Abstract
Fuelled by the obesity epidemic, there is considerable interest in the developmental origins of white adipose tissue (WAT) and the stem/progenitor cells from which it arises. While increased visceral fat mass is associated with metabolic dysfunction, increased subcutaneous WAT is protective. There are 6 visceral fat depots: perirenal, gonadal, epicardial, retroperitoneal, omental and mesenteric and it is a subject of much debate whether these have common developmental origins and whether this differs from subcutaneous WAT. Here we show that all 6 visceral WAT depots receive a significant contribution from cells expressing Wt1 late in gestation. Conversely, no subcutaneous WAT or brown adipose tissue (BAT) arises from Wt1 expressing cells. Postnatally, a subset of visceral WAT continues to arise from Wt1 expressing cells, consistent with the finding that Wt1 marks a proportion of cell populations enriched in WAT progenitors. We show all visceral fat depots have a mesothelial layer like the visceral organs with which they are associated and provide several lines of evidence that Wt1 expressing mesothelium can produce adipocytes. These results: reveal a major ontogenetic difference between visceral and subcutaneous WAT; pinpoint the lateral plate mesoderm as a major source of visceral WAT; support the notion that visceral WAT progenitors are heterogeneous; and suggest that mesothelium is a source of adipocytes.
Although there has been much progress recently in identifying adult WAT progenitors1-5 , the embryological origins of different WAT depots remain obscure. In a recent review this was highlighted as one of the big unanswered questions in the field6. Transcriptomic analysis of preadipocytes isolated from the stromal vascular component (SVF) of fat has revealed consistent differences in developmental gene expression between visceral depots and between visceral and subcutaneous fat7,8,9. However, in one study of human depots it was concluded that pre-adipocytes of mesenteric WAT had an expression profile closer to that of subcutaneous than omental pre-adipocytes. Similarly, transcriptome analysis of mouse adipose depots showed that the expression of some developmental genes was high in subcutaneous and perirenal WAT, but low in mesenteric WAT. It has been posited, from these and other similar studies, that each WAT depot could be considered a separate mini-organ. It was not possible to infer either a common or distinct origin for subcutaneous and visceral WAT or the different WAT depots.
The Wilms tumour gene, Wt1, is a major regulator of mesenchymal progenitors in the developing kidney and heart10,11. During development Wt1 expression is restricted mainly to the intermediate mesoderm, parts of the lateral plate mesoderm and tissues that derive from these including kidney, gonads, peri-/epicardium, spleen, mesentery, omentum and the mesothelial layer that lines the visceral organs and the body cavity (peritoneum). There is a striking relationship between these Wt1 expressing tissues and the location of the six visceral fat depots. Recently we showed that Wt1 is expressed in several visceral fat depots, but not in subcutaneous WAT or BAT12. These observations led us to hypothesise that some visceral fat may arise from Wt1 expressing cells during development. To test this, we carried out cell lineage analysis.
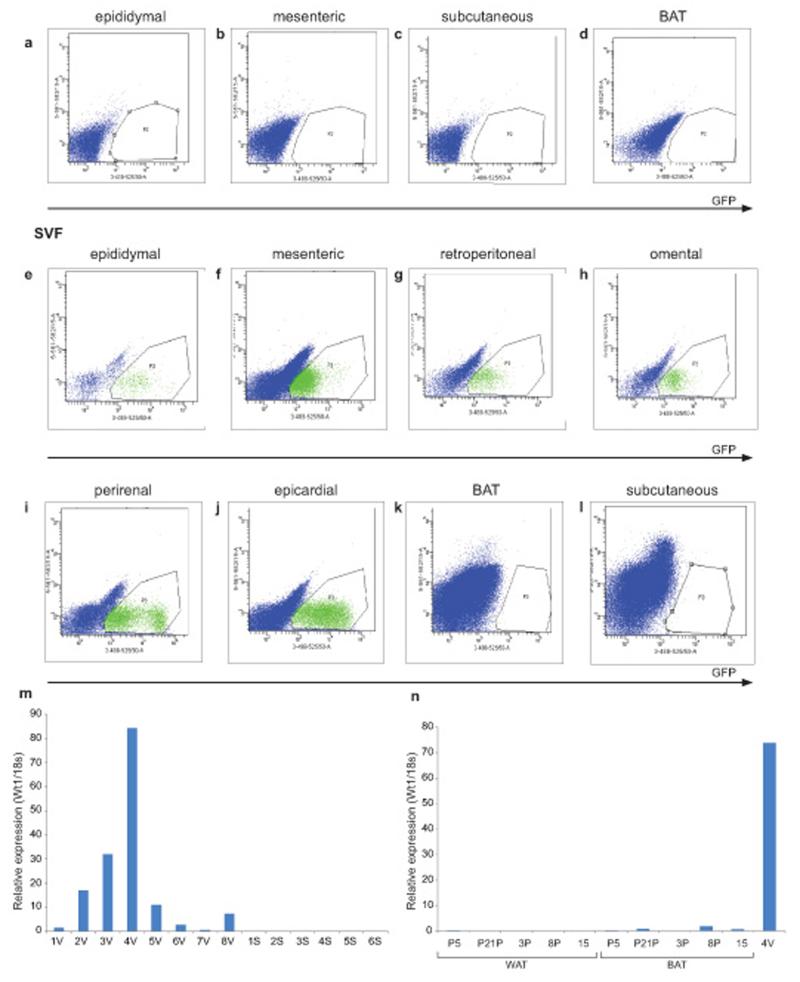
