Abstract
Background
Although it is now evident that normal cognition can occur despite significant AD pathology, few studies have attempted to characterize this discordance, or examine factors that may contribute to resilient brain aging in the setting of AD pathology.
Methods
More than 2,000 older persons underwent annual evaluation as part of participation in the Religious Orders Study or Rush Memory Aging Project. A total of 966 subjects who had brain autopsy and comprehensive cognitive testing proximate to death were analyzed. Resilience was quantified as a continuous measure using linear regression modeling, where global cognition was entered as a dependent variable and global pathology was an independent variable. Studentized residuals generated from the model represented the discordance between cognition and pathology, and served as measure of resilience. The relation of resilience index to known risk factors for AD and related variables was examined.
Results
Multivariate regression models that adjusted for demographic variables revealed significant associations for early life socioeconomic status, reading ability, APOE-ε4 status, and past cognitive activity. A stepwise regression model retained reading level (estimate = 0.10, SE = 0.02; p < 0.0001) and past cognitive activity (estimate = 0.27, SE = 0.09; p = 0.002), suggesting the potential mediating role of these variables for resilience.
Conclusions
The construct of resilient brain aging can provide a framework for quantifying the discordance between cognition and pathology, and help identify factors that may mediate this relationship.
Keywords: Cognitive activity, Neuropathology, Reading level, Reserve, Resilience
INTRODUCTION
Some people live for a century “sharp as a tack” while many others suffer varying degrees of cognitive and neurological deterioration with increased morbidity, dependence and mortality. Much is still being learned about the histopathological lesions associated with neurodegenerative dementias. Alzheimer’s disease (AD), cerebrovascular disease and other neurodegenerative disorders are associated with cascades of calcium dysregulation, apoptotic, inflammatory, oxidative stress and other destructive processes that lead to synaptic and neuronal loss. They also stimulate neuroplastic regenerative or reparative responses that may counteract or compensate for the degeneration. While clinicopathological correlation studies show significant associations of plaques, tangles, infarctions and other lesions with cognitive impairment, the relationships are imperfect, and it is increasingly recognized that some elderly persons may have abundant pathology and yet still are unimpaired. While such cases are the minority, it is evident that healthy cognition occurs amidst a spectrum of brain pathology - from those who remain cognitively intact and whose brains are relatively free of neurodegenerative disease or other pathological lesions to those who remain intact even with significant accumulations of pathology.
Normal cognition despite AD and/or vascular lesions was recognized over 80 years ago and has been described subsequently in case reports and series1. In recent years, increasing evidence for cognitive resilience or reserve has come from large-scale epidemiological studies. In community based studies of cognition with autopsy such as the Religious Orders Study (ROS) and its companion study, the Rush Memory and Aging Project (MAP), it has been found that a third of people with normal cognition (i.e., no dementia or mild cognitive impairment [MCI]) in their eighties and older can have densities of plaques and tangles that meet NIA-Reagan criteria for intermediate or even high likelihood of AD, as well as infarctions and Lewy bodies2. The Nun Study has also shown discordance of cognition with pathology as well as the important effects of education, diet, linguistic ability, and childhood positive emotion on cognitive function in late life3. The Baltimore Longitudinal Study of Aging4 and the Honolulu-Asia Aging Study5 have similarly reported a dissociation between plaques, tangles or other pathology and cognition, as have the 90+ Study6 and the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) in the oldest-old7.
Evidence from these and other studies suggests a paradigm shift in the consideration of the neurobiology of healthy aging and dementia. The standard paradigm for the study of cognition is to identify the presence of cognitive and/or functional decline and to attribute this to one of several common brain disease states, such as AD, vascular disease, Lewy body disease or any number of rare conditions, e.g., fronto-temporal dementia, progressive aphasia. Some findings support this traditional paradigm, at least in part. However, abundant neuropathology in persons without dementia is observed as well8, and the correlation between neuropathological lesions and cognition is modest, accounting only for about a quarter of the variance of cognition among older adults9,10. As such, the concept of resilient aging has emerged in recent years as a useful construct in characterizing such individuals who maintain intact cognitive function amidst evidence of significant AD pathology10.
