Abstract
Background
The natural history and the mechanisms behind the alteration of vaginal distension (VD) in a mouse model are not clear.
Objective
We examined the temporal sequelae of VD and pudendal nerve transection (PNT) on leak-point pressure (LPP) and the muscular and nerve components of the urethra in mice.
Design, setting, and participants
Seventy-two virgin female C57BL/6 mice were equally distributed into three groups. The VD group underwent VD for 1 h. The PNT group received bilateral PNT. A control group underwent sham VD.
Intervention
Each group was divided into four subgroups of six mice for measurement of LPP at 0, 4, 10, and 20 d after VD or PNT.
Measurements
LPP was measured. Morphology and neurofilament-immunoreactive nerve of the urethra were assessed.
Results and limitations
LPP was decreased at 0, 4, and 10 d but not at 20 d after VD. Decreased LPP persisted to 20 d in the PNT group. The external urethral striated muscle appeared wavy and disrupted in three mice at 0 d, in two mice at 4 d, in one mouse at 10 d, and in one mouse in 20 d after VD. The density of neurofilament-immunoreactive nerve in the urethra was reduced at 4 and 10 d after VD, but not at 20 d, and at 4, 10, and 20 d after PNT compared with the corresponding values of the sham VD group. The limitation of this animal model is that the pelvic floor structure of the mouse is different from that of female humans. Therefore, results of this study should be carefully applied to human subjects.
Conclusions
VD causes reversible stress urinary incontinence in female mice. Recovery of continence function following VD is associated with repair of the external urethral sphincter and reinnervation of the urethra. This mouse model will be useful for mechanistic investigation and targeting of therapeutic intervention by taking advantage of genetic manipulation.
Keywords: Vaginal distension, Pudendal nerve, Leak point pressure, Urethra, Neurofilament
1. Introduction
Stress urinary incontinence (SUI) is the most common type of urinary incontinence and affects approximately 25 million Americans of all ages, with disproportionally higher prevalence among women [1]. Birth trauma from vaginal delivery is one of the widely recognized risk factors in the genesis of SUI in women [1,2]. Specific mechanisms of birth trauma injury may include denervation damage, ischemia, and mechanical injuries to the muscular and nerve components of the lower urinary tract tissues [1,2]. The pudendal nerve innervates the external urethral sphincter (EUS) and is among the tissues that may be injured during vaginal delivery [3]. Over the last decade, animal models of SUI have increasingly been used to understand the pathogenesis of SUI [4,5]. Vaginal distension (VD) [6,7] and pudendal nerve transection (PNT) [8,9] have been used for creation of SUI in rats. Leak-point pressure (LPP) is used clinically to evaluate urethral competence and to determine the etiology of urine leakage. To take advantage of transgenic capabilities in mechanistic studies of SUI, we have recently created and reported a model of SUI by VD in mice [10]. In this study, we aimed to examine the temporal sequelae of VD and PNT on LPP and the muscular and nerve components of the urethra in mice.
2. Materials and methods
2.1. Experimental design
Seventy-two virgin female C57BL/6 strain mice (17.10 ± 0.13 g), aged 6–8 wk, were randomly distributed into three groups of 24 mice: (1) the VD group underwent VD for 1 h, using a modified 6-Fr Foley catheter with a balloon dilated to 0.3 ml; (2) the PNT group received bilateral PNT; and (3) the sham VD group received the vaginal accommodation of the urethral dilators, and then the insertion of the flattened 6-Fr Foley catheter in the vagina for 1 h. Each group was subsequently divided into four subgroups of six mice for measurement of LPP at 0, 4, 10, and 20 d, after VD or PNT. All mice underwent suprapubic tube implantation into the bladder 2 d before performing LPP. After measuring LPP, the animals were sacrificed and the urethras were removed for morphology and neurofilament-immunoreactive nerve assessment. All experimental protocols and procedures were approved by the Cleveland Clinic's Institutional Animal Care and Use Committee.