Before carrying out lineage analysis, it was first necessary to demonstrate that Wt1 is not itself expressed in adipocytes. Adipose tissue is composed of a mixed population of cells, including the mature adipocytes and the stromal vascular fraction (SVF). We isolated adipose depots from Wt1-GFP knockin mice and separated the tissues into mature adipocytes (floating) and the SVF by centrifugation. We showed that the Wt1 positive cells are not present in the floating mature adipocytes (Fig. 1a-d) but they were abundant in the SVF from epididymal, mesenteric, retroperitoneal, omental, epicardial, and perirenal WAT (Fig. 1e-l). We stained the floating layer with a lipid dye (LipidTox) and showed that all cells in this layer are LipidTox positive (Supplementary Fig. 1a). Wt1 positive cells were not found in the SVF obtained from subcutaneous WAT or BAT (Fig. 1e-l). We showed previously by Q-PCR that Wt1 mRNA is expressed in all the visceral fat depots analysed, including epididymal, mesenteric, and retroperitoneal WAT. Our FACS analysis reinforces these findings and also revealed Wt1-expressing cells in omental, epicardial and perirenal WAT. We also analysed WT1 expression in human adipose samples. As for mice, expression was observed in visceral (omental) WAT but levels were very low or undetectable in whole subcutaneous adipose tissue, and in subclavicular subcutaneous adipose enriched for BAT (confirmed by high levels of UCP-1 expression c.f. neighbouring white adipose tissues (an approximately 1687 fold increase of UCP-1 in the BAT, Supplementary Table 1) (Fig. 1m,n).
Figure 1. Wt1+ cells reside in the SVF in visceral WAT but not subcutaneous WAT or BAT depots.
Using the Wt1-GFP mouse model, representative FACS plots showing Wt1+ cells (GFP) are not detected in the mature adipocytes in the (a) epididymal, (b) mesenteric, (c) subcutaneous, or (d) BAT fat pad. In the SVF, Wt1+ cells are found in the (e) epididymal, (f) mesenteric, (g) retroperitoneal, (h) omental, (i) perirenal, and (j) epicardial depots; however, Wt1+ cells are not detected in the SVF from (k) BAT or (l) subcutaneous fat pads. GFP signal is indicated in the x-axis. Gates are chosen using cells from Wt1-GFP negative mice (n=3). (m) The level of WT1 mRNA in human visceral and subcutaneous fat is measured by Q-PCR (y-axis is expressed in arbitrary unit). V indicates ‘visceral’ (omental fat in this case). S indicates subcutaneous fat. (n), The level of WT1 mRNA from human ‘BAT’ and adjacent white adipose tissue (WAT) is measured by Q-PCR. Sample ‘4V’ is visceral fat which acts as a positive control (y-axis is expressed in arbitrary units).
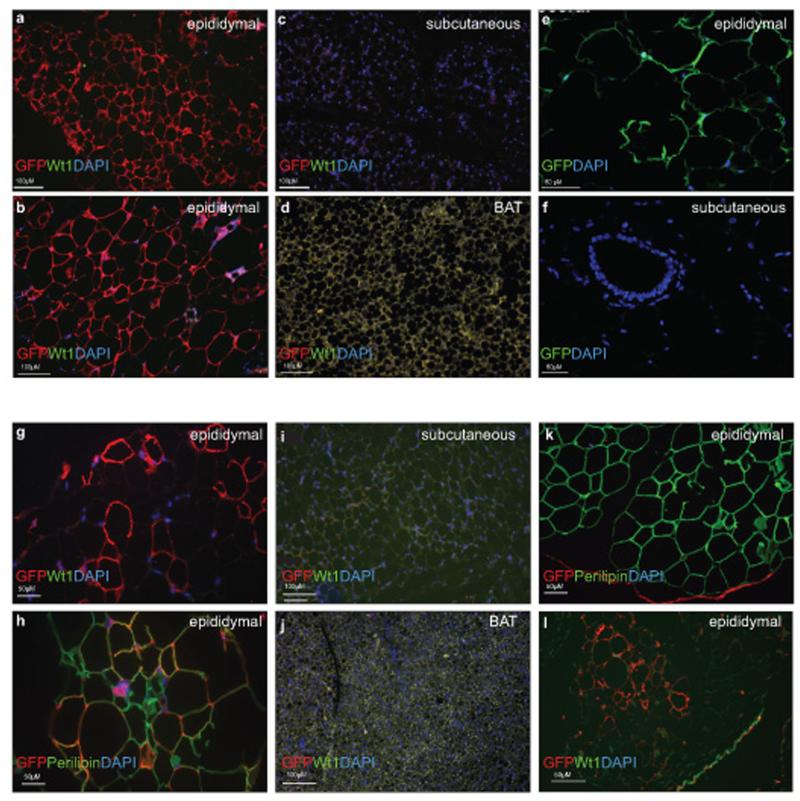
For cell lineage analysis, knock-in mice expressing tamoxifen-inducible Cre recombinase at the Wt1 promoter locus as described were crossed with the reporter; mTmG mice13. Prior to Cre-mediated recombination, Tomato is expressed ubiquitously under a pCA promoter. After Cre-mediated loxP recombination which excises Tomato, membranous GFP is expressed. Thus, this system allows genetic marking of CreER-expressing cells at the time of tamoxifen injection (driven by Wt1 promoter activity), and the subsequent tracing of these cells and their progeny. To determine whether adipose tissues may arise from Wt1-expressing cells during development, maternal injection of tamoxifen was performed at E14.5 (one dose) and the tissues analysed when the mice were 1.2 years old (n=4). Approximately 77% of mature adipocytes were Wt1+ lineage positive in the epididymal fat (Fig. 2a,b). Similarly, large numbers of adipocytes were positive in all visceral fat pads analysed including epicardial (66%), omental (47%), mesenteric (28%), retroperitoneal, and perirenal fat depots (Supplementary Fig. 2). However, there were no positive adipocytes derived from Wt1-expressing cells in the subcutaneous WAT and BAT (Fig. 2c,d).
