Abstract
The [PSI+] yeast prion is formed when Sup35 misfolds into amyloid aggregates. [PSI+], like other yeast prions, is dependent on the molecular chaperone Hsp104, which severs the prion seeds so that they pass on as the yeast cells divide. Surprisingly, however, overexpression of Hsp104 also cures [PSI+]. Several models have been proposed to explain this effect: inhibition of severing, asymmetric segregation of the seeds between mother and daughter cells, and dissolution of the prion seeds. First, we found that neither the kinetics of curing nor the heterogeneity in the distribution of the green fluorescent protein (GFP)-labeled Sup35 foci in partially cured yeast cells is compatible with Hsp104 overexpression curing [PSI+] by inhibiting severing. Second, we ruled out the asymmetric segregation model by showing that the extent of curing was essentially the same in mother and daughter cells and that the fluorescent foci did not distribute asymmetrically, but rather, there was marked loss of foci in both mother and daughter cells. These results suggest that Hsp104 overexpression cures [PSI+] by dissolution of the prion seeds in a two-step process. First, trimming of the prion seeds by Hsp104 reduces their size, and second, their amyloid core is eliminated, most likely by proteolysis.
INTRODUCTION
Saccharomyces cerevisiae [PSI+], the infectious misfolded amyloid form of the protein Sup35, is one of the better-characterized yeast prions (1–5). Sup35 is an essential protein with three domains, an N-terminal Asn/Gln-rich aggregation-prone domain, a middle domain, and a C-terminal domain that mediates translation termination. In [psi−] yeast cells, Sup35 is properly folded, whereas in [PSI+] yeast cells, most of the Sup35 is misfolded into amyloid seeds, resulting in significant readthrough at stop codons because of the absence of functional Sup35. The conversion of properly folded Sup35 into its misfolded amyloid conformation can occur either spontaneously or by seeding with misfolded Sup35. Once conversion occurs, the [PSI+] prion conformation continues to pass from generation to generation, with new seeds constantly being generated to maintain a steady-state level of seeds in the yeast population.
New seeds are produced when preexisting seeds are severed by Hsp104, a member of the triple-A ATPase family (6). Hsp104 is composed of six identical subunits that form a hexameric ring. Threading of Sup35 molecules through the pore of the Hsp104 hexamer severs the prion seeds in an ATP-dependent reaction (7). Inactivation of the Hsp104 ATPase activity inhibits its severing activity, which in turn cures [PSI+] and other prions by causing the seeds to dilute out as the cells divide (3, 8–14).
Surprisingly, unlike other yeast prions, the [PSI+] prion is also cured by overexpression of Hsp104 (3), and even though this was shown more than a decade ago, the mechanism of this curing is still not understood. However, it does appear that the mechanism of [PSI+] curing by Hsp104 overexpression is very different from the mechanism of curing by Hsp104 inactivation. First, the kinetics of [PSI+] curing when Hsp104 is overexpressed are very different from the kinetics of curing when Hsp104 is inactivated (8, 15, 16). Specifically, the time course of curing by Hsp104 overexpression does not show the long lag phase that is observed when curing is caused by the inactivation of Hsp104. This long lag phase is caused by prion seeds diluting out as the yeast cells divide. Second, there are different chaperone requirements for curing of [PSI+] by overexpression rather than inactivation of Hsp104. Increasing the level of Ssa1 inhibits curing of [PSI+] by the overexpression of Hsp104 (17) but does not affect curing by the inactivation of Hsp104 (8). The inhibitory effect of Ssa1 on curing by overexpression of Hsp104 was recently shown to be dependent on the cochaperone Sgt2, a protein that interacts with Sup35 as well as various other heat shock proteins and cytoplasmic tail-anchored proteins (18). In contrast to Ssa1, the Ssb members of the Hsp70 family support curing of [PSI+] by Hsp104 overexpression (19). Finally, Hsp90 and the cochaperones Sti1 and Cpr7 affect curing by Hsp104 overexpression but not by Hsp104 inactivation (15, 16).
One model that has been proposed for the curing of [PSI+] by the overexpression of Hsp104 is that it involves disaggregation or dissolution of the prion seeds (3, 4, 8). The role of Hsp104 in this dissolution process could be due to its severing activity, which reduces the size of the prion seeds and concomitantly increases the number of seeds. The dissolution model was supported by the presence of a large soluble Sup35 fraction in yeast lysates prepared from [PSI+] cells overexpressing Hsp104 (4). Further support came from in vivo studies showing that the green fluorescent protein (GFP)-labeled NM fragments of Sup35 (NMG), consisting of the N and M domains of Sup35, appeared diffuse in [PSI+] cells when Hsp104 was overexpressed (8). Alternatively, dissolution of the prion seeds might be due to a recently described activity of Hsp104 termed trimming, which reduces the size of the prion seeds by dissociating Sup35 from the end of a prion seed without producing a new seed (20). Trimming was first observed under conditions in which the severing activity of Hsp104 was inhibited by guanidine. We found that GFP-labeled Sup35 foci could not be detected in guanidine-treated cells by confocal microscopy in yeast cells that were still [PSI+]. This observation was supported by measuring the mobility of GFP-labeled Sup35 in the cytosol and also by measuring the soluble fraction of GFP-labeled Sup35 in cell lysates; both measurements showed an increase in the level of free Sup35. Therefore, in contrast to severing activity, trimming activity reduces the size of the prion seeds without increasing their number.
Recently, two other models have been proposed for the curing of [PSI+] by Hsp104 overexpression. One model proposes that the overexpression of Hsp104 blocks fragmentation of the amyloid fiber (21). This model was based on the observation that when Hsp104 was overexpressed, rather than being recruited to the NMG fibrils by Ssa1, it bound directly to the amyloid fiber. This is nonproductive binding since Hsp104 has to be recruited by Ssa1 to sever the prion fiber (7). The other model proposes that curing of [PSI+] by Hsp104 overexpression is due to asymmetric segregation of the prion seeds (22, 23). In this model, the overexpression of Hsp104 produces large seeds, which are retained in the mother cells upon cell division. This model predicts that the daughter cells, which inherit fewer seeds, should cure faster than the mother cells. Asymmetric segregation was recently shown to be the mechanism by which a mild heat shock cures [PSI+] prion (22).
Given the controversy over the mechanism of [PSI+] curing by Hsp104 overexpression, we have reexamined this curing process. We found that the kinetics of curing and the distribution of GFP-labeled Sup35 foci were not compatible with the inhibition-of-severing model, nor did we find any bias in curing between mother and daughter cells when cells were separated by fluorescence-activated cell sorting (FACS), as predicted by the asymmetric-segregation model of curing. Instead, by analyzing changes in GFP-labeled Sup35 during curing of [PSI+] by Hsp104 overexpression, we found that the trimming activity of Hsp104 is needed for the dissolution of the foci.
MATERIALS AND METHODS
Yeast strains, media, and growth conditions.
The 1074 [PSI+] yeast strain used in this study has NGMC, a construct in which GFP is inserted between the N and M domains of Sup35, which is composed of 3 domains (NMC), integrated into the genomic locus of SUP35. This strain was derived from wild-type strain 779-6A (MATa kar1-1 SUQ5 ade2-1 his3Δ202 leu2Δ1 trp1Δ63 ura3-52) (10). The STI1 deletion strain derived from 779-6A was described previously (16). Deletion of UMP1 was created by transforming strain 1074 by using a ump1::HIS3 disruption cassette that was PCR amplified from yeast deletion strain JD59 (24). Strains with mutant HSP104 genes were constructed as described previously (25). Plasmid transformations were performed as described previously (26). Yeast cells were grown at 30°C on synthetic dextrose (SD) medium (0.7% yeast nitrogen base, 2% glucose) with a complete supplement mixture or the appropriate amino acid dropout mixture for selection and maintenance of the particular plasmid. Synthetic galactose (SGal) medium contains both 2% galactose and 2% raffinose. To determine the prion phenotype, yeast cells were plated onto 1/2 yeast-peptone-dextrose (YPD) (0.5% yeast extract, 2% peptone, and 2% glucose) solid medium. Cultures were always maintained under active growing conditions (optical density at 600 nm [OD600] of ≤0.6) by periodic dilution with fresh medium.
Plasmids.
Plasmids pFL39-GAL-HSP104 and pFL39-GAL-HSP104-2KT are TRP1-based centromeric plasmids and have HSP104 under the control of the GAL1 promoter (8). Plasmid pRS314-rtHSP104 has HSP104, which is placed under the control of the tetracycline-inducible (TET-ON) expression system from pTET23 (27). To generate pRS314-rtHsp104, the tetracycline-inducible system was engineered into the centromeric pRS314 plasmid, and the PCR-amplified HSP104 coding region with the terminator region (positions −9 to +2962) was fused to the TET-ON promoter for the regulation of Hsp104 expression. The plasmids used to overexpress Hsp104(T160M) and a delta-N-terminal Hsp104 deletion mutant, Hsp104(Δ147), were described previously (28). pFL39-GAL-Hsp104(D184S) was generated to express Hsp104(D184S). All sequences were verified. The YEp105 (YEp pCUP1 Myc-Ub TRP1) plasmid used to express ubiquitin was a gift from Mark Hochstrasser (Yale University).
Curing experiments.