Cognitive functioning in late life is the ultimate readout of disease-related pathology, allostatic load and senescent processes in the setting of the brain’s ability to repair, resist or tolerate those processes through dynamic adaptive plasticity or reserve capacity. While the literature on the cellular and molecular mechanisms of general neuroplasticity11 and neuroplastic response to injury12 is vast and complex,13 studies that are directly germane to resilience in aging and neurodegeneration are limited so far. The goal of the present study is to utilize large, clinical and neuropathological cohorts from ROS and MAP studies to propose an innovative approach to conceptualizing resilient aging. The main objective is to operationalize resilient aging along intersecting vectors of cognition and pathology, where resilience represents an intact cognition in the setting of AD pathology. A related aim is to validate this construct using known risk factors for AD.
Another goal was to investigate relation of lifestyle factors, particularly cognitive activity, to resilience. There is now accumulating evidence that frequent participation in cognitively stimulating activities is associated with reduced risk of cognitive decline and dementia14. The extent to which cognitive activity contributes to resilient aging in the setting of AD pathology is less known.
METHODS
Participants
The study consisted of participants from two longitudinal clinical-pathological cohorts, Religious Order Study (ROS) and Memory and Aging Project (MAP). The ROS, started in 1994, enrolled Catholic priests, nuns, and brothers, from about 40 groups in 12 states. Participants were free of known dementia at enrollment, agreed to annual clinical evaluations, and signed both an informed consent and an Anatomic Gift Act form donating their brains at time of death. More detailed descriptions have been previously reported15. The MAP, started in 1997, enrolled older men and women from residential facilities across the metropolitan Chicago area, including subsidized senior housing facilities, retirement communities, and retirement homes, in addition to social service agencies and church groups. Participants were free of known dementia at baseline. Similar to ROS, all participants agreed to annual clinical evaluations and signed both an informed consent and an Anatomic Gift Act form donating their brains, spinal cords, and selected nerves and muscles to Rush investigators at the time of death. More detailed descriptions have been previously reported16.
As of April 2012, a total of 1,167 people have enrolled in ROS and completed the baseline evaluation. They have completed up to 18 years of annual follow-up. There have been 590 deaths with 554 brain autopsies (93.9%). In MAP, 1,571 people have enrolled and completed the baseline evaluation. They have completed up to 15 years of annual follow-up evaluations, and there have been 545 deaths and 439 brain autopsies (80.6%). In both studies, rate of participation in the annual clinical follow-up evaluations has exceeded 90% in survivors.
Clinical Evaluation
At baseline, participants had a uniform structured evaluation that was repeated annually by examiners blinded to previously collected data. Each participant underwent a uniform structured clinical evaluation that included a medical history, neurologic examination, and detailed cognitive testing. Dementia diagnosis was carried out by experienced clinicians using the results of this evaluation plus an in-person examination and the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association17. Details of the diagnostic procedure are described elsewhere16.
Assessment of Cognitive Function
Scores on 19 tests were used to create summary indices of global cognitive function and five specific cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability tests16. Episodic memory was assessed using seven tests: immediate and delayed recall of story A from Logical Memory, immediate and delayed recall of the East Boston Story, Word List Memory, Word List Recall, and Word List Recognition. Semantic memory was assessed using three tests: a 15-item version of the Boston Naming Test, Verbal Fluency, and a 15-item reading test. Working memory was assessed using three tests: Digit Span Forward, Digit Span Backward and Digit Ordering. Perceptual speed was assessed using four tests: Symbol Digit Modalities Test, Number Comparison, and two indices from a modified version of the Stroop Neuropsychological Screening Test. Visuospatial abilities were assessed using two tests: a 15-item version of Judgment of Line Orientation and a 16-item version of Standard Progressive Matrices. The composite measure of global cognitive function was based on all 19 neuropsychological tests. This was derived by converting the raw scores of individual tests to z-scores, using the mean and standard deviation from the baseline evaluation of the entire cohort, and averaging the z-scores. Thus, a higher score on the composite measure indicates better cognitive function. Detailed information on the individual tests and on the derivation and correlates of the composite measures is contained in previous publications15.
