Abstract
Mitochondria process local and global Ca2+ signals. Thereby the spatiotemporal patterns of mitochondrial Ca2+ signals determine whether the metabolism of these organelles is adjusted or cell death is executed. Mitochondrial Ca2+ channels of the inner mitochondrial membrane (IMM) actually implement mitochondrial uptake from cytosolic Ca2+ rises. Despite great efforts in the past, the identity of mitochondrial Ca2+ channels is still elusive. Numerous studies aimed to characterize mitochondrial Ca2+ uniport channels and provided a detailed profile of these great unknowns with important functions. This mini-review revisits previous research on the mechanisms of mitochondrial Ca2+ uptake and aligns them with most recent findings.
Keywords: Calcium signaling, ER Ca2+ release, grp75, IP3 receptor, Letm1, mCa1, mCa2, MiCa, MCU, Mitochondrial Ca2+ uniporter, Mitofusin, p38MAPK, Uncoupling protein, UCP2/3
1. Introduction
Mitochondria have been recently recognized as multifunctional organelles that elementary impact on many different signaling pathways, thus, putting a new complexion on the long-known cellular power houses [1–4]. One distinguished feature of mitochondria that became evident by the utilization of newly developed mitochondria-targeted protein-based Ca2+ sensors [5,6] is the organelle’s active involvement during physiological Ca2+ signaling [7–10].
Herein, mitochondria were discovered to be far more than a passive Ca2+ sink that stores Ca2+ ions as Ca2+-(poly)x-phosphate[11,12]. Indeed, mitochondrial Ca2+ handling turned out to represent a highly sophisticated mechanism that has multiple consequences for cells (Fig. 1A):
First, mitochondria themselves constitute a Ca2+ target as these organelles house several Ca2+-sensitive proteins among which key metabolic enzymes, such as the dehydrogenases of the Krebs-cycle, translate Ca2+ elevation in the mitochondrial matrix to increased respiration and ATP production [13–17].
Second, mitochondria crucially contribute to Ca2+ signaling by their ability to take up and release large amount of Ca2+ ions. By their potential to sequester cytosolic Ca2+, mitochondria are able to buffer Ca2+ in distinct region of the cell and keep spatial Ca2+ concentration low even under conditions of strong global Ca2+ mobilization upon cell stimulation, which significantly impacts on Ca2+-sensitive signal transduction within a cell [18–20]. Moreover, mitochondria are able to funnel Ca2+ to endoplasmic reticulum (ER) Ca2+ uptake sites [21,22] and accomplish ER Ca2+ replenishment during and after cell stimulation [22], an important process that ensures proper activity of the Ca2+-dependent ER chaperons of the protein folding machinery [23–26].
Third, an excessive mitochondrial Ca2+ load sweeps cells to death by triggering either apoptosis or necrotic cell death [27–29], thus, signifying that mitochondrial Ca2+ uptake in any case of cellular Ca2+ mobilization essentially needs to be precisely regulated [27–29].
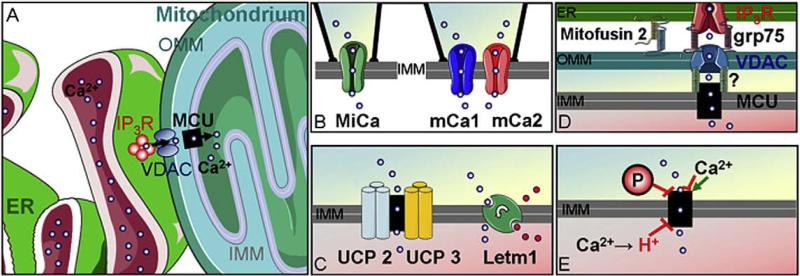
Fig. 1. Schematic illustration of mitochondrial Ca2+ uptake channels/carrier in the IMM, potential protagonists and the complexity if the mitochondrial environment.
(A) The complex environmental aspects of mitochondria in intact cells are illustrated. Local Ca2+ transfer from the ER via IP3 mediated Ca2+ release. VDAC as porines in the OMM deliver Ca2+ ions to the MCU or Ca2+ exchanger at the IMM. (B) Electrophysiological characterizations revealed distinct mitochondrial Ca2+ channels: the MiCa in COS-7 cells and mCa1 as well as mCa2 in human ventricular myocytes. (C) Overexpression and siRNA mediated knock-down of UCP2/3 suggest a fundamental importance of these proteins for mitochondrial Ca2+ uniport. A genome-wide RNAi screen identified Letm1 as a mitochondrial Ca2+/H+ antiporter that significantly contributes to mitochondrial Ca2+ uptake in the physiological range of cytosolic Ca2+ elevation. (D) ER-mitochondria contact sites are stabilized by mitofusin 2 and probably other, so far unknown, proteins. The chaperon grp75 was shown to link the IP3R to the VDAC in the OMM. Although proteins/factors that might link the VDAC to mitochondrial Ca2+ channels of the IMM have not been identified so far it is tempting to speculate that Ca2+ enters mitochondria via a Ca2+ tunnel spanning the OMM and IMM. (E) Evidence accumulated that kinases as well as Ca2+ and Ca2+-calmodulin differentially modulate the activities of mitochondrial Ca2+ channels.