[PSI+] strain 1074 was cured by overexpressing Hsp104 from either the GAL1 or the TET-ON promoter. When Hsp104 was expressed from the GAL1 promoter, cells grown in SD medium were shifted to SGal medium and continued to grow until they were cured. When Hsp104 was expressed from the TET-ON promoter, its expression was induced by the addition of 10 μg/ml doxycycline (Sigma), a derivative of tetracycline, in SD medium. The kinetics of curing was assayed by periodically plating culture aliquots onto 1/2 YPD plates. Colonies with any white sectors were counted as [PSI+] colonies.
FACS analysis of cells.
Cells were labeled for FACS analysis by using two different methods. The first method labeled bud scars to separate yeast cells based on age. Yeast cells grown in SGal medium for 5 generations to induce Hsp104 overexpression were washed in phosphate-buffered saline (PBS) and then incubated for 30 min at room temperature in 1 μg/ml Alexa-647 wheat germ agglutinin (WGA) (Invitrogen) in PBS. After washing twice in PBS, cells were sorted into two populations based on far-red fluorescence intensity by using the MoFlo cell sorter (Beckman Coulter). Cells were plated before and after sorting to determine the extent of curing.
The second method labeled the proteins on the yeast cell wall prior to the induction of Hsp104 overexpression. Yeast cells in SD medium were washed 3 times in PBS and then labeled (4 OD units of yeast) by using 10 mg/ml of sulfo-NHS-LC-biotin [sulfosuccinimidyl-6-(biotin-amido)hexanoate] (Pierce) for 30 min at room temperature (29). Cells were washed in Tris-EDTA buffer to quench labeling and then suspended at 0.1 OD units in 10 ml of SGal medium to induce Hsp104 expression. Cells were grown for 3 generations at 30°C, harvested, washed 3 times with PBS, and then suspended in 1 ml PBS. The cells were then fluorescently labeled by adding 20 μl of streptavidin-Dylight 488 (ThermoScientific) for 30 min at room temperature. Cells were washed with PBS and diluted 10-fold prior to running the mixture over the FACS instrument to collect nonfluorescent and fluorescent yeast cells. The yeast were plated before and after sorting on 1/2 YPD plates.
Imaging during curing by Hsp104 overexpression.
Cells were imaged on a Zeiss live confocal microscope by using a piezoelectric stage to obtain z-stacks with a 100× objective in 8-well 25-mm2 chambered coverslips (Lab-Tek). Focus intensity was measured by using Zeiss software. To count cells with foci and numbers of foci in a cell, cells at the indicated generations were fixed in 4% paraformaldehyde (Sigma) in PBS for 5 min at room temperature and scanned in z-stacks (16 slices at 0.4-μm intervals). To make the foci readily detectable by confocal microscopy, cells were stressed by incubating them in either medium containing 5 mM guanidine or water for 1 h prior to imaging. Microcolony analysis was performed by covering yeast cells with an agarose pad composed of 1% agarose in SD medium with and without 10 μg/ml doxycycline in 2-well 25-mm2 chambered coverslips (Lab-Tek). The yeast cells were imaged over time, as indicated in the text. The Metamorph program was used to obtain maximum pixel intensity when z-stacks were flattened into a single plane.
Western blotting.
To ensure that the same amount of protein was loaded into each well of the SDS gel, the protein concentration of yeast lysates was determined by the Bradford assay (30). Western blots were performed by using the following antibodies: rabbit anti-Hsp104 (Abcam), mouse anti-phosphoglycerate kinase (Invitrogen), and mouse anti-Myc (Sigma). The following secondary antibodies were used: donkey anti-rabbit IRDye 800 and donkey anti-mouse IRDye 680 (Li-Cor). Proteins were detected by using the Odyssey infrared system (Li-Cor Bioscience).
RESULTS
[PSI+] curing by overexpression of Hsp104 using the GAL1 promoter.
Our laboratory has been studying the curing of [PSI+] by confocal microscopy using yeast strain 1074, which expresses NGMC from the genomic SUP35 locus (20, 31, 32). In our previous study, we examined the curing of [PSI+] by the inactivation of Hsp104, and using the same methodology, we have now examined the curing of [PSI+] by Hsp104 overexpression. Along with imaging of the cells, the red/white colony plating assay was used to determine the prion phenotype; white or white/red-sectored colonies were scored as [PSI+], and completely red colonies were scored as [psi−]. First, the time course of [PSI+] curing was measured by growing [PSI+] yeast cells in SGal medium to induce the overexpression of Hsp104 from the GAL1 promoter, followed by plating of the yeast cells at different times. The results are plotted as the percentage of [PSI+] cells versus the number of generations (Fig. 1A). After a short lag, [PSI+] curing was relatively fast, but the rate of curing then gradually decreased as more cells were cured. This plot is consistent with 10 to 15% of the [PSI+] cells curing in each generation, as was established previously by using parental yeast strain 779-6A (16). The kinetics of curing of [PSI+] by overexpression is very different from that of curing by Hsp104 inactivation. When [PSI+] is cured by growing cells in guanidine to inactivate Hsp104, the plot of percent [PSI+] cells versus generations shows a long lag before the yeast cells start to cure. This lag has been shown to be due to dilution of the prion seeds when severing activity is inhibited, so curing occurs only once the prion seeds are diluted out by cell division (11, 13, 33).
FIG 1.
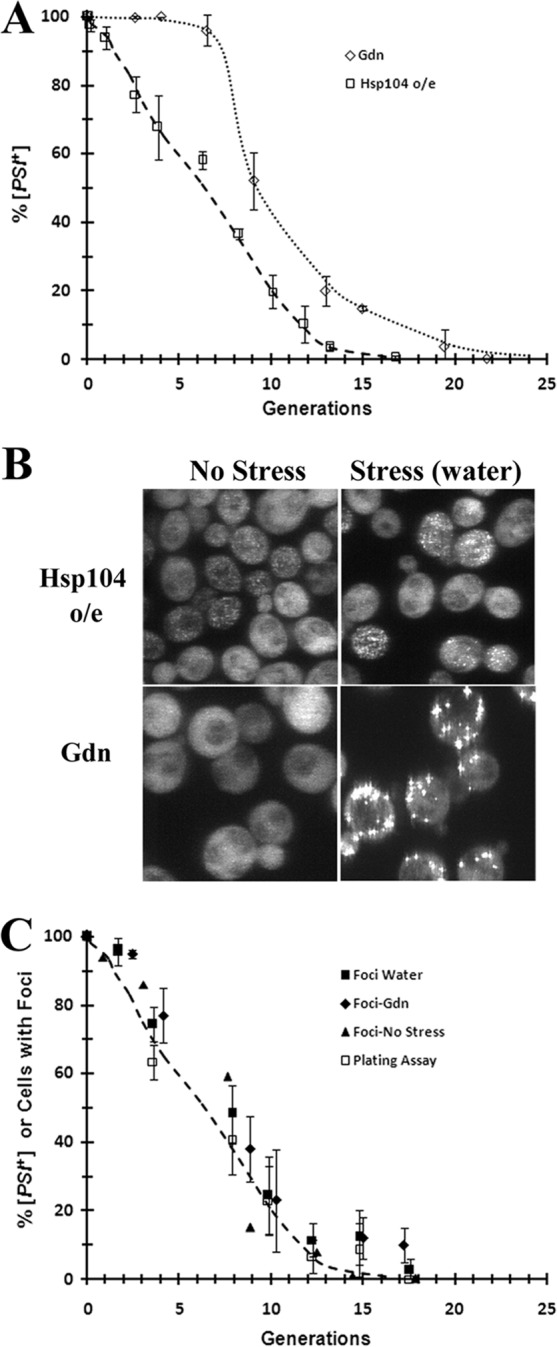
Curing of [PSI+] in strain 1074 when Hsp104 is overexpressed from the GAL1 promoter. (A) The percentage of [PSI+] cells and the percentage of cells with foci are plotted as a function of generation. Hsp104 overexpression (o/e) was induced by incubating cells in SGal medium. For comparison, [PSI+] was also cured at the same time by growing cells in SD medium in 5 mM guanidine (Gdn). Cells were plated at different generations on 1/2 YPD plates to determine the extent of [PSI+] curing by the red/white color assay. The data show the averages and the standard deviations from 3 independent experiments. (B) Confocal images of NGMC in yeast strain 1074 cells that were grown for 5 generations in SGal medium to overexpress Hsp104 or in SD medium with 5 mM guanidine. Images show cells before stress and after stressing them in water for 1 h. The same confocal settings were used for imaging of the cells. Images are projections of z-stacks. (C) Comparison of the percentage of [PSI+] cells and the percentage of cells with foci during curing of [PSI+] by overexpression of Hsp104. At the indicated times, cells were plated, imaged directly, or imaged after stressing in water or guanidine for 1 h. The percentages of [PSI+] cells were determined by a plating assay. z-stack confocal imaging was used to determine the number of cells with foci at different generations.
Next, we wanted to see if there is any difference between the distributions of foci when [PSI+] was cured by overexpression and inactivation of Hsp104. However, we first confirmed that cells with foci are a measure of [PSI+] cells during curing by Hsp104 overexpression, as was observed when [PSI+] was cured by Hsp104 inactivation (20). NGMC fluorescence was imaged by using confocal microscopy in cells overexpressing Hsp104 from the GAL1 promoter at the same time that cells were plated to determine the prion phenotype. NGMC has been shown to form foci in [PSI+] yeast cells but is diffuse in [psi−] yeast cells (20, 32). Figure 1B (top) shows fluorescence images of [PSI+] yeast cells grown for 5 generations in SGal medium. The yeast cells showed foci in the absence of stress, but the foci were much more apparent after stressing the cells for 1 h in water. This effect of stress is apparently due to an alteration of the balance of chaperones, since we previously observed that the overexpression of Ssa1 increased the intensity of the NGMC foci during the curing of [PSI+] in guanidine (20). By counting cells with foci during curing of [PSI+] by overexpression of Hsp104 at various times in both nonstressed and stressed cells, the percentage of cells with foci could be correlated with the extent of curing. The percentage of cells with foci when plotted as a function of generation time overlapped the curing curve (Fig. 1C).
