Abstract
There are limited data on the outcomes of autologous or allogeneic hematopoietic cell transplantation in diffuse large B-cell lymphoma transformed from follicular lymphoma. We analyzed transplant outcomes in 141 subjects with biopsy-proven diffuse large B-cell lymphoma transformed from follicular lymphoma reported to the Center for International Blood and Marrow Transplant Research from 1990–2009. Two groups were identified: autotransplant (N=108) and allotransplant (N=33). Fewer autotransplants were done for transformed follicular lymphoma in 2003–2009, with a shift favoring allotransplants. Autotransplant had 1 year non-relapse mortality of 8% (95% confidence intervals [CI] 4–14), 5 year progression free survival of 35% (95% CI 26–45), and 5 year overall survival of 50% (95% CI 40–59). Allotransplant had 1 year non-relapse mortality of 41% (95% CI 23–58), 5 year progression free survival of 18% (95% CI 6–35), and 5 year overall survival of 22% (95% CI 8–41). Autotransplant for transformed follicular lymphoma achieves sustained remission in a high proportion of subjects. The high non-relapse mortality of allotransplant obscured any benefit that might be associated with this transplant modality.
Keywords: transformed follicular lymphoma, transplant
Introduction
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma (NHL) in the western hemisphere.1,2 The histologic transformation of FL to diffuse large B-cell lymphoma (DLBCL) occurs in up to 30% of patients at 10 years.3–7 The rate of transformed FL (tFL) varies according to the definition of transformation (whether the definition includes only DLBCL, or also Burkitt lymphoma, grade 3B FL, and composite or discordant lymphomas), method of diagnosis (biopsy, cytology or clinical suspicion), duration of follow up, and inclusion of autopsy data.8
Compared with FL, in which the median survival is historically in the 10 year range without a plateau, histologic transformation of FL is usually associated with chemotherapy resistance and shorter survival after chemotherapy.9–13 There is no standard of care for tFL. Therapy for DLBCL transformed from FL is mainly based on guidelines for de novo advanced DLBCL. Because patients with tFL are typically excluded from FL and DLBCL clinical trials, there are limited data on the role of autologous or allogeneic hematopoietic cell transplantation. Most reports are small retrospective studies with brief follow up.14–20 Outcomes vary greatly because of different inclusion criteria.14–20 In addition, most of the studies were done before the availability of rituximab, an agent that has improved the outcomes of FL and DLBCL.14–20 We analyzed the outcomes of transplants for biopsy-proven transformation of FL to DLBCL in a larger patient cohort reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
Methods
Data Source
The CIBMTR comprises a voluntary network of more than 500 transplantation centers globally that submit comprehensive data on consecutive autotransplants and allotransplants to a centralized statistical center. The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. Protected health information during the performance of this observational research is collected and maintained in the CIBMTR's capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act (HIPAA). The observational research is conducted with a waiver of informed consent and in compliance with all applicable federal regulations regarding the protection of human research participants as assessed by the Institutional Review Board and the Privacy Officer at the Medical College of Wisconsin. Further details of the data source are described by Horowitz.21
Patient Population
Patients 18 years of age or older with FL at diagnosis by the World Health Organization classification, with subsequent biopsy-proven histologic transformation to DLBCL were included in this study.22 Pathology reports from the centers were reviewed in each case at the CIBMTR to confirm transformation to DLBCL. Histologic transformation to DLBCL was defined as large centroblasts diffusely infiltrating the lymph node and effacing the follicular architecture. No cases of composite or discordant lymphoma at diagnosis were included. Histologic transformation of other low-grade lymphomas, such as marginal zone lymphoma, chronic lymphocytic leukemia, was excluded. In addition, cases with transformation to histology other than DLBCL, such as Burkitt lymphoma, lymphoblastic lymphoma or Hodgkin lymphoma, were also excluded.
Subjects who had an initial single autotransplant or allotransplant for tFL were included. Subjects who had a prior transplant for FL before transformation or after transformation were excluded.
Study End Points and Definitions
The main objective of this study was to describe the outcomes of autotransplant and allotransplant for patients with DLBCL transformed from FL. The primary end point was overall survival (OS) and other end points of interest were the progression free survival (PFS), relapse/progression, non-relapse mortality (NRM), and the incidence of acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD). OS was defined as time to death after transplant. Death from any cause was considered an event. Surviving patients were censored at time of last follow up. PFS was defined as survival without disease relapse or progression after transplant. Relapse or progression of disease and death were events. Relapse/progression was defined as any new lesion after complete remission or increase in size of previously involved sites after transplant with NRM as a competing risk.23 NRM was defined as any death within the first 28 days of transplant or any death occurring after day 28 in the absence of disease relapse/progression. Relapse was a competing risk. Those who survived without relapse or progression were censored at last contact for PFS, progression/relapse and NRM. Acute GVHD was diagnosed by established criteria.24 Chronic GVHD was defined by standard criteria.25 The intensity of the conditioning regimen was defined by CIBMTR criteria.26 Related and unrelated donor (URD) transplant recipients were classified based on available HLA typing as previously described by Weisdorf et al.27
Statistical Methods
Univariate probabilities of OS and PFS for the autotransplant and allotransplant cohorts were calculated using the Kaplan-Meier estimator, with the variance estimated by Greenwood's formula.28 Variables considered for univariate analysis were age, Karnofsky performance status, presence of extranodal disease at transplant, prior use of rituximab, chemoresistance, interval from diagnosis of FL to transformation, and total body irradiation (TBI)-based conditioning. For allotransplant, additional variables tested were use of reduced intensity/non-myeloablative conditioning (RIC/NMAC), antithymocyte globulin (ATG), alemtuzumab, and donor source. Relapse/progression, NRM and the incidence of acute and chronic GVHD were estimated using cumulative incidence estimates to accommodate for competing risk.
