Abstract
A possible association between visual acuity (VA) and dementia was investigated in 2716 subjects who were aged between 53 and 102 at first visit and had varying degrees of dementia. Better VA was found to be significantly correlated with a lower dementia level (person coefficient range 0.146–0.261 over 10 years of follow-up, all correlations are significant, p<0.0001) as well as with a higher global cognitive score (person coefficient range −0.254 to −0.318 over 10 years of follow-up, all correlations are significant, p<0.0001), a grade encompassing 19 different cognitive tests. This correlation remained significant after adjustment for age, years of education, gender, use of ophthalmic drugs and years of follow-up.
Visual and cognitive impairments are common causes of disability worldwide and contribute profoundly both to personal distress and to the burden on care providers. The prevalence of these impairments is increasing rapidly with the ageing of the population.1 Previous studies have reported a possible association between visual disturbances and cognitive decline. Attempts to establish such an association are fraught with methodological issues, including the fact that cognitive functions are often measured by tests such as the Mini Mental State Examination (MMSE), the results of which may be skewed by impaired vision. While some studies have demonstrated that visually impaired subjects score lower on tasks that are not necessarily vision dependent,2 others have related lower cognitive scores only to difficulty in completion of the test.3 Yet other studies have excluded visually impaired individuals as being ‘non-testable’, perhaps overlooking the possibility that this population may have a higher rate of dementia.
On the other hand, some studies have pointed to a strong association between cognitive and visual decline. In one retrospective study, untreated poor vision was found to be associated with a ninefold risk of developing Alzheimer’s disease (AD) and a fivefold risk of developing cognitive impairment with no dementia.4 Vision impairment predicting cognitive and functional decline was also demonstrated in a cohort of older women aged 69 or older.5
A number of ocular pathologies have been specifically associated with reduced cognitive functions. Besides the fact that age is a main risk factor for both AD and age-related macular degeneration (AMD), these two conditions share several characteristics. Deposition of extracellular β-amyloid is seen both in the senile plaques of AD and in the drusen of AMD, and complement system components have been implicated in both disease processes.6
In its 16th report, the Age-Related Eye Disease Study group noted that even after adjustment for several possible confounding factors, mean scores of cognitive function measured by tests in its Cognitive Function Battery declined significantly with increased macular abnormalities and reduced visual acuity (VA).7 A smaller study, conducted in Korea, compared the global cognitive scores (GCSs) of elderly patients with and without AMD. VA was found to be significantly correlated with cognitive global function in patients with mild cognitive impairment as reflected by the MMSE score, more distinctly so in AMD patients. The subgroup of ‘geographic atrophy’ was associated with the worst cognitive function.8
In other studies, it was shown that cognitive impairment may be common in patients with glaucoma.9 Visual field defects compatible with a diagnosis of glaucoma were found to occur at a higher rate in elderly patients with AD in Europe10 and in Japan.11 Anatomical and histological similarities in thinning of the retinal nerve fibre layer and in retinal ganglion cell damage were observed both in AD and glaucoma.12,13 It is still a matter of debate, however, whether the association between reduced vision and cognitive decline signifies a common pathophysiological mechanism and whether a lack of sensory input contributes to the neurodegenerative process of AD.
Several studies have shown that treating visual impairment by cataract surgery improves cognitive function scores.14,15 An expansion of grey matter volume in the visual cortex was seen 6 weeks after cataract surgery, pointing to the possibility of cortical plasticity following visual improvement in adults.16
In the present study, we attempted to gain a better understanding of the possible association between vision impairment and cognitive function by analysing one of the largest used databases of elderly subjects suffering from various degrees of dementia and undergoing follow-up over a relatively long period.
SUBJECTS AND METHODS
The Religious Orders Study based at Rush University Medical Center in Chicago, Illinois, is a longitudinal clinical–pathologic cohort study of ageing and AD in a population of 1100 older Catholic nuns, priests and brothers who undergo annual medical and psychological evaluations and have agreed to donate their brains to science after death.17 The Memory and Aging Project at Washington University School of Medicine and Barnes-Jewish Hospital is a long-term epidemiologic clinical–pathologic study of cognitive functioning in more than 1200 ageing individuals from a range of backgrounds who undergo annual clinical evaluations and have agreed to donation of brain, spinal cord and muscle after death. For purposes of the present study, we extracted data accumulated over a follow-up period of approximately 18 years between 1994 and 2013 from the records of a total of 2716 subjects from the two studies. All of the study participants were aged between 53 and 102 at first visit.