Along with the different kinetics of curing, there were marked differences in NGMC fluorescence in yeast when [PSI+] yeast cells were cured by the overexpression of Hsp104 and by the inactivation of Hsp104 by 5 mM guanidine. Figure 1B shows images of yeast cells grown for 5 generations in SGal medium to overexpress Hsp104 or in guanidine to inactivate Hsp104 both before and after stress. In cells overexpressing Hsp104, the distribution of foci shows great heterogeneity in [PSI+] cells; there were cells with many foci and very little diffuse NGMC, cells with both diffuse NGMC and bright foci, and cells with only diffuse NGMC. In contrast, by stressing the guanidine-treated yeast cells, all of the cells had foci, and the numbers of foci per cell were similar in the population (Fig. 1B). Therefore, both the kinetics of curing and the distribution of NGMC foci in yeast cells are very different during curing of [PSI+] by overexpression and by inactivation of Hsp104. This in turn shows that the curing of [PSI+] by Hsp104 overexpression is not compatible with a recently proposed model suggesting that the overexpression of Hsp104 cures [PSI+] simply by inhibiting the severing of the prion seeds (21).
[PSI+] curing by overexpression of Hsp104 is not due to asymmetric segregation.
A possible explanation for Hsp104 overexpression curing [PSI+] faster than Hsp104 inactivation is that high concentrations of Hsp104 might increase the size of the prion seeds, which would lead to asymmetric segregation of the seeds. If there is asymmetric segregation of the seeds due to the retention of large seeds in the mother cells, the daughter cells would inherit fewer seeds and, in turn, cure faster than the mother cells. To determine whether asymmetric segregation of prion seeds during cell division is the mechanism by which the overexpression of Hsp104 cures [PSI+], we used FACS analysis to separate yeast cells based on age by sorting a partially cured [PSI+] yeast population based on the staining of bud scars. Specifically, [PSI+] yeast cells were grown for 5 generations in SGal medium to overexpress Hsp104, followed by using Alexa-647 WGA to label bud scars (34). The FACS plot shows the sorting of yeast cells based on the intensity of Alexa-647 fluorescence into cells with the lowest fluorescence intensity, representing newborn cells, and cells with the highest fluorescence intensity, representing the oldest cells (Fig. 2A). The sorting was successful, as indicated by the absence of bud scars in cells with the lowest fluorescence intensity, representing the newborn population, and numerous bright red bud scars, representing the old population (Fig. 2B). Importantly, the newborn cells had not yet divided, whereas the old population had undergone multiple divisions in SGal medium.
FIG 2.
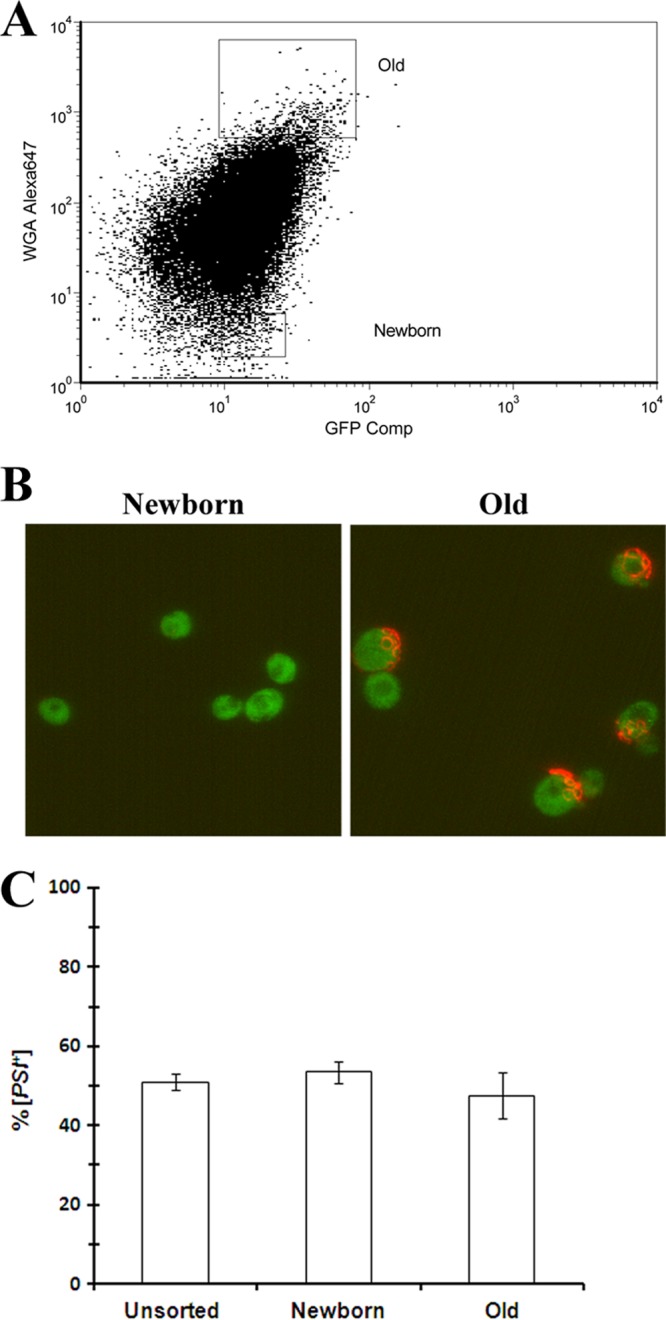
Analysis of asymmetric segregation of [PSI+] prion seeds by FACS. After labeling of the [PSI+] 1074 strain with Alexa-647 WGA, cells were grown for 5 generations in SGal medium to overexpress Hsp104 from the GAL1 promoter. After treatment of the cells with Alexa-647 WGA to stain bud scars, the yeast cells were sorted into two populations: newborn cells, cells with the lowest Alexa-647 fluorescence intensity, and old-age cells, cells with the highest Alexa-647 fluorescent intensity. (A) FACS plot of cells sorted based on the intensity of Alexa-647 WGA fluorescence. Cells were sorted into populations of the lowest and highest Alexa-647 intensities. (B) Alexa-647 WGA fluorescence of bud scars in the sorted populations. The images show NGMC (green) and Alexa-647 (red) fluorescence. Bud scars are visible only in the old population. (C) Percentages of [PSI+] cells before and after FACS analysis. The unsorted cells and the sorted cells were plated onto 1/2 YPD medium to determine the percentage of [PSI+] cells. The graph shows the averages and standard deviations from 3 independent experiments. Statistical analysis using the Student t test showed that there was no significant difference in the extent of [PSI+] curing among the different populations.
To determine the [PSI+] phenotype, we plated newborn, old, and unsorted cells. The plating assay showed no significant difference in the extent of curing between different populations of cells; unsorted, newborn, and old cells were all ∼50% cured (Fig. 2C). These results do not fit the asymmetric model of curing, which predicts that the old population would have a lower percentage of cured cells than the newborn population, since more seeds would have been retained in the mother cells. Therefore, the FACS data are not compatible with the asymmetric-segregation model for curing of [PSI+] yeast.
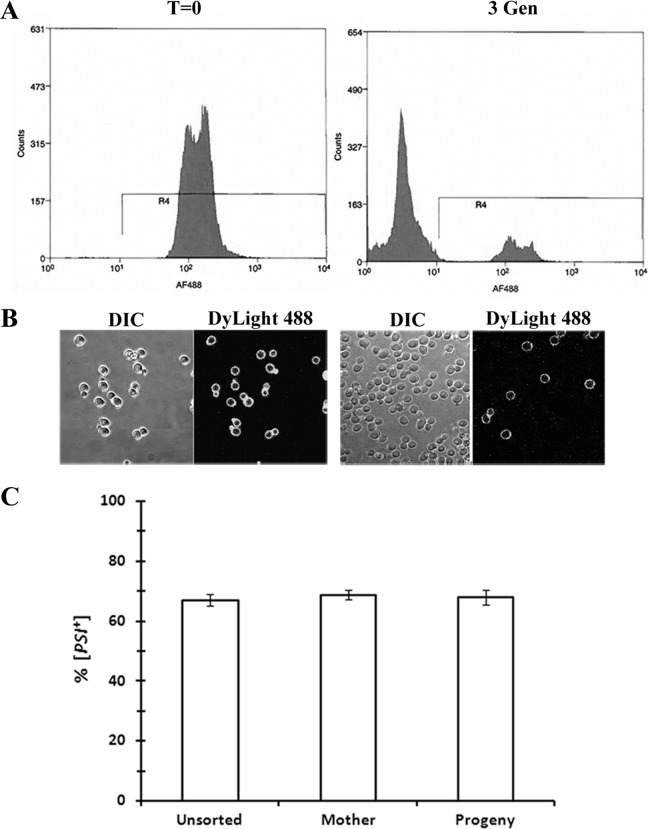
FACS analysis was also performed on parental strain 779-6A, which expresses wild-type Sup35. A different method was used to label yeast cells, which enables the cells to be sorted into mother cells and their progeny. First, [PSI+] yeast cells were incubated with NHS-biotin to label the proteins in the cell wall (29). An aliquot of the labeled population was fixed to prevent cell division prior to labeling with streptavidin-Dylight 488. Both the FACS plot (Fig. 3A, left) and fluorescence imaging (Fig. 3B, left) showed efficient labeling of this population of cells. The remainder of the biotin-labeled cells was grown in SGal medium to overexpress Hsp104 for 3 generations and then labeled with streptavidin-Dylight 488. At this time, the FACS plot (Fig. 3B, right) and confocal images (Fig. 3B, right) showed two populations of cells, the fluorescence-labeled population, the original mother cells, and the nonfluorescent population, the progeny of the labeled cells. The mother cells had undergone 3 divisions on average, whereas the progeny was composed of newborn and daughter cells that had undergone 1 or 2 divisions. Here again, the asymmetric-segregation model predicts that the fluorescent mother cells should cure more slowly than the nonfluorescent daughter cells. Contrary to this prediction, these two populations showed no significant difference in the percentage of cured cells, as determined by the plating assay (Fig. 3C). Therefore, these data show that the curing of [PSI+] by the overexpression of Hsp104 is not due to asymmetric segregation of the seeds between mother and daughter cells in parental strain 779-6A expressing wild-type Sup35, just as we found for strain 1074 expressing NGMC.