2.2. Vaginal distension
We followed our previously described method for VD in mice [10]. Under ketamine and xylazine anesthesia, vaginal accommodation of urethral dilators (Cardinal Health Company, Dublin, OH, USA) was achieved by sequentially inserting and removing increasing sizes of urethral dilators lubricated with Surgilube (Fougera, Melville, NY, USA). Then, a modified 6-Fr Foley catheter (Mentor, Tempe, AZ, USA) was lubricated and inserted into the vagina and was secured to the vaginal introitus with a 5/0 silk suture. To distend the vagina, 0.3 ml of distilled water was injected into the balloon [10]. After 1 h, the balloon was deflated and removed.
2.3. Pudendal nerve transection
PNT was performed similarly to the methods we described before in a rat model [8]. Under ketamine and xylazine anesthesia, a dorsal midline longitudinal incision was made in the skin between the bilateral ischium. After incision of bilateral lateral ventral sacrococcygeal muscles, the bilateral pudendal nerves in the ischiorectal fossa were located using an operating microscope. Then we cut a segment of the pudendal nerve on each side in the ischiorectal fossa. The muscle and skin incisions were closed separately with 4-0 vicryl sutures.
2.4. Leak-point pressure measurement
Two days before performing LPP, a suprapubic tube (PE-10 tubing) was implanted in the bladder [10]. LPP was measured according to previously described methods [8,10]. Mice were anesthetized with urethane (1 g/kg, ip). The bladder was emptied manually using Crede's maneuver, and then filled with room-temperature saline at 1 ml/h through the bladder catheter. The average bladder capacity of each mouse was determined after three to five voiding cycles. When half-bladder capacity was reached, gentle pressure with one finger was applied to the mouse's abdomen. Pressure was gently increased until urine leaked, at which time the externally applied pressure was rapidly removed. The peak bladder pressure was taken as the LPP. Five measurements were obtained on each animal, and the mean LPP was recorded. All of the LPP measurement procedures were performed by the same experienced investigator, who was blinded to the group of each animal.
2.5. Histologic examination, immunofluorescence staining, and quantification
The mice were sacrificed by overdose with isoflurane, and the middle one-third of the urethra was harvested. Half of each tissue sample was fixed in 10% buffered formalin for Masson's trichrome staining and the other half of each tissue sample was embedded in Tissue-Tek optimum cutting temperature (OCT) compound for immunofluorescence staining of neurofilament.
Sections 5-μm thick were obtained and stained with Masson's trichrome. The mean of the four regions of striated muscle in the urethra, near the two diagonal lines, was evaluated in detail in each mouse, as described before [11], using Image-Pro Plus v.5.1 image analysis software (Media Cybernetics Inc, Bethesda, MD, USA).
The OCT embedded frozen tissue was cut into 8-μm cryostat sections. The sections were incubated with a solution containing 3% normal sheep serum and 0.2% Triton-X-100 at room temperature and were kept overnight with rabbit antineurofilament-200–kDa protein antibody (AB 1982, 1:500; Chemicon, Temecula, CA, USA) at 4°C. The slides were then rinsed and incubated with fluorescein-conjugated Alexa 488 sheep antirabbit immunoglobulin G (1:5000; Molecular Probes, Eugene, OR, USA) for 1 h at room temperature. After brief rinsing, the preparation was mounted and examined with a fluorescence microscope.
The neurofilament 200–positive area and the whole cross-section tissue area of the urethra were measured using the following approach. Images of urethral tissue sections were acquired using a Leica DMR upright microscope (Heidelberg, Germany), ×10 objective, fluorescein isothiocyanate (FITC) filter cube (BP 480/40-nm excitation; BP 527/30-nm emission), MetaMorph v.6.3 acquisition software (Molecular Devices, Downingtown, PA, USA), and a MicroMax digital CCD camera (Princeton Instruments, Trenton, NJ, USA). Raster scans were performed across each tissue section using an eight-slide X-Y-Z–motorized stage (Prior Scientific, Rockland, MA, USA). Following background correction to remove illumination artifacts, the resulting image tiles were stitched together to produce high-resolution, large field-of-view images for quantitative analysis. The images were then analyzed with Image-Pro Plus v.5.1 image analysis software. The software can automatically distinguish regions stained with different colors and can accurately measure the areas by counting the pixels and converting pixels to number of square millimeters. The percentage of neurofilament 200 immunoreactive nerve fibers in the whole cross-section of the urethra tissue was measured.
