Abstract
Objectives
To evaluate the organ specificity of sine-wave electrical stimulation of the bladder through assessment of expression of Fos-immunoreactive (IR) cells in rat spinal cord regions.
Methods
Thirty-seven female Sprague-Dawley rats were divided into eight groups: sham stimulation; 5, 250, and 2,000 Hz stimulation with 1.5 mA or 2.0 mA intensity; and a group instilled with capsaicin in bladder. Using a recently developed bladder sensory threshold device, sine-wave electrical stimulation was applied for 90 min to rat bladder. Spinal cord was harvested after sacrifice. Fos-IR cells in the spinal regions of the medial dorsal horn, lateral dorsal horn, dorsal commissure (DCM), and sacral parasympathetic nucleus (SPN) were measured. The distributions of Fos-IR neurons were compared.
Results
The maximum expression of Fos-IR cells, induced by 250-Hz and 5-Hz stimulation of the bladder, was found at L6 of the spinal cord and was significantly higher than in the control group (p < 0.01). Stimulation with 2,000 Hz did not induce any Fos-IR cells. Fos-IR neurons were predominantly seen in the SPN region in response to 250-Hz stimulation and in the DCM region in response to 5-Hz stimulation. The numbers of positive neurons were similar to the numbers caused by capsaicin instillation.
Conclusions
Frequency-specific sine-wave electrical stimulation of the rat bladder induced the expression of Fos-IR cells in a neuroselective manner. The bladder sensory threshold device could be used for exploration of the pathophysiology of the diseases with disturbances of afferent pathway of the bladder.
Keywords: Bladder sensory threshold, Capsaicin, Fos, Spinal cord
INTRODUCTION
Disturbances of the autonomic afferent nerve fibers of the bladder are suspected in several common pathologies of the bladder, such as overactive bladder, diabetic bladder dysfunction, and neurogenic bladder1. The majority of the afferent impulses from the organs of the lower urinary tract in the rat that control urine storage and voiding are transferred via the pelvic nerve to the lumbar or sacral dorsal root ganglia, and finally to the L6-S1 segments of the spinal cord2. Those afferent fibers consist of small myelinated Aδ fibers and unmyelinated C fibers3. Aδ fibers have a mechano-sensitive role in monitoring normal bladder wall distention and contraction. C fibers are normally not mechano-sensitive to bladder distention. However, in neuropathic and/or inflammatory conditions, C fibers seem to acquire a new role: that of initiating urinary urge or bladder pain.
The Neurometer® electrostimulator (Neurotron, Inc., Baltimore, MD) can deliver sinusoid waveform constant alternating current stimuli to various cutaneous sites and has been used to assess thresholds of perception of sensory nerve conduction in humans and animals4,5. The Neurometer® can deliver stimuli at frequencies of 5, 250, and 2,000 Hz, which have been reported to selectively stimulate C, Aδ and large myelinated (Aβ) fibers, respectively, in humans6 and rats5,7. Recently, we developed the bladder sensory threshold (BST) device, which is implanted on the bladder mucosa in rats to assess bladder afferent nerve function8. We have shown that Neurometer®-BST stimulations at 5 or 250 Hz and measurements of bladder BST values in conscious rats, detected by a light startle response, are reproducible temporally and among different investigators8. Pretreatment with a desensitizing amount of resiniferatoxin, a potent stimulator/desensitizer of C fibers and some Aδ fibers9, resulted in higher BST values in response to Neurometer®-BST stimulation at 5 Hz, but not at 250 Hz8. Lidocaine, a more general nerve fiber desensitizer10, resulted in higher BST values at both 5 and 250 Hz stimulations8.
C-fos or Fos, an immediate early gene which can be activated by increased neuronal activity, allows an accurate quantitative and site specific evaluation of noiceptive input reaching the spinal cord from visceral organs. Previous studies demonstrated that noxious (chemical irritation) and non-noxious stimulation (bladder distension) of the rat lower urinary tract increased Fos expression in L6 spinal cord, including the superficial lateral horn (LDH) and medial dorsal horn (MDH), the dorsal commissure (DCM), and the sacral parasympathetic nucleus (SPN)11,12. Noxious stimulation of the bladder activated greater numbers of Fos expression in the MDH and DCM, whereas non-noxious stimulation induced greater expression in the SPN.11–13Very few Fos positive cells were identified after stimulation of the LDH12. Thus indicating the DCM as the site specific region for receipt of bladder noiceptive stimuli.
In the present study, we aim to examine whether frequency-specific sine wave eletrostimulation of the bladder provokes Fos expression in a site specific manner. If true, then the BST could be used for assessment of irregularities of afferent bladder sensation commonly seen in prevalent conditions.
MATERIALS AND METHODS
Experimental animals
All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Cleveland Clinic, the previous institution of the authors. Thirty-seven female Sprague-Dawley rats (~218–251g) were used in the experiments and maintained on ordinary laboratory chow and tap water ad libitum in a 12:12-hr light/dark cycle. Females were chosen for ease of transurethral catheterization, as there are no known effects of gender on afferent stimulation in the rat. The rats were divided into eight groups: electrode implantation only (n = 5), 5-Hz stimulation with 1.5 mA (n = 4) or 2.0 mA (n = 6); 250-Hz stimulation with 1.5 mA (n = 4) or 2.0 mA (n = 6); 2,000-Hz stimulation with 1.5 mA (n = 4) or 2.0 mA (n = 4); and a group infused with capsaicin in the bladder (1 mM; n = 4).