Substantial studies over the last decades demonstrated that Ca2+ channels and Ca2+ exchanger in the inner mitochondrial membrane (IMM) establish Ca2+ transfer across the organelle’s inner membrane, while the ion transfer across the outer mitochondrial membrane (OMM) was thought to represent a rather uncontrolled process [30–33]. However, the latter view has been changed recently as regulatory mechanisms of the main Ca2+-permeable channels in the OMM, the voltage-dependent anion selective channels (VDAC1, 2 and 3), have been demonstrated [34–36] and their functional involvement in apoptosis was described [37–41]. While with the VDAC family the proteins for the Ca2+ transfer across the OMM are most likely identified, the proteins that are responsible for Ca2+ movements across the IMM are not completely identified so far (Fig. 1A). However, these mitochondrial Ca2+-shuttling proteins have been functionally well characterized although in many studies isolated mitochondria were used (reviewed in [42]) and the translations of such results to respective processes in intact cells, where mitochondria are embedded into an highly interactive environment, need cautiousness [43]. In this review we consider these aspects and intend to summarize recent progresses in the identification of functional and structural properties of mitochondrial Ca2+ channels of the IMM.
2. Characterization and identification of mitochondrial Ca2+ channels
Energized, respiring mitochondria are naturally destined to sequester Ca2+ due to their great negative membrane potential of the IMM (ψmito) that establishes a strong driving force for Ca2+ uptake into this organelle [9,44]. Moreover, mitochondrial are capable to store high amounts of Ca2+ in the mitochondrial matrix by the formation of Ca2+-(poly)x-phosphates [12,31]. Mitochondrial Ca2+ uptake stimulates ATP production, but can also initiate cell death. Accordingly, the molecular mechanisms of mitochondrial Ca2+ uptake gained much attention during the last years.
2.1. Electrophysiological characterization of distinct mitochondrial Ca2+ channels
Recently, in two landmark publications that described the electrophysiological characterization of three highly selective Ca2+ channels in the IMM, which presumably account for the mitochondrial Ca2+ uniporter (MCU) phenomenon, were presented (Fig. 1B).
2.1.1. The MiCa
Applying the patch-clamp technique on mitoplasts (isolated mitochondria lacking their OMM) from COS-7 cells, the existence of a highly specific Ca2+ channel in the IMM has been convincingly demonstrated in 2004 by the laboratory of Clapham et al. [45]. This unique mitochondrial Ca2+ channel was referred to as MiCa. The electrophysiological characterization of MiCa showed that this channel is inwardly rectifying with a very high Ca2+ transport capacity making it very effective for Ca2+ uptake into energized mitochondria. These findings confirm visionary earlier studies in which the mitochondrial electrophoretic Ca2+ uniport was explored in isolated energized mitochondria [46,47] and reports that described the hexavalent cation ruthenium red (RR) and its related compound Ru360 as efficient inhibitors of the mitochondrial Ca2+ uniport in the nanomolar range [48]. Moreover, as expected for the MCU the permeability of MiCa to various divalent cations shows the following order: Ca2+ ≈ Sr2+ >> Mn2+ ≈ Ba2+, whereas MiCa is impermeable for Mg2+ ions. The monovalent ions K+ and Na+ do not contribute to the MiCa current in the presence of Ca2+, indicating the high Ca2+ selectivity of this mitochondrial Ca2+ channel. Notably, in this study 3–7 active channels per patch were found, which is a surprisingly high density of mitochondrial Ca2+ channels. Moreover, this study was of utmost significance as it was the first direct measurement of a mitochondrial Ca2+ channel in the IMM, thus, approving that mitochondrial Ca2+ uptake is indeed accomplished via a Ca2+ channel allowing the fast uniport of Ca2+ ions across the IMM (Fig. 1B).
2.1.2. The mCa1 and mCa2
In an outstanding work, two distinct mitochondrial Ca2+ channels have been recently electrophysiologically characterized in mitoplasts that were prepared from human ventricular myocytes [49]. These Ca2+-selective channels of the IMM are referred to as mCa1 and mCa2 and significantly differ in their single-channel amplitudes, opening times, open probabilities and their sensitivity to Ru360. Although the human mCa1 [49] share some characteristics such as its sensitivity to Ru360 with the MiCa [45] from COS-7 cells (an African Green Monkey SV40-transf’d kidney fibroblast cell line), the gating properties of both mitochondrial Ca2+ channels are considerable different, thus, possibly pointing to species and or tissue-specific heterogeneities among mitochondrial Ca2+ channels. Moreover, the coexistence of distinct mitochondrial Ca2+ currents, i.e. the Ru360-sensitive ImCa1 and the rather Ru360-insensitive ImCa2, in mitoplasts from the same origin (human ventricular myocytes) emphasize the need of different mitochondrial Ca2+ uptake pathways, which might be essential to properly integrate diverse cytosolic Ca2+ signals to mitochondrial Ca2+-induced metabolism in one given cell type. In this context the biophysical properties described for the mCa1 perfectly fit to the necessity of a mitochondrial Ca2+ channel facing the rapid and strong ER Ca2+ release sites. However, mCa2 with its lower single-channel amplitude, longer opening time and a higher open probability represents an ideal candidate to achieve efficient mitochondrial uptake of Ca2+ that rather slowly increases at mitochondrial Ca2+ uptake sites upon, e.g. Ca2+ entry via the store-operated Ca2+ entry (SOCE) pathway [2,50] (Fig. 1B). Notably, both mCa1 and mCa2 exhibit a high Ca2+ selectivity as it was reported for the MiCa. The relative divalent ion conductance of mCa1 and mCa2 for Sr2+, Mn2+, Ba2+ and Mg2+ has however not been tested so far.