Demographic data (race, gender and years of education), as well as cognitive data and data pertaining to VA, had been recorded at baseline (see tables 1 and 2). Thereafter, follow-up data on cognitive status and VA were recorded annually. Cognitive function was measured by a GCS, a summary of raw scores of 19 different tests estimating episodic memory, semantic memory, working memory, perceptual speed, neuropsychological screening and visuospatial ability.18 Scores were converted to standard scores, averaged and normalised. Each year, an estimated clinical diagnosis score termed the dementia level was awarded on a categorical scale over the five categories of ‘no cognitive impairment’, ‘mild cognitive impairment’, ‘mild cognitive impairment and other diagnoses’, ‘Alzheimer’s disease’ and ‘Alzheimer’s disease and other diagnoses’. Subjects who obtained an assessment in one of the ‘other diagnoses’ categories were excluded from analysis in order to avoid diagnoses with aetiologies common to both visual impairment and cognitive decline, such as cerebrovascular accident.
Table 1.
Patient demographics and baseline characteristics
| N | Mean | SD | Median | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Age | 2718 | 78.13 | 7.86 | 78.92 | 53.35 | 102.15 |
| Years of education | 2716 | 16.01 | 3.77 | 16.00 | 0.00 | 30.00 |
| Follow-up years | 2718 | 0.02 | 0.30 | 0.00 | 0.00 | 10.00 |
| Visual acuity | 2718 | 1.58 | 1.30 | 1.00 | 1.00 | 7.00 |
| Level of dementia | 2715 | 1.44 | 0.80 | 1.00 | 1.00 | 5.00 |
| Global score | 2718 | −0.00 | 0.64 | 0.10 | −3.99 | 1.46 |
Table 2.
Patient baseline characteristics
| N | % | |
|---|---|---|
| Gender | ||
| Women | 1940 | 71.43 |
| Men | 776 | 28.57 |
| Visual acuity | ||
| 1 | 2018 | 74.25 |
| 2 | 346 | 12.73 |
| 3 | 137 | 5.04 |
| 4 | 83 | 3.05 |
| 5 | 30 | 1.10 |
| 6 | 32 | 1.18 |
| 7 | 72 | 2.65 |
| Ophthalmic drugs | ||
| No | 2236 | 82.27 |
| Yes | 482 | 17.73 |
| Level of dementia | ||
| 1 | 1861 | 68.55 |
| 2 | 682 | 25.12 |
| 3 | 11 | 0.41 |
| 4 | 150 | 5.52 |
| 5 | 11 | 0.41 |
VA was tested with both eyes open. A card was held at a distance of 14 inches and measured at seven increments ranging from 20/40 or better (arbitrarily categorised as −1) to 20/400 or worse (categorised as −7).
The use of ophthalmic drops (including artificial tears and antiglaucoma and anti-inflammatory agents) was recorded, but no categorical distinction was made with regard to medication group.
Statistical methods
Pearson and Spearman correlation coefficients were used, as applicable, to assess relationships among severity of dementia, GCS and VA. These indices were calculated separately in each year of follow-up.
The GCS values obtained by patients treated with ophthalmic preparations and by untreated patients were compared by the use of Student t test, and their dementia levels were compared using the non-parametric Mann–Whitney test.
ANOVA with repeated measures (time points) was used to examine the effects of VA, age, years of education, gender, use of ophthalmic drugs and years of follow-up on the GCS. A mixed model was chosen for this analysis in order to account for the hierarchical structure of the data, time-dependent covariates and missing values.
All statistical analyses were performed using SAS for WindowsV.9.3.
RESULTS
A total of 2716 subject records were reviewed (1940 women, 776 men). The mean age of subjects at the beginning of follow-up was 78 (range 53–102).
Racial distribution was 92% white and 7% African-American. The remaining 1% was composed of Native Americans and Asians. The average number of years of education was 16 (15.6 years for women and 16.8 for men). The mean follow-up time was 4.4 years (range 0–18 years), and the mean number of visits was 6.5 (range 1–19).
At the first visit (baseline), 68.55% of subjects had no cognitive impairment, 25% had mild cognitive impairment and 5.5% had been diagnosed with AD. The average GCS was 0.00636. At the same time, VA of at least 20/40 was obtained by 74% of the subjects, 20/50 by 12% and 20/400 by only 2.6%.
Approximately one-half (51%), that is, 1122 of the subjects had ‘no cognitive decline’ at baseline, but were found to have progressed to some form of dementia during follow-up. Whereas only 124 of the subjects (4.5%) already had AD at the first visit, during follow-up 651 more subjects (23.95%) were diagnosed with AD (see table 3).
