Abstract
Purpose
We developed and tested the efficacy of an implantable bladder device which, when combined with the Neurometer®, can be used to assess fiber-specific afferent sensation of the bladder in the rat.
Materials and Methods
We developed an implantable bladder device which applies selective nerve fiber stimuli (250 Hz for small myelinated (Aδ), and 5 Hz for unmyelinated (C) fibers) to the bladder mucosa in the rat in order to obtain the bladder sensory perception threshold (SPT) values. We performed three experiments on fifty-five female Sprague-Dawley rats, examining the effects of our device on voiding habits; assessing the inter-observer reliability of SPT; and the effects of intravesical administration of resiniferatoxin and lidocaine on the SPT.
Results
The SPT values obtained by two blinded, independent observers were not different from one another (p= 0.41). The SPT values obtained at both stimulation frequencies remained constant for at least 3 weeks after device implantation. A significant increase in SPT values after instillation of resiniferatoxin (p = 0.02) was noted at a stimulus frequency of 5 Hz, whereas intravesical lidocaine led to an immediate increase in SPT at both 250 and 5 Hz. Device implantation led to an early decreased voided volume and increased frequency of voids, however these parameters returned to normal after 4 days.
Conclusions
Assessment of bladder afferent sensation with our newly developed device is feasible in rats, and provides sensory perception thresholds that appear to be fiber-type selective for autonomic bladder afferent nerves.
Keywords: Bladder, Sensory, Nerve, Rat
INTRODUCTION
Autonomic and sensory neuropathy is suspected in the pathogenesis of several diseases of the bladder including the overactive bladder and diabetic cystopathy 1,2. Despite strong evidence indicating the presence of increased or altered afferent autonomic sensitivity in these pathologies, to date, there exists no reliable way to measure the bladder manifestations of these conditions. The best tool that currently exists to evaluate bladder sensation is the filling phase of urodynamic studies, during which a conscious and cooperating patient will indicate when they feel the filling of their bladder. This is subject to numerous measurement biases and appears to lack the sensitivity or specificity to be used as a serious diagnostic tool for this indication. The ability to diagnose bladder sensory dysfunction in a more objective and convenient manner would likely be of use to the clinician.
Most of the afferent innervation of the bladder is conveyed by the pelvic and hypogastric nerves, which contain both myelinated (Aδ) and unmyelinated (C) axons. Aδ-fiber and some C-fiber afferents are mechanosensitive and respond to bladder filling by providing information about bladder wall tension and volume 3,4. Neuro-pathological conditions can selectively impair the functioning of specific subpopulation of nerve fibers while sparing the others 1,5. Therefore, diagnosing dysfunction in specific subpopulations of bladder afferent fibers in the neurogenic bladder, may be helpful in guiding therapeutic intervention. The Neurometer® electrodiagnostic stimulator (Neurotron, Inc, Baltimore, MD), through the application of a constant current neuroselective electrical stimulus, provides reliable measures of spinal sensory and peripheral sensory nerve function for both large and small myelinate, as well unmyelinated nerve fibers 6–9. The Neurometer® delivers sine-wave stimuli at frequencies of 2000, 250, and 5 Hz that have been shown to selectively stimulate large myelinated (Aβ), small myelinated (Aδ), and small unmyelinated (C) fibers, respectively 10,11. This easy to conduct electro diagnostic procedure has been used in the evaluation of bladder sensory function in humans 12. However, there is also interest in developing a method to evaluate bladder sensory function in experimental animals.
Our aim is to develop and test a prototype of a device that, in conjunction with the Neurometer®, would ultimately allow testing of subpopulations of bladder afferent fibers in the rat.
MATERIALS AND METHODS
Animals and Experimental Design
Fifty-five female Sprague-Dawley rats weighing approximately 300 gm were used. They were maintained on ordinary laboratory feeds and tap water ad librium in an animal facility with a 12:12h light/dark cycle. After the electrodes were implanted, the rats were used in three sets of experiments to assess: a) the effects of the device on voiding function; b) reliability over time and inter-reader variability of the SPT results; and c) the impact of resiniferatoxin and lidocaine instillation on bladder sensory perception threshold (SPT) values. All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Cleveland Clinic Foundation.
Device (electrode)
We developed a prototype implantable bladder device (Fig. 1). The device is made from Flexon™ suture, a stainless steel twisted, multistrand wire coated with PTFE (polytetrafluoroproethylene). The tip, which is placed in the bladder lumen, is a steel hook that is welded to the suture (Figure 1A). The hook is insulated with a polyester sleeve except for the tip which contacts the bladder mucosa with an surface area of < 1 mm2 (Figure 1B, 1C). The hook is attached to a silicone rubber disk (7mm diameter) that helps to secure the device to the bladder (Figure 1B). For sensory testing we used a battery operated Neurometer® (Neurotron, Inc., Baltimore, MD) capable of generating constant Alternating Current (AC) stimuli between 0.001 and 9.99 mA with quartz crystal calibrated sinusoid waveform stimuli at frequencies of 5 Hz, 250 Hz and 2000 Hz. In previous publications using the Neurometer, the response of subjects to stimuli was termed “current perception threshold (CPT)” 13–15. Instead, we use the term Sensory Perception Threshold (SPT) for the values obtained in response to bladder stimulation. Prior neuroanatomic and neurofucntional studies have failed to detect the presence of Aδ fibers in bladder innervation, as a result, we did not use the 2000 Hz stimulation frequency which is specific for this fiber type.