2.1.3. Ca2+ carriers and exchangers
Besides the Ca2+-permeable channels there are data describing the existence of Ca2+ carriers and exchangers in the IMM that are thought to achieve mitochondrial Ca2+ efflux under physiological conditions [51]. Notably, the mitochondrial Ca2+/Na+ exchanger (NCXmito) not only represent the main route for mitochondrial Ca2+ extrusion (for reviews see [2,51]) but may also contribute to mitochondrial Ca2+ uptake under certain conditions [43]. However, substantial novel findings regarding the physiological roles of the NCXmito would require the molecular identification of this Ca2+-shuttling protein.
2.2. Molecular identification of mitochondrial Ca2+ channels
Already more than 30 years ago, attempts to isolate and purify Ca2+ channels from isolated mitochondria were initiated [52,53]. However, despite many efforts the mitochondrial Ca2+ channels of the IMM have not been explicitly identified on the molecular level so far. The clarification of the molecular identities of mitochondrial Ca2+ channels is a great challenge for the future and would be essential for the understanding of mitochondrial Ca2+ signaling as a fundamental physiological process that essentially contributes to various intracellular signaling pathways. Moreover, recent studies demonstrated the great participation of mitochondrial Ca2+ uptake to various pathological processes [1,10,54,55] in, e.g. neurodegeneration [56,57] and cardio-vascular diseases [58,59], thus, pointing to mitochondrial Ca2+ channels as attractive targets for the development of novel therapeutic strategies against different diseases [60] (Fig. 1C).
2.2.1. Glycoproteins as early potential candidates for mitochondrial Ca2+ channels
Early studies in the 1970s and 1980s that aimed to isolate and purify the mitochondrial Ca2+ uniporter suggested that glycoproteins might be elementary involved in the transfer of Ca2 across the IMM [32,52,53,61]. Thereby, a almost 90% purified 18 and 75 kD fraction were isolated using affinity chromatography with labeled 103Ru360 and Ca2+ uptake into phospholipids vesicles reconstituted with such preparations could be observed [62]. However, the purification and subsequent identification of mitochondrial Ca2+ channels has turned out to be difficult and has not been accomplished yet. Notably, in course of these attempts antisera against mitochondrial glycoprotein preparations were obtained that inhibited the Ca2+ uniport in isolated liver mitoplasts and reconstituted phospholipids vesicles (reviewed in [33]), thus, indicating the potential importance of these findings.
2.2.2. The novel uncoupling proteins (UCPs) 2 and 3 are fundamental for mitochondrial Ca2+ uniport
Uncoupling is a process that dissipates the proton gradient across the IMM of energized mitochondria whereby heat instead of ATP is generated [63]. The UCP1, also referred to as thermogenin, is a protein of the IMM that accomplishes uncoupling of respiration from ATP production, while the exact molecular mechanism by which UCP1 funnels protons from the inter membrane space into the mitochondrial matrix is still debated [64]. After the identification of UCP1 the so-called novel UCP2 and UCP3 were discovered [65–67]. Although these proteins share certain sequence homology with UCP1, their contribution to uncoupling and thermoregulation under physiological conditions could not be confirmed so far [68]. However, recently the impact of protein overexpression and siRNA-mediated knock-down of UCP2 and UCP3 on mitochondrial Ca2+ uptake in intact endothelial cells was tested in response to physiological Ca2+ mobilization [69]. Surprisingly, these experiments revealed that both proteins are fundamental for mitochondrial Ca2+ uniport as the capacity as well as the velocity of mitochondrial Ca2+ sequestration strictly correlated with the expression level of UCP2 and UCP3. Expression of UCP2/3 mutants confirmed the important role of these proteins for mitochondrial Ca2+ uptake and pointed to the predicted inter membrane loop 2 (IML2) to be elementary for the mitochondrial Ca2+ transport function of these proteins. Notably, in the IML2 domain, UCP2 and UCP3 share high sequence homology whereas this sequence considerable differs from that of UCP1. Very recently we continued experiments with UCP3 mutants and discovered two distinct sites in the IML2 that are essential for the mitochondrial Ca2+ transport function of UCP3. Interestingly one site in the IML2 of UCP3 emerged to be specifically required for mitochondrial uptake of intracellularly released Ca2+, while another distinct position was essential for mitochondrial sequestration of entering Ca2+ (manuscript submitted). The obvious importance of UCP2/3 for mitochondrial Ca2+ uniport was further validated by experiments using isolated liver mitochondria from UCP2−/− mice [43,69]. In summary these data suggest that UCP2/3 are conductive subunits of a Ca2+-selective mitochondrial ion channel at the IMM, though further work is required to challenge this hypothesis (Fig. 1C).
