metabolism. 2011;96:E1866–E1870. doi: 10.1210/jc.2011-1339. [DOI] [PubMed] [Google Scholar]
- 34.Raja R., Kale S., Thorat D., Soundararajan G., Lohite K., Mane A. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of hif1alpha-mediated VEGF-dependent angiogenesis. Oncogene. 2013 Jun 3 doi: 10.1038/onc.2013.171. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 35.Tilli T.M., Franco V.F., Robbs B.K., Wanderley J.L., da Silva F.R., de Mello K.D. Osteopontin-c splicing isoform contributes to ovarian cancer progression. Molecular Cancer Research: MCR. 2011;9:280–293. doi: 10.1158/1541-7786.MCR-10-0463. [DOI] [PubMed] [Google Scholar]
- 36.Takafuji V., Forgues M., Unsworth E., Goldsmith P., Wang X.W. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26:6361–6371. doi: 10.1038/sj.onc.1210463. [DOI] [PubMed] [Google Scholar]
- 37.Waller A.H., Sanchez-Ross M., Kaluski E., Klapholz M. Osteopontin in cardiovascular disease: a potential therapeutic target. Cardiology in Review. 2010;18:125–131. doi: 10.1097/CRD.0b013e3181cfb646. [DOI] [PubMed] [Google Scholar]
- 38.Liaw L., Lombardi D.M., Almeida M.M., Schwartz S.M., deBlois D., Giachelli C.M. Neutralizing antibodies directed against osteopontin inhibit rat carotid neointimal thickening after endothelial denudation. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17:188–193. doi: 10.1161/01.atv.17.1.188. [DOI] [PubMed] [Google Scholar]
- 39.Bruemmer D., Collins A.R., Noh G., Wang W., Territo M., Arias-Magallona S. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. Journal of Clinical Investigation. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Psarras S., Mavroidis M., Sanoudou D., Davos C.H., Xanthou G., Varela A.E. Regulation of adverse remodelling by osteopontin in a genetic heart failure model. European Heart Journal. 2012;33:1954–1963. doi: 10.1093/eurheartj/ehr119. [DOI] [PubMed] [Google Scholar]
- 41.Kiefer F.W., Zeyda M., Gollinger K., Pfau B., Neuhofer A., Weichhart T. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010;59:935–946. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter G.K., Hauschka P.V., Poole A.R., Rosenberg L.C., Goldberg H.A. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochemical Journal. 1996;317(Pt 1):59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boskey A.L., Spevak L., Paschalis E., Doty S.B., McKee M.D. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcified Tissue International. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 44.Berezin A.E., Kremzer A.A. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis. 2013;229:475–481. doi: 10.1016/j.atherosclerosis.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Grau J.B., Poggio P., Sainger R., Vernick W.J., Seefried W.F., Branchetti E. Analysis of osteopontin levels for the identification of asymptomatic patients with calcific aortic valve disease. Annals of Thoracic Surgery. 2012;93:79–86. doi: 10.1016/j.athoracsur.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirose M., Tozawa K., Okada A., Hamamoto S., Higashibata Y., Gao B. Role of osteopontin in early phase of renal crystal formation: immunohistochemical and microstructural comparisons with osteopontin knock-out mice. Urological Research. 2012;40:121–129. doi: 10.1007/s00240-011-0400-z. [DOI] [PubMed] [Google Scholar]
- 47.Imano M., Satou T., Itoh T., Takeyama Y., Yasuda A., Peng Y.F. An immunohistochemical study of osteopontin in pigment gallstone formation. American Surgeon. 2010;76:91–95. [PubMed] [Google Scholar]
- 48.Denhardt D.T., Noda M. Osteopontin expression and function: role in bone remodeling. Journal of Cellular Biochemistry. Supplement. 1998;30-31:92–102. [PubMed] [Google Scholar]
- 49.Denhardt D.T., Noda M., O'Regan A.W., Pavlin D., Berman J.S. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. Journal of Clinical Investigation. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi S.T., Kim J.H., Kang E.J., Lee S.W., Park M.C., Park Y.B. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology (Oxford) 2008;47:1775–1779. doi: 10.1093/rheumatology/ken385. [DOI] [PubMed] [Google Scholar]