Figure 1.
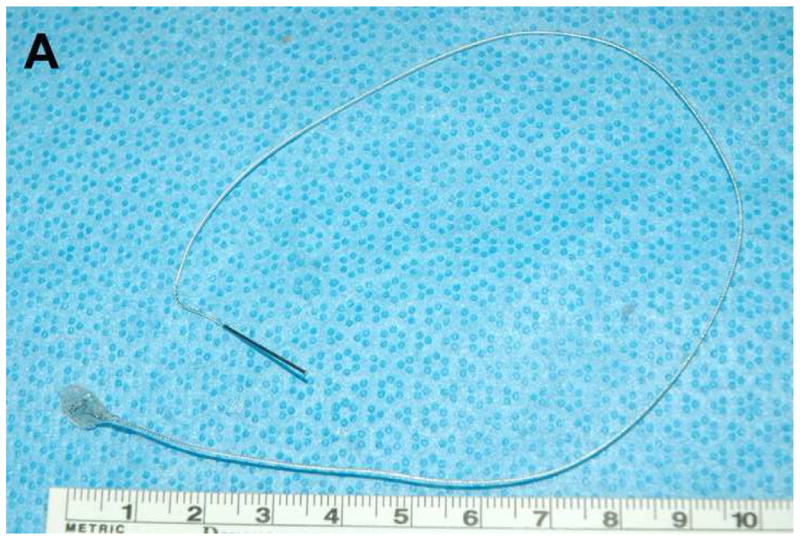
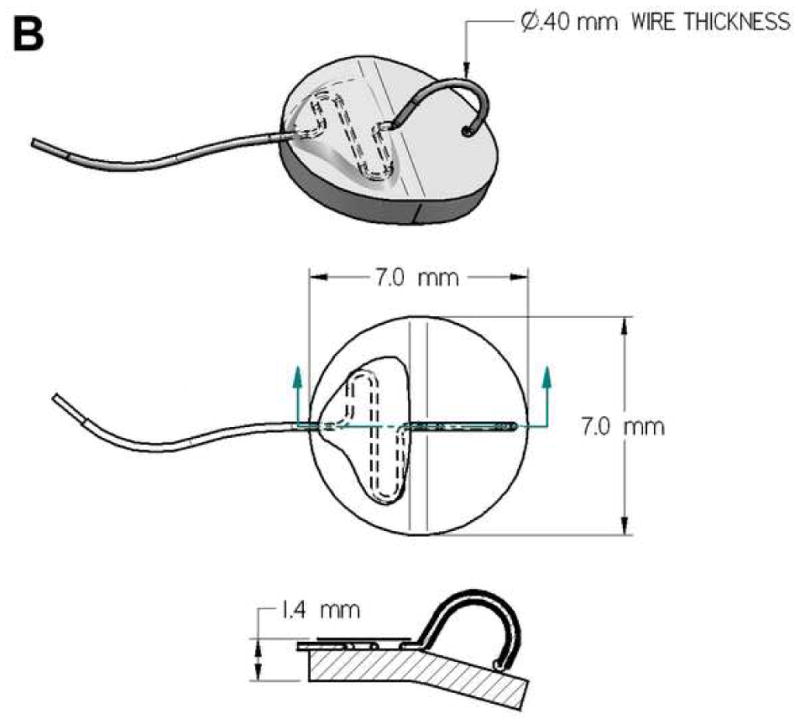
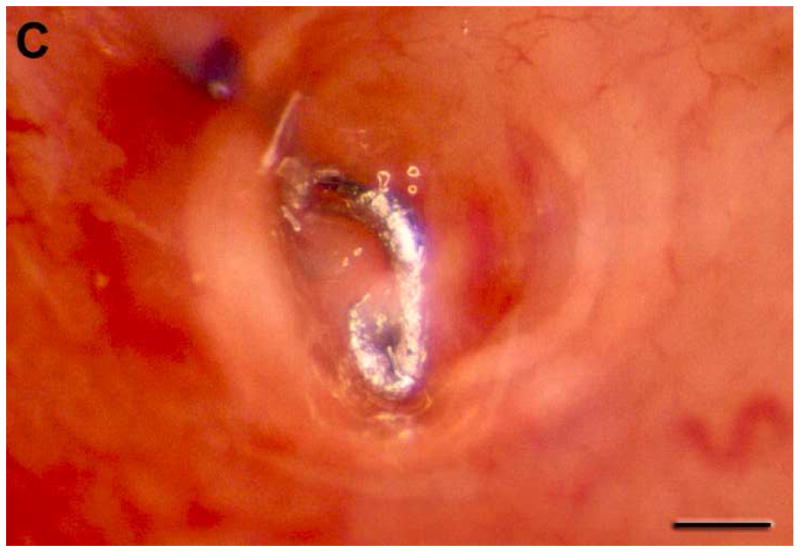
Neurometer and implanted bladder electrode. The size, configuration (A, B), including overall view (upper panel of Fig B), overhead view (middle panel of Fig B), side view (lower panel of Fig B), and position of the prototype catheter in the bladder (C, scale bar, 1 mm) developed to assess the neuroselective autonomic innervation of the bladder in a small animal model.