Figure 2. Extensive long term contribution of the Wt1+ cells, induced at E14.5 to mature adipocytes in epididymal WAT but not in subcutaneous WAT or BAT. Some Wt1+ cells in the adult adipose tissues can contribute to mature adipocytes in visceral but not subcutaneous WAT or BAT.
One dose of tamoxifen was given to pregnant animals at E14.5 and mice were harvested at 1.2-years old (n=3). Sections from various fat pads are stained with GFP antibody (red), Wt1-antibody (green), and DAPI (blue). a,b, epididymal fat pad; c, absence of Wt1-lineaged cells or endogenous Wt1-expressing cells in the subcutaneous and (d) BAT. In (e), immunostaining of sections of fat dissected from the constitutively active Wt1-Cre;R26RYFP indicates presence of the GFP positive adipocytes (indicate in green) in the visceral fat and absence of GFP positive cells in the subcutaneous WAT (f). (g), Wt1+ cells in the epididymal fat pad from the Wt1−CreERT2.mTmG model induced at 3-weeks old gave rise to mature adipocytes (GFP= red, endogenous Wt1= green, cell nuclei= DAPI, blue) when mice were harvested at 3 months-old. h, mature adipocytes are indicated by perilipin antibody staining (GFP= red, perilipin= green, cell nuclei= blue). i, Absence of GFP+ cells in the subcutaneous or BAT fat pad (j). k, The mesothelium structure in adipose tissue arises from Wt1+ cells (red). Mature adipocytes are stained with perilipin antibody (green). (l), Mesothelium in adipose tissues (epididymal) express endogenous Wt1 (indicated in green) and is Wt1-lineage positive (indicated in red).
We were interested in determining if the subcutaneous and visceral fat have different origins and when the separation might take place. During development, Wt1 is first expressed at E8.5 in the intermediate and lateral plate mesoderm in mice14. By E14.5, the formation of the body cavity has already taken place. To determine whether cells that are positive for Wt1 expression during E8.5-E14.5 can contribute to the subcutaneous or BAT adipo-lineages, we used a constitutively active Wt1−Cre;R26RYFP model15. No adipocytes that arise from Wt1-expressing cells were detected in subcutaneous WAT (Fig. 2f), while as expected adipocytes in visceral WAT that derived from Wt1-expressing cells were seen (Fig. 2e).
To determine whether any visceral fat may arise from Wt1 expressing cells postnatally during the growth phase, we induced the Cre by oral administration of tamoxifen to three-week old Wt1CreERT2; mTmG mice (n=3). GFP expression in adipose tissues was analysed when mice were three months-old. Membranous GFP signals were detected (while negative for endogenous Wt1) in epididymal fat pads using an anti-GFP antibody (Fig. 2g, indicated in red). A GFP signal was detected in some cells with mono-ocular lipid vacuoles, characteristic of mature adipocytes. Sections were co-stained with anti-perilipin antibody (an adipocyte marker, indicated in green) to verify that the GFP positive cells are adipocytes (Fig. 2h). No GFP signals were detected in the subcutaneous or BAT fat pads (Fig. 2i,j). To show that the absence of GFP signal was not due to the fixing method, we were able to stain the subcutaneous fat depot with an anti-RFP antibody (Supplementary Fig. 2f). In addition, we also tested if any Wt1-positive cells contributed to adipocytes in bone marrow. Surprisingly, we did not detect any Wt1+ cells in the bone marrow when mice were induced at embryonic stage (E14.5) or adult (Supplementary Fig. 1b&c).
Given the contribution of juvenile and adult Wt1 expressing cells to mature visceral adipocytes we next wanted to test whether Wt1 positive cells might express the cell surface marker sets that characterize progenitors. Recently two cell populations with the properties of adipocyte progenitors and preadipocytes have been isolated by FACS1,4; these are Lin−CD29+CD34+Sca1+CD24+ and Lin−CD29+CD34+Sca1+CD24− populations respectively (Fig. 3a). In subcutaneous WAT it was shown that the CD24− preadipocyte population arises from the CD24+ progenitor population and there is a shift from the latter to the former after birth4. We show that most of the Lin−Wt1+ cells in the adult adipose depots are in the Lin−CD31−CD29+CD34+ population (Fig. 3b,c,~90%). In addition, the majority of the Lin−Wt1+ cells is in the Lin−CD31−CD29+CD34+Sca1+CD24− population (from 60-90% depending on fat pad, Fig. 3c); while a smaller percentage of the Lin−CD31−Wt1+ cells are in the Lin−CD31−CD29+CD34+Sca1+CD24+ population, the highest level being in the omentum (Fig. 3c). Fig. 3d shows the percentage of cells in each population that are Wt1+. Epicardial and omental fat have the highest percentage of cells that are Wt1+ in both the Lin−CD31−CD29+CD34+Sca1+CD24− (60% and 40% respectively) and the Lin−CD31−CD29+CD34+Sca1+CD24+ (20% and 40% respectively) populations. Both omental and epicardial fat pads are particularly implicated in human disease risk16,17. We demonstrated that the Wt1+ cells in the SVF are capable of differentiating to adipocytes and muscle in vitro (Supplementary Fig. 3a,b). They have limited ability to form osteoblasts (Supplementary Fig. 3c). Interestingly, we also noticed a difference in the osteoblast-forming ability of SVFs between different fat pads (Supplementary Fig. 3d). SVFs from mesenteric, subcutaneous, and epicardial fat pads have stronger ability to differentiate to osteoblasts c.f. SVFs from epididymal and omental fat pads.