2.2.3. Letm1 as mitochondrial Ca2+/H+ antiporter contributing to mitochondrial Ca2+ uptake
As a result of a very recent siRNA screening to identify mitochondrial Ca2+ shuttling proteins in Drosophila S2 cells, Letm1 was identified as a Ca2+/H+ antiporter of the IMM, while respective candidates for mitochondrial Ca2+ channels have not been described [70]. Letm1 was previously associated with the Wolf–Hirschhorn syndrome, a complex congenital syndrome that is caused by a monoallelic deletion of chromosome 4 [71]. Although Letm1 has been referred to as a mitochondrial protein with unclear function, initially Letm1 was characterized to contribute to electroneutral K+/H+ exchange in mitochondria thereby controlling the mitochondrial K+ homeostasis and volume [72]. At a first glance the findings that Letm1 particularly contributes to mitochondrial Ca2+ uptake at low cytosolic Ca2+ raises (<1 μM), while at higher Ca2+ concentration another uptake pathway, presumably the MCU got activated [70], is surprising as one would rather expect that a Ca2+/H+ antiporter preferentially exports Ca2+ from mitochondria (Fig. 1C). However, these findings are in line with other reports suggesting the existence of MCU-independent uptake pathways that are presumably accomplished by mitochondrial exchangers working in their reversed mode [43,50,73,74].
2.2.4. Assembly of protein complexes that establish mitochondrial Ca2+ channels
Little is known whether mitochondrial Ca2+ channels are protein complexes or not. However our recent finding that an expression of human UCP2/3 was ineffective to affect mitochondrial uptake in yeast [69] suggest that additional proteins are necessary to constitute Ca2+-permeable channels in the IMM. Thus, it is reasonable to speculate that additional proteins/factors are necessary to reassemble the Ca2+ transport function of UCP2 and UCP3 in artificial or heterologous systems. In line with these findings and in analogy to the so called mitochondrial transition pore (MTP), a large conductance pore that upon opening makes the mitochondrial membranes suddenly permeable for molecules with a molecular weight up to appr. 1.5 kDa [75,76], it seems feasible that the mitochondrial Ca2+ uniport channels also exhibit multiprotein complexes of IMM and OMM proteins (Fig. 1D).
Overexpression of the adenine nucleotide translocase (ANT), which is also known to be a component of the MTP [77], was shown to significantly reduce mitochondrial Ca2+ uptake in intact cells [78]. Although the overexpression of ANT might cause MTP opening and, thus, depolarization of the IMM, it is tempting to speculate that the reduced mitochondrial Ca2+ signals in ANT overexpressing cells are at least in part, the result of a disturbed composition of a presumable mitochondrial Ca2+ channel complex. The most prominent candidate of a protein of the OMM that probably physically interact with proteins of the IMM to constitute a mitochondrial Ca2+ channel spanning the IMM and OMM is VDAC [79]. Overexpression of VDAC in HeLa cells and skeletal myotubes enhanced mitochondrial Ca2+ uptake, indicating that this OMM porines are involved in the transfer of cytosolic Ca2+ into the lumen of mitochondria [80]. Notably, the chaperone glucose-regulated protein 75 (grp75) was found to link the inositol 1,4,5-trisphosphate receptor (IP3R) to VDAC, which presumably enhances the transfer of Ca2+ from the ER towards mitochondria [81]. The exploration of the molecular basis of structural components of ER-mitochondria contact sites is currently a matter of intensive research [82,83]. Recently, mitofusin 2 was identified as a molecular component of such tethers that connect the ER with mitochondria, which was also elementary for mitochondrial Ca2+ uniport of Ca2+ that was mobilized from the ER [84]. The physical alliance between ER and mitochondria is also referred to as mitochondrial-associated ER membrane (MAM), which emerges to have important roles for Ca2+ signaling [83]. Interestingly, a recent study using electron tomography showed that in MAM the distance between ER and mitochondria is in the range of 10–25 nm, which would allow a direct interaction of proteins of the ER with proteins of the OMM [85]. Accordingly, it is reasonable that mitochondrial Ca2+ conducting proteins of the IMM might be assembled in a complex with MAM proteins, which substantially contribute to the gating of mitochondrial Ca2+ channels in intact cells (Fig. 1D).