Cox proportional hazards regression model was used to identify the risk factors significantly associated with treatment failure (1 – PFS) and overall mortality (1 – OS) for autotransplant. No multivariate analysis was considered for allotransplant due to small size of the study cohort. The variables considered in the multivariate models are listed in Table 2a. The assumption of proportional hazards for each factor in the Cox model was tested by adding a time-dependent covariate. The proportionality assumption was satisfied for each factor. A forward and backward stepwise model selection approaches were used to identify all significant risk factors.
Table 2a.
Univariate survival analysis for autologous hematopoietic cell transplantation
| Treatment Failure (1-PFS) | Overall Mortality (1-OS) | ||||
|---|---|---|---|---|---|
| Covariates | N | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| TBI based conditioning | |||||
| No | 84 | Reference | Reference | ||
| Yes | 24 | 1.27 (0.75–2.14) | 0.38 | 1.41 (0.82–2.44) | 0.22 |
| Chemo sensitivity | 0.38a | 0.32a | |||
| Sensitive | 90 | Reference | Reference | ||
| Resistant | 10 | 1.89 (0.90–3.97) | 0.09 | 1.98 (0.89–4.40) | 0.09 |
| Time from diagnosis to transformation | |||||
| < 1 year | 17 | Reference | Reference | ||
| ≥1 year | 91 | 0.69 (0.37–1.29) | 0.25 | 0.68 (0.35–1.31) | 0.25 |
| Age (years) | |||||
| > 60 | 39 | Reference | Reference | ||
| ≤ 60 | 69 | 1.06 (0.66–1.72) | 0.80 | 1.20 (0.71–2.02) | 0.50 |
| Karnofsky score | 0.50a | 0.71a | |||
| <90% | 35 | Reference | Reference | ||
| 90–100% | 68 | 0.76 (0.46–1.24) | 0.27 | 0.81 (0.48–1.37) | 0.43 |
| Extranodal disease prior to HCT | 0.25a | 0.66a | |||
| no | 75 | Reference | Reference | ||
| yes | 28 | 0.95 (0.56–1.60) | 0.85 | 0.84 (0.48–1.49) | 0.55 |
| Rituximab exposure prior to HCT | |||||
| no | 78 | Reference | Reference | ||
| yes | 30 | 0.99 (0.59–1.67) | 0.98 | 0.65 (0.35–1.20) | 0.17 |
multi degree-of-freedom overall test.
Abbreviations for tables 1,2: OS overall survival, PFS progression free survival, N number of patient evaluated, # number, dx diagnosis, 95% CI confidence interval, Prob probability, autoHCT autologous hematopoietic cell transplantation, alloHCT allogeneic hematopoietic cell transplantation without prior autoHCT, chemoRx chemotherapy, TBI total body irradiation, MAC myeloablative conditioning, RIC/NMAC reduced intensity conditioning/ non-myeloablative conditioning, FL follicular lymphoma, tFL transformed follicular lymphoma, BM bone marrow, PB peripheral blood, GVHD graft-versus-host disease, FK tacrolimus, CSA cyclosporine, TCD T cell depletion, ATG antithymocyte globulin, CAMPATH alemtuzumab, MRD matched related donor, URD unrelated donor, CR complete remission, PR partial remission, REL relapsed, PIF primary induction failure, NE non evaluable, KPS Karnofsky Performance status.
Results
Subject, Disease and Transplant Related Variables
Two groups reported to the CIBMTR from 1990–2009 were identified: 108 subjects underwent an autotransplant and 33 underwent an allotransplant without a prior autotransplant (Table 1). Median follow-ups were 85 and 64 months. Overall completeness index of the follow-up for the population at 5 years was 89%.29 Most autotransplants were from 1990–2002 compared with 2003–2009 for most allotransplants. Median interval from diagnosis of FL to tFL was 47 and 48 months. Disease variables at the time of diagnosis of tFL were unavailable. Median intervals from diagnosis of FL to transplant for auto- and allotransplant cohorts were similar (54 vs. 55 months) as was the interval from the diagnosis of tFL to transplant (6 vs. 8 months).
Table 1.