2.6. Data analysis
All data are expressed as the mean plus or minus standard error of the mean. Comparisons of measurements among the VD, PNT, and sham VD groups at the same time point were performed with the one-way analysis of variance (ANOVA) test. Bonferroni corrections were used to adjust for multiple pairwise comparisons, and p < 0.017 was established as statistically significant. Prism 4 (GraphPad Prism Software Inc, La Jolla, CA, USA) was used for all calculations.
3. Results
3.1. Leak-point pressure measurement
LPP was significantly decreased at 0, 4, and 10 d in the VD groups (10.88 ± 1.52, 10.04 ± 2.36, and 10.93 ± 2.11 cmH2O) compared with the sham VD group at the same time points (32.02 ± 4.30, 30.20 ± 5.90, and 26.72 ± 1.89 cmH2O). No significant differences were found in LPP at 20 d after VD versus the sham VD group, indicating spontaneous recovery of the continence mechanisms following VD in 20 d. LPP was significantly decreased at 0, 4, 10, and 20 d after PNT (6.85 ± 2.63, 5.01 ± 1.30, 6.90 ± 2.43, and 4.10 ± 0.62 cmH2O) compared with the sham VD group at the same time points (Fig. 1).
Fig. 1.

Leak-point pressure (LPP) values at 0, 4, 10, and 20 d after vaginal distension (VD), pudendal nerve transection (PNT), and sham VD groups. Results are expressed as the mean plus or minus standard error of the mean of six individual mice.
* Significantly different from corresponding value in the sham group (p < 0.01).
# Significantly different from corresponding value in the sham and VD groups (p < 0.01).
3.2. Histologic examination
Histologic examination of the midurethra showed a typical morphology consisting of the urothelium, then the underlying lamina propria, a layer of smooth muscle, and a thick layer of striated muscle (Fig. 2). The muscle fibers of urethral striated muscle appeared wavy and disrupted in three mice at 0 d, in two mice at 4 d, in one mouse at 10 d, and in one mouse at 20 d after VD. The connective tissues showed increase at 20 d after VD. The urethral striated muscle fiber showed atrophy after 10 d in the PNT group. The mean thickness of the four regions of striated muscle near the two diagonal lines, however, was not significantly different between groups at different time points (Fig. 3).
Fig. 2.