Device Implantation
The device was placed in the bladder of female rats under general anesthesia (Ketamine/Xylazine 100/10 mg/kg). A small lower abdominal incision was created and the bladder was delivered through the incision. A purse string stitch was placed on the posterior bladder wall using 5-0 chromic, a cystotomy was created, the device tip was placed within the bladder lumen, and the device was secured to the external surface of the bladder by tying the suture. The electrode lead wire was then passed subcutaneously to the nape of the rat’s neck using a specially designed trocar. The bladder was replaced within the peritoneal cavity and the abdominal wall fascia was closed, followed by skin closure.
Bladder Function Assessment
Fourteen rats were randomized to receive either device implantation (n=7), or sham procedure (n=7; abdominal incision without bladder manipulation). Both at 1 and 4 days after surgery, conscious rats were kept in Nalgene™ metabolic cages (Nalgene Co., Rochester, New York) for 25 hours. Rats were allowed to adapt to their new environment for one hour, then their 24-hour voiding behavior was recorded. Data was collected using PolyView data acquisition and analysis system (Astro-Med, Inc., West Warwick, RI). Parameters recorded included number of voids, mean voided volume and 24-hour urine output.
SPT Measurement
After device implantation, a skin patch dispersion electrode measuring 2 inch × 2 inch (SDE44; Neurotron, Inc., Baltimore, MD) was wrapped around the proximal tail, and both it and the bladder device were connected to the Neurometer® device. Rats were kept conscious during stimulation. Sine-wave stimuli (at 250 and 5 Hz) were then applied to the bladder mucosa of each rat at increasing intensity (increments of 0.05 mA) for 1 second on and 30 seconds off each stimulation until a light startle response was seen. The minimum intensity at which this response was seen was defined as the SPT. Rats were assigned numbers at the time of device implantation, and the order in which SPT measurements were obtained was determined by randomly selecting their number. Three measurements were obtained for each frequency, and the mean was used as the SPT value for that testing period.
Time-dependent and Inter-observer Variability of SPT Measurement
Twenty rats were divided into three groups. In the first two groups (n=14), in order to establish the day-today and week-to-week reliability of SPT values, SPT values were obtained on 3 consecutive days (n=8) and 3 consecutive weeks (n = 6). In the third group (n=6), the inter-observer reliability of the SPT measurement was examined 2 days after device implantation. Two investigators (RA and YY), each blinded to the results of the other, obtained SPT values on the same rats using the above mentioned technique.
Intravesical Instillation of Resiniferatoxin and Lidocaine
In the 3rd set of experiments, SPT values were obtained after intravesicle instillation of resiniferatoxin (Sigma Chemical Co., St. Louis, Missouri) or lidocaine 2 days after device implantation. Twenty one rats were randomly assigned to have their emptied bladders instilled with 0.3 ml of either resiniferatoxin (1000 μM solution, n=7), lidocaine (4% solution, n=7) or normal saline (n=7) for 30 minutes. Solutions were injected transurethrally using a syringe and a 22 Ga angiocatheter. During instillation, anesthesia was induced and maintained using 2% inhaled isoflurane. Conscious SPT measurements were obtained prior to, at 1 hour and 24 hours following instillation. The investigator obtaining the SPT values was blinded to the type of instillation.
Histology
In all experiments, animals were sacrificed 2 hours after testing in a carbon dioxide chamber and the portion of bladder surrounding the implanted electrode (approximately 5mm in diameter) was excised from each animal. The specimens were formalin-fixed, paraffin embedded, sectioned, and stained with hematoxylin and eosin. The bladders were all histologically examined. Representative images were captured with a digital camera mounted on a light microscope for examination.
Statistics
In the first experiment, the weight, mean voided volume, voiding interval and 24 hour urine output of the device and sham groups were compared using Wilcoxon-Mann-Whitney tests and a significance level of 0.05.
In the second experiment, for each stimulus frequency (5 Hz, 250 Hz), the SPT measurements were compared on day 1 to day 2, day 1 to day 3, and day 2 to day 3 using Wilcoxon matched pairs signed rank sum tests. To account for multiple comparisons between the different days, the significance level was adjusted to 0.05/3=0.017 to achieve an overall significance level of 0.05 (Bonferroni method). The same analysis was done for the SPT values obtained on 3 consecutive weeks.
In the third experiment, SPT values were compared before and after instillation using Wilcoxon sign-rank test. As in the prior experiment, to account for multiple comparisons between the different time points, the significance level was adjusted to 0.05/2=0.025.