Characteristics of patients with transformed diffuse large B-cell lymphoma from follicular lymphoma who received Hematopoietic Cell Transplant (HCT) from 1990–2009
| Auto HCT | Allo HCT | |
|---|---|---|
| Variable | N (%) | N (%) |
| Number of patients | 108 | 33 |
| Age, median (range) | 56 (19–74) | 49 (31–66) |
| Gender | ||
| Male | 65 (60) | 20 (61) |
| Female | 43 (40) | 13 (39) |
| Karnofsky score | ||
| < 90% | 35 (32) | 8 (24) |
| 90–100% | 68 (63) | 25 (76) |
| Missing | 5 (5) | 0 |
| Stage at diagnosis | ||
| I–II | 32 (30) | 9 (27) |
| III–IV | 72 (67) | 23 (70) |
| Missing | 4 (4) | 1 (3) |
| Disease status prior to HCT | ||
| CR1 | 9 (8) | 2 (6) |
| CR2 | 23 (21) | 7 (21) |
| Primary induction failure sensitive | 13 (12) | 7 (21) |
| Primary induction failure resistant | 3 (3) | 3 (10) |
| Relapsed sensitive | 39 (36) | 7 (21) |
| Relapsed resistant | 5 (5) | 5 (15) |
| Relapse untreated | 5 (5) | 1 (3) |
| Missing* | 11 (10) | 1 (3) |
| Chemosensitivity prior to HCT | ||
| Sensitive | 90 (83) | 23 (70) |
| Resistant | 10 (10) | 8 (24) |
| Untreated/unknown | 8 (7) | 2 (6) |
| Number of lines of chemo prior to HCT | ||
| 1–2 | 33(31) | 7(21) |
| ≥3 | 66(61) | 26(79) |
| Missing | 9(8) | 0 |
| Rituximab given between diagnosis and HCT | ||
| No | 78(72) | 11(33) |
| Yes | 30(28) | 22(67) |
| Known extranodal disease immediately prior to HCT | ||
| No | 75 (69) | 24 (73) |
| Yes | 28 (26) | 9 (27) |
| Missing | 5 (5) | 0 |
| Size of involved lymph nodes at HCT | ||
| Smaller than 5 cm | 18 (17) | 4 (12) |
| Equal to or greater than 5 cm | 14 (13) | 2 (6) |
| No lymphadenopathy at HCT | 35 (32) | 19 (58) |
| Missing | 41 (38) | 8 (24) |
| Interval between diagnosis and HCT (months) | 54 (6–347) | 55 (8–203) |
| Interval between diagnosis and transformation (months) | 47 (1–281) | 48 (1–173) |
| Interval between HCT and transformation (months) | 6 (2–76) | 8 (1–31) |
| Conditioning regimen | ||
| Myeloablative | 108 (100) | 20 (61) |
| Reduced intensity | 11 (33) | |
| Missing | 2 (6) | |
| Total body irradiation based conditioning | ||
| No | 84 (78) | 20 (61) |
| Yes | 24 (22) | 12 (36) |
| Missing | 0 | 1 (3) |
| Graft type | ||
| Bone marrow | 16 (15) | 10 (30) |
| Peripheral blood | 92 (85) | 23 (70) |
| Use of antithymocyte globulin (ATG)/Alemtuzumab | ||
| ATG/Alemtuzumab | 0 | 10 (30) |
| No ATG/Alemtuzumab | 0 | 23 (70) |
| Auto | 108 (100) | 0 |
| Type of donor | ||
| Matched related donor | 0 | 15(45) |
| Matched unrelated donor | 0 | 9(27) |
| Other | 0 | 9(27) |
| Autologous | 108 (100) | 0 |
| Year of transplant | ||
| 1990–1994 | 32 (30) | 1 (3) |
| 1995–2002 | 51 (47) | 9 (27) |
| 2003–2009 | 25 (23) | 23 (70) |
| Median follow-up of survivors (range), months | 85 (3–233) | 64 (3–97) |
Chemosensitivity known for 8 cases
Abbreviations for tables 1,2: OS overall survival, PFS progression free survival, N number of patient evaluated, # number, dx diagnosis, 95% CI confidence interval, Prob probability, autoHCT autologous hematopoietic cell transplantation, alloHCT allogeneic hematopoietic cell transplantation without prior autoHCT, chemoRx chemotherapy, TBI total body irradiation, MAC myeloablative conditioning, RIC/NMAC reduced intensity conditioning/ non-myeloablative conditioning, FL follicular lymphoma, tFL transformed follicular lymphoma, BM bone marrow, PB peripheral blood, GVHD graft-versus-host disease, FK tacrolimus, CSA cyclosporine, TCD T cell depletion, ATG antithymocyte globulin, CAMPATH alemtuzumab, MRD matched related donor, URD unrelated donor, CR complete remission, PR partial remission, REL relapsed, PIF primary induction failure, NE non evaluable, KPS Karnofsky Performance status.
Rituximab was given pretransplant in 28% and 61% of the cohorts (Table 1). Radioimmunotherapy with 90Y-ibritumomab tiuxetan or 131I-tositumomab was given to 5 subjects (1 autotransplant [0.9%] and 4 [12%] allotransplant recipients). Most of the subjects were responsive to chemotherapy before autotransplant (83%) and allotransplant (70%). However patients with poor risk features at transplant were also included in this study. Chemoresistance at transplant was present in 10% of autotransplant and 24% of allotransplant patients. Median numbers of lines of chemotherapy were 3 and 4 indicating a heavily pretreated group. Further, bulky lymphadenopathy of 5 cm or more was present at transplant in 13% of autotransplant and 6% of allotransplant patients. In addition, extranodal disease at transplant was present in 26% of the autotransplant group and 27% of the allotransplant group indicating a sizeable fraction of patients with poor risk features at transplant. Cytogenetic data were not available.
Carmustine, etoposide, cytarabine, melphalan (BEAM) was used in 27% and cyclophosphamide, carmustine, etoposide (CBV) in 29% of autotransplant recipients. Most allotransplant recipients (61%) received myeloablative conditioning (MAC) with cyclophosphamide, total body irradiation (Cy/TBI). The remaining subjects had RIC/NMAC, primarily fludarabine-2 Gy TBI (Flu-TBI), fludarabine-cyclophosphamide (Flu-Cy), or fludarabine-melphalan (Flu-Mel).
Transplantation Outcomes
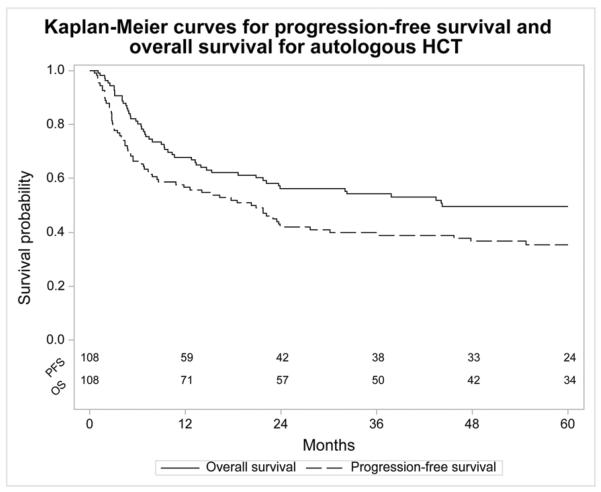
The autotransplant cohort had a 1 year NRM of 8% (95% CI 4–14), 5 year probability of relapse/progression of 54% (95% CI 44–63), 5 year PFS of 35% (95% CI 26–45), and 5 year OS of 50% (95% CI 40–59). There was a plateau after 40 months at 39% (95% CI 30–48) for PFS and 53% (95% CI 43–63) for OS (Figure 1). Causes of death included relapse/progression (41%) and second cancers (4%).