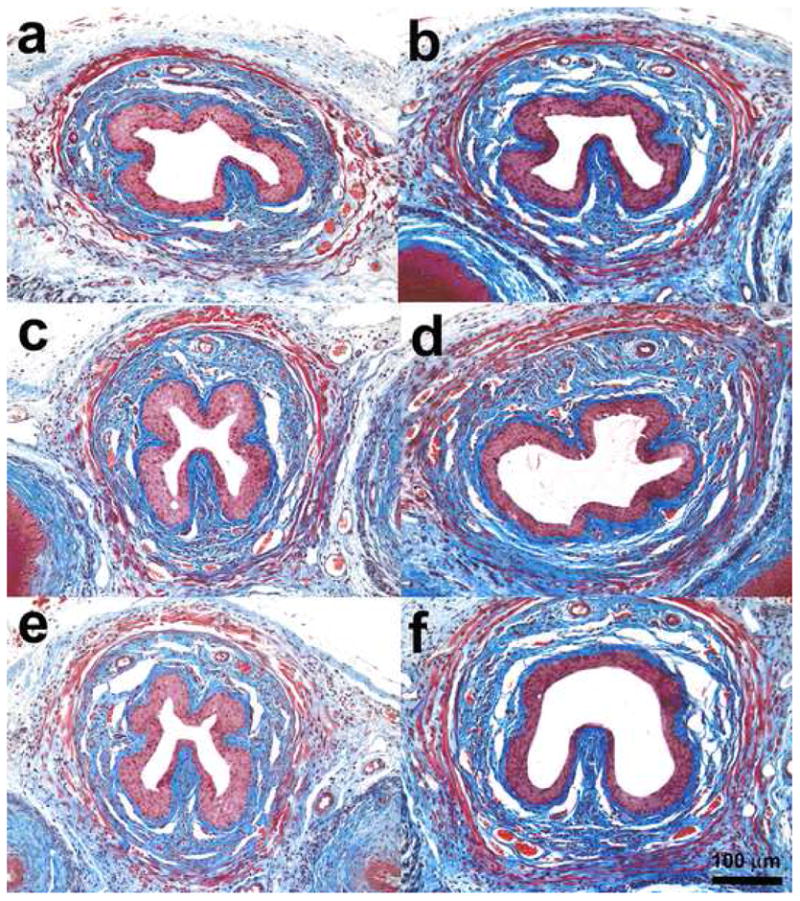
Images (Masson's trichrome staining) of transverse sections of the midurethra at (a) 0 d after vaginal distension (VD), (b) 4 d after VD, (c) 10 d after VD, (d) 20 d after VD, (e) 4 d after pudendal nerve transection, and (f) 4 d after sham VD.
Fig. 3.

The width of urethral striated muscle from the mean of the four regions of striated muscle, near the two diagonal lines, in midurethra in vaginal distension (VD), pudendal nerve transection (PNT), and sham VD groups. Results are expressed as the mean plus or minus standard error of the mean of four to six individual mice.
3.3. Immunofluorescence staining of neurofilaments
Neurofilament-immunoreactive nerves were seen in both urothelium and muscle layers but predominantly within the smooth muscle and striated muscle (Fig. 4). The density of immunoreactive neurofilaments in the urethra was significantly reduced at 4 and 10 d after VD (2.61 ± 0.05, 2.88 ± 0.19%) and at 4, 10, and 20 d after PNT (2.45 ± 0.11, 2.27 ± 0.28, 2.57 ± 0.65%) compared with the corresponding values of the sham VD group (Fig. 5). There was no significant difference, however, in the density of neurofilament at 20 d after VD and at 20 d after sham VD (Fig. 5), indicating that recovery of the continence function may be related to reinnervation.
Fig. 4.
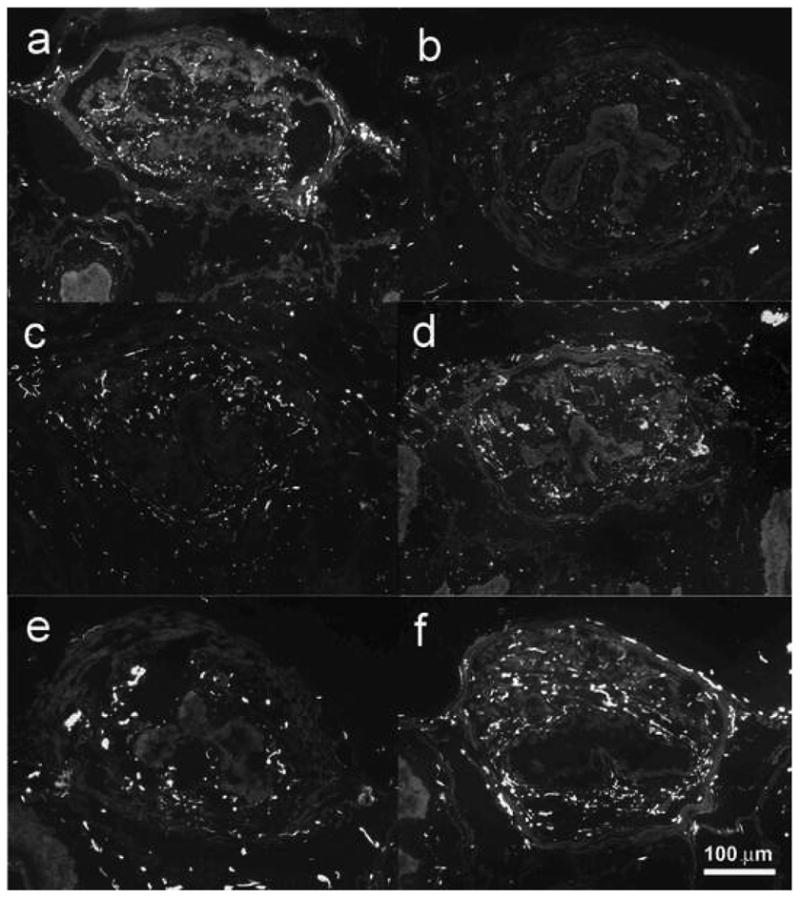
Representative images of neurofilament-immunoreactive positive nerves in transverse sections of the midurethra at (a) 0 d after vaginal distension (VD), (b) 4 d after VD, (c) 10 d after VD, (d) 20 d after VD, (e) 4 d after pudendal nerve transection, and (f) 4 d after sham VD.