RESULTS
Bladder Function Assessment
Table 1 presents the median and range for the implanted bladder electrode (at 1 and 4 days) and sham groups for mean volume voided, 24-hour urine output and number of voids in 24 hours. There is no statistically significant difference in rat weight or 24 hour urine output between the groups. However, device-implanted rats had a significantly lower mean voided volume and voided more frequently 1 day after electrode implantation. The differences in voiding behavior became insignificant 4 days after implantation.
Table 1.
Voiding parameters in sham-operated rats and device implanted rats at 1 and 4 days.
| Variable | Group (n=7)
|
||||
|---|---|---|---|---|---|
| Sham | Device (1 day) | p value | Device (4 days) | p value | |
| Mean volume voided (ml) | 0.61 (0.47, 0.85) | 0.37 (0.22, 0.49) | 0.003* | 0.59 (0.37, 0.89) | 0.62 |
| 24 hour urine output (ml) | 9.1 (4.25, 18.62) | 8.01 (5.27, 14.7) | 0.90 | 7.83 (4.5, 15.13) | 0.43 |
| Number of voids in 24 hours | 15 (9, 22) | 24 (19, 31) | 0.006* | 12.5 (10, 17) | 0.25 |
Values are shown as median (range).
Achieved statistical significance compared to sham group
Time-dependent and Inter-observer Variability in SPT Measurement
SPT values obtained on the same 6 rats by 2 investigators blinded to the results of the other were consistent (Table 2). In addition, the rats showed constant sensory stimulus thresholds with respective stimulations over a 3-day (Figure 2A), as well as over a 3- week period (Figure 2B). There was no statistically significant difference between SPT values between the time points for each of the frequencies.
Table 2.
Inter-observer reliability. SPT values obtained on 6 rats by 2 investigators.
| Rat | Frequency (Hz) | Investigator #1 SPT value (mA) |
Investigator #2 SPT value (mA) |
Difference SPT value (mA) |
|---|---|---|---|---|
| 1 | 250 | 0.28 | 0.27 | 0.01 |
| 5 | 0.21 | 0.22 | 0.01 | |
| 2 | 250 | 0.29 | 0.30 | 0.01 |
| 5 | 0.36 | 0.32 | 0.04 | |
| 3 | 250 | 0.27 | 0.27 | 0 |
| 5 | 0.21 | 0.22 | 0.01 | |
| 4 | 250 | 0.26 | 0.27 | 0.01 |
| 5 | 0.16 | 0.17 | 0.01 | |
| 5 | 250 | 0.36 | 0.35 | 0.01 |
| 5 | 0.20 | 0.18 | 0.02 | |
| 6 | 250 | 0.27 | 0.28 | 0.01 |
| 5 | 0.17 | 0.18 | 0.01 |
The differences of SPT values between two investigators were analyzed by using Wilcoxon matched pairs signed rank sum tests: p = 0.41
Figure 2.
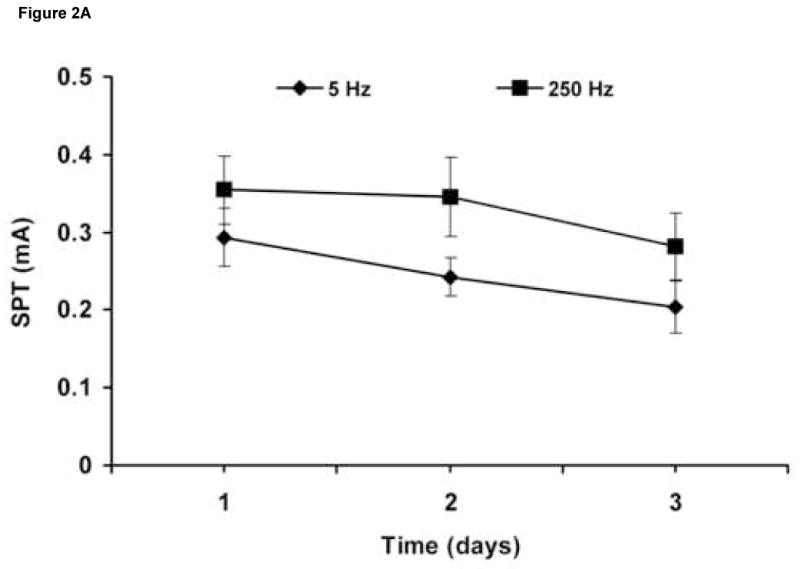
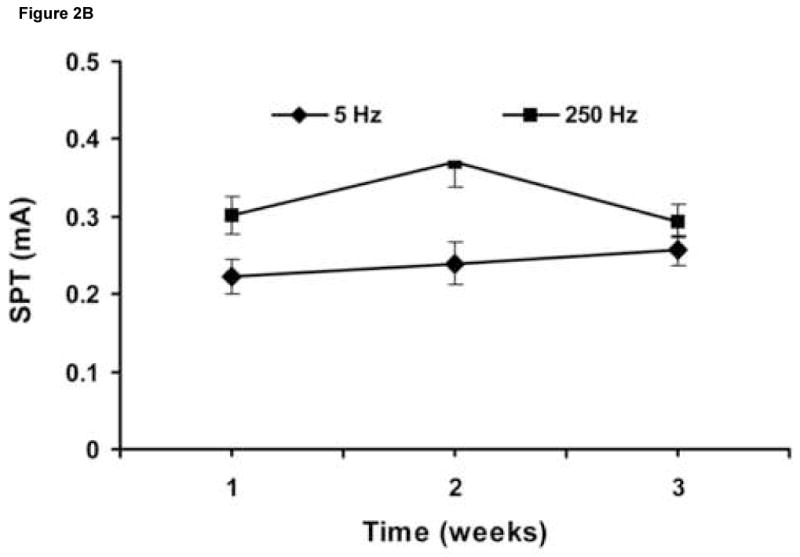
SPT values obtained over 3 consecutive days (n = 8) (A), and over 3 consecutive weeks (n = 6) (B). Results are expressed as means ± S.D.