3. Modulation of mitochondrial Ca2+ channels
3.1. Phosphorylation of mitochondrial Ca2+ channels
First conspicuous evidence that mitochondrial Ca2+ channels are targets of kinases came from the observation that an inhibition of the p38 mitogen-activated kinase (MAPK) with SB 202190 increased mitochondrial Ca2+ uptake in response to cell stimulation with an IP3 generating agonist [86]. At a first glance this finding would indicate that mitochondrial Ca2+ channels exhibit serine/threonine-phosphorylation sites that, once phosphorylated negatively regulated the channel’s activity. Because other inhibitors of the p38MAPK failed to mimic the effect of SB 202190 [87], while structural related compounds without affecting this kinase activity, such as plant flavanoids, enhanced mitochondrial Ca2+ loading [88], the contribution of p38MAPK to the regulation of mitochondrial Ca2+ uniport was questioned and an alternative explanation suggesting that compounds such as SB 202190 directly bind to mitochondrial Ca2+ channels was discussed. However, the group of András Spät subsequently convincingly demonstrated that siRNA mediated knock-down of p38MAPK efficiently and specifically increased mitochondrial Ca2+ uptake upon cell stimulation with an IP3 generating agonist [89]. Form this study and their subsequent intriguing work [90,91], the authors concluded that p38MAPK, novel isoforms of the protein kinase C (PKC) family and protein kinase D play central roles in the regulation of mitochondrial Ca2+ channels to prevent mitochondrial Ca2+ overload by explosive IP3 mediated ER Ca2+ mobilization and hence protect cells from cell death. Further studies also point to different PKC isoforms that putatively modulate mitochondrial Ca2+ uptake channels: overexpression of PKCβ in HeLa cells was shown to reduce mitochondrial Ca2+ uptake, whereas overexpression of PKCζ increased mitochondrial Ca2+ transients upon cell stimulation with an IP3 generating agonist [92] (Fig. 1E).
3.2. Regulation of mitochondrial Ca2+ channels by Ca2+
Early Ca2+ uptake studies with isolated mitochondria indicated that mitochondrial Ca2+ channels are activated by Ca2+ [93]. These experiments suggest a slow and allosteric activation of the mitochondrial Ca2+ uniport by Ca2+. In contrast, whole mitoplast patch-clamp experiments exclude a role for Ca2+ in activating mitochondrial Ca2+ channels [45], as a Na+ conductance in the absence of Ca2+ was recorded, indicating that Ca2+ is not essential for MiCa activity. The inconsistency of such datasets show that depending on how isolated mitochondria/mitoplasts have been prepared and depending on the overall experimental conditions an methods used to measure the functioning of mitochondrial Ca2+ channels, clearly different, even contradictory results can be obtained [94] vs. [43]. Moreover, the Ca2+ sensitivity of mitochondrial Ca2+ channels might be linked to signaling proteins such as calmodulin, which are fragilely or transiently associated with mitochondrial Ca2+ channels in intact cells. Accordingly, mitochondrial isolation might lead to a loss of these kind of interactions as such procedures are simply too invasive. This view is supported by findings that mitochondrial Ca2+ uniport is partially a Ca2+-calmodulin-gated process using permeabilized RBL-1 cells [95]. This study describes that mitochondrial Ca2+ uniport is activated via a Ca2+-calmodulin dependent mechanism, whereas cytosolic Ca2+ subsequently leads to an inactivation of mitochondrial Ca2+ channels, preventing further Ca2+ uptake by these organelles. The role of calmodulin in facilitating the activity of the mitochondrial Ca2+ uniport was further confirmed by Csordas and Hajnoczky [96]. However, a biphasic regulation of mitochondrial Ca2+ uptake channels points to complex mechanisms that tune the transit of Ca2+ across the IMM in order to avoid fatal mitochondrial Ca2+ overload during intracellular Ca2+ signaling. Moreover, Moreau and Parekh continued their intriguing studies and reported recently that the Ca2+-dependent inactivation of the mitochondrial Ca2+ uniporter is linked to proton-fluxes through the ATP-synthase [97], providing not only a mechanism of autoregulation of ATP synthesis but also a reasonable feedback mechanism between mitochondrial Ca2+ up-take and the organelle’s metabolic function.
In isolated liver and heart mitochondria a so-called rapid mode of mitochondrial Ca2+ upake (RaM) exists [98]. This process allows a fast pulsatile uptake of large amounts of Ca2+ but only for a short period of time, because RaM was shown to be quickly inactivated by Ca2+ via Ca2+ binding to an external site [99]. Notably, RaM is also sensitive to RR and Ru360, hence, it is possible that RaM is not a distinct mitochondrial Ca2+ channel but rather reflects a certain state of mitochondrial Ca2+ uniport channel(s) (Fig. 1E).
4. Conclusion
Recently promising progresses in the identification and characterization of mitochondrial Ca2+ transporters has been accomplished. Nevertheless, despite numerous studies convincingly characterize functional and structural aspects of mitochondrial Ca2+ uptake, our current picture on the actual proteins being involved remains rather vague. Intriguingly, the existence of various distinct mitochondrial Ca2+ channels that accomplish the transfer of Ca2+ across the IMM have been reported, and it is tempting to speculate that a species- and tissue-specific diversities of mitochondrial Ca2+ channels exists. Nevertheless, the experimental conditions and techniques used to characterize mitochondrial Ca2+ fluxes have to be taken into consideration if a general assertion is made. Thus, despite recent progresses, the identification of the molecular components of mitochondrial Ca2+ channels remains a challenging task in molecular physiology and awaits further intensive investigation.