Figure 1.
Kaplan-Meier curves for progression free survival and overall survival for autologous hematopoietic cell transplantation
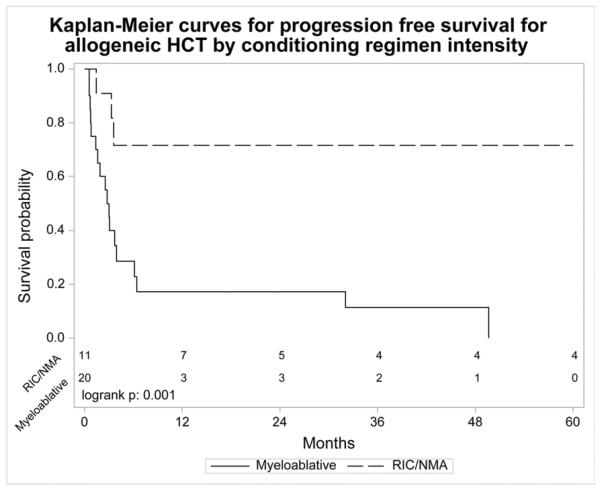
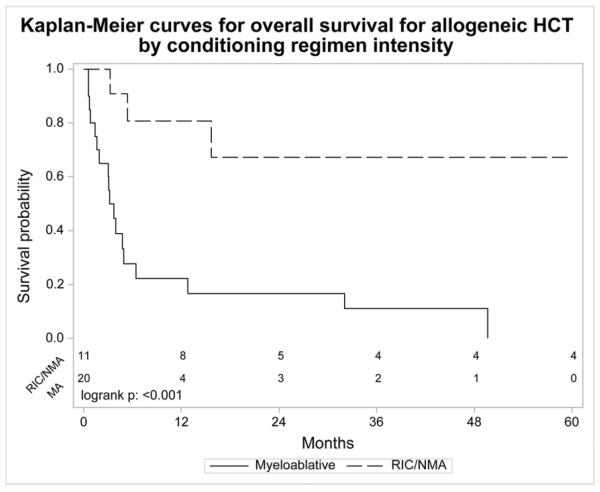
The allotransplant cohort had a 1 year NRM of 41% (95% CI 23–58), 5 year probability of relapse/progression of 33% (95% CI 17–50), 5 year PFS of 18% (95% CI 6–35) and 5 year OS of 22% (95% CI 8–41). The cumulative incidences of grades II–IV and III–IV aGVHD at day 100 were 42% (95% CI 26–59) and 27% (95% CI 14–42), respectively. The cumulative incidence of cGVHD at 1 year was 26% (95% CI 13–43). The major causes of death were organ failure (24%), relapse/progression (18%) and GVHD (12%). For MAC allotransplant, 3 year PFS was 11% (95% CI 1–30) and 3 year OS was 11% (95% CI 1–29). Three year PFS was 48% (95% CI 18–79) and 3 year OS was 67% (95% CI 35–93) for NMAC/RIC allotransplant. One year NRM for MAC allotransplant was 57% (95% CI 31–77). Patients who underwent RIC/NMAC allotransplant did not experience NRM within 5 years but 5 of the 13 (38%) patients died of relapse/progression.
Univariate Analysis
For autotransplant, age, Karnofsky performance status, presence of extranodal disease at HCT, rituximab use pre-transplant, time from diagnosis of FL to tFL of ≥1 year vs. < 1 year, chemosensitivity, or TBI conditioning did not have a statistically significant impact on PFS/OS (Table 2a). Notably, subjects with chemotherapy resistant disease achieved a 3 year OS of 27% (95% CI 7–59) after autotransplant.
For allotransplant, age, Karnofsky performance status, presence of extranodal disease at HCT, pre-transplant rituximab use, time from diagnosis of FL to tFL of ≥1 year vs. < 1 year, chemosensitivity, use of ATG or alemtuzumab or donor source did not have a statistically significant impact on PFS/OS (Table 2b). Subjects with chemotherapy resistant disease achieved a 3 year OS of 21% (95% CI 0–62) after allotransplant. The 3 year PFS was 11% (95% CI 1–30) for MAC and 48% (95% CI 18–79) for RIC/NMAC, p=0.001. The 3 year OS was 11% (95% CI 1–29) for MAC and was 67% (95% CI 35–93) for RIC/NMAC, p<0.001. RIC/NMAC was associated with significantly higher PFS and OS compared to MAC (Table 2b and Figure 2a and 2b).
Table 2b.