Effects of Intravesical Resiniferatoxin or Lidocaine
Intravesical saline administration had no effect on SPT values at any frequency (Figure 3A). Whereas, intravesical resiniferatoxin led to a significant increase in SPT values at 5 Hz, 24 hours after instillation (Figure 3B). Lidocaine led to significantly higher SPT values 1 hour after instillation at both 250 and 5 Hz, which returned to baseline after 24 hours (Figure 3C).
Figure 3.
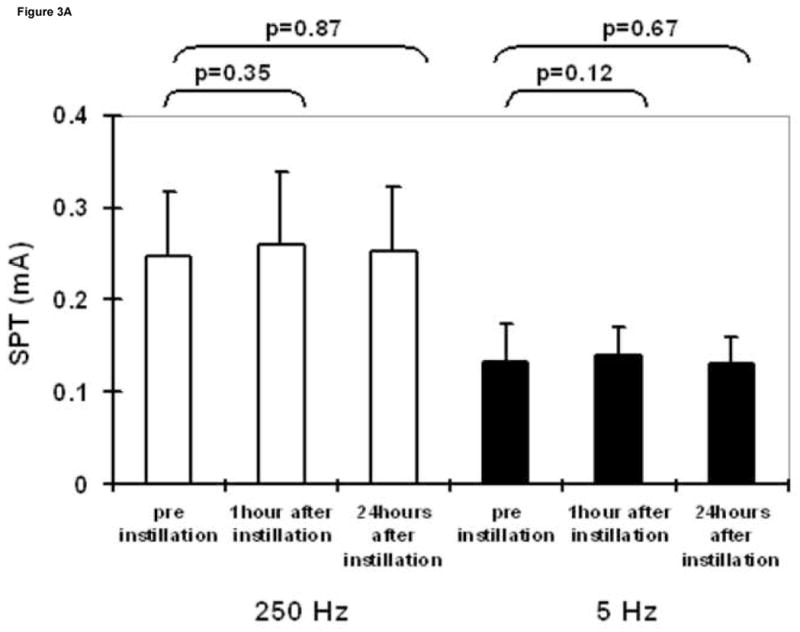
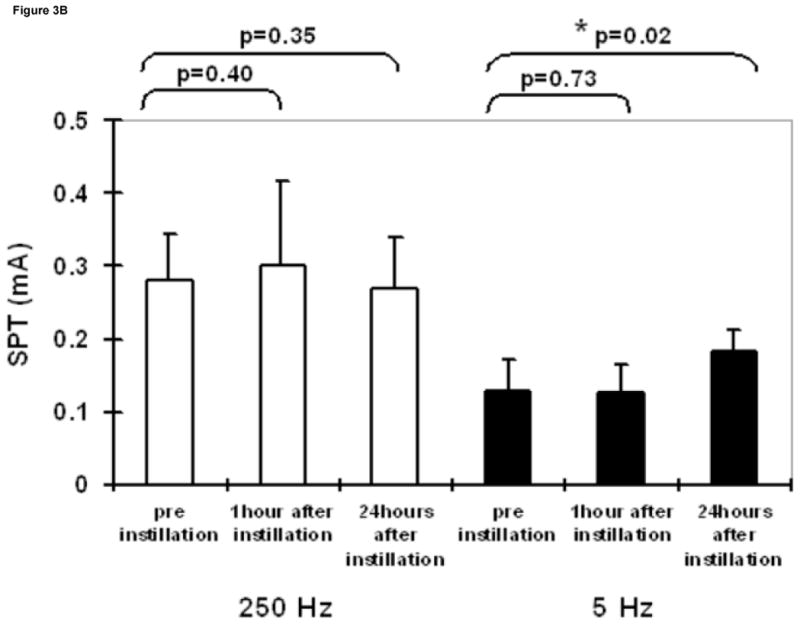
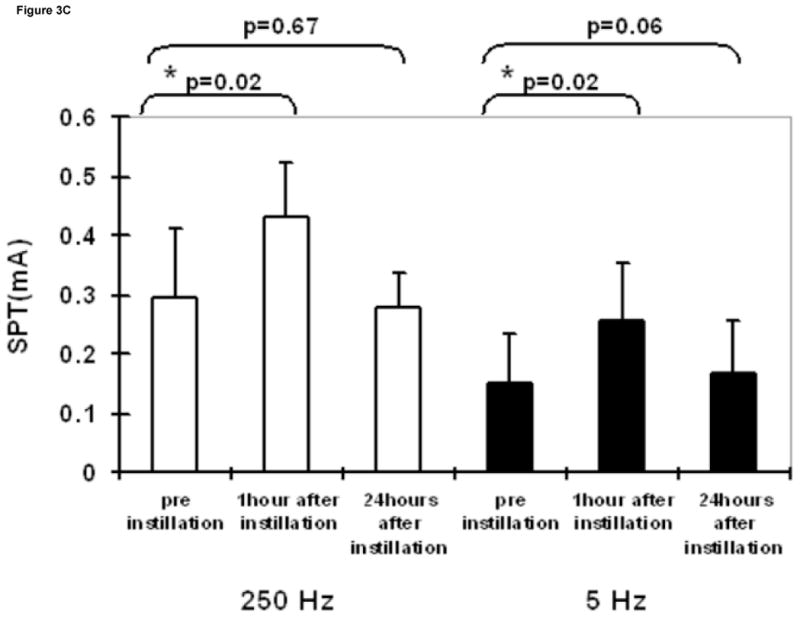
SPT values at 250 and 5 Hz prior to, one hour following, and 24 hours following intravesical administration of saline (A), resiniferatoxin (1 μM solution) (B), and lidocaine (4% solution) (C). Results are expressed as means ± S.D. *significantly different from the corresponding value before instillation (p <0.025).