Acknowledgements
The author’s research on mitochondrial ion homeostasis is funded by the Austrian Science Funds (FWF, P20181-B05, 21857-B18 and F3010-B05).
Abbreviations
- ANT
adenine nucleotide translocase
- ER
endoplasmic reticulum
- grp75
glucose-regulated protein 75
- IML2
inter membrane loop 2
- IMM
inner mitochondrial membrane
- IP3R
inositol 1,4,5-trisphosphate receptor
- MAM
mitochondrial-associated ER membrane
- MAPK
mitogen-activated kinase
- mCa/MiCa
mitochondrial Ca2+ channel
- MCU
mitochondrial Ca2+ uniporter
- MTP
mitochondrial transition pore
- NCXmito
mitochondrial Ca2+/Na+ exchanger
- OMM
outer mitochondrial membrane
- PKC
protein kinase C
- RaM
rapid mode of mitochondrial Ca2+ uptake
- RR
ruthenium red
- SOCE
store-operated Ca2+ entry
- UCP
uncoupling protein
- VDAC
voltage-dependent anion selective channels
References
- [1].Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [2].Graier WF, Frieden M, Malli R. Mitochondria and Ca2+ signaling: old guests, new functions. Pflugers Arch. 2007;455:375–396. doi: 10.1007/s00424-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr. Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- [4].Soubannier V, McBride H. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim. Biophys. Acta. 2008;1793:154–170. doi: 10.1016/j.bbamcr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- [5].Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+ Proc. Natl. Acad. Sci. USA. 2001;98:3197–3202. doi: 10.1073/pnas.051636098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- [7].Brini M. Ca2+ signalling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium. 2003;34:399–405. doi: 10.1016/s0143-4160(03)00145-3. [DOI] [PubMed] [Google Scholar]
- [8].Campanella M, Pinton P, Rizzuto R. Mitochondrial Ca2+ homeostasis in health and disease. Biol. Res. 2004;37:653–660. doi: 10.4067/s0716-97602004000400022. [DOI] [PubMed] [Google Scholar]
- [9].Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology. 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- [10].Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nicholls DG. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur. J. Biochem. 1974;50:305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- [12].Carafoli E. Historical review: mitochondria and calcium: ups and downs of an unusual relationship. Trends Biochem. Sci. 2003;28:175–181. doi: 10.1016/S0968-0004(03)00053-7. [DOI] [PubMed] [Google Scholar]
- [13].McCormack JG, Denton RM. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem. J. 1979;180:533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McCormack JG, Denton RM. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+ Biochem. J. 1984;218:235–247. doi: 10.1042/bj2180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- [16].Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Malli R, Frieden M, Osibow K, Graier WF. Mitochondria efficiently buffer subplasmalemmal Ca2+ elevation during agonist stimulation. J. Biol. Chem. 2003;278:10807–10815. doi: 10.1074/jbc.M212971200. [DOI] [PubMed] [Google Scholar]
- [19].Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J. Cell Sci. 2004;117:6327–6337. doi: 10.1242/jcs.01559. [DOI] [PubMed] [Google Scholar]
- [20].Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu. Rev. Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- [21].Arnaudeau S, Kelley WL, Walsh JV, Jr., Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- [22].Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the ER. J. Biol. Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- [23].Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- [24].Michalak M, Mariani P, Opas M. Calreticulin, a multifunctional Ca2+ binding chaperone of the endoplasmic reticulum. Biochem. Cell Biol. 1998;76:779–785. doi: 10.1139/bcb-76-5-779. [DOI] [PubMed] [Google Scholar]
- [25].Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- [26].Osibow K, Frank S, Malli R, Zechner R, Graier WF. Mitochondria maintain maturation and secretion of lipoprotein lipase in the endoplasmic reticulum. Biochem. J. 2006;396:173–182. doi: 10.1042/BJ20060099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roy SS, Hajnóczky G. Calcium, mitochondria and apoptosis studied by fluorescence measurements. Methods. 2008;46:213–223. doi: 10.1016/j.ymeth.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- [30].Nicholls DG. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem. J. 1978;176:463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- [32].Saris NE, Allshire A. Calcium ion transport in mitochondria. Methods Enzymol. 1989;174:68–85. doi: 10.1016/0076-6879(89)74011-8. [DOI] [PubMed] [Google Scholar]
- [33].Saris NE, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry. 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- [34].Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J. Biol. Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- [35].Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem. J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hajnoczky G, Csordas G, Yi M. Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium. 2002;32:363–377. doi: 10.1016/s0143416002001872. [DOI] [PubMed] [Google Scholar]