Univariate survival analysis for allogeneic hematopoietic cell transplantation
| PFS | OS | ||||
|---|---|---|---|---|---|
| Covariates | N | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| TBI based conditioning | |||||
| No | 20 | Reference | Reference | ||
| Yes | 12 | 1.07 (0.46–2.49) | 0.88 | 0.93 (0.40–2.18) | 0.87 |
| Chemo sensitivity | |||||
| Sensitive | 23 | Reference | Reference | ||
| Resistant | 8 | 1.36 (0.52–3.52) | 0.53 | 1.30 (0.50–3.37) | 0.59 |
| Time from diagnosis to transformation | |||||
| Less than a year | 9 | Reference | Reference | ||
| A year or greater | 24 | 0.43 (0.18–1.03) | 0.06 | 0.53 (0.23–1.25) | 0.15 |
| Age (years) | |||||
| > 50 | 15 | Reference | Reference | ||
| ≤ 50 | 18 | 0.66 (0.29–1.50) | 0.32 | 0.64 (0.28–1.45) | 0.28 |
| Karnofsky score | |||||
| <90 % | 8 | Reference | Reference | ||
| 90–100% | 25 | 0.88 (0.34–2.24) | 0.79 | 0.80 (0.31–2.05) | 0.64 |
| Extranodal disease pre HCT | |||||
| no | 24 | Reference | Reference | ||
| yes | 9 | 2.21 (0.91–5.37) | 0.08 | 2.06 (0.86–4.96) | 0.11 |
| Rituximab exposure pre HCT | |||||
| no | 11 | Reference | Reference | ||
| yes | 22 | 1.28 (0.52–3.11) | 0.59 | 1.40 (0.57–3.40) | 0.46 |
| ATG/Alemtuzumab | |||||
| ATG and/or Alemtuzumab | 10 | Reference | Reference | ||
| No ATG and/or Alemtuzumab | 23 | 1.51 (0.59–3.84) | 0.39 | 1.66 (0.65–4.23) | 0.29 |
| Conditioning regimen intensity | 0.01a | 0.007a | |||
| Myeloablative | 20 | Reference | Reference | ||
| RIC/NMAC | 11 | 0.16 (0.04–0.54) | 0.004 | 0.14 (0.04–0.49) | 0.002 |
| Disease status at transplant | 0.24a | 0.31a | |||
| CR | 8 | Reference | Reference | ||
| PIF/REL | 21 | 1.07 (0.38–2.98) | 0.90 | 0.90 (0.32–2.52) | 0.84 |
| Donor type | 0.53a | 0.29a | |||
| HLA identical sibling | 12 | Reference | Reference | ||
| URD | 18 | 0.95 (0.39–2.32) | 0.92 | 0.82 (0.34–2.01) | 0.67 |
multi degree-of-freedom overall test.
Abbreviations for tables 1,2: OS overall survival, PFS progression free survival, N number of patient evaluated, # number, dx diagnosis, 95% CI confidence interval, Prob probability, autoHCT autologous hematopoietic cell transplantation, alloHCT allogeneic hematopoietic cell transplantation without prior autoHCT, chemoRx chemotherapy, TBI total body irradiation, MAC myeloablative conditioning, RIC/NMAC reduced intensity conditioning/ non-myeloablative conditioning, FL follicular lymphoma, tFL transformed follicular lymphoma, BM bone marrow, PB peripheral blood, GVHD graft-versus-host disease, FK tacrolimus, CSA cyclosporine, TCD T cell depletion, ATG antithymocyte globulin, CAMPATH alemtuzumab, MRD matched related donor, URD unrelated donor, CR complete remission, PR partial remission, REL relapsed, PIF primary induction failure, NE non evaluable, KPS Karnofsky Performance status.
Figure 2.
Kaplan-Meier curve for (a.) progression free survival and (b.) overall survival for allogeneic hematopoietic cell transplantation by conditioning regimen intensity.
Multivariate analysis
Cox proportional hazards regression model was used to identify risk factors significantly associated with the treatment failure (1 – PFS) and overall mortality (1 – OS) for autotransplant. With a 5% significance level, no risk factors were significant. No multivariate analysis was considered for allotransplant due to the small size of the study cohort.
Discussion
In this study we determined that autotransplant for tFL results in durable remissions in a high proportion of subjects. There was an unexpected high NRM in the allotransplant recipients that may have obscured any benefit seen with allotransplant. It is interesting that clinicians seemingly favored the use of allotransplants in the 2003–2009 era. As this is a retrospective study it is impossible to know why clinicians chose autotransplant or allotransplant. The pre-transplant variables were comparable in terms of risk factors and markers of poor prognosis. Availability of a good donor is not the explanation as more than half the patients did not have a matched sibling. Perhaps this reflects a perception that autotransplant would be less effective at producing long-term remission for tFL in the rituximab era. There are limited data on the impact of prior rituximab on outcomes of autotransplant for tFL.16,30 We found a 5 year PFS of 35% (95% CI 26–45) and 5 year OS of 50% (95% CI 40–59) after autotransplant for tFL, with a seemingly similar benefit in those with prior rituximab therapy. However, only 28% received rituximab prior to autotransplant, so firm conclusions regarding the impact of prior rituximab awaits larger studies. There was a plateau for PFS and OS, suggesting that a subset may be cured with autotransplant, although longer follow up in needed to determine if these patients remain disease free.
Our results are similar to those of the European Bone Marrow Transplant (EBMT) registry report of 50 tFL subjects from the pre-rituximab era (Table 3).17 There is only one phase 2 study in tFL, also pre-rituximab, reporting a 5 year 0S of 47% (95% CI 29–65) with autotransplant, which compared favorably to those not transplant eligible.31 More recently the Canadian Blood and Marrow Transplant Group (CBMTG) reported a 5 year OS of 61% (standard error 7%) with rituximab-chemotherapy alone without transplant. However, a third of this subset had received no therapy for FL before histological transformation and a quarter had limited stage tFL, a highly favorable subset likely contributing to these results.32 Even in the pre-rituximab era, watchful waiting before transformation and limited stage of tFL were significant predictors of long-term survival (median survival 81 months), albeit with a continuous risk of relapse and without a plateau on the survival curve.3,5,33 In contrast, we demonstrated a plateau on the survival curve with autotransplant in a heavily pretreated cohort. A key limitation to analyses such as this is that without a prospective comparison, it is not certain that autotransplantation is a better approach than nontransplant therapies. However, the durability of the response is an important advantage of autotransplant for tFL. Likewise, in the largest study of radioimmunotherapy for tFL, the response rates were high but their durability was poor with 5 year PFS of only 17%.34
Table 3.