Electrode implantation and electrical stimulation
The bladder BST electrode is made from Flexon™ suture, a twisted multi-strand stainless steel wire coated with olytetrapuluoroproethylene [8]. With the rats under general anesthesia (urethane 1.2 g/kg), the electrode was implanted in the posterior bladder through a middle abdominal incision. In addition, fur was removed from part of the neck for placement of a separate dispersion electrode (SDE 44, Neurotron Inc. Baltimore, MD). Both electrodes were connected to the Neurometer® device. Electrical stimulation was applied via the Neurometer® to the bladder for 90 min at 2,000 Hz, 250 Hz or 5 Hz. In the electrode implantation only group, electrode was implanted but no stimulation was applied.
Intravesical capsaicin
Rats were anesthetized (urethane 1.2 g/kg). A 24-g catheter was inserted into the bladder through the urethra and the bladder was emptied. Capsaicin solution (0.5 ml of a 1 mM solution) was injected through the catheter and kept inside the bladder for 30 min14. Mineral oil was applied around the urethral orifice to reduce the effect of capsaicin on the perineal skin and vaginal mucosa.
Tissue harvest and immunohistochemistry staining of Fos protein
Two hours after onset of electrical or chemical stimulation, rats were perfused via the intracardiac route with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde and rats were sacrificed. The spinal cord was removed and immersed in 4% paraformaldehyde overnight. Spinal cords were sectioned at 40 µm on a freezing microtome and stored in cryoprotective solution. Eight to twelve sections from each spinal cord segment (L1–L6, and S1) were prepared for immunohistochemistry staining of Fos protein. The stored sections were rinsed with PBS and incubated in c-fos antiserum (1:15000, Oncogene, Cambridge, MA) for 80 hr, then in secondary antibody (1:600, Vector Laboratories, Burlingame, CA) for 2 hr. The presence of Fos protein was revealed by the avidin-biotin complex method (Vector Laboratories, Burlingame, CA) and visualized by diaminobenzidine. After staining, sections were dehydrated using ethanol, followed by xylene, and placed under a coverslip.
Stained sections were captured with Q Capture Pro (QImaging Corp., Surrey, BC, Canada) software using an Olympus photomicroscope and digital images of whole cross sections of the spinal cord were saved for analysis. The numbers of positive cells were counted with Image-Pro 5.1 image analysis software (Media Cybernetics, Silver Spring, MD). Counts of Fos immunoreactive (Fos-IR cells) per spinal cord level are presented as average cell numbers of eight to twelve sections from each spinal cord segment. Four spinal cord regions MDH, LDH, dorsal commissure DCM, and lateral laminae V–VII including the sacral parasympathetic nucleus SPN were divided as previously reported11,12. Cells exhibiting c-fos immunoreactivity in four spinal cord regions were counted. The distribution of Fos-IR cells in four spinal cord regions was expressed as percentage of Fos-IR cells in each region (MDH, LDH, DCM, and SPN) to the total c-fos-positive cells in four regions at the L6 spinal cord level.
Statistics
The values were expressed as mean ±the standard deviation of the mean. Comparisons between the number of Fos-IR cells in control or stimulated situations were made by one way analysis of variance, followed by Newman-Keuls multiple comparison. the standard deviation and p < 0.05 was considered as significant (Graph pad 4.0 software).
RESULTS
Distribution of Fos-IR cells in L1-S1 spinal cord segments after bladder stimulation
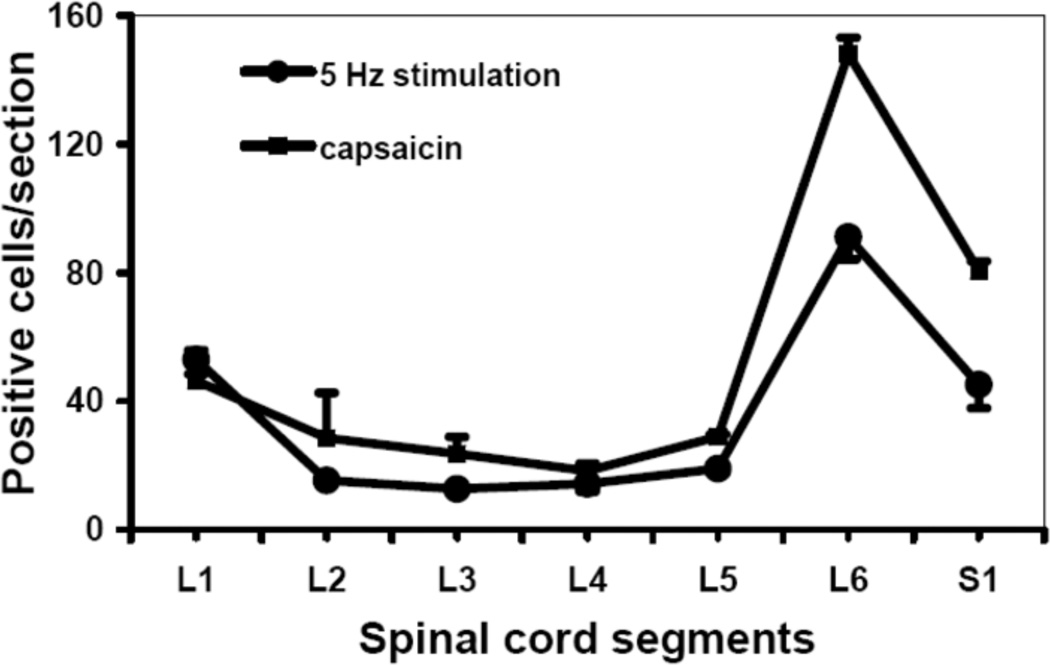
The distribution of Fos-IR cells in rat spinal cord segments L1 to S1 was determined after application of two different types of stimuli to the wall of the bladder: Neurometer®-BST stimulation at 5 Hz and intravesical infusion of the C-fiber selective activator and neurotoxin capsaicin. The two types of stimuli yielded similar distribution patterns of Fos-IR expression, with a major peak in L6 and lesser expression in S1 and L1. This pattern suggests that Neurometer®-BST stimulation at 5 Hz is similar to that caused by apsaicin and thus is selective for C-fibers (Fig. 1).