- 51.Klein-Nulend J., Roelofsen J., Semeins C.M., Bronckers A.L., Burger E.H. Mechanical stimulation of osteopontin mRNA expression and synthesis in bone cell cultures. Journal of Cellular Physiology. 1997;170:174–181. doi: 10.1002/(SICI)1097-4652(199702)170:2<174::AID-JCP9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 52.Chellaiah M.A., Kizer N., Biswas R., Alvarez U., Strauss-Schoenberger J., Rifas L. Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Molecular Biology of the Cell. 2003;14:173–189. doi: 10.1091/mbc.E02-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asou Y., Rittling S.R., Yoshitake H., Tsuji K., Shinomiya K., Nifuji A. Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology. 2001;142:1325–1332. doi: 10.1210/endo.142.3.8006. [DOI] [PubMed] [Google Scholar]
- 54.Lund S.A., Wilson C.L., Raines E.W., Tang J., Giachelli C.M., Scatena M. Osteopontin mediates macrophage chemotaxis via alpha4 and alpha9 integrins and survival via the alpha4 integrin. Journal of Cellular Biochemistry. 2013;114:1194–1202. doi: 10.1002/jcb.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denhardt D.T., Mistretta D., Chambers A.F., Krishna S., Porter J.F., Raghuram S. Transcriptional regulation of osteopontin and the metastatic phenotype: evidence for a Ras-activated enhancer in the human OPN promoter. Clinical & Experimental Metastasis. 2003;20:77–84. doi: 10.1023/a:1022550721404. [DOI] [PubMed] [Google Scholar]
- 56.Gao C., Guo H., Mi Z., Wai P.Y., Kuo P.C. Transcriptional regulatory functions of heterogeneous nuclear ribonucleoprotein-U and -A/B in endotoxin-mediated macrophage expression of osteopontin. Journal of Immunology. 2005;175:523–530. doi: 10.4049/jimmunol.175.1.523. [DOI] [PubMed] [Google Scholar]
- 57.Hijiya N., Setoguchi M., Matsuura K., Higuchi Y., Akizuki S., Yamamoto S. Cloning and characterization of the human osteopontin gene and its promoter. Biochemical Journal. 1994;303(Pt 1):255–262. doi: 10.1042/bj3030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo X., Zhang Y.P., Mitchell D.A., Denhardt D.T., Chambers A.F. Identification of a Ras-activated enhancer in the mouse osteopontin promoter and its interaction with a putative ETS-related transcription factor whose activity correlates with the metastatic potential of the cell. Molecular and Cellular Biology. 1995;15:476–487. doi: 10.1128/mcb.15.1.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao W., Wang L., Zhang L., Yuan C., Kuo P.C., Gao C. Differential expression of intracellular and secreted osteopontin isoforms by murine macrophages in response to toll-like receptor agonists. Journal of Biological Chemistry. 2010;285:20452–20461. doi: 10.1074/jbc.M110.110312. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Ogawa D., Stone J.F., Takata Y., Blaschke F., Chu V.H., Towler D.A. Liver X receptor agonists inhibit cytokine-induced osteopontin expression in macrophages through interference with activator protein-1 signaling pathways. Circulation Research. 2005;96:e59–67. doi: 10.1161/01.RES.0000163630.86796.17. [DOI] [PubMed] [Google Scholar]
- 61.Zhao W., Wang L., Zhang M., Wang P., Zhang L., Yuan C. NF-kappaB- and AP-1-mediated DNA looping regulates osteopontin transcription in endotoxin-stimulated murine macrophages. Journal of Immunology. 2011;186:3173–3179. doi: 10.4049/jimmunol.1003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oyama Y., Akuzawa N., Nagai R., Kurabayashi M. PPARgamma ligand inhibits osteopontin gene expression through interference with binding of nuclear factors to A/T-rich sequence in THP-1 cells. Circulation Research. 2002;90:348–355. doi: 10.1161/hh0302.105098. [DOI] [PubMed] [Google Scholar]
- 63.Giachelli C.M., Lombardi D., Johnson R.J., Murry C.E., Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. American Journal of Pathology. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- 64.Weber G.F., Zawaideh S., Hikita S., Kumar V.A., Cantor H., Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. Journal of Leukocyte Biology. 2002;72:752–761. [PubMed] [Google Scholar]
- 65.Crawford H.C., Matrisian L.M., Liaw L. Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. Cancer Research. 1998;58:5206–5215. [PubMed] [Google Scholar]
- 66.McKee M.D., Nanci A. Secretion of osteopontin by macrophages and its accumulation at tissue surfaces during wound healing in mineralized tissues: a potential requirement for macrophage adhesion and phagocytosis. Anatomical Record. 1996;245:394–409. doi: 10.1002/(SICI)1097-0185(199606)245:2<394::AID-AR19>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 67.O'Regan A.W., Chupp G.L., Lowry J.A., Goetschkes M., Mulligan N., Berman J.S. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. Journal of Immunology. 1999;162:1024–1031. [PubMed] [Google Scholar]