Figure 3. Wt1+ cells in adult adipose tissues express adipose progenitor surface markers.
a, A schematic representation of the FACS strategy taken from Rodeheffer et al is shown1. b, Dot plots showing representative FACS staining profiles and gating of adipose SVF from adult Wt1-GFP mice. Lin−and CD31− populations (‘P8’) from live singlets are selected. The Lin−CD31− population is further separated on the basis of expression of CD34 and CD29. The CD34 and CD29 double positive cells are further analysed based on the expression of Sca1 and CD24. The majority of the Wt1-GFP positive cells (indicated in green) are in the (Lin−CD31−CD34+CD29+Sca1+CD24−) population. c, The percentage of each populations in (Lin−CD31−GFP+) from different fat pads is shown (n=4. Data represent the mean ±s.e.m). d, The percentage of the cells in each population that are Wt1-GFP positive. Epi= epididymal, Mes= mesenteric, RP= retroperitoneal, and OM= omentum. (n=4. Data represent the mean ±s.e.m.). (e), Effect of deleting Wt1 on the percentage of different populations of adipose progenitors. FACS analysis of percentage of adipose progenitors in fat pad from 3-weeks old female WGER mice (CAGGCreERT2.Wt1loxp/GFP) that were injected with tamoxifen and harvested at day 7 post-injection (n=4 for each group. Data represent the mean ±s.e.m. and one-tailed Student’s t-tests were used to assess statistical significance. *P< 0.0.5). Cre-negative (CAGG+/+.Wt1loxp/GFP) littermates injected with tamoxifen are included as the control.
To determine if Wt1 has a role in regulating the behavior of adipose progenitors, we deleted Wt1 using a ubiquitous tamoxifen-inducible model. In this model, one allele of the Wt1 locus is floxed by loxP sites and the other allele is a GFP knockin that disrupts Wt1 function (CAGG−CreERT2; Wt1loxp/GFP). We performed the deletion in 3-week old mice and harvested the tissues 7 days after the first dose of tamoxifen (n=4 for each group). We saw a trend of reduction in the % of GFP cells in the Lin−CD31−CD29+CD34+Sca1+CD24− population in all fat pads analysed; however the difference was only statistically significant in epididymal (p=0.015, Fig. 3e) and omental fat (p=0.042, Fig. 3e), but not in the mesenteric and epicardial fat (Fig. 3e). Although there was a reduction in the % of Lin−CD31−CD29+CD34+Sca1+CD24+ cells that were GFP+ in the epididymal and epicardial fat, this was not statistically significant.
Like other visceral organs that are covered by a layer of Wt1-expressing mesothelium, we also observed that the visceral adipose depots are lined by a GFP-positive mesothelium. Fig. 2k shows epididymal fat at 6.5 months following tamoxifen induction at 4 months where GFP is indicated in red and the adipocyte marker perilipin is marked in green. This mesothelial layer still expressed endogenous Wt1 and GFP 1.2 years after being induced at E14.5 (Fig. 2l). We also observed a Wt1-positive and cytokeratin-positive mesothelial layer in the other visceral fat depots (Supplementary Fig. 4a). The importance of the mesothelium as a source of mesenchymal progenitors for interstitial cells/structures during development has been demonstrated in the heart, lung, gut, and liver10,15,18-23. Furthermore, the peritoneum and its extensions, the mesentery and omentum are essentially mesothelial structures which develop fat depots. Therefore, we hypothesized that the mesothelium might be able to contribute to the adipocytes which often physically attach to the visceral organs. Consistent with this idea, in the lineage tracing model, a short pulse of tamoxifen (induced at E14.5 and analysed at E16.5) only labeled mesothelial cells at the site where future fat pads will be formed (Supplementary Fig. 4b). Before postnatal day 4, adipocytes are not yet formed in the thin, translucent membrane-like structure adjacent to the testes which later becomes the epididymal fat pad24. Han et al developed an ex-vivo culture system for this epididymal appendage and showed that it can produce adipocytes, following the normal time course of fat production in vivo. Furthermore, they showed that if this structure was removed in vivo, epididymal fat was no longer produced. We reasoned that this structure looks very similar to a mesothelium and hence it would express Wt1. Therefore we cultured this layer to see if it expresses Wt1, a major marker for the mesothelium, and to determine if Wt1 expressing cells contribute to mature adipocytes. Using pups from the Wt1−CreERT2;mTmG line, this layer of ‘mesothelium’ was dissected from postnatal day 3 pups and cultured ex vivo in medium containing 4-OH tamoxifen and adipogenic medium. Time lapse video showed that all cells in the mesothelium expressed RFP at the beginning and no GFP signals were detected. After 5.5-9.5 hours, many cells expreseds GFP and some of these started to migrate out from the mesothelium (Supplementary Video 1). No GFP cells were detected in the Cre-negative mesothelium from the Wt1−CreERT2; mTmG pups (Supplementary Video 2). After 110 hours, some cells that migrated out started to produce lipid droplets. The mesothelial nature of the epididymal appendage was confirmed by immunohistochemistry using mesothelin antibody (Supplementary Fig. 5a). Wt1-GFP embryo stained with the mesothelin antibody was used a positive control (Supplementary Fig. 5b).