FIG 3.
Analysis of asymmetric segregation of [PSI+] prion seeds by FACS. The [PSI+] 779-6A strain was labeled with NHS-biotin and streptavidin-Dylight 488 as described in Materials and Methods. (A) FACS plot of cells sorted based on the fluorescence intensity of streptavidin-Dylight 488. (Left) Cells at time zero that were fixed with paraformaldehyde immediately after labeling of the cells with NHS-biotin. (Right) Biotinylated cells were grown for 3 generations in SGal medium. This yielded two populations of cells, a fluorescently labeled population of cells (the original labeled population) and a nonfluorescent population of cells (the progeny of the labeled population). Yeast cells were incubated with streptavidin-Dylight 488 to detect biotinylated cells. (B) Differential interference contrast (DIC) and fluorescence images of samples used for sorting. The left panel shows fixed biotinylated cells (time zero), and the right panel shows cells grown for 3 generations in SGal medium. (C) Percentage of [PSI+] cells before and after FACS analysis. Unsorted and sorted cells were plated onto 1/2 YPD medium to determine the percentage of [PSI+] cells. The sorted population labeled “Mother” indicates the initial labeled cells, and the population labeled “Progeny” indicates the cells grown during incubation for 3 generations in SGal medium after labeling. The graph shows the averages and standard deviations for 3 independent experiments. Statistical analysis using the Student t test showed that there was no significant difference in the extent of [PSI+] curing among the different populations.
Distribution of NGMC foci during curing of [PSI+] by Hsp104 overexpression.
Since FACS analysis shows that curing of [PSI+] by Hsp104 overexpression is not due to asymmetric segregation of the seeds, the distribution of seeds should be proportional to the relative volume of the mother cells and the daughter cells. We tested this prediction by imaging the foci in daughter-mother pairs during the curing of [PSI+] by Hsp104 overexpression. To identify mother-daughter pairs, [PSI+] cells were grown for ∼5 generations in SGal medium to induce Hsp104 overexpression prior to immobilizing the cells on chamber slides with concanavalin A. To prevent cells from being in close proximity to one another, a very small number of cells was added to the slide. After 3 h in SGal medium, the immobilized cells were stressed, followed by z-stack confocal imaging to determine the number of NGMC foci in each cell. The NGMC foci, which have been shown to be equivalent to the prion seeds in stressed cells (20), were then counted in mother-daughter pairs.
Examination of 200 daughter-mother pairs showed that NGMC was completely diffuse in 46% of the pairs, while there were too many seeds to accurately count for 14% of the pairs. In the remaining 80 daughter-mother pairs (40% of the population), the number of foci in mother and daughter cells was counted for each pair. Even though there was a marked heterogeneity in the number of foci in the different mother-daughter pairs, with the total number of foci in pairs ranging from 15 to 220, the ratio of the number of foci in the daughter relative to that in its mother showed very little variability. We find that the ratio of the number of foci in the daughter cell relative to the number in its mother was 0.64 ± 0.14, while the ratio of the volume of the daughter cell relative to that of the mother cell was 0.66 (14). Therefore, the seeds were distributing according to the relative volumes of the daughter and the mother cells. Therefore, as predicted by the FACS data, there is no evidence of asymmetric segregation of the seeds between the mother and the daughter cells during the curing of [PSI+] by the overexpression of Hsp104.
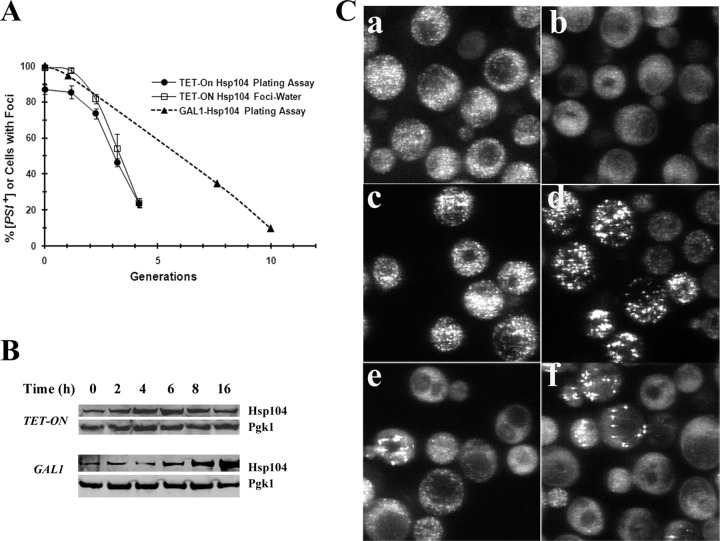
Since our data do not fit with either the asymmetric-distribution model or the inhibition-of-severing model for curing of [PSI+] by Hsp104 overexpression, Hsp104 overexpression must cure [PSI+] by the dissolution of the prion seeds. To obtain images of this dissolution process, we monitored a cell as it formed a microcolony. When a single [PSI+] cell overexpressing Hsp104 forms a microcolony, the dissolution model predicts that at least some microcolonies should contain cells with no foci. Since large microcolonies frequently have problems of multiple layers of overlapping cells (35), we wanted to obtain curing in relatively few generations. Therefore, we overexpressed Hsp104 from different promoters to determine which promoter provided the fastest curing of [PSI+]. We found that the curing of [PSI+] was much faster when Hsp104 was overexpressed by using the TET-ON promoter than when it was overexpressed by using the GAL1 promoter (Fig. 4A). More than 50% of the cells were cured in 3 generations when Hsp104 was overexpressed from the TET-ON promoter, whereas it took 5 generations to obtain comparable curing when Hsp104 was overexpressed from the GAL1 promoter. To understand the difference in the rates of curing of [PSI+] when Hsp104 was expressed from these promoters, Western blot analysis was performed to measure the levels of Hsp104 expression (Fig. 4B). Even though the final level of Hsp104 expression from the GAL1 promoter was 4- to 5-fold higher than that with the TET-ON promoter, the induction of Hsp104 with the TET-ON promoter was much faster than that with the GAL1 promoter, which is consistent with the higher rate of curing by the TET-ON promoter.
FIG 4.
Curing of [PSI+] in strain 1074 when Hsp104 is overexpressed from the TET-ON promoter. (A) The percentage of [PSI+] cells and the percentage of cells with foci were plotted as a function of generation. Hsp104 overexpression was induced by adding 10 μg/ml doxycycline to yeast cells grown in SD medium. Cells were plated at different times onto 1/2 YPD plates to determine the extent of [PSI+] curing by the red/white color assay. The percentage of cells with foci was determined from the confocal images of cells at the indicated generations after incubation in water for 1 h. The curing of [PSI+] by the overexpression of Hsp104 from the GAL1 promoter is shown for comparison (dashed line). The time points show the averages and standard deviations from 3 independent experiments. (B) Western blot of Hsp104 measured in yeast before and after induction of Hsp104 for the indicated times. Doxycycline was added to induce the overexpression of Hsp104 from the TET-ON promoter, and galactose was added to induce the overexpression of Hsp104 from the GAL1 promoter. Pgk1 was used as an internal loading control. (Ca and b) Fluorescence images of nonstressed cells prior to the induction of Hsp104 (a) and after the addition of doxycycline for 1 generation (b). (c to f) Fluorescence images of stressed cells prior to the induction of Hsp104 (c) and after the addition of doxycycline for 1 generation (d), 2 generations (e), and 3 generations (f). Cells were stressed by incubating them in water for 1 h. The same confocal settings were used for imaging of the cells. Images are projections of z-stacks obtained by using the Metamorph program.
Prior to the microcolony analysis, cells were also imaged by confocal microscopy before and after stress to determine if we could observe a decrease in the size of the foci before complete dissolution took place. As expected, the foci were clearly visible before the induction of Hsp104; the foci were 25% brighter than the background fluorescence (Fig. 4Ca). However, after one generation of Hsp104 induction, it was not possible to resolve the foci from the background fluorescence in the majority of cells (Fig. 4Cb). Nevertheless, as shown by the effect of stress, all of the cells had foci, and two-thirds of the cells had >100 foci per cell, almost the same as that prior to the induction of Hsp104, when 90% of the cells had >100 foci per cell (Fig. 4Cc and d). Therefore, after the induction of Hsp104 for one generation, many foci could not be detected in the absence of stress, indicating that the level of GFP fluorescence in these foci was too low to be detected by confocal microscopy. Since GFP fluorescence is quenched only by acidic pH (36), the reduction in GFP intensity indicates that there were fewer GFP molecules and, thus, less NGMC in the foci. In agreement with this conclusion, we previously showed that the intensity of the foci correlated with their diffusional mobility, which is a measure of their molecular weight (20).