Neuropathologic Evaluation
A standard protocol was used for brain removal, sectioning and preserving of tissue, and quantifying AD pathology and cerebral infarctions, as described in detail elsewhere18. To briefly describe here, brains were removed in a standard fashion and cut coronally into 1-cm slabs that were visually inspected and photographed. After slabs were fixed in 4% paraformaldehyde for 3–21 days, they were dissected into blocks that were embedded in paraffin and cut into 6-µm sections. Bielschowsky silver stain was used to visualize neuritic plaques, diffuse plaques, and neurofibrillary tangles in five brain regions: midfrontal gyrus, superior temporal gyrus, inferior parietal gyrus, entorhinal cortex, and the CA1 sector of the hippocampus. A measure we labeled “global AD pathology” was based on counts of neuritic plaques, diffuse plaques, and neurofibrillary tangles identified by a modified Bielschowsky silver stain in 5 brain regions, with standard scores averaged across pathology types and regions. Composite summary measures of the percent area occupied by amyloid- and the density of neurofibrillary tangles were developed by averaging the values for each lesion for all regions assessed.
Neuropathologic diagnoses were established by a board-certified neuropathologist blinded to age and all clinical data. NIA-Reagan,19 Braak,20 and CERAD21 classifications were scored. Infarctions were documented along with their age, volume (in mm3), side, and location. Lewy bodies were identified with antibodies to α-synuclein, as described elsewhere22.
Assessment of Covariates
Demographic Variables
These included age at death, gender, years of education, and race. Early life Socioeconomic status was based on four indicators – parental education (mean years of schooling completed by the participant’s mother and father), paternal occupation (the father’s principal occupation coded according to perceived prestige), total number of children in the family, and community-level socioeconomic status23. These indicators were converted to z scores and averaged to yield a composite index, with higher z-scores indicating higher socioeconomic status.
Medical comorbities
Participants in the ROS and MAP studies undergo a comprehensive medical history interview at baseline evaluation, which includes numerous self-report questions pertaining to various diseases. Medical conditions such as head trauma, hypertension, heart condition, hypothyroidism, and cancer were rated as absent or present (0 or 1) as determined by self-report; stroke was diagnosed based on self-report plus clinical examination, as previously described.16 Participants were asked to bring all prescription and over-the-counter medications to the visit; these medications were inspected, identified, and coded using the Medi-Span ® system (Medi-Span, Inc., Indianapolis, IN).24
Reading level
This was assessed at baseline using a modified form of the National Adult Reading Test25. Participants were asked to read aloud a series of words with atypical spelling–sound correspondences (e.g., epitome, impugn).
Past Cognitive activity
We used previously established composite measure of past cognitive activity26. At baseline, persons were asked about time typically spent in 7 common activities that involve information processing as a central component: viewing television; listening to radio; reading newspapers; reading magazines; reading books; playing games such as cards, checkers, crosswords, or other puzzles; and going to museums. Frequency of participation in each activity was rated on a 5-point scale: every day or about every day (5 points); several times a week (4 points); several times a month (3 points); several times a year (2 points); and once a year or less (1 point). The questionnaire included 30 items, with 11 items about childhood (3 items for age 6 and 8 items for age 12), 10 items about young adulthood (age 18), and 9 items about middle age (age 40). Responses to each item were averaged to yield the composite measure.
APOE genotyping
Blood was collected with acid citrate dextrose anticoagulant and stored at room temperature. Lymphocyte separation was performed within 24 hours of collection. DNA was extracted from approximately 2–3 million cells, and genotyping was performed by an investigator blinded to all clinical and postmortem data as previously described27.
Statistical Analyses
Descriptive statistics were used to show the demographic characteristics of participants. As described in greater detail below, studentized residuals were generated from a linear regression model of global cognition variable on global pathology. This residual served as an outcome variable. Analyses involved two steps. We first conducted four separate multiple linear regression, in which the residual was separately regressed against each of the four predictor variables of interest (early life socioeconomic status, reading level, presence of APOEe4 alleles, and post cognitive, with additional terms to control for age, gender, race and years of education. To determine the relative contribution of each predictor variable, we then performed stepwise regression with the 4 demographic variables forced in first, and then entering the variables using a selection criterion of p < 0.05. Statistical significance was determined by an alpha level of p < 0.01. All statistical tests were two-sided. Statistical analyses were performed using SAS (SAS Institute, Cary, NC)28.
RESULTS
Participant Characteristics
At the time of these analyses, a data from autopsies of 966 participants from MAP and ROS cohorts were available. Participant characteristics for the entire group are shown in Table 1.
Table 1.