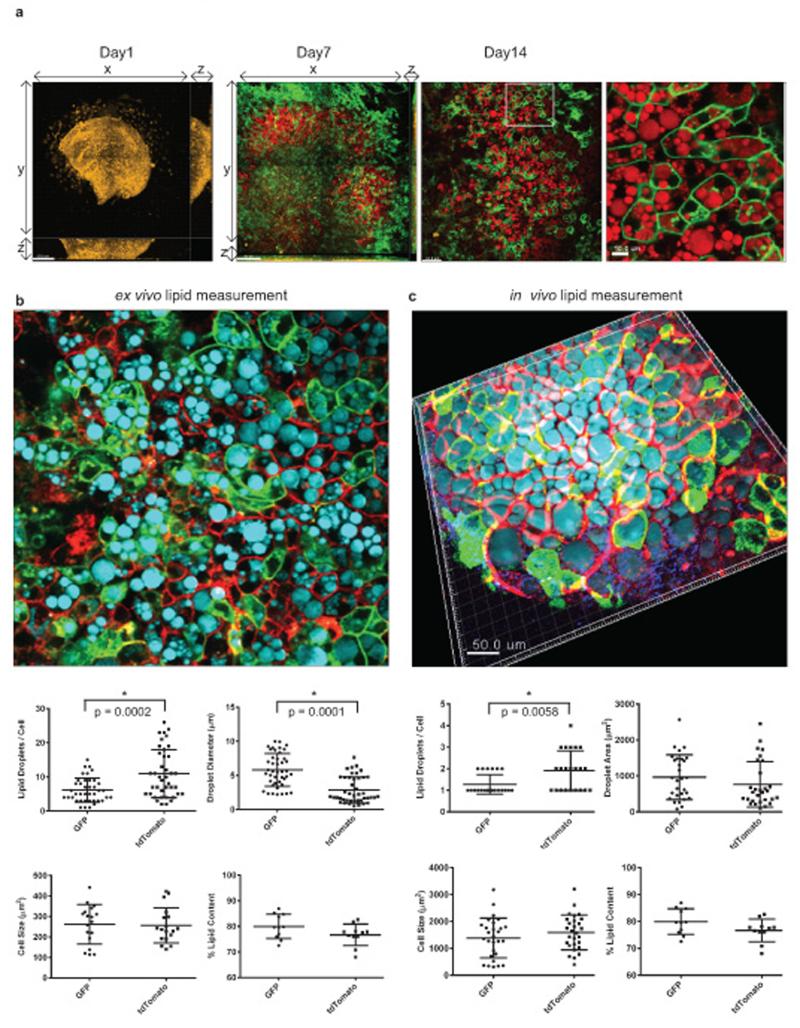
We then used multiphoton microscopy to further characterize the lipid droplet-containing cells in the center of the explant culture. Lipid droplets generate strong CARS (Coherent anti-Stokes Raman scattering) signals (indicated in red). The explant was imaged on day 1, day 7, and day 14 after culturing with adipogenesis medium. It is clearly seen that there are Wt1-derived cells from the epididymal appendage (i.e. cells with membranous GFP signal) that are capable of generating lipid containing adipocytes (Fig. 4a). We confirmed that the lipid droplets-filled cells were adipocytes by staining with FABP4 and perilipin antibodies (Supplementary Fig. 5c,d). From results obtained from both the explant culture system and in vivo lineage tracing in adult mice, there are Wt1-derived and non-Wt1-derived adipocytes. We were interested in this heterogeneity and in knowing if the Wt1-derived adipocytes (i.e. GFP+) differ from the other adipocytes (RFP+). In the ex vivo system (Fig. 4b), preliminary results suggested that Wt1-lineage adipocytes had fewer but larger lipid droplets per cell c.f. RFP+ adipocytes. Similarly, in the in vivo epididymal fat there were fewer droplets in the GFP+ cells than the RFP+ cells but there was no significant difference in droplet size. The cell size and % of lipid content did not differ between GFP+ or RFP+ adipocytes either in the ex vivo or in vivo systems (Fig. 4c).
Figure 4. Characterising an ex vivo model of mesothelium/epididymal appendage differentiation into adipocytes using multiphoton microscopy.
a, Multiphoton microscope images showing epididymal appendage explants (Wt1CreERT2;mTmG/+) cultured in adipogenesis differentiation medium at Day 1, Day 7, and Day 14. Membranous GFP signal is indicated in green. CARS is used to detect lipid (indicated in red). An enlarged image of Day 14 is also included. Lipid droplet analysis of the Wt1-derived adipocytes (green) and the neighbouring adipocytes (red) from the explants culture system (b) or from epididymal fat pad induced by tamoxifen in vivo (c). (green = GFP, red = tdTomato, cyan = CARS). Analysis includes quantification of number of lipid droplets / cell, quantification of lipid droplet diameter, quantification of cell size, and quantification of % lipid content / cell. Statistics source data for (c) can be found in Supplementary Table 5.