Prior to the microcolony analysis, we determined the percentage of cells with foci in cells after stress at different generations to correlate imaging with the extent of curing. The foci in the z-stack confocal images were counted at different generation times (Fig. 4Cc to f). When the percentages of cells with foci were plotted on the same graph as the curing curve, there were slightly more cells with foci than [PSI+] cells (Fig. 4A). This suggests that a small amount of curing was occurring on the plate. This is probably due to low levels of Hsp104 overexpression prior to the addition of doxycycline because of the leakiness of the TET-ON promoter in yeast (37). These results again confirm that the foci are equivalent to the prion seeds.
Having established that the TET-ON promoter gives rapid curing, microcolony analysis was first performed on control cells, either [PSI+] or [psi−] cells, before the induction of Hsp104 expression (Fig. 5A). As expected, the cells in the microcolony grown from noninduced [PSI+] yeast had fine foci, which increased in intensity after the yeast cells were stressed. In contrast, the cells in the microcolony grown from [psi−] yeast had diffuse NGMC fluorescence in both the presence and absence of stress. Next, we imaged 50 microcolonies formed from a mother-daughter pair of [PSI+] cells grown in doxycycline for 5 h (∼2 to 3 generations) to induce Hsp104 overexpression. Figure 5B shows representative images of 3 microcolonies, before stress and after the cells were stressed in guanidine. The cells in colony 1 showed diffuse NGMC fluorescence prior to stress, but after stress, cells showed numerous foci, indicating that curing had not yet occurred. The cells in colony 2 also showed diffuse NGMC fluorescence prior to stress, but only a few foci were visible after stress, indicating that these cells were either all cured or almost all cured. Interestingly, the cells in colony 3 appeared heterogeneous; there were both cured and noncured cells, indicating that curing is a random process with no bias in curing between mother and daughter cells, consistent with the FACS data. Of 50 microcolonies, 36% resembled colony 1 in that all the cells had many foci, 40% resembled colony 2 in that the cells had at most a few foci, and 24% resembled colony 3 in that the cells showed heterogeneity in the number of foci. Therefore, consistent with the dissolution model, overall, the microcolonies showed a reduction in the number of foci.
FIG 5.
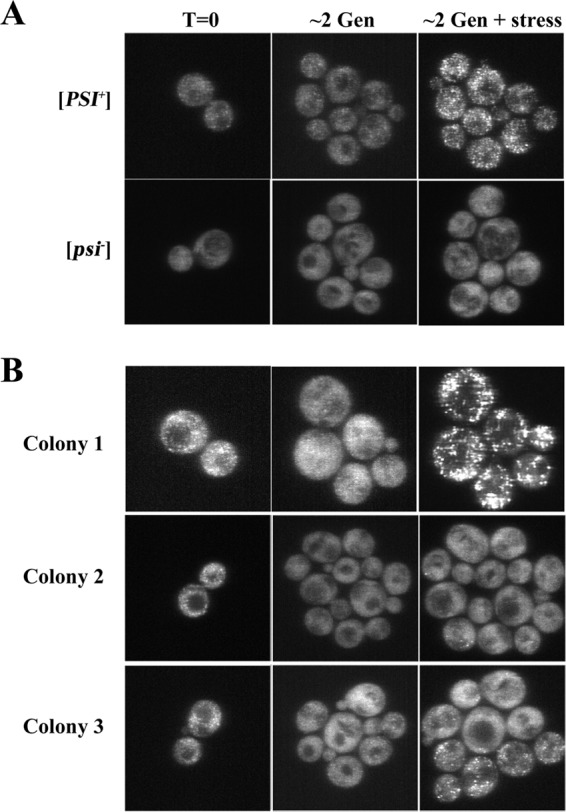
Confocal images of NGMC in microcolonies of strain 1074 yeast cells carrying pRS314-rtHSP104. Yeast cells were added to a chamber slide and then covered with an SD medium agarose pad without or with 10 μg/ml doxycycline. Images were taken at 0 h and 5 h (2 to 3 generations) and after an additional hour with 5 mM guanidine to stress the cells. (A) A microcolony was grown from either [PSI+] yeast (top row) or [psi−] yeast (bottom row) in the absence of doxycycline. (B) Microcolonies (colonies 1 to 3) were grown from [PSI+] yeast in the presence of 10 μg/ml doxycycline to induce the overexpression of Hsp104 from the TET-ON promoter. The same confocal settings were used for imaging of the cells. Images are maximum-intensity projections of z-stacks.
To confirm this conclusion, we actually determined the average number of foci per cell before and after Hsp104 overexpression. The foci had to be counted in stressed cells because in the absence of stress, most foci could not be detected due to the high background fluorescence, whereas after stress, the fluorescence intensity of the foci increased to 50 to 75% above the background level. Before the induction of Hsp104, >90% of the cells (n = 60) had 100 to 150 foci per cell. After Hsp104 overexpression, there was a marked decrease in the average number of foci per cell in all 50 microcolonies. There were 15 microcolonies with an average of <10 foci per cell, 20 microcolonies with an average of between 10 and 20 foci per cell, and 15 microcolonies with an average of between 21 and 50 foci per cell. Therefore, during curing of [PSI+] by Hsp104 overexpression, there was a loss of foci, as predicted by the dissolution model.
Trimming activity of Hsp104, but not severing activity, is necessary but not sufficient for curing of [PSI+] by Hsp104 overexpression.
Since our imaging showed a decrease in the intensity of the NGMC foci during curing, there appears to be a decrease in the number of NGMC molecules per seed. This in turn might be caused by an increased severing of the prion seeds by high concentrations of Hsp104. To determine whether this is the case, guanidine was used to inhibit severing. First, we established that guanidine effectively inhibits severing even when Hsp104 is overexpressed by measuring the kinetics of [PSI+] curing by guanidine in the absence and presence of Hsp104 overexpression. This was done by using a Δsti1 strain derived from 779-6A because the overexpression of Hsp104 cures [PSI+] very poorly in this strain (15, 16), so almost all of the curing that we observed would be caused by the added guanidine. As shown in Fig. 6A, the kinetics of [PSI+] curing by guanidine are very similar in the presence and absence of overexpressed Hsp104. In both plots, there was a long lag period when the seeds were diluted out by cell division prior to curing. The curing in yeast overexpressing Hsp104 was slightly faster than that in yeast with endogenous Hsp104, which is probably due to the small amount of curing caused by the overexpression of Hsp104 in the Δsti1 strain (Fig. 6A, dashed line). However, this is a small effect, and importantly, the data show that guanidine is able to effectively inhibit the severing of the prion seeds in cells overexpressing Hsp104.
FIG 6.

Curing of [PSI+] by Hsp104 overexpression when severing is inhibited by guanidine. (A) Curing of [PSI+] by guanidine inactivation in the presence and absence of high concentrations of Hsp104. By using a Δsti1 strain derived from 779-6A, [PSI+] yeast was transformed with the vector control or a plasmid expressing Hsp104 from the GAL1 promoter. [PSI+] cells were cured in SGal medium with and without guanidine. Curing data are from yeast cells grown in 5 mM guanidine with endogenous levels of Hsp104 or in yeast cells overexpressing (o/e) Hsp104. The curing of [PSI+] in yeast cells overexpressing Hsp104 in the absence of guanidine is shown by the dashed line. (B) Curing of [PSI+] by Hsp104 overexpression from the GAL1 promoter was performed in the presence and absence of 5 mM guanidine in strain 1074. The solid symbols are data obtained in the presence of guanidine, and the open symbols are data obtained in the absence of guanidine. (C) Curing of [PSI+] by Hsp104 overexpression from the GAL1 promoter was performed in the presence and absence of guanidine in strain 779-6A. The solid symbols are data obtained in the presence of guanidine, and the open symbols are data obtained in the absence of guanidine. Data points were obtained from three independent experiments under each experimental condition.
Next, the curing of [PSI+] by the overexpression of Hsp104 was measured in the presence and absence of guanidine to determine the contribution of severing to this process in both strain 1074 and parental strain 779-6A. If severing activity is playing a major role in the curing of [PSI+] by Hsp104 overexpression, the rate of curing should slow down in the presence of guanidine; if guanidine completely inhibited severing, there should be a long lag before curing occurs as the seeds are diluted out by cell division. As shown in Fig. 6B and C, guanidine slightly delayed (1 to 2 generations) the rate of curing of [PSI+] in strain 1074 and did not significantly affect the rate of [PSI+] curing by Hsp104 overexpression in strain 779-6A. Importantly, since the kinetics of curing of [PSI+] by Hsp104 overexpression follows the same time course as that obtained in the absence of guanidine, curing in the presence of guanidine is not due to an inhibition of severing. Therefore, the severing activity of overexpressed Hsp104 makes at most a minor contribution to the curing of [PSI+] by Hsp104 overexpression.