Fig. 5.
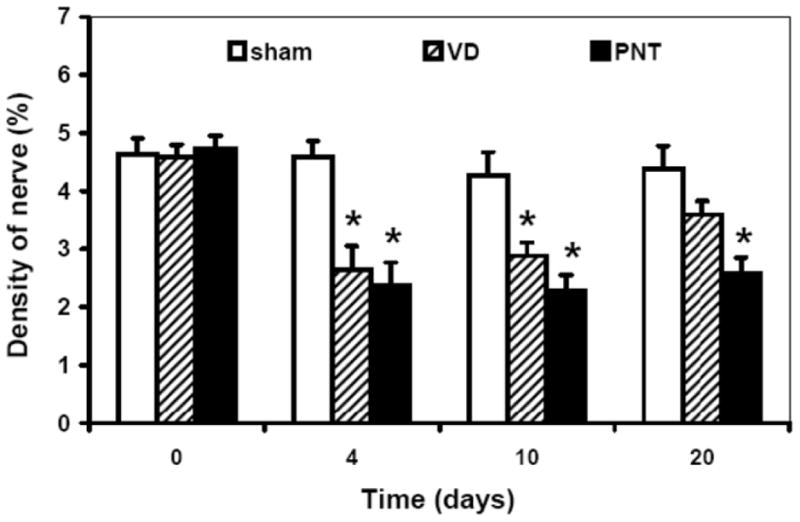
The density of neurofilament-immunoreactive positive nerves in transverse sections of the midurethra in vaginal distension (VD), pudendal nerve transection (PNT), and sham VD groups. Results are expressed as the mean plus or minus standard error of the mean of four to six individual mice.
* Significantly different from corresponding value in the sham group (p < 0.01).
4. Discussion
Birth trauma resulting from vaginal delivery is one of the widely recognized risk factors in the genesis of SUI in women. Foldspang et al [12] found that two-thirds of women who were 30–44 yr old with SUI had at least one vaginal delivery. Postpartum incontinence is typically attributed to pathophysiologic changes that occur as a result of delivery, such as nerve or muscle injury or damage to the urethra and its suspensory structures [1,9]. This damage occurs as a direct result of the large ratio of the baby's head to the birth canal in humans. Pressure to the vaginal sidewall can reach 240 cmH2O during the peak of contractions [13], which is sufficient to cause microcirculatory ischemia to muscle and nerve tissue if it is maintained for >30 min [14]. The stretch injury also causes alteration of the composition and function of the lower urinary tract tissues. Other factors implicated in the etiology of incontinence include fetal size, prolonged second-stage labor, existence of prepregnancy incontinence, and antenatal bladder neck mobility [15].