Studies on outcomes of autologous hematopoietic cell transplantation for follicular lymphoma with histologic transformation
| STUDY Years of HCT |
N | AGE Median (range) years | tFL Definition | PFS | OS | NRM | COMMENTS |
|---|---|---|---|---|---|---|---|
| PRE RITUXIMAB ERA | |||||||
| Williams17 EBMT Retrospective 1982–1995 |
50 auto HCT | 45 (28–61) | FL→DLBCL or any high grade lymphoma | 2y 40% 5y 30% |
2y 60% 5y 51% |
18% | Improved survival if chemosensitive at HCT. Same outcomes for FL, de novo relapsed DLBCL and transformed lymphoma in matched controlled analysis. |
| Friedberg18 Retrospective 1982–1997 |
27 auto HCT | 44 (29–58) | FL→DLBCL CLL→DLBCL |
5y 46% | 5y 58% | 0 | All relapses after autoHCT were DLBCL. tFL within 18 months from diagnosis had better OS. |
| Eide31 Prospective Phase 2 1991–2007 |
30 auto HCT | 55 (31–65) | FL or MZL →DLBCL or between DLBCL and BL, composite lymphoma | 5y 32% | 5y 47% | 7% | Plateau on PFS after 40 months at 30%. |
| RITUXIMAB ERA | |||||||
| Hamadani16 Retrospective 1991–2007 |
24 auto HCT | 56 (47–68) | FL→DLBCL | 3y 40% | 3y 52% | 8% | Improved PFS in 62% of patients with rituximab in treatment course. Chemosensitivity at HCT had no effect on outcomes after HCT. |
| Ban-Hoefen30 Retrospective 1998–2010 |
18 auto HCT | 58 (40–65) | FL or MZL→DLBCL | 2y 59% | 2y 82% | 0 | Improved outcomes compared to historical pre-rituximab cohorts.17 |
| Villa32 CBMTG Retrospective 2001–2010 |
97 auto HCT | 56 (32–66) | FL→DLBCL or BL | 5y 55% | 5y 57% | 5% | AutoHCT had better PFS/OS than rituximab-chemoRx. |
| Current study Retrospective 1990–2009 |
108 auto HCT | 56 (19–74) | FL→DLBCL | 5y 36% | 5y 50% | 8% | No impact of chemosensitivity or prior rituximab use on outcomes. |
Abbreviations for Tables 3 and 4: OS overall survival, PFS progression free survival, NRM non-relapse-mortality, N number of patients evaluated, Y year, autoHCT autologous hematopoietic cell transplantation, alloHCT allogeneic hematopoietic cell transplantation, chemoRx chemotherapy, TBI total body irradiation, ChemoR chemoresistance, MAC myeloablative conditioning, RIC reduced intensity conditioning, NMAC non-myeloablative conditioning, FL follicular lymphoma, tFL transformed follicular lymphoma, DLBCL diffuse large B cell lymphoma, BL Burkitt lymphoma, SLL small lymphocytic lymphoma, MZL marginal zone lymphoma, EBMT European Bone Marrow Transplant Registry, CBMTG Canadian Blood and Marrow Transplant Group, CIBMTR Center for International Blood and Marrow Transplant Research, MRD matched related donor, URD unrelated donor.
In our study, RIC/NMAC allotransplant provided improved PFS and OS compared with MAC (Table 2b and Figure 2). The high NRM following MAC allotransplant influenced these outcomes and so cannot be recommended. RIC/NMAC allotransplant maybe the best strategy to solve the problem of NRM in allotransplant since the low NRM with RIC/NMAC did not obscure the potential benefits of allotransplant. Firm conclusions about the role of RIC/NMAC allotransplant in tFL await prospective comparisons with autotransplant. Nonetheless, outcomes of allotransplant for tFL appear to be inferior to FL. In the study by Khoury et al, NMAC allotransplant for FL lead to a 5 year OS of 85% (95% CI 71–93) with a plateau on the survival curve, implying a curative potential and stronger graft versus lymphoma effect.36 In a recent CIBMTR analysis, GVHD was associated with lower relapse in FL but not de novo DLBCL and this effect was more prominent with RIC than MAC.37 Similar to de novo DLBCL, graft versus lymphoma effects seem to be less effective in transformed DLBCL than in FL.38
This study has the limitations of any retrospective study such as inherent patient selection bias as to the type of transplant performed. The comparison of autotransplant with allotransplant is biased since most autotransplants were performed a decade before allotransplants. Further, the number of allotransplant patients is low and the results of RIC/NMAC significantly better than with MAC making a direct comparison of allotransplant with autotransplant difficult. Nonetheless this multicenter study is the largest to our knowledge to describe transplant outcomes specifically in biopsy-proven DLBCL transformed from FL. Other studies have included heterogeneous low grade lymphomas transforming to a variety of high grade lymphomas, all of which can influence transplant outcomes and make the results difficult to interpret (Tables 3 and 4).17–20,30–32 Several conclusions can be made from our study. Firstly, allotransplant with RIC/NMAC had improved survival compared to MAC, but any potential benefit from MAC was obscured by the high NRM. The precise role of allotransplant awaits prospective comparison of RIC/NMAC allotransplant with autotransplant. Secondly, autotransplant provides durable survival for tFL irrespective of age, early or late histologic transformation, extranodal disease at transplant or prior rituximab use. The outcomes of autotransplant seem to be more durable than published data on non-transplant therapies and merits a prospective study to definitively answer this question.7,31,32,33,34
Table 4.