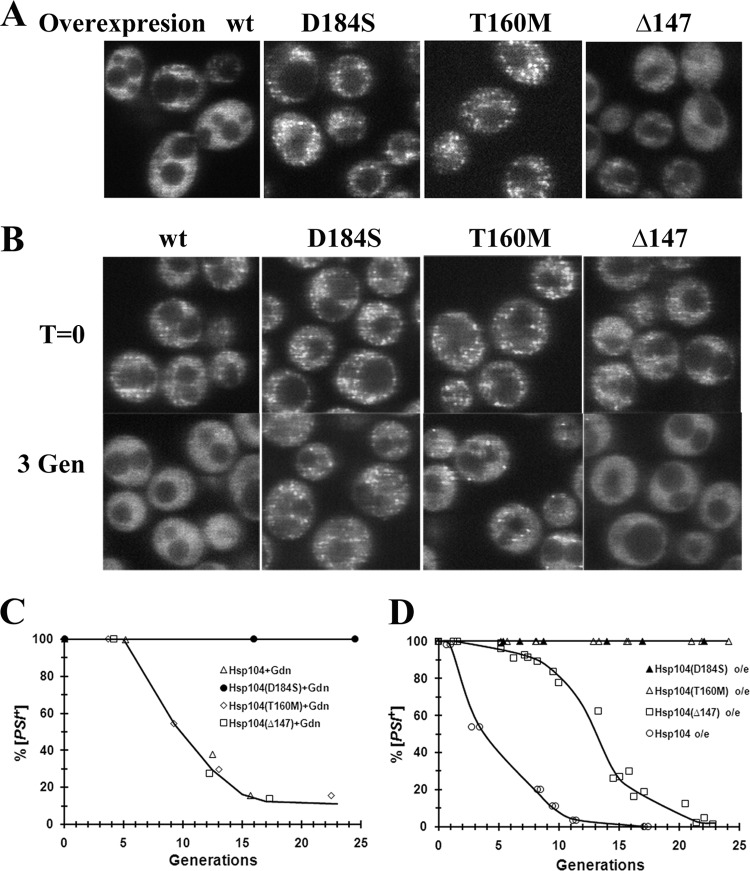
Having ruled out that the reduction in the number of NGMC molecules in the foci is due to the severing action of Hsp104, we examined whether this reduction is due to the trimming activity of Hsp104. Trimming activity reduces the number of molecules in the prion seeds, perhaps by removing molecules from the end of the prion amyloid, but, in contrast to the severing activity, does this without increasing the number of seeds (20). To determine whether trimming is important for the curing of [PSI+] by Hsp104 overexpression, we screened for Hsp104 mutants that propagate [PSI+] but do not trim the seeds. Different Hsp104 mutants were overexpressed overnight in strain 1074, followed by confocal imaging. We found two Hsp104 mutants, Hsp104(D184S) and Hsp104(T160M), that, when overexpressed, caused a marked increase in the fluorescence intensity of the NGMC foci. We validated that these mutants do not trim by integrating these mutants into the genomic HSP104 locus of strain 1074. The expression of endogenous levels of either Hsp104(D184S) or Hsp104(T160M) caused an increase in the intensity of the NGMC foci (Fig. 7B), and stress caused no further increase in intensity. The mutants differed in that the [PSI+] strain expressing Hsp104(T160M) was cured by 5 mM guanidine, whereas, as observed previously (25), yeast cells expressing endogenous levels of Hsp104(D184S) are resistant to curing in guanidine (Fig. 7B and C). However, this difference had no effect on our results; we found that the overexpression of either Hsp104(D184S) or Hsp104(T160M) did not cure [PSI+] (Fig. 7D), which confirms that the curing of [PSI+] by Hsp104 overexpression is dependent on the trimming activity of Hsp104.
FIG 7.
Different Hsp104 mutants affect the trimming of NGMC foci and the curing of [PSI+] yeast. (A) Confocal images of NGMC in [PSI+] yeast cells overexpressing different Hsp104 mutants in strain 1074. The following Hsp104 mutants were overexpressed from the GAL1 promoter: Hsp104(D184S), Hsp104(T160M), and Hsp104(Δ147). Yeast cells were grown overnight in SGal medium prior to imaging. Cells were imaged by using identical settings. wt, wild type. (B) Confocal images of [PSI+] yeast strains expressing Hsp104, Hsp104(D184S), Hsp104(T160M), and Hsp104(Δ147) in yeast cells derived from parental strain 1074. The Hsp104 mutants were integrated into the HSP104 genomic locus. Images shown are cells in SD medium (time zero) or after growth for 3 generations in 5 mM guanidine (3 Gen). (C) Curing of [PSI+] by 5 mM guanidine in yeast cells expressing endogenous amounts of the indicated Hsp104 mutants. (D) Curing of [PSI+] by overexpressing the indicated Hsp104 mutants from the GAL1 promoter.
Even though trimming appears necessary for the curing of [PSI+] by Hsp104 overexpression, trimming alone is apparently not sufficient for curing. In our screen for mutants that did not trim, we observed that overnight overexpression of the N-terminal deletion mutant of Hsp104, Hsp104(Δ147), caused a marked reduction in the number of detectable foci, suggesting that it had trimming activity. Surprisingly, however, this mutant was very poor at curing when it was overexpressed. Overexpression of this mutant cured [PSI+] in strain 1074 only after >10 generations (Fig. 7D), and [PSI+] was not cured at all when this mutant was overexpressed in yeast strain 779-6A (28). We confirmed that Hsp104(Δ147) has trimming activity by showing that there was a loss of detectable foci when [PSI+] was cured by guanidine in yeast cells expressing endogenous levels of this mutant (Fig. 7B). These results suggest that the trimming activity of Hsp104 is not sufficient to cure [PSI+] by Hsp104 overexpression.
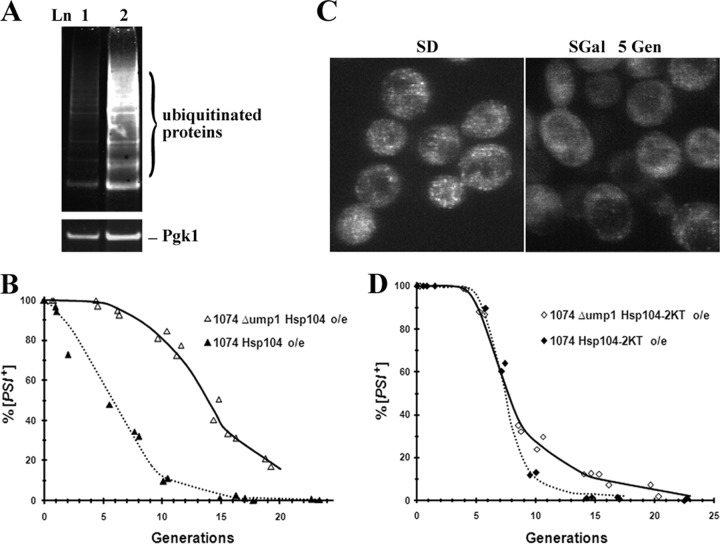
Since the rate of curing of [PSI+] by Hsp104 overexpression is increased by ubiquitination (16) and decreased by deletion of the ubiquitin-conjugating enzyme Ubc4 (38), we examined whether a defect in the proteasome machinery delays the curing of [PSI+] by Hsp104 overexpression using a UMP1 deletion strain. The deletion of UMP1 prevents the proper maturation of the 20S proteasome, which impairs the degradation of ubiquitinated cargo (24). This was confirmed by Western blot analyses showing much more ubiquitinated cargo in lysates prepared from the UMP1 deletion strain than in lysates from the parental strain (Fig. 8A). Interestingly, the rate of curing of [PSI+] by the overexpression of Hsp104 was markedly reduced in the UMP1 deletion strain compared to the parental strain (Fig. 8B), but this was not because trimming was absent in this strain. Imaging of NGMC in the UMP1 deletion strain showed that before the induction of Hsp104, NGMC foci were apparent in [PSI+] yeast cells, but after the induction of Hsp104 for 5 generations, NGMC appeared diffuse in many cells, even though the yeast cells were still [PSI+] (Fig. 8C). Therefore, prior to curing of [PSI+], there is trimming of the NGMC foci by Hsp104 in the UMP1 deletion strain, but this is not sufficient to cause curing. Proteasome activity is also necessary.
FIG 8.
Effect of inhibiting proteasome activity on curing of [PSI+] by overexpression of Hsp104 or the dominant negative Hsp104 mutant (Hsp104-2KT). (A) Western blot of ubiquitinated cargo in cells grown overnight in 10 μM copper to express Myc-tagged ubiquitin in yeast strain 1074 (lane 1) and the Δump1 strain derived from yeast strain 1074 (lane 2). The Western blot was probed with an anti-Myc antibody. Pgk1 was used as an internal loading control. (B) [PSI+] was cured by overexpression of Hsp104 from the GAL1 promoter in strain 1074 and the Δump1 strain. The percentages of [PSI+] cells are plotted as a function of generations. Data are from 2 independent experiments. (C) Confocal images of NGMC in [PSI+] yeast cells of the Δump1 strain grown in either SD medium or SGal medium for 5 generations to overexpress Hsp104. (D) [PSI+] was cured by overexpression of the dominant negative Hsp104 mutant Hsp104-2KT from the GAL1 promoter in strain 1074 and the Δump1 strain. The percentages of [PSI+] cells are plotted as a function of generation time. Data are from 2 independent experiments.