Animal models of SUI have been used over the last decade to investigate the pathogenesis of SUI [4]. The rat VD model was used to simulate the damage that occurs in the pelvic floor during vaginal delivery of children [6,16]. To take advantage of future utilization of transgene and knockout technology, we recently reported successful induction of SUI in mice by VD [10]. That study showed that a modified 6-Fr Foley catheter with balloon inflated to 0.3 ml would provide a SUI model comparable to what had been described in the female rat [6]. Subsequently, we aimed to examine the time course of LPP change after VD and to compare it to a durable model of SUI (ie, PNT) [4]. Additionally, we were interested in exploring the possible mechanisms of these changes, specifically the extent of damage to muscle and nerve components in the urethra following VD.
The present study showed that LPP was significantly decreased at 0, 4, and 10 d after VD compared with sham VD. LPP, however, was not significantly different at 20 d after VD compared with sham VD, indicating the recovery of continence. LPP was significantly lower at 0, 4, 10, and 20 d after PNT compared with sham VD, confirming that PNT is a durable SUI model.
The mechanisms of LPP decrease and recovery involve EUS injury and repair in VD animals. The contraction of EUS is very important to maintaining urinary continence during elevation of abdominal pressure [16]. Histologic results in our study demonstrated the EUS of some VD animals was disrupted and wavy. The number of mice with disrupted and wavy urethral sphincter was three, two, one, and one at 0, 4, 10, and 20 d, respectively, indicating the injury and healing process over time after VD. We cannot preclude the presence of ultrastructural damage of EUS in animals, which did not show obvious disruption by gross examination. A recent clinical study of the ultrastructural characteristics of the detrusor and urethral musculature in women with urinary incontinence showed that urethral smooth muscle cells are separated by several microns with almost no direct cell-to-cell communication [17].
The mechanisms of LPP decrease and recovery also involve nerve degeneration and regeneration in the urethra in VD animals. Neurofilament 200 mainly stained A fiber, which are myelinated afferent or efferent fibers of the peripheral nervous system [18]. The present study showed that the density of neurofilaments in the urethra was significantly reduced at 4 and 10 d after VD and at 4, 10, and 20 d after PNT compared with sham VD. Therefore, we propose that a reduced nerve density, reflecting a partial loss of innervation, is another key reason for the impaired continence mechanism following VD. The pudendal nerve, which innervates the EUS, courses through Alcock's canal and is particularly vulnerable to stretch and crush injury during childbirth. Nerve injury can lead to functional denervation of muscles with a variety of degenerative changes in muscle morphology and biochemistry, resulting in decreased urethral closure ability and symptoms of SUI. Allen et al observed that vaginal delivery in nulliparous women was associated with partial denervation of the pelvic floor [3]. The density of neurofilaments 20 d after VD was not significantly different from sham VD, suggesting that reinnervation of the urethra occurs >20 d following VD. Previous study showed that bilateral pudendal nerve crush in the female rat induced mild SUI, lasting for 2 wk, and neuroregeneration happened 14 d after pudendal nerve crush in rats [19]. Regeneration of nerves was also observed in 80% of women 6 mo after delivery. Women submitted to elective cesarean section, however, demonstrated no electromyographic changes before and after childbirth [3]. These studies support our results.