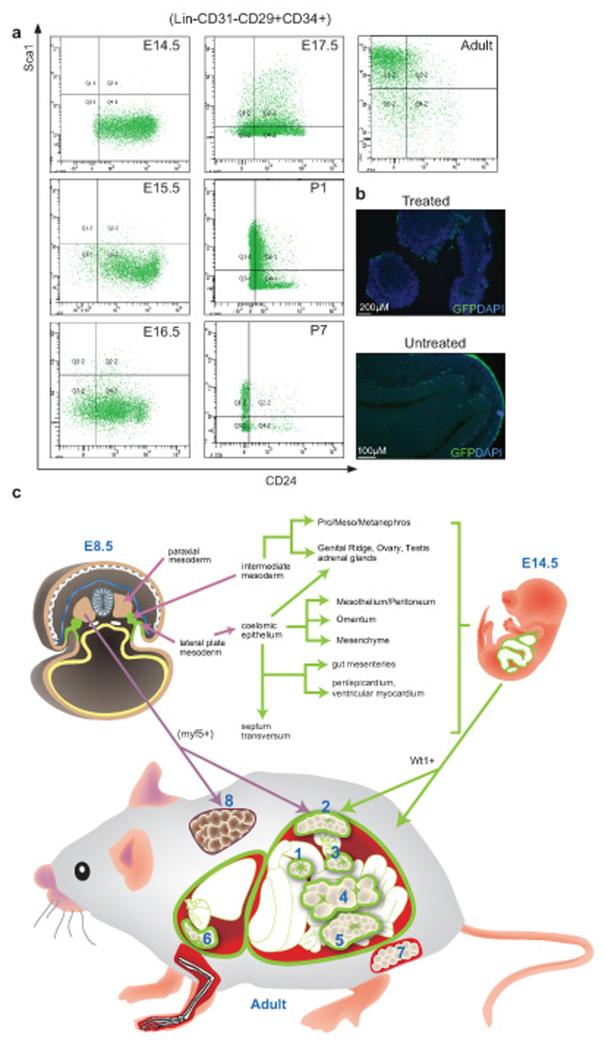
We hypothesized that mesothelium might be a source of visceral adipocytes and hence we were interested in knowing if mesothelium during development shares the cell surface marker expression pattern of adipose progenitors and preadipocytes seen in the adult fat pads. FACS analysis was carried out on GFP+ mesothelial cells removed by gentle collagenase treatment (Fig. 5b). We chose E14.5 as a starting point as this is the stage at which embryos were induced in the previous lineage tracing analysis (Fig. 2). At E14.5, the mesothelium from Wt1-GFP embryos is Lin−CD31−CD34+CD29+CD24+Sca1−. The expression pattern remains unchanged at E15.5 and E16.5. There is an emergence of Lin−CD31−CD34+CD29+CD24+Sca1+ population of cells at E17.5. At P1 and P7, there was a reduction in cells with CD24+ expression and the population of Sca1+ cells increased. In adult, the majority of cells are Lin−CD31−CD34+CD29+Sca1+CD24− (Fig. 5a). Hence the mesothelium expresses cell surface markers characteristic of WAT progenitors and preadipocytes and there is a gradual shift in the pattern that broadly resembles that described for subcutaneous WAT4.
Figure 5. FACS profiling of mesothelium and mesothelium-derived cells by adipose progenitor markers.
(a) Mesothelium and the mesothelium-derived cells collected from the Wt1-GFP line at different developmental stages and adult were stained with adipose progenitor surface markers. Representative FACS plots showing GFP+ cells stained with the markers. (b) Immunofluorescence staining showing the mesothelium staining at the surface of visceral organs (E15.5), with and without collagenase treatment. GFP is indicated in green and cell nuclei are stained with DAPI (blue). (c) Summary of Wt1-expressing WATs and their possible origins during development. In E8.5, Wt1 is first expressed in the intermediate mesoderm and lateral plate mesoderm (highlighted in green). Wt1+ mesenchymal cells from intermediate mesoderm later give rise to pro/meso/metanephros, genital ridge, ovary, testis, and adrenal glands. Wt1-expressing coelomic epithelium (derived from lateral plate mesoderm) contributes to mesothelium, mesenchyme, and also genital ridge, ovary, testis, and adrenal glands. At E9.5, Wt1 expression is also found in septum transversum, which contributes to gut mesenteries, hepatic sinusoids, peri/epicardium, ventricular myocardium see note above, and diaphragm. At E14.5, Wt1 is expressed in the developing kidney, gonads, adrenal glands, spleen, omentum, mesenteries, the mesothelial layer of all visceral organs (including epicardium and pericardium), and the peritoneum, as well as mesenchyme located near these tissues; the mesothelium and mesenchyme are represented in green. In the adult, we found Wt1 expression in all the visceral fat pads including omentum (1), retroperitoneal (2), perirenal (3), mesenteric (4), epididymal (5) in the abdominal cavity, and epicardial (6) in the thoracic cavity. Its expression is not detected in the subcutaneous (7) and BAT (8) which are both outside the body cavity. It has been shown previously that the myf5+ population of cells in the paraxial mesoderm contributes to BAT as well as retroperitoneal WAT26. The origin of subcutaneous WAT (7) is currently not known.