- 68.Zhu B., Suzuki K., Goldberg H.A., Rittling S.R., Denhardt D.T., McCulloch C.A. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. Journal of Cellular Physiology. 2004;198:155–167. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki K., Zhu B., Rittling S.R., Denhardt D.T., Goldberg H.A., McCulloch C.A.G. Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts. Journal of Bone and Mineral Research. 2002;17:1486–1497. doi: 10.1359/jbmr.2002.17.8.1486. [DOI] [PubMed] [Google Scholar]
- 70.Philip S., Bulbule A., Kundu G.C. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-kappa B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. Journal of Biological Chemistry. 2001;276:44926–44935. doi: 10.1074/jbc.M103334200. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto N., Sakai F., Kon S., Morimoto J., Kimura C., Yamazaki H. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. Journal of Clinical Investigation. 2003;112:181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsui Y., Rittling S.R., Okamoto H., Inobe M., Jia N., Shimizu T. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:1029–1034. doi: 10.1161/01.ATV.0000074878.29805.D0. [DOI] [PubMed] [Google Scholar]
- 73.Ophascharoensuk V., Giachelli C.M., Gordon K., Hughes J., Pichler R., Brown P. Obstructive uropathy in the mouse: role of osteopontin in interstitial fibrosis and apoptosis. Kidney International. 1999;56:571–580. doi: 10.1046/j.1523-1755.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 74.Liaw L., Birk D.E., Ballas C.B., Whitsitt J.S., Davidson J.M., Hogan B.L.M. Altered wound healing in mice lacking a functional osteopontin gene (spp1) Journal of Clinical Investigation. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashkar S., Weber G.F., Panoutsakopoulou V., Sanchirico M.E., Jansson M., Zawaideh S. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 76.Chabas D., Baranzini S.E., Mitchell D., Bernard C.C.A., Rittling S.R., Denhardt D.T. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed M., Kundu G.C. Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast cancer cells. Molecular Cancer. 2010;9:101. doi: 10.1186/1476-4598-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Das R., Philip S., Mahabeleshwar G.H., Bulbule A., Kundu G.C. Osteopontin: it's role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression. IUBMB Life. 2005;57:441–447. doi: 10.1080/15216540500159424. [DOI] [PubMed] [Google Scholar]
- 79.Lin Y.H., Yang-Yen H.F. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. Journal of Biological Chemistry. 2001;276:46024–46030. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- 80.Courter D.L., Lomas L., Scatena M., Giachelli C.M. Src kinase activity is required for integrin AlphaVbeta3-mediated activation of nuclear factor-kappaB. Journal of Biological Chemistry. 2005;280:12145–12151. doi: 10.1074/jbc.M412555200. [DOI] [PubMed] [Google Scholar]
- 81.O'Regan A.W., Hayden J.M., Berman J.S. Osteopontin augments CD3-mediated interferon-{gamma} and CD40 ligand expression by T cells, which results in IL-12 production from peripheral blood mononuclear cells. Journal of Leukocyte Biology. 2000;68:495–502. [PubMed] [Google Scholar]
- 82.Rossger K., Charpin-El-Hamri G., Fussenegger M. A closed-loop synthetic gene circuit for the treatment of diet-induced obesity in mice. Nature Communications. 2013;4:2825. doi: 10.1038/ncomms3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahlqvist E., Osmark P., Kuulasmaa T., Pilgaard K., Omar B., Brons C. Link between GIP and osteopontin in adipose tissue and insulin resistance. Diabetes. 2013;62:2088–2094. doi: 10.2337/db12-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gualillo O., Gonzalez-Juanatey J.R., Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends in Cardiovascular Medicine. 2007;17:275–283. doi: 10.1016/j.tcm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Kiefer F.W., Zeyda M., Todoric J., Huber J., Geyeregger R., Weichhart T. Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008;149:1350–1357. doi: 10.1210/en.2007-1312. [DOI] [PubMed] [Google Scholar]