- [37].Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- [38].Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- [39].Rostovtseva TK, Antonsson B, Suzuki M, Youle RJ, Colombini M, Bezrukov SM. Bid, but Not Bax, Regulates VDAC Channels. J. Biol. Chem. 2004;279:13575–13583. doi: 10.1074/jbc.M310593200. [DOI] [PubMed] [Google Scholar]
- [40].Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- [41].Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–193. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- [42].Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- [43].Trenker M, Fertschai I, Malli R, Graier WF. UCP2/3 - likely to be fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 2008;10:1237–1240. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- [45].Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- [46].Bernardi P, Paradisi V, Pozzan T, Azzone GF. Pathway for uncoupler-induced calcium efflux in rat liver mitochondria: inhibition by ruthenium red. Biochemistry. 1984;23:1645–1651. doi: 10.1021/bi00303a010. [DOI] [PubMed] [Google Scholar]
- [47].Reed KC, Bygrave FL. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem. J. 1974;140:143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].de Jesús García-Rivas G, Guerrero-Hernández A, Guerrero-Serna G, Rodríguez-Zavala JS, Zazueta C. Inhibition of the mitochondrial calcium uniporter by the oxo-bridged dinuclear ruthenium amine complex (Ru360) prevents from irreversible injury in postischemic rat heart. FEBS J. 2005;272:3477–3488. doi: 10.1111/j.1742-4658.2005.04771.x. [DOI] [PubMed] [Google Scholar]
- [49].Michels G, Khan IF, Endres-Becker J, Rottlaender D, Herzig S, Ruhparwar A, Wahlers T, Hoppe UC. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 2009;119:2435–2443. doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- [50].Demaurex N, Poburko D, Frieden M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochim. Biophys. Acta. 2009;1787:1383–1394. doi: 10.1016/j.bbabio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- [51].Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- [52].Sottocasa G, Sandri G, Panfili E, De Bernard B, Gazzotti P, Vasington FD, Carafoli E. Isolation of a soluble Ca2+ binding glycoprotein from ox liver mitochondria. Biochem. Biophys. Res. Commun. 1972;47:808–813. doi: 10.1016/0006-291x(72)90564-5. [DOI] [PubMed] [Google Scholar]
- [53].Panfili E, Sandri G, Sottocasa GL, Lunazzi G, Liut G, Graziosi G. Specific inhibition of mitochondrial Ca2+ transport by antibodies directed to the Ca2+-binding glycoprotein. Nature. 1976;264:185–186. doi: 10.1038/264185a0. [DOI] [PubMed] [Google Scholar]
- [54].Duchen MR. Mitochondria and Ca2+ in cell physiology and pathophysiology. Cell Calcium. 2000;28:339–348. doi: 10.1054/ceca.2000.0170. [DOI] [PubMed] [Google Scholar]
- [55].Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [56].Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- [57].Vercesi AE, Kowaltowski AJ, Oliveira HCF, Castilho RF. Mitochondrial Ca2+ transport, permeability transition and oxidative stress in cell death: implications in cardiotoxicity, neurodegeneration and dyslipidemias. Front. Biosci. 2006;11:2554–2564. doi: 10.2741/1990. [DOI] [PubMed] [Google Scholar]
- [58].Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ. Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- [59].Liu T, O’Rourke B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J. Bioenerg. Biomembr. 2009;41:127–132. doi: 10.1007/s10863-009-9216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ralph SJ, Low P, Dong L, Lawen A, Neuzil J. Mitocans: mitochondrial targeted anti-cancer drugs as improved therapies and related patent documents. Recent Pat. Anticancer Drug Discov. 2006;1:327–346. doi: 10.2174/157489206778776952. [DOI] [PubMed] [Google Scholar]
- [61].Mironova GD, Sirota TV, Pronevich LA, Trofimenko NV, Mironov GP, Grigorjev PA, Kondrashova MN. Isolation and properties of Ca2+-transporting glycoprotein and peptide from beef heart mitochondria. J. Bioenerg. Biomembr. 1982;14:213–225. doi: 10.1007/BF00751016. [DOI] [PubMed] [Google Scholar]
- [62].Zazueta C, Zafra G, Vera G, Sanchez C, Chavez E. Advances in the purification of the mitochondrial Ca2+ uniporter using the labeled inhibitor 103Ru360. J. Bioenerg. Biomembr. 1998;30:489–498. doi: 10.1023/a:1020546331217. [DOI] [PubMed] [Google Scholar]
- [63].Argyropoulos G, Harper M-E. Uncoupling proteins and thermoregulation. J. Appl. Physiol. 2002;92:2187–2198. doi: 10.1152/japplphysiol.00994.2001. [DOI] [PubMed] [Google Scholar]
- [64].Nicholls DG. The physiological regulation of uncoupling proteins. Biochim. Biophys. Acta. 2006;1757:459–466. doi: 10.1016/j.bbabio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- [65].Affourtit C, Crichton PG, Parker N, Brand MD. Novel uncoupling proteins. Novartis Found Symp. 2007;287:70–80. discussion 80–91. [PubMed] [Google Scholar]
- [66].Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- [67].Nedergaard J, Matthias A, Golozoubova V, Jacobsson A, Cannon B. UCP1: the original uncoupling protein–and perhaps the only one? New perspectives on UCP1, UCP2, and UCP3 in the light of the bioenergetics of the UCP1-ablated mice. J. Bioenerg. Biomembr. 1999;31:475–491. doi: 10.1023/a:1005400507802. [DOI] [PubMed] [Google Scholar]