We also examined whether the proteasome machinery affects the curing of [PSI+] by the inactivation of Hsp104. As shown in Fig. 8D, the rate of curing of [PSI+] by the overexpression of the dominant negative Hsp104 mutant (Hsp104-2KT) in the UMP1 deletion strain is similar to that of the parent stain. Therefore, the proteasome has a role in the curing of [PSI+] by Hsp104 overexpression but not in the curing of [PSI+] by Hsp104 inactivation.
DISCUSSION
It has long been recognized that the overexpression of Hsp104 cures the [PSI+] prion but fails to cure other yeast prions. Several models have been proposed to explain how the overexpression of Hsp104 cures [PSI+] (15). The asymmetric-segregation model proposes that each time a yeast cell divides, there is a greater retention of seeds in the mother cell than in the daughter, thus leading to the curing of the daughter cells (39). In contrast, the dissolution model proposes that depolymerization or digestion of the seeds causes the loss of the seeds, while cell division plays only a passive role by diluting out any remaining seeds (3, 4, 8). Recently, another model, which proposed that the overexpression of Hsp104 cures [PSI+] by inhibiting the severing of the seeds, was described (21). This model is based on the observation that high concentrations of Hsp104 bind directly via their N termini to the prion fibers instead of Hsp70/Hsp40 recruiting Hsp104 to the prion fibers, which is necessary for severing (40).
We tested the asymmetric-segregation model by labeling either cell wall proteins or bud scars and then using FACS to sort the yeast cells into populations of mother and daughter cells. Regardless of the labeling method, both strain 779-6A expressing wild-type Sup35 and strain 1074 expressing NGMC showed the same extent of curing in mother and daughter cell populations. In addition, we determined that the ratio of the numbers of foci between daughter and mother cells was 2:3, the ratio predicted from the relative volumes of the daughter and mother cells (14). These results strongly suggest that the curing of [PSI+] by Hsp104 overexpression is not due to asymmetric segregation of the prion seeds between mother and daughter.
The data also do not fit with the recently proposed model that [PSI+] curing by Hsp104 overexpression is due to an inhibition of severing of the prion seeds (21). First, curing of [PSI+] by Hsp104 overexpression does not show the long lag observed when [PSI+] is cured by inactivating Hsp104. During the lag phase, the seeds are being diluted out by cell division. In addition, the GFP-labeled Sup35 foci show a very heterogeneous distribution during the curing of [PSI+] by Hsp104 overexpression, whereas the distribution of foci appears much more homogeneous when curing is done by the inactivation of Hsp104. In addition, our data are not compatible with the inhibition of severing leading to large dead-end aggregates that are retained in the mother cell, since this would produce asymmetric segregation of the prion seeds.
Ruling out the asymmetric segregation model and the inhibition-of-severing model, this leaves the dissolution model as the mechanism for the curing of [PSI+] by Hsp104 overexpression. To reduce the size of the seeds, it is possible that the overexpression of Hsp104 caused excessive severing. However, this was ruled out by showing that the curing of [PSI+] was not significantly affected by the addition of 5 mM guanidine, which inhibits the severing activity of Hsp104. Furthermore, increased severing would produce an increased number of seeds, which is counterproductive to the curing of [PSI+]. Instead, our data support that the dissolution of foci is caused by the trimming of the prion seeds. First, we observed that there was a decrease in the GFP intensity of the foci, which indicates a reduction in the number of NGMC molecules in the foci. Second, by using different Hsp104 mutants, we observed that mutants that do not trim the NGMC foci also do not cure [PSI+] when overexpressed.
Our results show that the trimming activity of Hsp104 is important for curing of [PSI+] by Hsp104 overexpression. Mutants of Hsp104 that did not trim the NGMC foci did not cure [PSI+] when overexpressed. On the other hand, our data are consistent with the overexpression of Hsp104 causing a partial block in severing, since we previously observed that the trimming activity of Hsp104 was prominent only when the severing of the prion seeds was inhibited by guanidine (20). In addition, since guanidine had little effect on the curing of [PSI+] by Hsp104 overexpression, it is possible that the severing activity was already inhibited by the overexpression of Hsp104. If inhibition of severing varies among cells overexpressing Hsp104, this would contribute to the heterogeneity in the number of foci per cell and the time course of [PSI+] curing.
The GFP-labeled Sup35 foci in partially cured yeast showed cell-to-cell variation in the loss of foci, which is consistent with the kinetics of [PSI+] curing by the overexpression of Hsp104. At this time, it is not known whether foci are lost with the same time constant in a given cell. Since the seeds have been shown to be highly dynamic due to Hsp104-dependent remodeling activity (41), individual foci may vary in a given cell, which may lead to different rates of dissolution. In addition, since Hsp104 was being overexpressed from centromeric plasmids in this study, the number of plasmids per cell may range from 1 to 3 (42), which may also contribute to differences in the rate of dissolution.
Our results fit a 2-step dissolution model, as shown in Fig. 9. First, the trimming activity of Hsp104 dissociates molecules from the ends of the seeds to produce the prion core. It is not clear whether trimming removes just monomers or also oligomers of Sup35 that then dissociate into monomers. The second step in the curing of [PSI+] by Hsp104 overexpression is dependent on the proteasome machinery. One possibility is that high concentrations of Hsp104 in combination with other chaperones present the amyloid core to the proteasome for digestion. Another possibility is that digestion of the amyloid core requires a balance of chaperones, which requires the proteasome to digest one or more chaperones to achieve this balance. Finally, there is the possibility that once the proteasome balances the chaperones, high Hsp104 concentrations then depolymerize the core. Although both trimming and proteolysis appear necessary for the curing of [PSI+] by Hsp104 overexpression, the relative contribution of these two factors to the curing process is not yet clear. Finally, we are left with the puzzle of why overexpression of the N-terminal deletion mutant, which trims the prion seeds, does not cure [PSI+]. An understanding of why this mutant does not cure is necessary to fully understand the mechanism of curing of [PSI+] by Hsp104 overexpression.
FIG 9.

Model of curing of [PSI+] by Hsp104 overexpression. Curing of [PSI+] is shown in this model as a 2-step process. First, Hsp104 reduces the number of molecules in the prion fiber by trimming Sup35 monomers from the ends of the fiber. The second step involves dissolution of the prion core, which is dependent on high Hsp104 concentrations, multiple chaperones, and the proteasome. The function of the proteasome may be to digest the core and/or to control the balance of chaperones.
Our model is supported by the in vitro data showing that high concentrations of Hsp104 disassembled amyloid fibers composed of the NM domain of Sup35 into small fragments that were unable to nucleate new seeds (43). It also fits with a previous fractionation study in which a large soluble pool of Sup35 was present in lysates prepared from [PSI+] yeast cells overexpressing Hsp104 (4). This model is also consistent with the observation that the overexpression of Ssa1 inhibits curing by the overexpression of Hsp104 (17), in that increased concentrations of Ssa1 inhibited the trimming of the prion seeds by Hsp104 (20). However, it does not account for the observation that the SDS-resistant polymeric subunits, which make up the seeds, became larger in [PSI+] cells during curing by overexpression of Hsp104 (44). As was pointed out in that study, the size of the SDS-resistant aggregates may increase, while at the same time, there is an overall decrease in the size of the seeds (44).
Interestingly, there are many factors that are required for [PSI+] curing by Hsp104 overexpression that are not required for [PSI+] propagation. In addition to the N-terminal deletion mutant of Hsp104, which was shown to propagate [PSI+] but not cure when overexpressed (28), truncation of the carboxy-terminal dimerization domain from Sis1 inhibits [PSI+] curing by Hsp104 overexpression (45). The chaperones Sti1, Cpr7, and Hsp90 are also needed for the curing of [PSI+] by Hsp104 overexpression but not for [PSI+] propagation (15, 16). The chaperones, which are needed only for the curing of [PSI+] by Hsp104 overexpression, but not for propagation, may be involved in presenting the amyloid core to the proteasome machinery. Since [PSI+] curing by Hsp104 overexpression is sensitive to a large cohort of chaperones, slight differences in their concentrations may lead to [PSI+] cells curing at different rates after the induction of Hsp104 overexpression. Our observation that, given the right balance of chaperones, the trimming activity of Hsp104 in combination with proteasome activity rids the cell of amyloid suggests that trimming is an important physiological function of Hsp104 in vivo.
ACKNOWLEDGMENTS
We thank Christine Moomau for technical assistance and the National Heart, Lung, and Blood Institute Flow Cytometry Core Facility.