Participant Characteristic
| Characteristic | Entire Group (N = 966) |
|---|---|
| Age at death (mean ± SD) | 87.9 ± 6.7 |
| Gender (% female) | 616, 64% |
| Years of education (mean ± SD) | 16.5 ± 3.7 |
| Race (number white, % white,) | 938 (97%) |
| APOE ε4 allele present, (number, %,) | 249/939, 27% |
| National Adult Reading Test (mean ± SD) | 12.9 ± 3.8 |
| Early life socioeconomic status* (mean ± SD) | −0.007 ± 0.7 |
| Cognition status at death (n = 964) | |
| Normal cognition, % | 308, 32% |
| MCI (single & multiple domain), % | 242, 25% |
| Dementia, % | 414, 43% |
| Medical comorbities at enrollment in study | |
| Hypertension (n, %) | N/962, 59% |
| Stroke (n, %) | N/960 24% |
| Head injury (n, %) | N/ 964 9% |
| Thyroid disease (n, %) | N/965, 23% |
| Heart condition (n, %) | N/965, 24% |
| Cancer (n, %) | N/965, 42% |
APOE = Apolipoprotein E; SES = socioeconomic status; MCI = mild cognitive impairment. SD = standard deviation
Early life SES indicates a composite index, with higher scores indicating higher SES.
Operationalization of Resilience
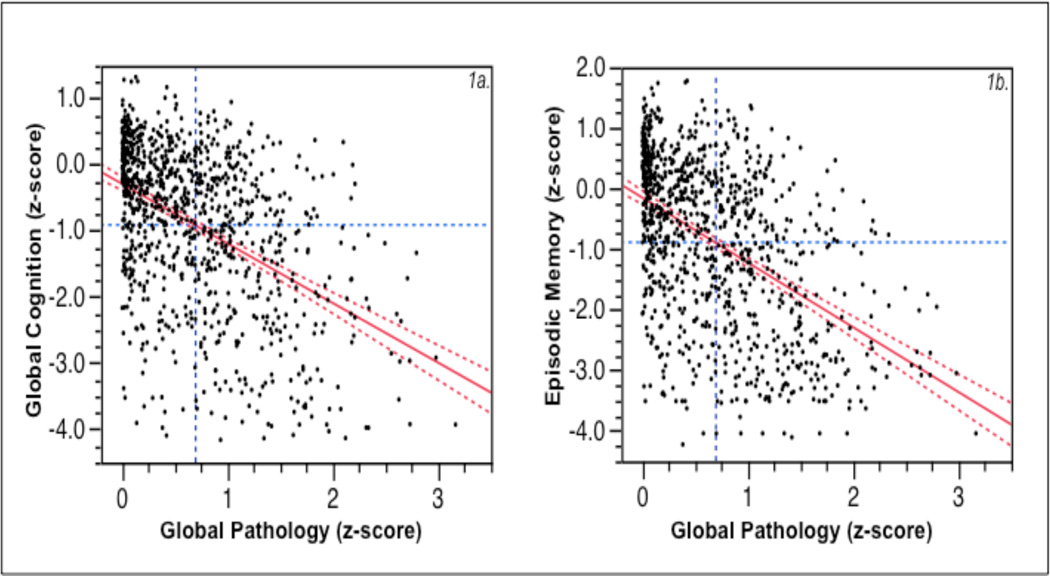
Resilience represented the discordance between predicted cognition and pathology. A scatterplot plot of relationship between global pathology and global cognition is depicted in Figure 1a. While the figure shows a relationship between AD pathology and cognition (estimate = −0.90, SE = 0.06; p < .0001), there is also a marked variability. As expected, some individuals showed high cognition and relatively low AD pathology (Healthy), while others showed low cognition and high pathology (AD-Dementia) (see also29). Of particular interest, however, are those with discordance between cognition and pathology, especially those with relatively intact cognition despite significant AD pathology. The construct of resilient aging is used to operationalize this phenomenon.
Figure 1.
a: Scatterplot of global cognition (composite z-score of 19 tests in psychometric battery) versus global pathology (composite z-score of quantitative measures of neuritic plaques, diffuse plaques, and neurofibrillary tangles), with regression line and its confidence interval superimposed. Blue dashed lines are at the mean values of global cognition (horizontal line) and global pathology (vertical line) for the entire cohort (n = 966). Figure 1b is similar, expect that episodic memory score replaces the global cognition score.