Figure 1.
Fos-IR cells in L1 to S1 spinal cord sections after bladder stimulation. Following Neurometer®-BST stimulation of the bladder mucosa at 5 Hz (2.0 mA, 90 min, n=6), or intravesical capsaicin treatment (1 mM, 30 min, n=4), Fos was detected by immunohistochemistry and the numbers of Fos-immunoreactivecells per section were counted.
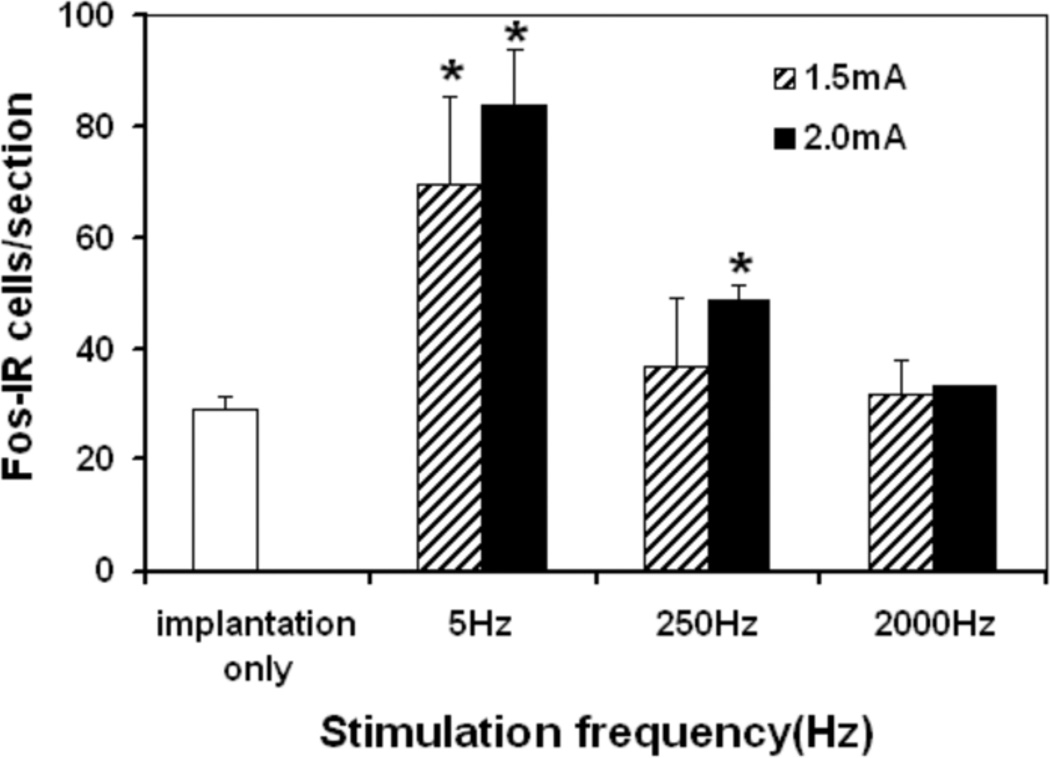
Frequency-dependent induction of Fos-IR cells in L6 spinal cord
Implantation of the electrode induced a small amount of Fos-IR cells in L6 spinal cord (Fig. 2). Compared to electrode implantation only, 5-Hz stimulation increased Fos-IR cells significantly at 1.5 and 2.0 mA intensities (p<0.01), while 250-Hz stimulation increased Fos-IR cells significantly only at the relatively high 2.0 mA intensity (p <0.01) (Fig. 2). However, 2,000 Hz stimulation did not induce Fos-IR cells significantly beyond electrode implantation at either intensity (Fig. 2).
Figure 2.
Histogram showing Fos-immunoreactive cells per section at L6 following: electrode implantation only (n=5), 5 Hz stimulated at 1.5 mA (n=4) and 2.0 mA (n=6), 250 Hz stimulated at 1.5 mA (n=4) and 2.0 mA (n=6), 2000 Hz stimulated at 1.5 mA, and 2. 0 mA (n=4).
* Significantly different when compared with electrode implantation only group, (p<0.01).