Studies on outcomes of allogeneic HCT for follicular lymphoma with histologic transformation
| Study N Years of HCT |
Age Median (range) years | tFL definition | Median lines of chemoRx before HCT |
Conditioning Donor |
PFS | OS | NRM | Comments |
|---|---|---|---|---|---|---|---|---|
| Ramadan19 N=40 1989–2005 |
44 (28–57) | FL,SLL, MZL → intermediate or higher grade lymphoma, composite lymphoma | 3 chemoR 20% |
MAC MRD 25 URD 15 |
5y 23% | 5y 23% | 3y 36% | No impact on outcomes of composite vs. transformed lymphomas, URD vs. MRD. Performance of HCT within a year of diagnosis had better outcomes. |
| Rezvani20 N=62 of which 16 had TL 1998–2006 |
54 (33–66) | FL, SLL, MZL→aggressive NHL in 16 patients, remainder had low grade lymphoma. | 6 ChemoR 23% |
NMAC 2Gy TBI +/− FLU MRD 34 URD 28 |
3y 21% for TL 3y 43% for FL |
3y 18% for TL 3y 52% for FL |
3y 42% | Better outcomes for indolent lymphomas vs. transformed lymphoma, no impact of chemoresistance at HCT. 27 patients had prior autoHCT. |
| Hamadani35 N=8 1999–2007 |
56 (44–63) | FL→DLBCL | 4 ChemoR 38% |
MAC 6 RIC 2 MRD 5 URD 3 |
4y 56% | 4y 66% | 25% | No disease relapse after 1 year |
| Villa32 CBMTG N=22 2001–2010 |
48 (32–57) | FL→DLBCL, BL | 3 ChemoR 18% |
MAC >95% MRD14 URD 7 MMRD 1 |
5y 45% | 5y 45% | 5y 23% | 2 had prior autoHCT for FL No difference in OS between alloHCT and autoHCT. |
| Current study N=33 1990–2009 |
49 (31–66) | FL→DLBCL | 4 ChemoR 35% |
MAC 20 RIC/NMAC 5/6 MRD 12 URD 21 |
5y 18% | 5y 22% | iy 41% 5y 49% |
No impact of chemoresistance, at HCT or URD on outcomes. Better 3y PFS/OS with RIC/NMAC (48%/67% vs. 11%) than with MAC. |
Abbreviations for Tables 3 and 4: OS overall survival, PFS progression free survival, NRM non-relapse-mortality, N number of patients evaluated, Y year, autoHCT autologous hematopoietic cell transplantation, alloHCT allogeneic hematopoietic cell transplantation, chemoRx chemotherapy, TBI total body irradiation, ChemoR chemoresistance, MAC myeloablative conditioning, RIC reduced intensity conditioning, NMAC non-myeloablative conditioning, FL follicular lymphoma, tFL transformed follicular lymphoma, DLBCL diffuse large B cell lymphoma, BL Burkitt lymphoma, SLL small lymphocytic lymphoma, MZL marginal zone lymphoma, EBMT European Bone Marrow Transplant Registry, CBMTG Canadian Blood and Marrow Transplant Group, CIBMTR Center for International Blood and Marrow Transplant Research, MRD matched related donor, URD unrelated donor.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U10 HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Morton LM, Wang SS, Devesa SS, Hartage P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novelli S, Briones J, Sierra J. Epidemiology of lymphoid malignancies: last decade update. Springer plus. 2013;2(1):70. doi: 10.1186/2193-1801-2-70. epub 2013 Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma. J Clin Oncol. 2008;26(32):5165–5169. doi: 10.1200/JCO.2008.16.0283. [DOI] [PubMed] [Google Scholar]
- 4.Bastion Y, Sebban C, Berger F, et al. Incidence, predictive factors, and outcomes of lymphoma transformation in follicular lymphoma patients. J Clin Oncol. 1997;15(4):1587–1594. doi: 10.1200/JCO.1997.15.4.1587. [DOI] [PubMed] [Google Scholar]
- 5.Gine E, Montoto S, Bosch F, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) and the histologic subtype are the most important factors to predict histologic transformation in follicular lymphoma. Ann Oncol. 2006;17(10):1539–1545. doi: 10.1093/annonc/mdl162. [DOI] [PubMed] [Google Scholar]
- 6.Montoto S, Davies AJ, Matthews J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25(17):2426–2433. doi: 10.1200/JCO.2006.09.3260. [DOI] [PubMed] [Google Scholar]
- 7.Conconi A, Ponzio C, Lobetti-Bodoni C, et al. Incidence, risk factors and outcome of histologic transformation in follicular lymphoma. Br J Haematol. 2012;157(2):188–196. doi: 10.1111/j.1365-2141.2012.09054.x. [DOI] [PubMed] [Google Scholar]
- 8.Garvin AJ, Simon RM, Osborne CK, Merrill J, Young RC, Berard CW. An autopsy study of histologic progression in non-Hodgkin's lymphoma. 192 cases from the National Cancer Institute. Cancer. 1983;52(3):393–398. doi: 10.1002/1097-0142(19830801)52:3<393::aid-cncr2820520302>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Cohen Y, Da'as N, Libster D, Amir G, Berrebi A, Polliack A. Large cell transformation of chronic lymphocytic leukemia and follicular lymphoma during or soon after treatment with fludarabine-rituximab containing regimens: natural history or therapy related complication. Eur J Haematol. 2002;68(2):80–83. doi: 10.1034/j.1600-0609.2002.01599.x. [DOI] [PubMed] [Google Scholar]
- 10.Solal-Celigny P, Bellei M, Marchesselli L, et al. Watchful waiting in low-tumor burden follicular lymphoma in the rituximab era: results of an F2-study database. J Clin Oncol. 2012;30(31):3848–3853. doi: 10.1200/JCO.2010.33.4474. [DOI] [PubMed] [Google Scholar]
- 11.Czuczman MS, Vose JM, Witzig TE, et al. The differential effect of lenalidomide monotherapy in patients with relapsed or refractory transformed non-Hodgkin lymphoma of distinct histologic origin. Br J Haematol. 2011;154(4):477–481. doi: 10.1111/j.1365-2141.2011.08781.x. [DOI] [PubMed] [Google Scholar]
- 12.Molica S. A systematic review on Richter syndrome: what is the published evidence? Leuk Lymphoma. 2010;51(3):415–421. doi: 10.3109/10428190903515192. [DOI] [PubMed] [Google Scholar]