We quantified resilience as a continuous measure in a linear regression modeling, where global cognition was entered as a dependent variable and global pathology was an independent variable. As pathology and cognition are continuous variables, this approach minimizes the need for artificial dichotomization, and keeps continuous variables continuous30. From the regression model, studentized residuals were then generated. The residual represents the difference between observed and predicted values, and serves as a measure of the discordance between pathology and expected cognition based on the central regression line, which we refer to as the Resilience Index. Thus, higher scores on the residual (or the Resilience Index) indicate better cognitive function than expected based on the amount of AD pathology, whereas low scores indicate cognitive frailty with worse cognitive function than expected.
Demographic Variables
An initial model was fit consisting of each of the demographic variables (age, gender, education, and race), with Resilience Index as the outcome variable. Results indicated that age at death was significantly associated with resilience, where older age was associated with less resilience (estimate = −0.03, SE = 0.004; p < 0.0001). Education was also significant, such that more years of education completed were related to higher resilience (estimate = 0.03, SE = 0.009; p = 0.0009). On the other hand, resilience was not significantly associated with gender (estimate = 0.03, SE = 0.07; p = 0.74) or race (estimate = −0.13, SE = 0.09; p = 0.19). All subsequent analyses adjusted for these demographic variables.
Early Life Socioeconomic status
In a linear model adjusted for the demographic variables, there was a significant association between resilience and early life socioeconomic status, where individuals with high levels of early life socioeconomic status showed more resilience (Table 2).
Table 2.
Relation of Resilience Index to Early Life Socioeconomic Status, Reading level, APOE Genotype, and Past Cognitive Activitya
| Separate Models | Joint model | |||
|---|---|---|---|---|
| Variables | Estimate (SE)b | p value | Estimate (SE)b |
p value |
| Early life socioeconomic status | 0.1251 (0.0447) | 0.005 | 0.0261 (0.0709) | 0.7128 |
| Reading level | 0.0649 (0.0086) | <. 0001 | 0.1031 (0.0182) | <. 0001 |
| APOE-ε4 status | −0.1191 (0.0360) | 0.001 | −0.1356 (0.1112) | 0.2234 |
| Past cognitive activity | 0.31 (0.08) | 0.0006 | 0.2689 (0.0876) | 0.0023 |
Separate linear regression models, adjusted for age, gender, education, and race.
The estimates are unstandardized regression coefficients (SE = standard error).
Reading Level
This measure was also significantly associated with the Resilience Index, in that individuals with higher scores on the Reading Test showed greater resilience (Table 2). In a model that also included early life socioeconomic status, reading level remained significantly associated with the Resilience Index, (estimate = 0.06, SE = 0.008; p < 0.0001) whereas early life socioeconomic status was no longer significant, (estimate = 0.06, SE = 0.05; p = 0.16).
APOE Genotype
We also examined whether the APOE genotype, a known risk factor for AD, was sensitive to our model of resilience. We observed a significant association, where the presence of APOE-ε4 allele was associated with less resilience (Table 2).
Past Cognitive Activity
Cognitive activity data were available for a subset of participants (n = 389). In a model that adjusted for the demographic variables, past cognitive activity was significantly associated with resilience, such that individuals with more frequent participation in cognitively stimulating activities showed higher resilience (Table 2).
In addition, we conducted a stepwise regression analysis using a model that included all the variables in Table 2 (early life socioeconomic status, reading level, APOE genotype, and past cognitive activity). The stepwise regression (that adjusted for the demographic variables) retained reading level (estimate = 0.10, SE = 0.02; p < 0.0001) and past cognitive activity (estimate = 0.27, SE = 0.09; p = 0.002), further indicating the strong association of these variables with resilience.
Resilience Index for Memory
Further, as also pointed out to us by a Reviewer, given that the effect AD pathology is likely to be more robust in the memory domain, we generated a Resilience Index for memory, where we were entered episodic memory as dependent variables in the regression model. The composite score for episodic memory was generated using seven tests as described above: immediate and delayed recall of story A from Logical Memory, immediate and delayed recall of the East Boston Story, Word List Memory, Word List Recall, and Word List Recognition. Figure 1b shows the leverage plot for the relationship between global pathology and episodic memory. As expected, there was a significant relationship between AD pathology and episodic memory (estimate = −1.07, SE = 0.06; p < .0001). We repeated analyses in Table 2 using Resilience Index-Memory as an outcome variable. There were significant associations between Resilience Index-Memory and reading level (estimate = 0.04, SE = 0.01; p < 0.0001), past cognitive activity (estimate = 0.19, SE = 0.08; p = 0.026), while early life socioeconomic status did not reach significance (estimate = 0.08, SE = 0.05; p = 0.09). Further, APOE genotype showed an even more robust relationship (estimate = −0.12, SE = 0.04; p < 0.0008), suggesting that memory-specific resilience is strongly related to APOE status.