- 86.Chapman J., Miles P.D., Ofrecio J.M., Neels J.G., Yu J.G., Resnik J.L. Osteopontin is required for the early onset of high fat diet-induced insulin resistance in mice. PLoS One. 2010;5:e13959. doi: 10.1371/journal.pone.0013959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samuvel D.J., Sundararaj K.P., Li Y., Lopes-Virella M.F., Huang Y. Adipocyte-mononuclear cell interaction, toll-like receptor 4 activation, and high glucose synergistically up-regulate osteopontin expression via an interleukin 6-mediated mechanism. Journal of Biological Chemistry. 2010;285:3916–3927. doi: 10.1074/jbc.M109.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu C., Kumar P.A., Sun J., Aggarwal A., Fan Y., Sperling M.A. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. Journal of Biological Chemistry. 2013;288:15725–15735. doi: 10.1074/jbc.M113.460212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Omar B., Banke E., Guirguis E., Akesson L., Manganiello V., Lyssenko V. Regulation of the pro-inflammatory cytokine osteopontin by GIP in adipocytes – a role for the transcription factor NFAT and phosphodiesterase 3B. Biochemical and Biophysical Research Communications. 2012;425:812–817. doi: 10.1016/j.bbrc.2012.07.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christensen B., Klaning E., Nielsen M.S., Andersen M.H., Sorensen E.S. C-terminal modification of osteopontin inhibits interaction with the alphaVbeta3-integrin. Journal of Biological Chemistry. 2012;287:3788–3797. doi: 10.1074/jbc.M111.277996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kollmer M., Buhrman J.S., Zhang Y., Gemeinhart R.A. Markers are shared between adipogenic and osteogenic differentiated mesenchymal stem cells. Journal of Developmental Biology and Tissue Engineering. 2013;5:18–25. doi: 10.5897/JDBTE2013.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeyda M., Gollinger K., Todoric J., Kiefer F.W., Keck M., Aszmann O. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology. 2011;152:2219–2227. doi: 10.1210/en.2010-1328. [DOI] [PubMed] [Google Scholar]
- 93.Gomez-Ambrosi J., Catalan V., Ramirez B., Rodriguez A., Colina I., Silva C. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. Journal of Clinical Endocrinology and Metabolism. 2007;92:3719–3727. doi: 10.1210/jc.2007-0349. [DOI] [PubMed] [Google Scholar]
- 94.Ahmad R., Al-Mass A., Al-Ghawas D., Shareif N., Zghoul N., Melhem M. Interaction of osteopontin with il-18 in obese individuals: implications for insulin resistance. PLoS One. 2013;8:e63944. doi: 10.1371/journal.pone.0063944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarac F., Basoglu O.K., Gunduz C., Bayrak H., Biray Avci C., Akcicek F. Association of osteopontin and tumor necrosis factor-alpha levels with insulin resistance in obese patients with obstructive sleep apnea syndrome. Journal of Endocrinological Investigation. 2011;34:528–533. doi: 10.3275/7287. [DOI] [PubMed] [Google Scholar]
- 96.You J.S., Ji H.I., Chang K.J., Yoo M.C., Yang H.I., Jeong I.K. Serum osteopontin concentration is decreased by exercise-induced fat loss but is not correlated with body fat percentage in obese humans. Molecular Medicine Reports. 2013;8:579–584. doi: 10.3892/mmr.2013.1522. [DOI] [PubMed] [Google Scholar]
- 97.Bertola A., Deveaux V., Bonnafous S., Rousseau D., Anty R., Wakkach A. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes. 2009;58:125–133. doi: 10.2337/db08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schaller G., Aso Y., Schernthaner G.H., Kopp H.P., Inukai T., Kriwanek S. Increase of osteopontin plasma concentrations after bariatric surgery independent from inflammation and insulin resistance. Obesity Surgery. 2009;19:351–356. doi: 10.1007/s11695-008-9532-9. [DOI] [PubMed] [Google Scholar]
- 99.Komorowski J., Jankiewicz-Wika J., Kolomecki K., Cywinski J., Piestrzeniewicz K., Swietoslawski J. Systemic blood osteopontin, endostatin, and E-selectin concentrations after vertical banding surgery in severely obese adults. Cytokine. 2011;55:56–61. doi: 10.1016/j.cyto.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 100.Riedl M., Vila G., Maier C., Handisurya A., Shakeri-Manesch S., Prager G. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2008;93:2307–2312. doi: 10.1210/jc.2007-2383. [DOI] [PubMed] [Google Scholar]
- 101.Nakamachi T., Nomiyama T., Gizard F., Heywood E.B., Jones K.L., Zhao Y. PPARalpha agonists suppress osteopontin expression in macrophages and decrease plasma levels in patients with type 2 diabetes. Diabetes. 2007;56:1662–1670. doi: 10.2337/db06-1177. [DOI] [PubMed] [Google Scholar]