Histology
Figure 4 is a representative image showing the defect created by the implanted device. Review of the bladder specimens revealed evidence of mild inflammatory changes and edema in the area surrounding the implanted device 1 day after implantation. Small submucosal hematomas were observed in a few specimens. However, these changes appeared to disappear after 4 days after implantation. The mucosal layer appeared well preserved and was without significant pathological change.
Figure 4.
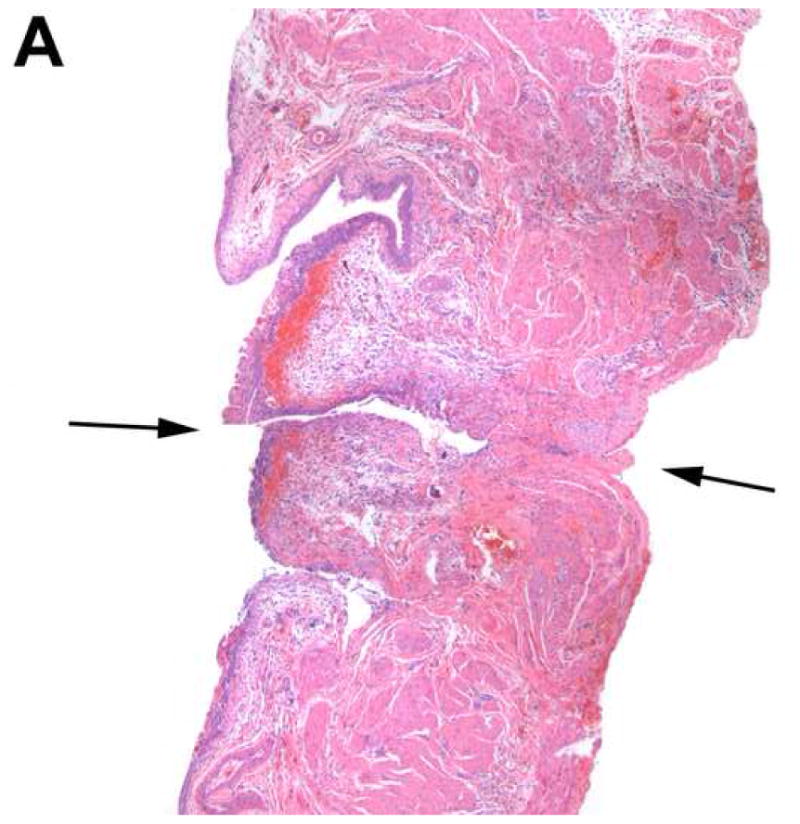
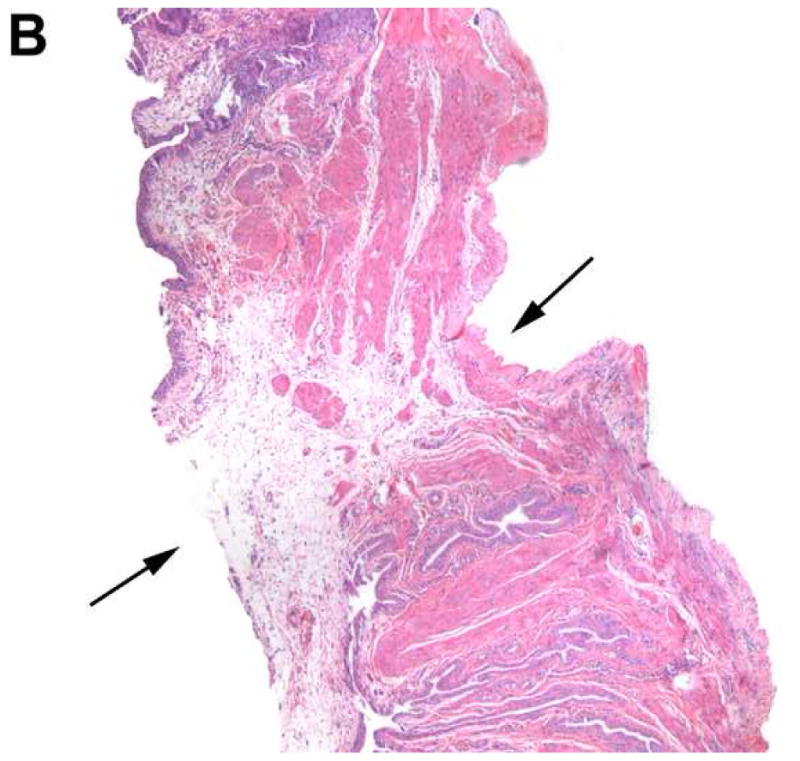
Histology of the bladder in a device-implanted rat. H & E staining of the bladder under light microscopy (40×) illustrates the defect created by the device (black arrow) and mild inflammatory changes in adjacent areas 1 day (A) and 4 days (B) after implantation.