Distribution of Fos-IR cells following electrical stimulation
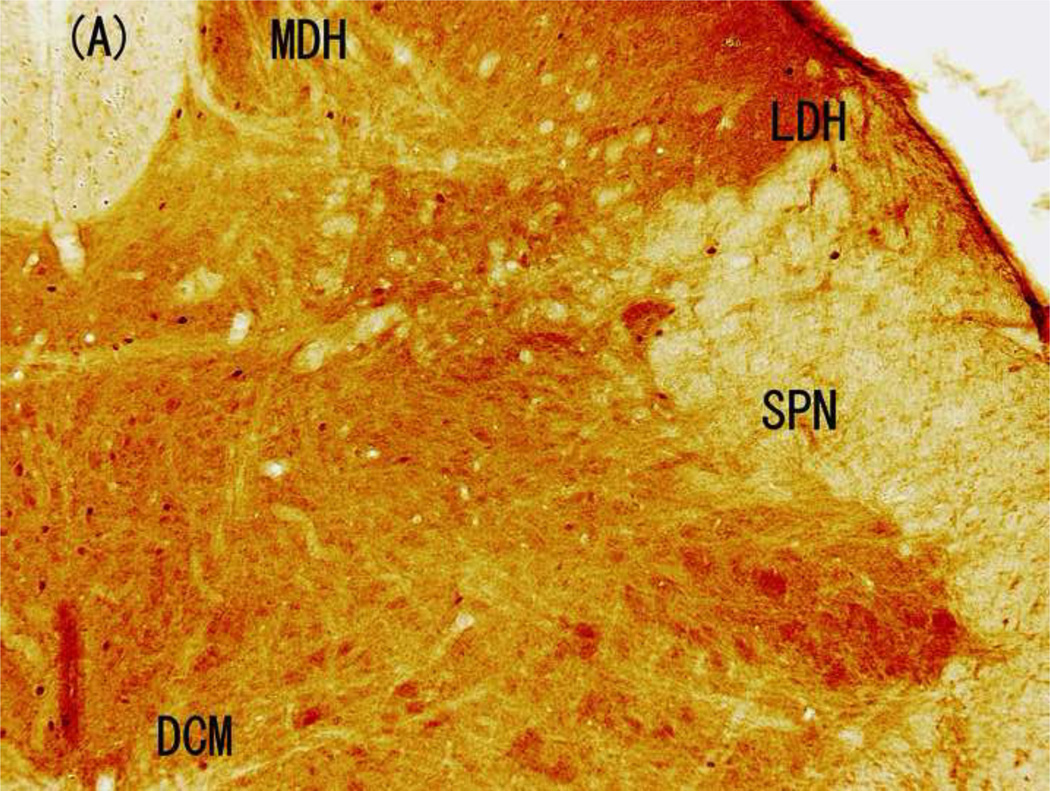
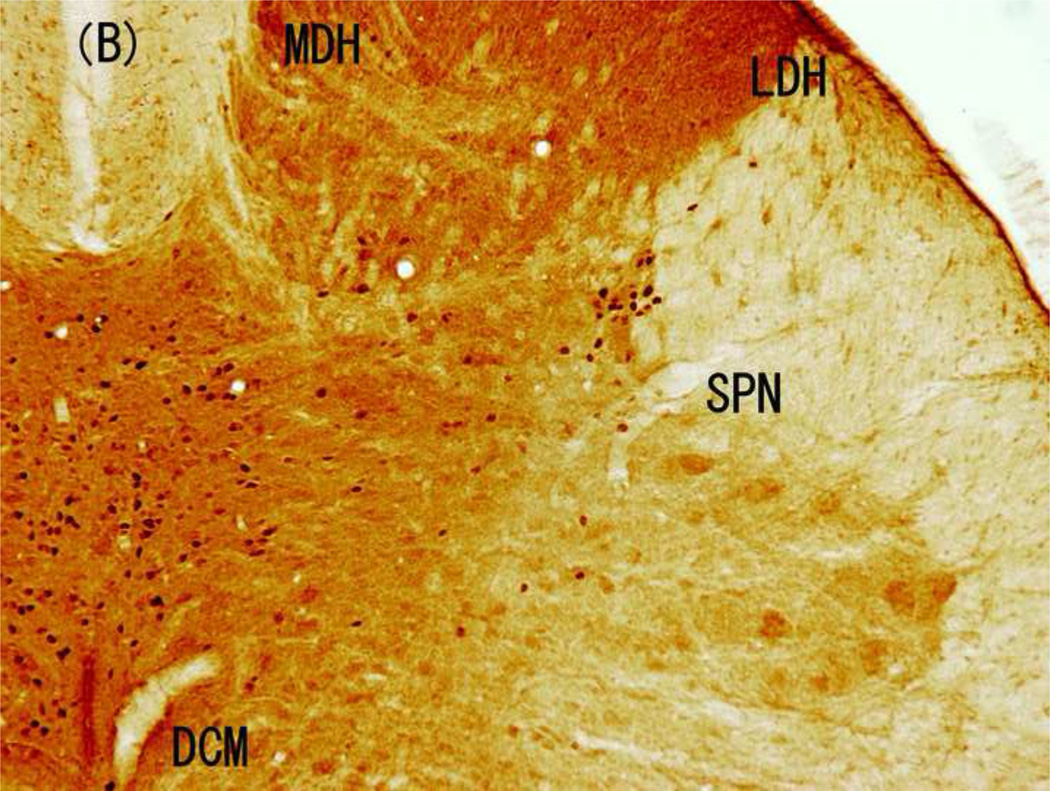
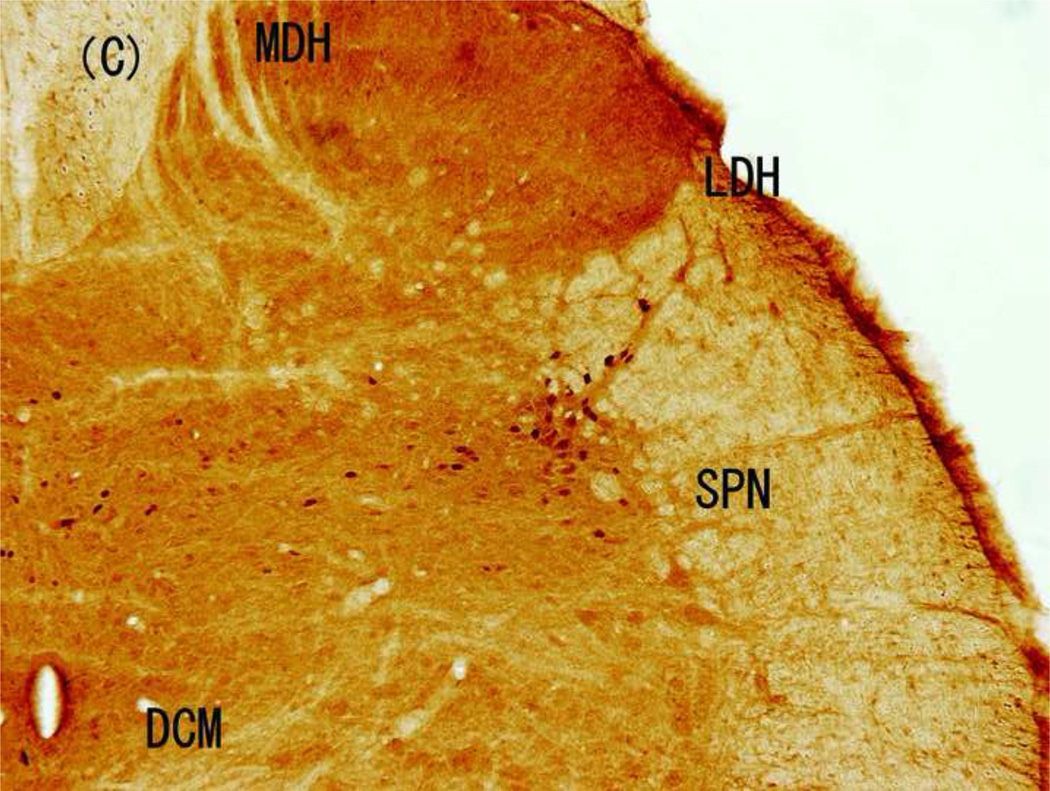
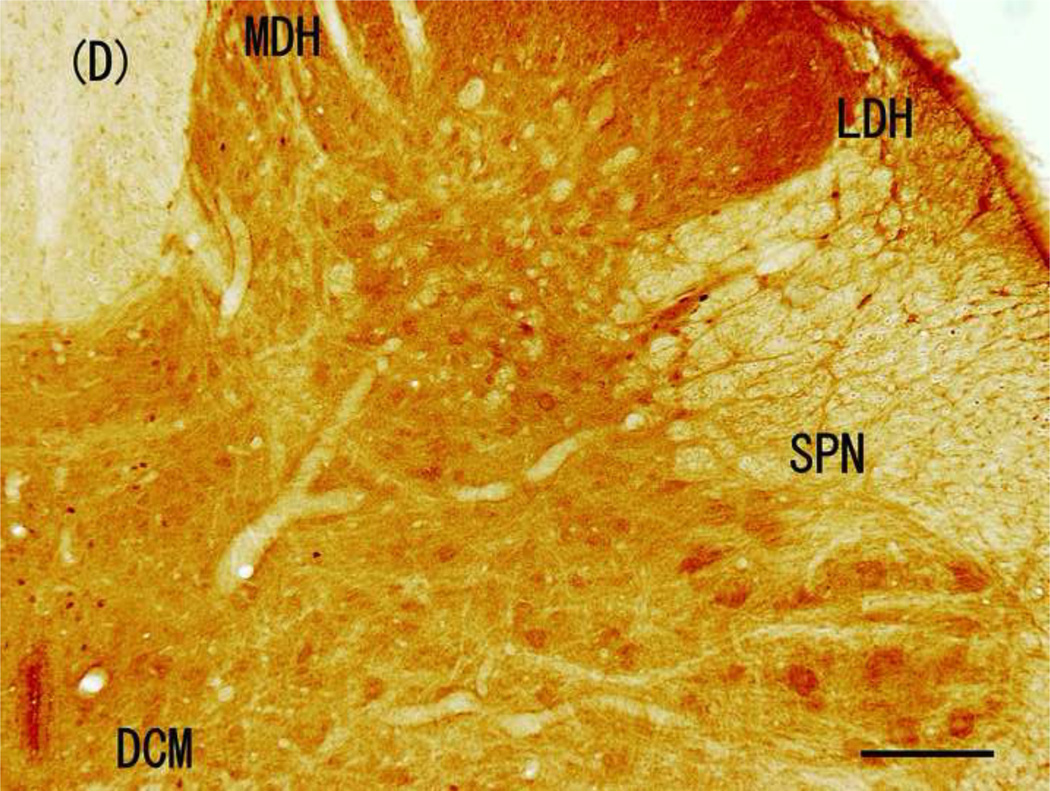
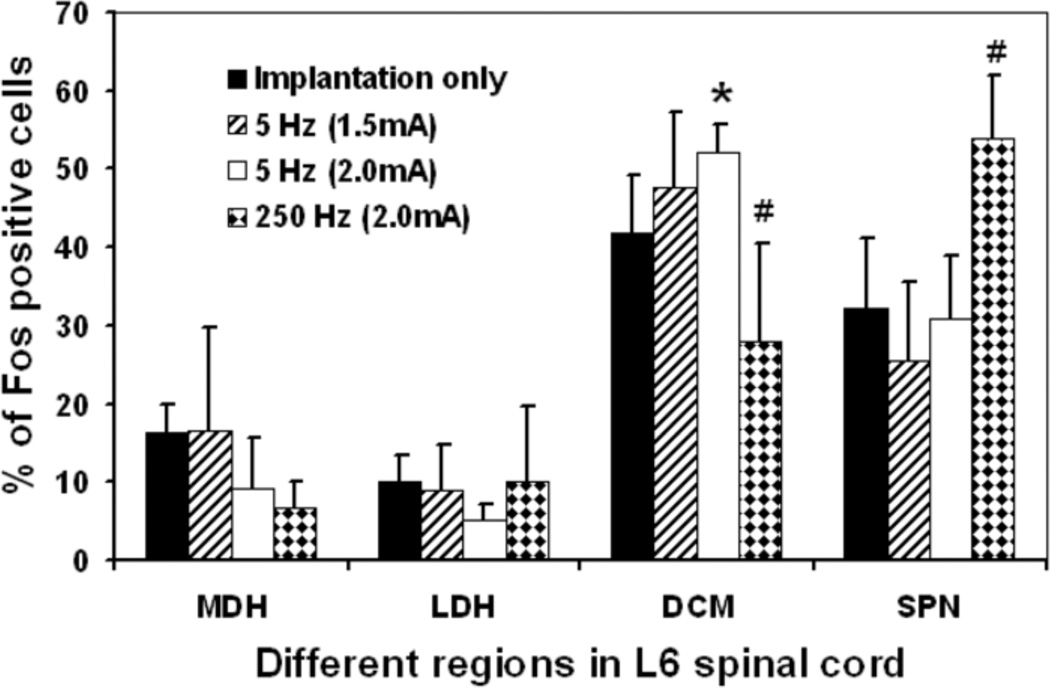
The distribution of Fos-IR cells varied obviously in response to the different electrical stimulations (Fig. 3 and 4). Implantation of the electrode only induced small, but different amounts of Fos-IR cells in four regions of L6 spinal cord: 16.2 ± 3.8 % Fos-IR cells in MDH, 9.9 ± 3.4 % in LDH, 32.1 ± 9.0 % in SPN, with most of them (41.7 ± 7.5%) in DCM (Fig. 4 and 5). Similarly, the highest amount of Fos-IR cells in response to 5-Hz stimulation at 2.0 mA was also in the DCM. However, 250-Hz (2.0 mA) stimulation induced the highest expression of Fos-IR cells in the SPN (Fig. 3 and 4).