DISCUSSION
The main objective of the present study was to conceptualize resilience in its dynamic form using clinico-neuropthaological indices. We present an innovative approach where, using a large neuropathological cohort, we operationalize resilience as discordance between cognition and pathology. While it has been known for decades that the relationship between neuropathology and cognition is imperfect, and that there are in fact some individuals who remain cognitively intact despite sustaining significant brain damage, there has been less focus on quantifying this discordance and on conceptualizing resilience as a continuous outcome measure. Even though dichotomous diagnoses for neurodegenerative diseases are based on statistical thresholds for the densities of lesions, pathology and cognition are continuous variables. As such, artificial dichotomization of continuous variables can have potential limitations, including loss of power, spurious statistical significance, and decrease in reliability31. In the present study, we sought to quantify resilience as a continuous variable using studentized residuals in a linear regression model. This approach has the advantages of keeping continuous variables (i.e., global cognition and global pathology) continuous, thus providing an index by which the “discordance” to be quantified.
It is also important to note the distinction between resilient brain aging and resilience as commonly used in psychological setting. Psychological resilience usually refers to individual’s ability to cope in the face of adversity. It represents person’s ability to “bounce back” from various stressful experiences, including trauma, loss, chronic conditions, and natural disasters32. Psychological resilience is commonly assessed using a variation of resilience scales to determine individuals’ responses to various life challenges presented from childhood to older age, and there currently exists a wealth of data using this approach33. The concept of resilient brain aging, on the other hand, examines the relationship between cognitive and pathological processes. For AD, this represents individual’s ability to sustain a relatively normal cognitive function despite evidence of significant AD pathology. The allied concepts of "resilience" and "reserve" are well recognized in clinical medicine34. The present study contributes to this field by utilizing large epidemiological neuropathological cohort to propose a model that can help quantify resilience as an index, which represents the discordance between neuropthaology and cognition. Such an index doesn't require dichotomization of variables, and could prove useful in examining factors (neurobiological or environmental) that account for the variance in cognitive function in the setting of AD pathology.
We also examined the extent to which, the resilience index, as developed, would be sensitive to known risk factors for AD and other related variables. Significant association was observed between education and resilience, such that higher level of education was related to greater resilience. This finding is consistent with accumulating evidence showing that education mitigates the impact of pathology on clinical expression of dementia35, and can differentiate individuals who remain cognitively intact despite sustaining equivalent amount of pathology (on biomarkers of both Aβ plaque deposition and neuronal degeneration) as those with dementia36. We also observed an association between resilience and reading level, a measure considered to be a good estimator of premorbid function, and relatively resistant to brain injury and neurodegenerative diseases37. Our findings are consistent with this, in that individuals with higher reading level were able to resist the deleterious effects of brain damage in AD and remained cognitively intact despite the significant pathology. In addition, consistent with previous findings14,38, we observed a significant association between past cognitive activity and resilience, such that higher resilience was related to greater participation in cognitively stimulating activities earlier in life. To our knowledge, this is the largest neuropathological cohort to demonstrate the relationship between past cognitive activity and resilience amidst significant AD pathology.
On the other hand, age and presence of any APOE-ε4 allele were inversely related to resilience, such that older age and the presence of APOE-ε4 allele indicated lower resilience. That is, old age and APOE-ε4 status decreased individuals’ ability to remain cognitively intact (resilient) in the setting of AD pathology. This suggests, for example, that individuals carrying the APOE-ε4 allele show more cognitive impairment in the setting of AD pathology compared to those sustaining equivalent amount of pathology, but not carrying the allele. This important to note because it suggests that cognitive impairment in the setting of AD pathology is not fully explained by the AD pathology per se, but that old age and APOE-ε4 status may contribute to this effect.
It is important to recognize limitations of this study. While the rich clinical and neuropathological data from the large ROS and MAP cohorts presented an opportunity to quantify resilience as described above, the use of mostly white volunteer cohorts who agreed to annual evaluations and post-mortem organ donation limits generalizability to the general older population. Self-report assessments of cognitive activity may also be subject to recall bias, particularly in persons with cognitive difficulties. The past cognitive ability data were available for a subset of participants, thus limiting the number of participants included in the final multivariate model. We also examined a relatively limited number of potential mediators of resilience and further work will need to include other promising lifestyle, personality, and genetic factors.