DISCUSSION
Afferent bladder innervation consists of myelinated Aδ-fibers and unmyelinated C-fibers. Normal voiding has been shown to be dependent on Aδ-fiber bladder afferents, whereas C-fiber afferents are responsible for bladder nociceptive responses 16,17. The previous and successful use of Neurometer® and measurement of current perception threshold (CPT) in cutaneous afferent sites 13–15 makes this technique an attractive modality to assess the status of the specific afferent nerve fibers in the bladder or other visceral organs.
In one of the first applications of this technology in visceral organ, Ukimura and colleagues evaluated human bladder mucosal sensory function by obtaining CPT measures in healthy volunteers and patients with neuropathic bladders 12. They showed quantitative neuroselective measurement of CPT values in the human bladder mucosal function was feasible. This was accomplished using a 5 French transurethral bladder catheter electrode. CPT measures obtained at 250 and 5 Hz, reflecting Aδ and C fiber function, followed the clinical expectations by demonstrating significant hyperesthesia in patients with upper motor neuroron lesions and hypoesthesia in patients with infra-sacral neural injury, and diabetes. These results seemed consistent with other clinical findings in these patients. In exploration of utility of this technique in assessment of response to therapeutic interventions on bladder sensory dysfunction, Yokoyama et al., obtained CPT measures for C and Aδ-fibers before and after treatment of patients with idiopathic detrusor overactivity with resiniferatoxin (RTX), a specific C-fiber neurotoxin that leads to fiber desensitization 1. Mean CPT values at 5 Hz, increased significantly in all patients who showed clinical improvement after treatment.
Recently, a technique described measuring the neuroselective CPT for peripheral sensation (plantar surface) in animals 10. The article suggested that the Neurometer provided selective stimulation of the three subsets of nerve fibers in rats (Aβ, Aδ and C-fibers), as it does in humans. They defined the CPT value as the minimum intensity at which the rat vocalized or startled. This method has been used by other groups for the assessment of analgesic agents in rat models of acute and neuropathic pain 18. However, there has not been a report of the use of the Neurometer to assess the sensory function in visceral organs, such as urinary bladder, in experimental animal.
In our study, we established a technique to measure bladder SPT values using our newly developed device using the startle response in rats. Our results showed consistent SPT values for all frequencies when measurements are obtained on three consecutive days and three consecutive weeks with excellent inter-observer reliability, supporting the reliability and reproducibility of our technique.
Consistent with the previous report in humans 1, in our study, a significant increase in the SPT was obtained only for 5 Hz after intravesical instillation of resiniferatoxin solution, which is known to cause desensitization of C-fiber afferents 19, after 24 hours, whereas normal saline had no effects on the SPT. However, intravesical instillation of lidocaine, which blocks all the subpopulation of nerve fibers 20, induced short-term increases in SPT at both 5 Hz and 250 Hz stimulation frequencies. These data support the fact that the stimulation is selective for the different types of bladder sensory nerve fibers.
In order to explore the effects of the implanted electrode on bladder function, we observed the voiding habits of rats after the implantation. Although the implantation had no significant effect on 24-hour urine output, it did decrease the mean micturition volume and increase the number of voids in the short-term. This is possibly related to surgical factors during implantation.. However, these changes in the animals’ voiding behavior are no longer present 4 days after device implantation.
The results of our study demonstrate the proof of concept and feasibility of use of our device, which is used in conjunction with the Neurometer® for the assessment of afferent function of the bladder in rats. These results warrant future studies to explore the potential use of this device in the investigation of diseases of the bladder in which afferent innervation appears to play a role.
CONCLUSION
We present a technique of measuring the bladder sensory perception threshold using the Neurometer® electrostimulator with an implantable bladder device in rats. This technique, which allows the selective assessment of subsets of bladder sensory nerve fibers in rats (i.e. Aδ and C fibers), will contribute to further understanding the bladder sensory dysfunction in various pathologic conditions such as acute and interstitial cystitis, bladder outlet obstruction, overactive bladder, and spinal cord injury in experimental rat models. In addition, this novel technique may be an effective tool to test the efficacy of new therapeutic drugs for bladder sensory dysfunction in experimental rat models.