Table 3.
Dementia levels at first follow-up and last follow-up
| Dementia level | At first follow-up | At last follow-up | ||
|---|---|---|---|---|
| Number | Per cent | Number | Per cent | |
| 1 | 2203 | 81.05 | 1081 | 39.77 |
| 2 | 381 | 14.02 | 961 | 35.36 |
| 3 | 10 | 0.37 | 25 | 0.92 |
| 4 | 117 | 4.30 | 582 | 21.41 |
| 5 | 7 | 0.26 | 69 | 2.54 |
Subjects who had ‘no cognitive decline’ at baseline had a mean VA of 1.4 (median of 1, corresponding to a VA of 20/40 or better), whereas subjects diagnosed with AD had a mean VA of 2.27 (also with a median of 1).
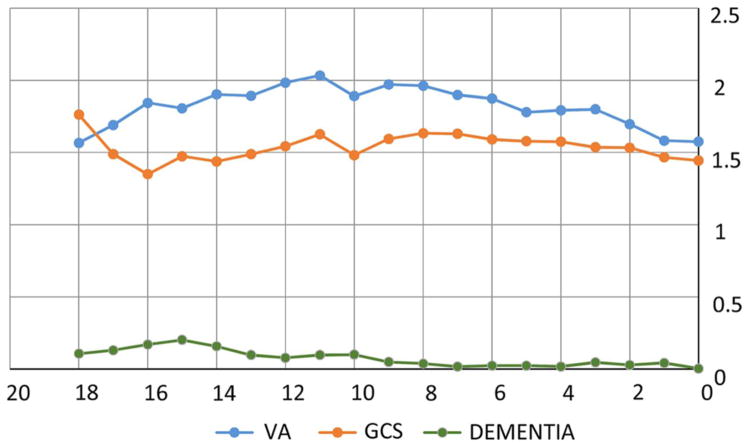
Changes in severity of VA, GCS and dementia over time are recorded in figure 1.
Figure 1.
Changes in visual acuity (VA), global cognitive score (GCS) and dementia over 18 years of follow-up. Access the article online to view this figure in colour.
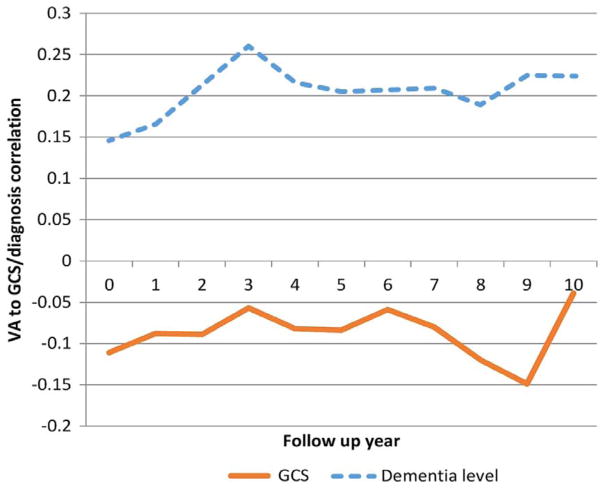
Pearson correlation coefficient was calculated in each year of follow-up and demonstrated a low but significant correlation between VA and level of dementia. The trend is depicted in figure 2.
Figure 2.
Correlation between visual acuity (VA) and dementia level (global cognitive score (GCS)) over 10 years of follow-up. The GCS shows a negative correlation with VA because a higher VA score (as arbitrarily determined in this study) signifies a lower VA. This relationship is maintained during the first 7 follow-up years, as higher VA can be seen to be correlated with better (higher) GCS. Access the article online to view this figure in colour.
The correlation was strongest during the first 3 years of follow-up years. It then remained stable until the 7th follow-up year and weakened thereafter.
Similarly, better baseline VA was significantly associated with a lower level of dementia and a higher GCS at the end of follow-up.
ANOVA with repeated measures using the mixed model (shown in table 4) was used to assess the relationship between GCS levels and parameters such as VA, age at first visit, years of education and gender. The main result, when adjusted for age at first visit, number of years of education, gender and length of follow-up, was a significant positive correlation between VA and GCS (better VA with better GCS).
Table 4.