- 13.McNamara C, Davis J, Dyer M, et al. Guidelines on the investigation and management of follicular lymphoma. Br J Haematol. 2012;156(4):446–467. doi: 10.1111/j.1365-2141.2011.08969.x. [DOI] [PubMed] [Google Scholar]
- 14.Schouten HC, Bierman PJ, Vaughan WP, et al. Autologous bone marrow transplantation in follicular non-Hodgkin's lymphoma before and after histologic transformation. Blood. 1989;74(7):2579–2584. [PubMed] [Google Scholar]
- 15.Chen C, Crump M, Tsang R, Stewart AK, Keating A. Autotransplants for histologically transformed follicular non-Hodgkin's lymphoma. Br J Haematol. 2001;113(1):202–208. doi: 10.1046/j.1365-2141.2001.02705.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamadani M, Benson DM, Lin TS, Porcu P, Blum KA, Devine SM. High-dose therapy and autologous stem cell transplantation for follicular lymphoma undergoing transformation to diffuse large B-cell lymphoma. Eur J Haematol. 2008;81(6):425–431. doi: 10.1111/j.1600-0609.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams CD, Harrison CN, Lister TA, et al. High-dose therapy and autologous stem-cell support for chemosensitive transformed low-grade follicular non-Hodgkin's lymphoma: a case-matched study from the European Bone Marrow Transplant Registry. J Clin Oncol. 2001;19(3):727–735. doi: 10.1200/JCO.2001.19.3.727. [DOI] [PubMed] [Google Scholar]
- 18.Friedberg JW, Neuberg D, Gribben JG, et al. Autologous bone marrow transplantation after histologic transformation of indolent B cell malignancies. Biol Blood Marrow Transplant. 1999;5(4):262–268. doi: 10.1053/bbmt.1999.v5.pm10465106. [DOI] [PubMed] [Google Scholar]
- 19.Ramadan KM, Connors JM, Al-Tourah AJ, et al. Allogeneic SCT for relapsed composite and transformed lymphoma using related and unrelated donors: long- term results. Bone Marrow Transplant. 2008;42(9):601–608. doi: 10.1038/bmt.2008.220. [DOI] [PubMed] [Google Scholar]
- 20.Rezvani A, Storer B, Marris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin's lymphoma. J Clin Oncol. 2008;26(2):211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz M. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42(Suppl 1):S1–S2. doi: 10.1038/bmt.2008.101. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and genetics of tumors of hematopoietic and lymphoid tissues. 1 ARC Press; Lyon, France: 2001. World Health Organization classification of tumors. [Google Scholar]
- 23.Cheson B, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphoma. J Clin Oncol. 1999;17(4):1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 25.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 26.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantor AB. Projecting the standard error of the Kaplan-Meier estimator. Stat Med. 2001;20(14):2091–2097. doi: 10.1002/sim.856. [DOI] [PubMed] [Google Scholar]
- 29.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359(9314):1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 30.Ban-Hoefen M, Kelly JL, Bernstein SH, et al. High-dose therapy and autologous stem cell transplant for transformed non-Hodgkin lymphoma in the rituximab era. Leuk Lymphoma. 2012;53(5):827–832. doi: 10.3109/10428194.2011.631637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eide MB, Lauritzsen GF, Kvalhelm G, et al. High dose chemotherapy with autologous stem cell support for patients with histologically transformed B-cell non-Hodgkin lymphoma. A Norwegian multi center phase II study. Br J Haematol. 2011;152(5):600–610. doi: 10.1111/j.1365-2141.2010.08519.x. [DOI] [PubMed] [Google Scholar]
- 32.Villa D, Crump M, Panzarella T, et al. Autologous and allogeneic stem-cell transplantation for transformed follicular lymphoma: a report of the Canadian Blood and Marrow Transplant Group. J Clin Oncol. 2013;31(9):1164–1171. doi: 10.1200/JCO.2012.44.0693. [DOI] [PubMed] [Google Scholar]
- 33.Yuen AR, Kamel OW, Halpern J, Horning SJ. Long-term survival after histologic transformation of low grade follicular lymphoma. J Clin Oncol. 1995;13(7):1726–1733. doi: 10.1200/JCO.1995.13.7.1726. [DOI] [PubMed] [Google Scholar]
- 34.Fisher RI, Kaminski MS, Wahl RL, et al. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low grade and transformed non-Hodgkin's lymphoma. J Clin Oncol. 2005;25(30):7565–7573. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 35.Hamadani M, Awan FT, Elder P, et al. Feasibility of allogeneic hematopoietic stem cell transplantation for follicular lymphoma undergoing transformation to diffuse large B-cell lymphoma. Leuk Lymphoma. 2008;49(10):1893–1898. doi: 10.1080/10428190802270902. [DOI] [PubMed] [Google Scholar]
- 36.Khouri IF, McLaughlin P, Saliba RM, et al. Eight year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(2):5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbano-Ispizua A, Pavletic SZ, Flowers MED, et al. Association of graft vs. host disease (GVHD) with a lower relapse/progression rate after allogeneic hematopoietic stem cell transplantation (HSCT) with reduced intensity conditioning in patients with follicular and mantle cell lymphoma: A CIBMTR analysis [abstract] Blood. 2013;122:2093. [Google Scholar]
- 38.Lazarus HM, Zhang MJ, Carreras J, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol Blood Marrow Transplant. 2010;16(1):35–45. doi: 10.1016/j.bbmt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]