The present study suggested that unlike PNT, which directly damages the pudendal nerve, VD causes injury to the EUS and its innervation simultaneously, and 20 d after injury represents an early time point for functional recovery after VD in mice. The muscle recovery and nerve recovery can promote each other. The repaired muscle can promote regeneration of the nerve by increasing the production of some neurotrophins, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-4 (NT-4) [20–22]. Reinnervation of some muscles results in restoration, to some degree, of the original muscle fiber structure and contractile activity, accompanied by a reversal of some previous biochemical alterations [23]. A recent study has shown that VD-induced expression of monocyte chemotactic protein-3, a chemotactic agent in stem cell homing, as well as its receptor, CC chemokine receptor 1 [24], might initiate the repair of the damaged muscle and nerve through stem cell homing response; however, the functional recovery may not mean a full structural recovery. Clinically, women undergo an anatomic or neuromuscular injury during childbirth, but they remain clinically asymptomatic as long as there is compensation by other components of the continence mechanism. With the loss of some muscle strength or innervation to the urethral sphincter due to aging or other injuries, the symptoms of SUI become apparent, usually many years after the first delivery. A clinical study showed an increase in the prevalence of moderate to severe SUI after vaginal delivery from 2% to 12% 10 yr after delivery [25].
This study has some limitations. First, the pelvic floor structure of mouse is different from that of female humans; therefore, results of this study should be carefully applied to human subjects. Second, the present study cannot discriminate which component of the pudendal nerve (afferent or efferent, automatic or somatic) is damaged. Further study is needed to illustrate which component of pudendal nerve is damaged during VD. Third, intrinsic sphincter deficiency and urethral hypermobility are two main pathophysiologic mechanisms of SUI. Our study did not observe the effects of VD on the periurethra tissue, which might be another mechanism of VD-induced incontinence.
5. Conclusions
Both VD and PNT caused SUI in female mice, as measured by LPP; however, VD causes reversible SUI in female mice. LPP recovered 20 d after VD. Such recovery is temporally associated with repair of the EUS and reinnervation of the urethra. This observation, in addition to our model of SUI in mice, could be used for mechanistic investigation of SUI and targeting of therapeutic intervention.
Take-home message.
Birth trauma is a recognized risk factor of stress urinary incontinence. We examined the natural history and the mechanisms behind the alteration of simulated childbirth in mouse. Recovery of continence function following simulated childbirth involves urethra muscle and nerve repair.
Acknowledgments
The authors thank Dr. Amit Vasanji (Department of Biomedical Engineering, Cleveland Clinic) for assistance with quantification of the density of neurofilament-immunoreactive nerve terminals and Dr. Jay Reeder for critical review.
Funding/Support and role of the sponsor: The study was supported by the following grants: NIH-NIDDK-DK02631 and the Young Investigator Award of the National Kidney Foundation.
Footnotes
Author contributions: Firouz Daneshgari had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lin, Liu, Daneshgari
Acquisition of data: Lin, Li, Xiao
Analysis and interpretation of data: Lin, Liu, Daneshgari
Drafting of the manuscript: Liu, Daneshgari
Critical revision of the manuscript for important intellectual content: Liu, Daneshgari
Statistical analysis: Lin
Obtaining funding: Daneshgari
Administrative, technical, or material support: Xiao
Supervision: Liu, Daneshgari
Other (specify): none
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Retzky SS, Rogers RM., Jr Urinary incontinence in women. Clin Symp. 1995;47:2–32. [PubMed] [Google Scholar]