Mixed model analysis for associations with global cognitive score
| Effect | Estimate | SE | Pr>|t| |
|---|---|---|---|
| Visual acuity | −0.03312 | 0.002021 | <0.0001 |
| Age at baseline | −0.03897 | 0.001614 | <0.0001 |
| Years of education | 0.04537 | 0.003354 | <0.0001 |
| Women | 0.1209 | 0.02734 | <0.0001 |
| Years of follow-up | −0.04553 | 0.001789 | <0.0001 |
| Ophthalmic drops | 0.01016 | 0.007030 | 0.1489 |
Each of the secondary parameters (listed in table 4) was also significantly correlated with GCS. A higher (better) GCS was correlated with younger age, shorter follow-up, female gender and more years of education. There was no correlation between GCS and the use of ophthalmic drugs.
Because of the high dropout rate after the first 10 years, the full cohort analysis was repeated twice, once for subjects with at least 4 years of follow-up and once for subjects who were followed up for at least 10 years. Similar results were obtained in each case.
DISCUSSION
As the fastest growing population sector in most countries is said to be the oldest old (>85 years of age),19 dealing with the consequences of longevity takes on a growing importance. One consequence is dementia, the prevalence of which according to a US population-based study increases with age from 5.0% at the age of 71–79 to 37.4% of those aged 90 and older.20
An association between visual impairment and cognitive decline has been previously described in a number of studies4,5,21 but up to now has not yet been characterised. The fact that both pathologies become more prevalent with older age raises the possibility that they share some common pathophysiological aspects, and thus that one may lead to the other.
The present large cohort study incorporates a follow-up of up to 18 years in an elderly population with various levels of cognitive function. We found an interrelationship between VA, dementia level and GCS, regardless of whether the analyses were conducted in terms of a single variable or in the context of age, gender or education. Moreover, the correlation was consistent whether applied to the entire cohort or as a subanalysis of subjects with at least a 4-year or a 10-year period of follow-up. The correlation coefficient was low, reflecting the fact that dementia is a multifactorial entity. Various pathophysiological, environmental and behavioural factors have previously been associated with the occurrence and natural history of cognitive decline.22–25
In contrast to some reports that the role of sensory function increases in importance with advancing age and may explain most differences in intelligence among the very old,26,27 we found the correlation between VA and cognitive function to be stronger at earlier stages of follow-up and to show a slight decrease over time. This observation may have two possible explanations. One is the increased variability of cognitive function with advancing age, as previously described.28,29 The other is an increasing role of factors other than vision that contribute to cognitive decline in older age.
The strength of this study derives from its very large population and long follow-up. However, it also has several limitations:
VA was measured in large increments that are not consistent with the LogMAR scale.
There is a potential for decreased VA to adversely affect the ability of some individuals to complete certain tasks used in neuropsychological testing.
Although a long follow-up period was noted for some subjects, there was a substantial dropout after the 7th year and the average follow-up was 4.48 years.
Where visual impairment was present, its aetiology was not recorded.
Lack of optimal correction of VA is very common among the elderly and this lack is a major cause of limitation of activities of daily living in this population.30 As visual impairment in most cases is treatable, at least to some extent, and since visual rehabilitation has been shown to improve several aspects of cognitive function even in elders with low vision,31 it is imperative to further validate and define the association of visual impairment and cognitive decline.
Our findings might become more useful if the ability to predict visual deterioration in the elderly is substantiated.32 This study, given its large cohort, further establishes this association and discloses its relevance at an earlier point than previously thought. An interesting possible implication is that early intervention towards vision correction may reduce the severity of dementia and its associated functional decline.
Acknowledgments
We thank the Rush Alzheimer’s Disease Center, which conducted the Religious Orders Study and the Rush Memory and Aging Project, with support from National Institute on Aging grants P30AG10161 and R01AG17917 and from the Illinois Department of Public Health.
Footnotes
Contributors
As the corresponding author, I hereby certify that each author has participated sufficiently in the intellectual content and the writing of the work to take public responsibility for it. I also confirm that neither of the authors have any relationships/conditions/circumstances that present potential competing interests. Sivan Elyashiv, MD.
Competing interests
None.
Ethcs approval
Rush institutional review board.
Provenance and peer review
Not commissioned; externally peer reviewed.