Few reports have addressed the discordance of AD phenotype and cognition, and the neurobiological correlates of resilient brain aging. Troncoso and colleagues reported preserved numbers and larger neuronal sizes in the hippocampus and other brain regions in their asymptomatic AD cases compared to normal, MCI and clinical AD cases in the BLSA and Nun Study cohorts.39,40 Studies of animal models also report the role of hippocampal brain-derived neurotrophic factor (BDNF) expression in resilience to chronic stress. Numerous studies have also indicated a link between the presence of BDNF and cognitive and affective disorders. The protective role of neurotrophins in the setting of resilient brain aging, however, is less understood.
Other studies suggest intermediate changes in select presynaptic proteins in preclinical or "early" AD.4,41 In a recent study, we measured immunolabled neurons, atrocytes, postynaptic spines and presynaptic vesicle protein expression in three groups: pathological AD with normal cognition ("AD-Resilient"), pathological AD with AD-typical dementia ("AD-Dementia)" and pathologically normal with normal cognition ("Normal Comparison"). We found that the AD-Resilient group exhibited preserved densities of presynaptic terminals and dendritic spines similar to the Normal Comparison group, and increased densities of astrocytes compared to both the AD-Dementia and Normal Comparison groups. Astrocyte responses to injury are diverse and regulated along context-specific spectra of molecular expression, morphological changes and neurochemical production. In insidious conditions like AD, there is increased expression of GFAP and hypertrophy of astrocyte cell bodies and processes. Cortical astrocytes maintain brain health through structural and biochemical support to neurons and endothelial cells, maintain extracellular ion balance, provide nutrients, regulate cortical blood flow, transmitter uptake and release, modulate synaptic neurotransmission and provide trophic and detoxifying support in response to injury.42 As such, astroglial activation may play an ameliorative role for brain functioning in the setting of AD pathology. Further work is needed to understand the mechanisms underlying resilient brain aging in the setting of pathology.
Nonetheless, the present study contributes to the field in several important ways. The cohort consisted of more than 900 well-characterized older adults with clinical and neuropathological data, assessed using previously established neuropathological and psychometric measures. The study also identified and characterized resilient individuals who remained cognitively intact, despite significant AD pathology. While it has long been recognized that there is discordance in the relationship between cognitive function and underlying AD pathology, to our knowledge, this is the first study that attempted to quantify this discordance in a meaningful way by characterizing resilience across the spectra of cognition and pathology. As such, the concept of resilience can provide a framework for understanding mechanisms that underlie healthy aging amidst disease-related pathology. It can also prove useful in clinical practice, particularly in identifying individuals who are at a greater risk of developing dementia for clinical trials (e.g., those with low resilience), and in designing prevention and treatment strategies that allow older adults to age successfully despite significant AD pathology. Further, the demonstration that resilience is associated with modifiable risk factors suggests that lifestyle interventions have the potential to allow some individuals to remain cognitively intact despite significant AD pathology.
Acknowledgements & Funding
This work was supported by grants from the National Institute on Aging (P30AG10161, R01AG039478, R01AG17917, R01AG15819, P30AG10124). We are deeply indebted to all the volunteers in the Rush Religious Order Study and the Memory and Aging Project, and the staff engaged in subject assessment, autopsy, and brain banking at Rush University Medical Center.
Footnotes
Disclosure Statement
The authors report no conflicting interests.