Acknowledgments
This study was supported by grants from NIH, NIDDK, KO8 DK02631, U01-DK61018-02S1, and R41 DK074987-01 and a Juvenile Diabetes Research Foundation International Postdoctoral Fellowship (G. Liu).
Glossary
- SPT
sensory perception threshold
- CPT
current perception threshold
- RTX
resiniferatoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yokoyama T, Nozaki K, Fujita O, Nose H, Inoue M, Kumon H. Role of C afferent fibers and monitoring of intravesical resiniferatoxin therapy for patients with idiopathic detrusor overactivity. J Urol. 2004;172:596. doi: 10.1097/01.ju.0000132769.71014.b5. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Daneshgari F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol. 2005;288:F1220. doi: 10.1152/ajprenal.00449.2004. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol. 2001;166:1951. [PubMed] [Google Scholar]
- 4.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27:141. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, et al. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J Urol. 2002;168:1259. doi: 10.1016/S0022-5347(05)64636-8. [DOI] [PubMed] [Google Scholar]
- 6.Falci S, Best L, Bayles R, Lammertse D, Starnes C. Dorsal root entry zone microcoagulation for spinal cord injury-related central pain: operative intramedullary electrophysiological guidance and clinical outcome. J Neurosurg. 2002;97:193. doi: 10.3171/spi.2002.97.2.0193. [DOI] [PubMed] [Google Scholar]
- 7.Katims JJ, Naviasky EH, Ng LK, Rendell M, Bleecker ML. New screening device for assessment of peripheral neuropathy. J Occup Med. 1986;28:1219. [PubMed] [Google Scholar]
- 8.Baron GC, Irving GA. Effects of tourniquet ischemia on current perception thresholds in healthy volunteers. Pain Pract. 2002;2:129. doi: 10.1046/j.1533-2500.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakai T, Tomiyasu S, Yamada H, Sumikawa K. Evaluation of allodynia and pain associated with postherpetic neuralgia using current perception threshold testing. Clin J Pain. 2006;22:359. doi: 10.1097/01.ajp.0000178222.85636.00. [DOI] [PubMed] [Google Scholar]
- 10.Kiso T, Nagakura Y, Toya T, Matsumoto N, Tamura S, Ito H, et al. Neurometer measurement of current stimulus threshold in rats. J Pharmacol Exp Ther. 2001;297:352. [PubMed] [Google Scholar]
- 11.Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1:13. doi: 10.1186/1744-8069-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ukimura O, Ushijima S, Honjo H, Iwata T, Suzuki K, Hirahara N, et al. Neuroselective current perception threshold evaluation of bladder mucosal sensory function. Eur Urol. 2004;45:70. doi: 10.1016/j.eururo.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Kho HS, Kim YK, Lee SW, Chung SC. Reliability and characteristics of current perception thresholds in the territory of the infraorbital and inferior alveolar nerves. J Orofac Pain. 2000;14:286. [PubMed] [Google Scholar]
- 14.Ro LS, Chen ST, Tang LM, Hsu WC, Chang HS, Huang CC. Current perception threshold testing in Fabry’s disease. Muscle Nerve. 1999;22:1531. doi: 10.1002/(sici)1097-4598(199911)22:11<1531::aid-mus7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Takekuma K, Ando F, Niino N, Shimokata H. Age and gender differences in skin sensory threshold assessed by current perception in community-dwelling Japanese. J Epidemiol. 2000;10:S33. doi: 10.2188/jea.10.1sup_33. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura N, de Groat WC. Neural control of the lower urinary tract. Int J Urol. 1997;4:111. doi: 10.1111/j.1442-2042.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 17.Chuang YC, Fraser MO, Yu Y, Beckel JM, Seki S, Nakanishi Y, et al. Analysis of the afferent limb of the vesicovascular reflex using neurotoxins, resiniferatoxin and capsaicin. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1302. doi: 10.1152/ajpregu.2001.281.4.R1302. [DOI] [PubMed] [Google Scholar]
- 18.Akada Y, Mori R, Kato Y, Yamasaki F, Mochizuki H. Analgesic properties of the novel compound M43068 in rat models of acute and neuropathic pain. Eur J Pharmacol. 2005;523:46. doi: 10.1016/j.ejphar.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Apostolidis A, Gonzales GE, Fowler CJ. Effect of intravesical Resiniferatoxin (RTX) on lower urinary tract symptoms, urodynamic parameters, and quality of life of patients with urodynamic increased bladder sensation. Eur Urol. 2006;50:1299. doi: 10.1016/j.eururo.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Sakai T, Tomiyasu S, Yamada H, Ono T, Sumikawa K. Quantitative and selective evaluation of differential sensory nerve block after transdermal lidocaine. Anesth Analg. 2004;98:248. doi: 10.1213/01.ANE.0000093232.72967.76. [DOI] [PubMed] [Google Scholar]