Figure 3.
Brightfield photographs from sections of L6 in control group and groups stimulated with 5, 250, and 2000 Hz. (A) Control; (B) 5-Hz stimulation; (C) 250-Hz stimulation; and (D) 2,000-Hz stimulation. Scale bar, 200 µM. Abbreviations: LDH, superficial lateral horn; MDH, medial dorsal horn; DCM, dorsal commissure; SPN, sacral parasympathetic nucleus.
Figure 4.
Histogram showing the distribution expressed as percentages of Fos-IR cells in four regions of the L6 spinal cord (MDH, LDH, DCM, and SPN) in rats following electrode implantation only (n = 5) or any of these electrical stimulation: 5-Hz stimulated at 1.5 mA (n=4), 5-Hz stimulated group 2.0 mA (n=6), and 250-Hz stimulated at 2.0 mA (n=6). Cells activated by electrical stimulation were estimated by subtracting the cells induced by electrode implantation only. Abbreviations: LDH, superficial lateral horn; MDH, medial dorsal horn; DCM, dorsal commissure; SPN,sacral parasympathetic nucleus.
* Significantly different when compared with implantation only, (p<0.01).
# Significantly different when compared with implantation only, 5-Hz stimulation at 1.5 mA and 2.0 mA, (p<0.01).
DISCUSSIONS
Both human and animal urinary bladder afferents are composed of Aδ and C fibers3. Aδ fibers transfer impulses from normal bladder filling, thus conveying information about the sense of bladder fullness (distention). C-fibers have higher mechanical thresholds than Aδ fibers. C-fibers can become hypermechano-sensitive in various pathologies induced by inflammation, chemical mediators, or noxious stimulations1.