To confirm the mesothelial nature of the cells used for the FACS analysis, we used Q-PCR to measure the mRNA levels of two validated mesothelial markers, Msln and Upk3b25, in E14.5 and adult preparations. The levels of Msln and Upk3b mRNA were approximately two orders of magnitude higher in the GFP+ cells than in the GFP negative cells from the collagenase-treated layer at both E14.5 and adult stages (Supplementary Table 2a). Importantly the GFP+ cells after a brief pulse of tamoxifen in the lineage tracing system, and GFP+ cells isolated from the epididymal appendage used for explant showed similar absolute levels of mesothelial marker mRNA expression to the validated mesothelia taken at E14.5 and adult (Supplementary Table 2b,c).
One major conclusion from this study is that visceral and subcutaneous WAT, the so-called bad and good fat, have different origins during development. At least 30-80% (depending on the depot) of visceral adipocytes in 14 month old mice have arisen from cells that express Wt1 between E14.5-16.5 (the likely duration of action of tamoxifen). Given that only a single dose of tamoxifen has been administered, Cre-mediated recombination is unlikely to be complete and some fat progenitors will arise outside this timeframe, these percentages are likely to be under-estimates. Taken together, these data suggest that Wt1 marks true long-term adipocyte progenitors late in gestation. We find no contribution of Wt1 expressing cells to subcutaneous WAT or BAT during embryogenesis or in postnatal life in the fat pads analysed. This conclusion is also supported by experiments using a constitutive Wt1Cre for the lineage analysis. The question arises as to the exact location and nature of the Wt1 expressing adipose progenitors/pre-adipocytes. Over the past few years, it has become evident that the mesothelium is an abundant source of mesenchymal cells that contribute to a variety of tissues and cell types10,15,18-23. Here we provide evidence that the mesothelium is a source for at least some visceral WAT progenitors/ pre-adipocytes. Firstly, we show that visceral WAT has a Wt1-expressing mesothelial layer. Secondly, in the region where visceral fat will form a short pulse of tamoxifen at E14.5 in the lineage tracing model predominantly labels mesothelial cells as revealed by immunohistochemistry and Q-PCR on sorted cells. Thirdly, using an ex-vivo culture system, we show that an epididymal mesothelial appendage can produce adipocytes from Wt1-expressing cells. Finally, Wt1+ mesothelial cells express cell surface markers characteristic of WAT progenitors late in gestation and this shifts postnatally to a cell surface marker pattern characteristic of pre-adipocytes; thus approximating a scenario reported for subcutaneous WAT4. The mesothelium has its origins in the lateral plate mesoderm and we propose that this is the source of some adipose progenitors in all visceral WAT depots. Some visceral WAT may also arise from Wt1 expressing cells derived from the intermediate mesoderm. BAT has been shown to arise from paraxial mesoderm and, consistent with these different mesodermal origins, we find no contribution of Wt1 expressing cells to the BAT lineage. This leaves open the question of the developmental origins of subcutaneous fat. It seems reasonable to hypothesise that the source will be outside the peritoneum. The diagram in Fig. 5c explains these conclusions and speculations.
A second conclusion from the lineage tracing is that Wt1 contributes to a subset of adipocytes and that the proportion varies between visceral depots. Support for this idea of progenitor heterogeneity comes from the study of Sanchez-Gurmaches et al26 who showed that 50% of retroperitoneal fat arises from myf5 expressing cells but at most only a few percent of epididymal adipocytes arise from this source. This ties in nicely with our demonstration that the majority of epididymal fat arises from Wt1+ progenitors, whereas fewer than 50% of retroperitoneal adipocytes derive from Wt1+ progenitors. In the adult, the majority of Wt1 positive cells in the SVF express markers characteristic of pre-adipocytes. The proportion of pre-adipoctyes expressing Wt1 varies from 10-60% between depots, again supporting the idea of heterogeneity. Similarly, depending on the depot, Wt1+ cells comprise 4-40% of the adult progenitor population, being most abundant in omental and epicardial fat. All this raises the interesting question of whether the adipocytes arising from Wt1+ progenitors/pre-adipocytes have different properties from the adipocytes arising from the Wt1− progenitors/pre-adipocytes. Our first experiments to address this issue using multiphoton microscopy on in vivo and ex-vivo epididymal fat suggest that the adipocytes arising from Wt1 progenitors/ pre-adipocytes have fewer droplets. More work will be required to determine if the Wt1-derived adipocytes have different metabolic properties than the non-Wt1-derived adipocytes. Our preliminary data suggest that Wt1 is not only a marker for visceral fat progenitors but may also function in their regulation. More work will be needed in mice and in culture to investigate this and the mechanism involved.