- 2.Altman D, Ekstrom A, Forsgren C, Nordenstam J, Zetterstrom J. Symptoms of anal and urinary incontinence following cesarean section or spontaneous vaginal delivery. Am J Obstet Gynecol. 2007;197:512–7. doi: 10.1016/j.ajog.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 3.Allen RE, Hosker GL, Smith AR, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97:770–9. doi: 10.1111/j.1471-0528.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 4.Hijaz A, Daneshgari F, Sievert KD, Damaser MS. Animal models of female stress urinary incontinence. J Urol. 2008;179:2103–10. doi: 10.1016/j.juro.2008.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez LV, Chen S, Jack GS, de Almeida F, Lee KW, Zhang R. New objective measures to quantify stress urinary incontinence in a novel durable animal model of intrinsic sphincter deficiency. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1332–8. doi: 10.1152/ajpregu.00760.2004. [DOI] [PubMed] [Google Scholar]
- 6.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143–51. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 7.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002;90:403–7. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 8.Hijaz A, Daneshgari F, Huang X, et al. Role of sling integrity in the restoration of leak point pressure in the rat vaginal sling model. J Urol. 2005;174:771–5. doi: 10.1097/01.ju.0000164721.52278.29. [DOI] [PubMed] [Google Scholar]
- 9.Boyles SH, Li H, Mori T, Osterweil P, Guise JM. Effect of mode of delivery on the incidence of urinary incontinence in primiparous women. Obstet Gynecol. 2009;113:134–41. doi: 10.1097/AOG.0b013e318191bb37. [DOI] [PubMed] [Google Scholar]
- 10.Lin YH, Liu G, Daneshgari F. A mouse model of simulated birth trauma induced stress urinary incontinence. Neurourol Urodyn. 2008;27:353–8. doi: 10.1002/nau.20509. [DOI] [PubMed] [Google Scholar]
- 11.Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25:388–96. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- 12.Foldspang A, Mommsen S, Lam GW, Elving L. Parity as a correlate of adult female urinary incontinence prevalence. J Epidemiol Community Health. 1992;46:595–600. doi: 10.1136/jech.46.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rempen A, Kraus M. Measurement of head compression during labor: preliminary results. J Perinat Med. 1991;19:115–20. doi: 10.1515/jpme.1991.19.1-2.115. [DOI] [PubMed] [Google Scholar]
- 14.Gelberman RH, Szabo RM, Williamson RV, Hargens AR, Yaru NC, Minteer-Convery MA. Tissue pressure threshold for peripheral nerve viability. Clin Orthop Relat Res. 1983:285–91. [PubMed] [Google Scholar]
- 15.Burgio KL, Zyczynski H, Locher JL, Richter HE, Redden DT, Wright KC. Urinary incontinence in the 12-month postpartum period. Obstet Gynecol. 2003;102:1291–8. doi: 10.1016/j.obstetgynecol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Kamo I, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol. 2003;285:R356–65. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- 17.Hale DS, Benson JT, Brubaker L, Heidkamp MC, Russell B. Histologic analysis of needle biopsy of urethral sphincter from women with normal and stress incontinence with comparison of electromyographic findings. Am J Obstet Gynecol. 1999;180:342–8. doi: 10.1016/s0002-9378(99)70211-5. [DOI] [PubMed] [Google Scholar]
- 18.Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakamoto K, Smith GM, Storer PD, Jones KJ, Damaser MS. Neuroregeneration and voiding behavior patterns after pudendal nerve crush in female rats. Neurourol Urodyn. 2000;19:311–21. doi: 10.1002/(sici)1520-6777(2000)19:3<311::aid-nau11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Hasan W, Zhang R, Liu M, Warn JD, Smith PG. Coordinate expression of NGF and alpha-smooth muscle actin mRNA and protein in cutaneous wound tissue of developing and adult rats. Cell Tissue Res. 2000;300:97–109. doi: 10.1007/s004410000175. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, Chen LS, Miyauchi Y, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 22.Sakuma K, Watanabe K, Sano M, et al. A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res. 2001;907:1–19. doi: 10.1016/s0006-8993(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg HA, Hood DA. Blood flow, mitochondria, and performance in skeletal muscle after denervation and reinnervation. J Appl Physiol. 1994;76:859–66. doi: 10.1152/jappl.1994.76.2.859. [DOI] [PubMed] [Google Scholar]
- 24.Wood HM, Kuang M, Woo L, et al. Cytokine expression after vaginal distension of different durations in virgin Sprague-Dawley rats. J Urol. 2008;180:753–9. doi: 10.1016/j.juro.2008.03.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman D, Ekstrom A, Gustafsson C, Lopez A, Falconer C, Zetterstrom J. Risk of urinary incontinence after childbirth: a 10-year prospective cohort study. Obstet Gynecol. 2006;108:873–8. doi: 10.1097/01.AOG.0000233172.96153.ad. [DOI] [PubMed] [Google Scholar]