The Neurometer® electrostimulator has been used to assess current perception threshold values of afferent nerve fibers, allowing clinical diagnosis of hyperesthesia or hypoesthesia in various peripheral neuropathies. The neuroselectivities of the 5, 250, and 2,000 Hz frequencies of Neurometer® stimuli for C, Aδ, and Aβ fibers, respectively, have been established at cutaneous sites in humans6 and rats5,7. Interestingly, the feasibility of fiber-type-selective assessment of bladder sensory thresholds was first reported in the clinical setting using a transurethral electrode in conjunction with the Neurometer® 15–17. Those reports indicated the potential utility of the Neurometer® in assessing quantitative hypersensitivities of Aδ or C fibers, which are involved in common bladder pathologies.
Motivated by the cited reports and given the significant need for a device by which afferent functions of the bladder in small rodents can be evaluated, we have developed 10 the BST device, which can be used with the Neurometer® to assess sensory perception in the bladder of conscious rats8. In our first report, in addition to demonstrating the feasibility of use of the BST device in the conscious rat, we showed that intravesical infusion of resiniferatoxin caused a significant increase in the bladder sensory threshold values in response to 5-Hz stimulation, but not to 250-Hz stimulation8. Those data suggested that stimulation at 5 Hz markedly stimulates C fibers.
Our present data suggests that Neurometer®-BST stimulation of rat bladder wall at the 5-Hz frequency induced the same pattern of expression of Fos-IR cells in spinal cord segments L1-S1 as intravesical capsaicin infusion. These data indicate that nerve stimulation by Neurometer®-BST device is limited to bladder afferents. Furthermore, our findings indicated that Fos-IR cells in the L6 spinal cord segment induced by intravesical infusion of capsaicin and Neurometer®-BST stimulation at 5 Hz (1.5, and 2.0 mA) and 250 Hz (2.0 mA), but not at 2,000 Hz, are consistent with the presence of C and Aδ fibers, but not Aβ fibers, in the bladder3. These data represent the entire response of all stimulated neurons including those directly stimulated in the immediate area of the electrode as well as those in the diffuse adjacent areas. The 90-minute stimulation period was sufficient to assume diffusion may have occurred. This may explain how the effects of electrostimulation were beyond htat of a simple single point stimulation of the bladder wall.
The demonstration of distinct patterns of Fos-IR cell expression in four regions (MDH, LDH, DCM and SPN) of the L6 spinal cord in rats after 1% acetic acid (activate C fiber) or distension with saline solution (activate Aδ fiber mostly) stimulation of the bladder provided a model for distinguishing bladder afferent activity unmyelinated C fibers or myelinated Aδ fibers roughly12. Birder et al reported that 1% acetic acid stimulation induced 50.3% Fos-IR cells in the DCM and saline solution stimulation induced 52.3% Fos-IR cells in the SPN12. In the present study, the highest percentages of Fos-IR cells among regions of the L6 spinal cord was in the DCM (53.5%) after 5 Hz (1.5 mA) Neurometer®-BST stimulation of the rat bladder and 54.5% in the SPN after 250 Hz (2.0 mA) stimulation. These data correlate well with the report by Birder et al12 and suggest that the stimulation might be in a neuroselective manner. In future studies, we plan to include additional co-staining techniques in order to differentiate motor versus interneurons for Fos positive cells in the SPN.
Afferent pathways from the lower urinary tract (bladder and urethra) pass through the pelvic, hypogastric or pudendal nerve. It has been reported that, when bilateral pelvic and pudendal nerves were transected, Fos-IR cells in response to intravesical infusion of 1% acetic acid was not observed at L6 spinal cord [12]. Selective transections of either the pelvic or pudendal nerve reduced the percentage of Fos-IR cells to 39% or 47% of control, respectively, and changed the distribution of Fos-IR cells in the four spinal cord regions11. Birder et al [12] showed that the pudendal nerve activated a greater level of Fos-IR cells in the DCM (46%) and MDH (34%), whereas the pelvic nerve activated a greater level of positive cells in the DCM (48%) and SPN (33%). The pelvic nerve carries the afferent signal from the bladder body, whereas the somatic pudendal nerve carries the afferent signal from the distal urethra. Our study revealed that electrical stimulation induced Fos-IR cells predominantly in the DCM or SPN, which suggests electrical stimulation by the electrode implanted in the bladder wall stimulate only the pelvic nerve, not the pudendal nerve.
Previous reports have shown that Fos-IR cells also occur in response to noxious and non-noxious stimulation of the bladder18,19. This response can be explained by the innervations of the hypogastric nerve to the bladder from L1. The hypogastric nerve carries the afferent signal from the bladder neck, whereas the pelvic nerve carries the afferent input from the bladder body. In fact, our study revealed that 5-Hz stimulation induced Fos-IR cells in L6 as well as L1. Although the electrode was implanted in the bladder body in our study, it is conceivable that electrical stimulation from the electrode has the potential to excite the hypogastric nerve via an electrical current reaching the bladder neck through intravesical urine, which is thought to be a good conductor of electrical current.