REFERNCES
- 1.Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968 Sep-Oct;7(2):331–356. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- 2.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006 Jun 27;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 3.Iacono D, Markesbery WR, Gross M, et al. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009 Sep 1;73(9):665–673. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien RJ, Resnick SM, Zonderman AB, et al. Neuropathologic Studies of the Baltimore Longitudinal Study of Aging (BLSA) J Alzheimers Dis. 2009 Aug 3; doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White L. Brain Lesions at Autopsy in Older Japanese-American Men as Related to Cognitive Impairment and Dementia in the Final Years of Life: A Summary Report from the Honolulu-Asia Aging Study. J Alzheimers Dis. 2009 Aug 3; doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 6.Head E, Corrada MM, Kahle-Wrobleski K, et al. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol Aging. 2009 Jul;30(7):1125–1134. doi: 10.1016/j.neurobiolaging.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009 May 28;360(22):2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 8.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 9.Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008 Sep;65(9):1211–1217. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewbank DC, Arnold SE. Cool with plaques and tangles. N Engl J Med. 2009 May 28;360(22):2357–2359. doi: 10.1056/NEJMe0901965. [DOI] [PubMed] [Google Scholar]
- 11.Loebrich S, Nedivi E. The function of activity-regulated genes in the nervous system. Physiol Rev. 2009 Oct;89(4):1079–1103. doi: 10.1152/physrev.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Filippo M, Tozzi A, Costa C, et al. Plasticity and repair in the post-ischemic brain. Neuropharmacology. 2008 Sep;55(3):353–362. doi: 10.1016/j.neuropharm.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Berlucchi G, Buchtel HA. Neuronal plasticity: historical roots and evolution of meaning. Exp Brain Res. 2009 Jan;192(3):307–319. doi: 10.1007/s00221-008-1611-6. [DOI] [PubMed] [Google Scholar]
- 14.Stern C, Munn Z. Cognitive leisure activities and their role in preventing dementia: a systematic review. Int J Evid Based Healthc. 2010 Mar;8(1):2–17. doi: 10.1111/j.1744-1609.2010.00150.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: overview and change in cognitive and motor speed. Aging Neuropschol Cogn. 2004;11:280–303. [Google Scholar]
- 16.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer's Disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004 Apr 13;62(7):1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 19.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995 May-Jun;16(3):271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- 21.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991 Apr;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 22.McKeith IG, Galasko D, Kosaka K, et al. Concensus guidlines for the clinical and pathological diagnosis of dementia with Lewy bodies (DLB) Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RS, Scherr PA, Bienias JL, et al. Socioeconomic characteristics of the community in childhood and cognition in old age. Exp Aging Res. 2005 Oct-Dec;31(4):393–407. doi: 10.1080/03610730500206683. [DOI] [PubMed] [Google Scholar]
- 24.Medi-Span Inc. Master Drug Data Base Documentation Manual. Medi-Span: Indianapolis I, Medi-Span Inc. IN; 1995. [Google Scholar]
- 25.Nelson HE. The National Adult Reading Test. Windsor, England: The NFER-Publishing Co. Ltd.; 1982. [Google Scholar]
- 26.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007 Nov 13;69(20):1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 27.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003 Jan 28;60(2):246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute Inc. SAS/STAT User’s Guide Version 8. Cary NSI; 2000. [Google Scholar]
- 29.Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE. Cognition and neuropathology in aging: multidimensional perspectives from the rush religious orders study and rush memory and aging project. Curr Alzheimer Res. 2011 Jun 1;8(4):336–340. doi: 10.2174/156720511795745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. The cost of dichotomization. Appl Psychol Meas. 1983;7:249–253. [Google Scholar]
- 32.Karoly P, Ruehlman LS. Psychological "resilience" and its correlates in chronic pain: findings from a national community sample. Pain. 2006 Jul;123(1–2):90–97. doi: 10.1016/j.pain.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Lamond AJ, Depp CA, Allison M, et al. Measurement and predictors of resilience among community-dwelling older women. J Psychiatr Res. 2008 Dec;43(2):148–154. doi: 10.1016/j.jpsychires.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riudavets MA, Iacono D, Resnick SM, et al. Resistance to Alzheimer's pathology is associated with nuclear hypertrophy in neurons. Neurobiol Aging. 2007 Oct;28(10):1484–1492. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006 Jul-Sep;20(3) Suppl 2:S63–S68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- 36.Negash S, Xie S, Davatzikos C, et al. Cognitive and Functional Resilience Despite Molecular Evidence of Alzheimer’s Disease Pathology. Alzheimer’s and Dementia. doi: 10.1016/j.jalz.2012.01.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jefferson AL, Gibbons LE, Rentz DM, et al. A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. J Am Geriatr Soc. 2011 Aug;59(8):1403–1411. doi: 10.1111/j.1532-5415.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003 Jun 19;348(25):2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 39.West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer's disease. Neurobiol Aging. 2004 Oct;25(9):1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Iacono D, O'Brien R, Resnick SM, et al. Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol. 2008 Jun;67(6):578–589. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(8):933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010 Jan;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]