- [68].Nedergaard J, Cannon B. The “novel” uncoupling proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp. Physiol. 2003;88:65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- [69].Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L. LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum. Mol. Genet. 2008;17:201–214. doi: 10.1093/hmg/ddm297. [DOI] [PubMed] [Google Scholar]
- [72].Froschauer E, Nowikovsky K, Schweyen RJ. Electroneutral K+/H+ exchange in mitochondrial membrane vesicles involves Yol027/Letm1 proteins. Biochim. Biophys. Acta. 2005;1711:41–48. doi: 10.1016/j.bbamem.2005.02.018. [DOI] [PubMed] [Google Scholar]
- [73].Graier WF, Trenker M, Malli R. Mitochondrial Ca2+, the secret behind the function of uncoupling proteins 2 and 3? Cell Calcium. 2008;44:36–50. doi: 10.1016/j.ceca.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Parekh AB. Mitochondrial regulation of store-operated CRAC channels. Cell Calcium. 2008;44:6–13. doi: 10.1016/j.ceca.2007.12.006. [DOI] [PubMed] [Google Scholar]
- [75].Lemasters JJ. The mitochondrial permeability transition and the calcium, oxygen and pH paradoxes: one paradox after another. Cardiovasc. Res. 1999;44:470–473. doi: 10.1016/s0008-6363(99)00368-5. [DOI] [PubMed] [Google Scholar]
- [76].Lemasters JJ, Theruvath TP, Zhong Z, Nieminen A-L. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Baines CP. The molecular composition of the mitochondrial permeability transition pore. J. Mol. Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wieckowski MR, Szabadkai G, Wasilewski M, Pinton P, Duszyński J, Rizzuto R. Overexpression of adenine nucleotide translocase reduces Ca2+ signal transmission between the ER and mitochondria. Biochem. Biophys. Res. Commun. 2006;348:393–399. doi: 10.1016/j.bbrc.2006.07.072. [DOI] [PubMed] [Google Scholar]
- [79].Gonçalves RP, Buzhysnskyy N, Scheuring S. Mini review on the structure and supramolecular assembly of VDAC. J. Bioenerg. Biomembr. 2008;40:133–138. doi: 10.1007/s10863-008-9141-2. [DOI] [PubMed] [Google Scholar]
- [80].Rapizzi E, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Szabadkai G, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rizzuto R, et al. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim. Biophys. Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- [85].Csordas G, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Montero M, Lobaton CD, Moreno A, Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. FASEB J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- [87].Montero M, Lobaton CD, Gutierrez-Fernandez S, Moreno A, Alvarez J. Modulation of histamine-induced Ca2+ release by protein kinase C: effects on cytosolic and mitochondrial [Ca2+] peaks. J. Biol. Chem. 2003;278:49972–49979. doi: 10.1074/jbc.M308378200. [DOI] [PubMed] [Google Scholar]
- [88].Montero M, Lobaton CD, Hernandez-Sanmiguel E, Santodomingo J, Vay L, Moreno A, Alvarez J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem. J. 2004;384:19–24. doi: 10.1042/BJ20040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Szanda G, Koncz P, Rajki A, Spät A. Participation of p38 MAPK and a novel-type protein kinase C in the control of mitochondrial Ca2+ uptake. Cell Calcium. 2008;43:250–259. doi: 10.1016/j.ceca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- [90].Koncz P, Szanda G, Fülöp L, Rajki A, Spät A. Mitochondrial Ca2+ uptake is inhibited by a concerted action of p38 MAPK and protein kinase D. Cell Calcium. 2009;46:122–129. doi: 10.1016/j.ceca.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [91].Spät A, Fülöp L, Koncz P, Szanda G. When is high-Ca2+ microdomain required for mitochondrial Ca+ uptake? Acta Physiol. 2009;195:139–147. doi: 10.1111/j.1748-1716.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- [92].Pinton P, Leo S, Wieckowski MR, Di Benedetto G, Rizzuto R. Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. J. Cell Biol. 2004;165:223–232. doi: 10.1083/jcb.200311061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kröner H. Ca2+ ions, an allosteric activator of calcium uptake in rat liver mitochondria. Arch. Biochem. Biophys. 1986;251:525–535. doi: 10.1016/0003-9861(86)90360-7. [DOI] [PubMed] [Google Scholar]
- [94].Brookes PS, et al. UCPs – unlikely calcium porters. Nat. Cell Biol. 2008;10:1235–1237. doi: 10.1038/ncb1108-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Moreau B, Nelson C, Parekh AB. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr. Biol. 2006;16:1672–1677. doi: 10.1016/j.cub.2006.06.059. [DOI] [PubMed] [Google Scholar]
- [96].Csordas G, Hajnoczky G. Plasticity of mitochondrial calcium signaling. J. Biol. Chem. 2003;278:42273–42282. doi: 10.1074/jbc.M305248200. [DOI] [PubMed] [Google Scholar]
- [97].Moreau B, Parekh AB. Ca2+-dependent inactivation of the mitochondrial Ca2+ uniporter involves proton flux through the ATP synthase. Curr. Biol. 2008;18:855–859. doi: 10.1016/j.cub.2008.05.026. [DOI] [PubMed] [Google Scholar]
- [98].Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim. Biophys. Acta. 2001;1504:248–261. doi: 10.1016/s0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- [99].Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J. Biol. Chem. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]