Supplementary Material
Acknowledgements
We thank Dr Paul Perry and Mr Matt Pearson (MRC HGU, IGMM, Edinburgh) for assistance with image capturing and time-lapse videoing, Mr Craig Nicol and Dr Laura Lettice for assistance with graphic design and publication (MRC HGU, IGMM, Edinburgh), Lynne Ramage (University/BHF Centre for Cardiovascular Science, Edinburgh) for technical assistance with human adipose tissues, Dr Rita Carmona and Dr Elena Cano (University of Malaga, Malaga) for assistance with immunostaining, Miss Elisabeth Freyer (MRC HGU, IGMM, Edinburgh) and Dr Martin Waterfall (School of Biological Sciences, Edinburgh University, Edinburgh) for assistance of running the FACS facility. Human sample collection was funded by the EU FP7 programme, the British Heart Foundation and the Medical Research Council. This work has been supported by a Medical Research Council core grant to the Human Genetics Unit (Edinburgh), grant BFU2011-25304 (MINECO, Spain), grant BFU2012-25304, and Wellcome Trust (091374/z/10/z). AS was supported by grants from ARC ( SL120120304626), ANR (ADSTEM) and FRM (DEQ20090515425).
Footnotes
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. pii: S0092-8674(08)01194-X ; doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 2.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. pii: 1156232 ; doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran KV, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. pii: S1550-4131(12)00012-5 ; doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. pii: ncb2696 ; doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry R, et al. Esrrg functions in early branch generation of the ureteric bud and is essential for normal development of the renal papilla. Hum Mol Genet. 2011;20:917–926. doi: 10.1093/hmg/ddq530. pii: ddq530 ; doi: 10.1093/hmg/ddq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. 2012;8:55–66. doi: 10.1007/s12015-011-9242-x. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- 7.Macotela Y, et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. pii: db11-1753 ; doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchkonia T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–307. doi: 10.1152/ajpendo.00202.2006. pii: 00202.2006 ; doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, et al. Adipose depots possess unique developmental gene signatures. Obesity. 2010;18:872–878. doi: 10.1038/oby.2009.512. pii: oby2009512 ; doi: 10.1038/oby.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Estrada OM, et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2010;42:89–93. doi: 10.1038/ng.494. pii: ng.494 ; doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essafi A, et al. A wt1-controlled chromatin switching mechanism underpins tissue-specific wnt4 activation and repression. Dev Cell. 2011;21:559–574. doi: 10.1016/j.devcel.2011.07.014. pii: S1534-5807(11)00306-6 ; doi: 10.1016/j.devcel.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chau YY, et al. Acute multiple organ failure in adult mice deleted for the developmental regulator Wt1. PLoS Genet. 2011;7:e1002404. doi: 10.1371/journal.pgen.1002404. pii: PGENETICS-D-11-00778 ; doi: 10.1371/journal.pgen.1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 14.Chau YY, Hastie ND. The role of Wt1 in regulating mesenchyme in cancer, development, and tissue homeostasis. Trends Genet. 2012;28:515–524. doi: 10.1016/j.tig.2012.04.004. pii: S0168-9525(12)00068-6 ; doi: 10.1016/j.tig.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. pii: 132/23/5317 ; doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 16.Rosito GA, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. pii: CIRCULATIONAHA.107.743062 ; doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 17.Ding J, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. pii: ajcn.2008.27358 ; doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ijpenberg A, et al. Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev Biol. 2007;312:157–170. doi: 10.1016/j.ydbio.2007.09.014. pii: S0012-1606(07)01353-X ; doi: 10.1016/j.ydbio.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Rinkevich Y, et al. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012 doi: 10.1038/ncb2610. pii: ncb2610 ; doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Que J, et al. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. pii: 0808649105 ; doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmona R, Cano E, Mattiotti A, Gaztambide J, Munoz-Chapuli R. Cells Derived from the Coelomic Epithelium Contribute to Multiple Gastrointestinal Tissues in Mouse Embryos. PLoS One. 2013;8:e55890. doi: 10.1371/journal.pone.0055890. pii: PONE-D-12-11792 ; doi: 10.1371/journal.pone.0055890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano E, Carmona R, Munoz-Chapuli R. Wt1-expressing progenitors contribute to multiple tissues in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2013;305:L322–332. doi: 10.1152/ajplung.00424.2012. pii: ajplung.00424.2012 ; doi: 10.1152/ajplung.00424.2012. [DOI] [PubMed] [Google Scholar]
- 24.Han J, et al. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. pii: 138/22/5027 ; doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 25.Kanamori-Katayama M, et al. LRRN4 and UPK3B are markers of primary mesothelial cells. PLoS One. 2011;6:e25391. doi: 10.1371/journal.pone.0025391. pii: PONE-D-11-13799 ; doi: 10.1371/journal.pone.0025391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Gurmaches J, et al. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16:348–362. doi: 10.1016/j.cmet.2012.08.003. pii: S1550-4131(12)00325-7 ; doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosen N, et al. The Wilms’ tumor gene WT1-GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis. Leukemia. 2007;21:1783–1791. doi: 10.1038/sj.leu.2404752. pii: 2404752 ; doi: 10.1038/sj.leu.2404752. [DOI] [PubMed] [Google Scholar]
- 28.del Monte G, et al. Differential Notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ Res. 2011;108:824–836. doi: 10.1161/CIRCRESAHA.110.229062. pii: CIRCRESAHA.110.229062 ; doi: 10.1161/CIRCRESAHA.110.229062. [DOI] [PubMed] [Google Scholar]
- 29.Wessels A, et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol. 2012;366:111–124. doi: 10.1016/j.ydbio.2012.04.020. pii: S0012-1606(12)00209-6 ; doi: 10.1016/j.ydbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.