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), the National Heart, Lung, and Blood Institute, and the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published ahead of print 14 March 2014
REFERENCES
- 1.Wickner RB. 1994. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 264:566–569. 10.1126/science.7909170 [DOI] [PubMed] [Google Scholar]
- 2.Patino MM, Liu JJ, Glover JR, Lindquist S. 1996. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273:622–626. 10.1126/science.273.5275.622 [DOI] [PubMed] [Google Scholar]
- 3.Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. 1995. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [PSI+]. Science 268:880–884 [DOI] [PubMed] [Google Scholar]
- 4.Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. 1996. Propagation of the yeast prion-like [PSI+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 15:3127–3134 [PMC free article] [PubMed] [Google Scholar]
- 5.Serio TR, Lindquist SL. 1999. [PSI+]: an epigenetic modulator of translation termination efficiency. Annu. Rev. Cell Dev. Biol. 15:661–703. 10.1146/annurev.cellbio.15.1.661 [DOI] [PubMed] [Google Scholar]
- 6.Romanova NV, Chernoff YO. 2009. Hsp104 and prion propagation. Protein Pept. Lett. 16:598–605. 10.2174/092986609788490078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tessarz P, Mogk A, Bukau B. 2008. Substrate threading through the central pore of the Hsp104 chaperone as a common mechanism for protein disaggregation and prion propagation. Mol. Microbiol. 68:87–97. 10.1111/j.1365-2958.2008.06135.x [DOI] [PubMed] [Google Scholar]
- 8.Wegrzyn RD, Bapat K, Newnam GP, Zink AD, Chernoff YO. 2001. Mechanism of prion loss after Hsp104 inactivation in yeast. Mol. Cell. Biol. 21:4656–4669. 10.1128/MCB.21.14.4656-4669.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moriyama H, Edskes HK, Wickner RB. 2000. [URE3] prion propagation in Saccharomyces cerevisiae: requirement for chaperone Hsp104 and curing by overexpressed chaperone Ydj1p. Mol. Cell. Biol. 20:8916–8922. 10.1128/MCB.20.23.8916-8922.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung G, Masison DC. 2001. Guanidine hydrochloride inhibits Hsp104 activity in vivo: a possible explanation for its effect in curing yeast prions. Curr. Microbiol. 43:7–10. 10.1007/s002840010251 [DOI] [PubMed] [Google Scholar]
- 11.Ferreira PC, Ness F, Edwards SR, Cox BS, Tuite MF. 2001. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol. Microbiol. 40:1357–1369. 10.1046/j.1365-2958.2001.02478.x [DOI] [PubMed] [Google Scholar]
- 12.Eaglestone SS, Ruddock LW, Cox BS, Tuite MF. 2000. Guanidine hydrochloride blocks a critical step in the propagation of the prion-like determinant [PSI+] of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 97:240–244. 10.1073/pnas.97.1.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ness F, Ferreira P, Cox BS, Tuite MF. 2002. Guanidine hydrochloride inhibits the generation of prion “seeds” but not prion protein aggregation in yeast. Mol. Cell. Biol. 22:5593–5605. 10.1128/MCB.22.15.5593-5605.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne LJ, Cole DJ, Cox BS, Ridout MS, Morgan BJT, Tuite MF. 2009. The number and transmission of [PSI+] prion seeds (propagons) in the yeast Saccharomyces cerevisiae. PLoS One 4:e4670. 10.1371/journal.pone.0004670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moosavi B, Wongwigkarn J, Tuite MF. 2010. Hsp70/Hsp90 co-chaperones are required for efficient Hsp104-mediated elimination of the yeast [PSI+] prion but not for prion propagation. Yeast 27:167–179. 10.1002/yea.1742 [DOI] [PubMed] [Google Scholar]
- 16.Reidy M, Masison DC. 2010. Sti1 regulation of Hsp70 and Hsp90 is critical for curing of Saccharomyces cerevisiae [PSI+] prions by Hsp104. Mol. Cell. Biol. 30:3542–3552. 10.1128/MCB.01292-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newnam GP, Wegrzyn RD, Lindquist SL, Chernoff YO. 1999. Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol. Cell. Biol. 19:1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiktev DA, Patterson JC, Muller S, Bariar B, Pan T, Chernoff YO. 2012. Regulation of chaperone effects on a yeast prion by cochaperone sgt2. Mol. Cell. Biol. 32:4960–4970. 10.1128/MCB.00875-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernoff YO, Newnam GP, Kumar J, Allen K, Zink AD. 1999. Evidence for a protein mutator in yeast: role of the Hsp70-related chaperone ssb in formation, stability, and toxicity of the [PSI] prion. Mol. Cell. Biol. 19:8103–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park YN, Morales D, Rubinson EH, Masison D, Eisenberg E, Greene LE. 2012. Differences in the curing of [PSI+] prion by various methods of Hsp104 inactivation. PLoS One 7:e37692. 10.1371/journal.pone.0037692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkler J, Tyedmers J, Bukau B, Mogk A. 2012. Hsp70 targets Hsp100 chaperones to substrates for protein disaggregation and prion fragmentation. J. Cell Biol. 198:387–404. 10.1083/jcb.201201074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newnam GP, Birchmore JL, Chernoff YO. 2011. Destabilization and recovery of a yeast prion after mild heat shock. J. Mol. Biol. 408:432–448. 10.1016/j.jmb.2011.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helsen CW, Glover JR. 2012. Insight into molecular basis of curing of [PSI+] prion by overexpression of 104-kDa heat shock protein (Hsp104). J. Biol. Chem. 287:542–556. 10.1074/jbc.M111.302869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos PC, Hockendorff J, Johnson ES, Varshavsky A, Dohmen RJ. 1998. Ump1p is required for proper maturation of the 20S proteasome and becomes its substrate upon completion of the assembly. Cell 92:489–499. 10.1016/S0092-8674(00)80942-3 [DOI] [PubMed] [Google Scholar]
- 25.Jung G, Jones G, Masison DC. 2002. Amino acid residue 184 of yeast Hsp104 chaperone is critical for prion-curing by guanidine, prion propagation, and thermotolerance. Proc. Natl. Acad. Sci. U. S. A. 99:9936–9941. 10.1073/pnas.152333299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gietz RD, Schiestl RH. 2007. Large-scale high-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2:38. 10.1038/nprot.2007.15 [DOI] [PubMed] [Google Scholar]
- 27.Park Y-N, Morschhauser J. 2005. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot. Cell 4:1328–1342. 10.1128/EC.4.8.1328-1342.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung G-C, Masison DC. 2006. N-terminal domain of yeast Hsp104 chaperone is dispensable for thermotolerance and prion propagation but necessary for curing prions by Hsp104 overexpression. Genetics 173:611–620. 10.1534/genetics.106.056820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park PU, McVey M, Guarente L. 2002. Separation of mother and daughter cells. Methods Enzymol. 351:468–477. 10.1016/S0076-6879(02)51865-6 [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Wu YX, Jung G, Tutar Y, Eisenberg E, Greene LE, Masison DC. 2005. Role for Hsp70 chaperone in Saccharomyces cerevisiae prion seed replication. Eukaryot. Cell 4:289–297. 10.1128/EC.4.2.289-297.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene LE, Park YN, Masison DC, Eisenberg E. 2009. Application of GFP-labeling to study prions in yeast. Protein Pept. Lett. 16:635–641. 10.2174/092986609788490221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne LJ, Cox BS, Cole DJ, Ridout MS, Morgan BJ, Tuite MF. 2007. Cell division is essential for elimination of the yeast [PSI+] prion by guanidine hydrochloride. Proc. Natl. Acad. Sci. U. S. A. 104:11688–11693. 10.1073/pnas.0701392104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Contreras R. 2004. The bud scar-based screening system for hunting human genes extending life span. Ann. N. Y. Acad. Sci. 1019:355–359. 10.1196/annals.1297.061 [DOI] [PubMed] [Google Scholar]
- 35.Yang Z, Hong JY, Derkatch IL, Liebman SW. 2013. Heterologous gln/asn-rich proteins impede the propagation of yeast prions by altering chaperone availability. PLoS Genet. 9:e1003236. 10.1371/journal.pgen.1003236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsien RY. 1998. The green fluorescent protein. Annu. Rev. Biochem. 67:509–544. 10.1146/annurev.biochem.67.1.509 [DOI] [PubMed] [Google Scholar]
- 37.Belli G, Gari E, Piedrafita L, Aldea M, Herrero E. 1998. An activator/repressor dual system allows tight tetracycline-regulated gene expression in budding yeast. Nucleic Acids Res. 26:942–947. 10.1093/nar/26.4.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen KD, Chernova TA, Tennant EP, Wilkinson KD, Chernoff YO. 2007. Effects of ubiquitin system alterations on the formation and loss of a yeast prion. J. Biol. Chem. 282:3004–3013. 10.1074/jbc.M609597200 [DOI] [PubMed] [Google Scholar]
- 39.Helsen CW, Glover JR. 2012. A new perspective on Hsp104-mediated propagation and curing of the yeast prion [PSI (+)]. Prion 6:234–239. 10.4161/pri.19913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tipton KA, Verges KJ, Weissman JS. 2008. In vivo monitoring of the prion replication cycle reveals a critical role for Sis1 in delivering substrates to Hsp104. Mol. Cell 32:584–591. 10.1016/j.molcel.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satpute-Krishnan P, Langseth SX, Serio TR. 2007. Hsp104-dependent remodeling of prion complexes mediates protein-only inheritance. PLoS Biol. 5:e24. 10.1371/journal.pbio.0050024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan BE, Mount RC, Hadfield C. 1996. Determination of plasmid copy number in yeast. Methods Mol. Biol. 53:193–203 [DOI] [PubMed] [Google Scholar]
- 43.Shorter J, Lindquist S. 2006. Destruction or potentiation of different prions catalyzed by similar Hsp104 remodeling activities. Mol. Cell 23:425–438. 10.1016/j.molcel.2006.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kryndushkin DS, Alexandrov IM, Ter-Avanesyan MD, Kushnirov VV. 2003. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J. Biol. Chem. 278:49636–49643. 10.1074/jbc.M307996200 [DOI] [PubMed] [Google Scholar]
- 45.Kirkland PA, Reidy M, Masison DC. 2011. Functions of yeast Hsp40 chaperone Sis1p dispensable for prion propagation but important for prion curing and protection from prion toxicity. Genetics 188:565–577. 10.1534/genetics.111.129460 [DOI] [PMC free article] [PubMed] [Google Scholar]