- 102.Kiefer F.W., Neschen S., Pfau B., Legerer B., Neuhofer A., Kahle M. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia. 2011;54:2132–2142. doi: 10.1007/s00125-011-2170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kwon H.J., Won Y.S., Yoon W.K., Nam K.H., Kim D.Y., Kim H.C. The role of osteopontin in d-galactosamine-induced liver injury in genetically obese mice. Toxicology and Applied Pharmacology. 2010;242:344–351. doi: 10.1016/j.taap.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 104.Sahai A., Malladi P., Melin-Aldana H., Green R.M., Whitington P.F. Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;287:G264–273. doi: 10.1152/ajpgi.00002.2004. [DOI] [PubMed] [Google Scholar]
- 105.Sahai A., Malladi P., Pan X., Paul R., Melin-Aldana H., Green R.M. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;287:G1035–G1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 106.Fischer J.W., Tschope C., Reinecke A., Giachelli C.M., Unger T. Upregulation of osteopontin expression in renal cortex of streptozotocin-induced diabetic rats is mediated by bradykinin. Diabetes. 1998;47:1512–1518. doi: 10.2337/diabetes.47.9.1512. [DOI] [PubMed] [Google Scholar]
- 107.Kelly D.J., Wilkinson-Berka J.L., Ricardo S.D., Cox A.J., Gilbert R.E. Progression of tubulointerstitial injury by osteopontin-induced macrophage recruitment in advanced diabetic nephropathy of transgenic (mRen-2)27 rats. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association – European Renal Association. 2002;17:985–991. doi: 10.1093/ndt/17.6.985. [DOI] [PubMed] [Google Scholar]
- 108.Susztak K., Bottinger E., Novetsky A., Liang D., Zhu Y., Ciccone E. Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes. 2004;53:784–794. doi: 10.2337/diabetes.53.3.784. [DOI] [PubMed] [Google Scholar]
- 109.Lorenzen J., Shah R., Biser A., Staicu S.A., Niranjan T., Garcia A.M. The role of osteopontin in the development of albuminuria. Journal of the American Society of Nephrology: JASN. 2008;19:884–890. doi: 10.1681/ASN.2007040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan X., Sano M., Lu L., Wang W., Zhang Q., Zhang R. Plasma concentrations of osteopontin, but not thrombin-cleaved osteopontin, are associated with the presence and severity of nephropathy and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovascular Diabetology. 2010;9:70. doi: 10.1186/1475-2840-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li C., Yang C.W., Park C.W., Ahn H.J., Kim W.Y., Yoon K.H. Long-term treatment with ramipril attenuates renal osteopontin expression in diabetic rats. Kidney International. 2003;63:454–463. doi: 10.1046/j.1523-1755.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 112.Joseph S.B., Castrillo A., Laffitte B.A., Mangelsdorf D.J., Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature Medicine. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 113.Tachibana H., Ogawa D., Matsushita Y., Bruemmer D., Wada J., Teshigawara S. Activation of liver X receptor inhibits osteopontin and ameliorates diabetic nephropathy. Journal of the American Society of Nephrology: JASN. 2012;23:1835–1846. doi: 10.1681/ASN.2012010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Katakam A.K., Chipitsyna G., Gong Q., Vancha A.R., Gabbeta J., Arafat H.A. Streptozotocin (STZ) mediates acute upregulation of serum and pancreatic osteopontin (OPN): a novel islet-protective effect of OPN through inhibition of STZ-induced nitric oxide production. Journal of Endocrinology. 2005;187:237–247. doi: 10.1677/joe.1.06411. [DOI] [PubMed] [Google Scholar]
- 115.Lyssenko V., Eliasson L., Kotova O., Pilgaard K., Wierup N., Salehi A. Pleiotropic effects of GIP on islet function involve osteopontin. Diabetes. 2011;60:2424–2433. doi: 10.2337/db10-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chiocchetti A., Orilieri E., Cappellano G., Barizzone N., D' Alfonso S., D' Annunzio G. The osteopontin gene +1239A/C single nucleotide polymorphism is associated with type 1 diabetes mellitus in the Italian population. International Journal of Immunopathology and Pharmacology. 2010;23:263–269. doi: 10.1177/039463201002300124. [DOI] [PubMed] [Google Scholar]
- 117.Marciano R., D'Annunzio G., Minuto N., Pasquali L., Santamaria A., Di Duca M. Association of alleles at polymorphic sites in the osteopontin encoding gene in young type 1 diabetic patients. Clinical Immunology. 2009;131:84–91. doi: 10.1016/j.clim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 118.McClean P.L., Irwin N., Hunter K., Gault V.A., Flatt P.R. (Pro(3))GIP[mPEG]: novel, long-acting, mpegylated antagonist of gastric inhibitory polypeptide for obesity-diabetes (diabesity) therapy. British Journal of Pharmacology. 2008;155:690–701. doi: 10.1038/bjp.2008.317. [DOI] [PMC free article] [PubMed] [Google Scholar]