The potential limitations of our study are related to the need for placement of an electrode on the bladder that by itself could interfere with afferent activity of the bladder and the fact that we did not include any groups of animals in which no manipulation (implantation or stimulation) was done. Thus, we assessed Fos-IR cells following electrode implantation only. Electrode implantation caused a distribution pattern of Fos-IR cells similar to that caused by 5-Hz stimulation (at both 1.5 and 2.0 mA), but different from that caused by 250-Hz stimulation. Since implantation itself has potential minor inflammatory effects on the bladder wall, it is reasonable to suggest that implantation of the BST can cause a C-fiber-like pattern of Fos-IR cells.
In conclusion, we stimulated bladder afferent fibers with various intensities of sine-wave electrical stimulation by our newly developed BST device and assessed the Fos-IR cells in comparison to intravesical infusion of capsaicin or electrode implantation only. The distribution of Fos-IR cells at L6 was increased in a neuroselective manner and was significantly different between stimulation with 250 Hz versus 5 Hz. The results of stimulation with 5 Hz corresponds to the response resulted from C-fiber activation by capsaicin. The results of 250-Hz stimulation corresponded to the reported response from the normal bladder distention, which is mainly controlled by the mechano-sensitive Aδ fibers. Our results demonstrate that sine-wave electro-stimulation of the bladder is a feasible method by which we can evaluate the function of bladder afferent nerve fibers. The primary targets for future studies would include examination of altered afferent bladder function in pathological conditions, such as overactive bladder, neurogenic bladder, and diabetic cystopathy.
ACKNOWLEDGEMENTS
We thank Kerry O. Grimberg, PhD for her medical editorial assistance. The experiments were performed at the Lerner Research Institute, The Cleveland Clinic, Cleveland, OH. Drs. Liu and Daneshgari are currently at the Departmnet of Urology, Case Western Reserve University, Cleveland, OH. Dr. Yamada is in the Department of Urology, Kyoto Prefectural University of Medicine, Kyoto, Japan.
GRANTS
This study was supported by grants from NIH-NIDDK-DK070004-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002;59:43–50. doi: 10.1016/s0090-4295(01)01637-5. [DOI] [PubMed] [Google Scholar]
- 2.Steers WD, Ciambotti J, Etzel B, Erdman S, de Groat WC. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol. 1991;310:401–410. doi: 10.1002/cne.903100309. [DOI] [PubMed] [Google Scholar]
- 3.Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the Rat's urinary bladder. J Neurophysiol. 2000;84:1924–1933. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- 4.Masson EA, Boulton AJ. The Neurometer: validation and comparison with conventional tests for diabetic neuropathy. Diabet Med. 1991;8(Spec No):S63–S66. doi: 10.1111/j.1464-5491.1991.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 5.Kiso T, Nagakura Y, Toya T, Matsumoto N, Tamura S, Ito H, Okada M, Yamaguchi T. Neurometer measurement of current stimulus threshold in rats. J Pharmacol Exp Ther. 2001;297:352–356. [PubMed] [Google Scholar]
- 6.Katims JJ, Long DM, Ng LK. Transcutaneous nerve stimulation. Frequency and waveform specificity in humans. Appl Neurophysiol. 1986;49:86–91. [PubMed] [Google Scholar]
- 7.Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1:13. doi: 10.1186/1744-8069-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abouassaly R, Liu G, Yamada Y, Ukimura O, Daneshgari F. Efficacy of a novel device for assessment of autonomic sensory function in the rat bladder. J Urol. 2008;179:1167–1172. doi: 10.1016/j.juro.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 10.Gokin AP, Philip B, Strichartz GR. Preferential block of small myelinated sensory and motor fibers by lidocaine: in vivo electrophysiology in the rat sciatic nerve. Anesthesiology. 2001;95:1441–1454. doi: 10.1097/00000542-200112000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. J Neurosci. 1992;12:4878–4889. doi: 10.1523/JNEUROSCI.12-12-04878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993;265:R326–R333. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- 13.Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J Neurobiol. 1995;26:403–412. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- 14.Cruz F, Avelino A, Coimbra A. Desensitization follows excitation of bladder primary afferents by intravesical capsaicin, as shown by c-fos activation in the rat spinal cord. Pain. 1996;64:553–557. doi: 10.1016/0304-3959(95)00157-3. [DOI] [PubMed] [Google Scholar]
- 15.Ukimura O, Ushijima S, Honjo H, Iwata T, Suzuki K, Hirahara N, Okihara K, Mizutani Y, Kawauchi A, Miki T. Neuroselective current perception threshold evaluation of bladder mucosal sensory function. Eur Urol. 2004;45:70–76. doi: 10.1016/j.eururo.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama T, Nozaki K, Fujita O, Nose H, Inoue M, Kumon H. Role of C afferent fibers and monitoring of intravesical resiniferatoxin therapy for patients with idiopathic detrusor overactivity. J Urol. 2004;172:596–600. doi: 10.1097/01.ju.0000132769.71014.b5. [DOI] [PubMed] [Google Scholar]
- 17.Kenton K, Simmons J, FitzGerald MP, Lowenstein L, Brubaker L. Urethral and bladder current perception thresholds: normative data in women. J Urol. 2007;178:189–192. doi: 10.1016/j.juro.2007.03.032. discussion 192. [DOI] [PubMed] [Google Scholar]
- 18.Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1027–R1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- 19.Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2000;279:R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]