References
- 1.Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–34. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jefferis JM, Collerton J, Taylor JP, et al. The impact of visual impairment on Mini-Mental State Examination Scores in the Newcastle 85+ study. Age Ageing. 2012;41:565–8. doi: 10.1093/ageing/afs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jefferis JM, Taylor JP, Collerton J, et al. The association between diagnosed glaucoma and cataract and cognitive performance in very old people: cross-sectional findings from the Newcastle 85+ study. Ophthalmic Epidemiol. 2013;20:82–8. doi: 10.3109/09286586.2012.757626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers MA, Langa KM. Untreated poor vision: a contributing factor to late-life dementia. Am J Epidemiol. 2010;171:728–35. doi: 10.1093/aje/kwp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohno-Matsui K. Parallel findings in age-related macular degeneration and Alzheimer’s disease. Prog Retin Eye Res. 2011;30:217–38. doi: 10.1016/j.preteyeres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Cognitive impairment in the Age-Related Eye Disease Study AREDS Report No. 16. Arch Ophthalmol. 2006;124:537– 43. doi: 10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo SJ, Park KH, Ahn J, et al. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology. 2012;119:2094–101. doi: 10.1016/j.ophtha.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Yochim BP, Mueller AE. Prevalence of cognitive impairment, depression, and anxiety symptoms among older adults with glaucoma. J Glaucoma. 2012;21:250–4. doi: 10.1097/IJG.0b013e3182071b7e. [DOI] [PubMed] [Google Scholar]
- 10.Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur Neurol. 2002;47:165–8. doi: 10.1159/000047976. [DOI] [PubMed] [Google Scholar]
- 11.Tamura H, Kawakami H, Kanamoto T, et al. High frequency of open-angle glaucoma in Japanese patients with Alzheimer’s disease. J Neurol Sci. 2006;246:79–83. doi: 10.1016/j.jns.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Hedges TR, III, Perez Galves R, Speigelman D, et al. Retinal nerve fiber layer abnormalities in Alzheimer’s disease. Acta Ophthalmol Scand. 1996;74:271–5. doi: 10.1111/j.1600-0420.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 13.Blanks JC, Torigoe Y, Hinton DR, et al. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–84. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 14.Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am J Ophthalmol. 2008;146:404–9. doi: 10.1016/j.ajo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Tamura H, Tsukamoto H, Mukai S, et al. Improvement in cognitive impairment after cataract surgery in elderly patients. J Cataract Refract Surg. 2004;30:598–602. doi: 10.1016/j.jcrs.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Lou AR, Madsen KH, Julian HO, et al. Postoperative increase in grey matter volume in visual cortex after unilateral cataract surgery. Acta Ophthalmol. 2013;91:58–65. doi: 10.1111/j.1755-3768.2011.02304.x. [DOI] [PubMed] [Google Scholar]
- 17.Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20(3 suppl 2):S63–8. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Boyle PA, Buchman AS, Wilson RS, et al. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66:1339–44. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slavin MJ, Brodaty H, Sachdev PS. Challenges of diagnosing dementia in the oldest old population. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt051. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyes-Ortiz CA, Kuo YF. Near vision impairment predicts cognitive decline: data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc. 2005;53:681–6. doi: 10.1111/j.1532-5415.2005.53219.x. [DOI] [PubMed] [Google Scholar]
- 22.Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–45. doi: 10.2147/CLEP.S30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey DA. Alzheimer’s disease, dementia, mild cognitive impairment and the menopause: a ‘window of opportunity’? Womens Health (Lond Engl) 2013;9:279–90. doi: 10.2217/whe.13.22. [DOI] [PubMed] [Google Scholar]
- 24.Cheng ST, Chow PK, Song YQ, et al. Mental and physical activities delay cognitive decline in older persons with dementia. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 25.Karp A, Paillard-Borg S, Wang HX, et al. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 26.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–55. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 27.Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychol Aging. 1997;12:410–32. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- 28.Christensen H, Mackinnon AJ, Korten AE, et al. An analysis of diversity in the cognitive performance of elderly community dwellers: individual differences in change scores as a function of age. Psychol Aging. 1999;14:365–79. doi: 10.1037//0882-7974.14.3.365. [DOI] [PubMed] [Google Scholar]
- 29.Nelson EA, Dannefer D. Aged heterogeneity: fact or fiction? The fate of diversity in gerontological research. Gerontologist. 1992;32:17–23. doi: 10.1093/geront/32.1.17. [DOI] [PubMed] [Google Scholar]
- 30.Daien V, Pérès K, Villain M, et al. Visual impairment, optical correction, and their impact on activity limitations in elderly persons: the POLA study. Arch Intern Med. 2011;171:1206–7. doi: 10.1001/archinternmed.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitson HE. A low-vision rehabilitation program for patients with mild cognitive deficits. JAMA Ophthalmol. 2013;131:912–19. doi: 10.1001/jamaophthalmol.2013.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig DE. Factors accounting for the 4-year change in acuity in patients between 50 and 80 years. Optom Vis Sci. 2013;90:620–7. doi: 10.1097/OPX.0b013e318296ac4d. [DOI] [PMC free article] [PubMed] [Google Scholar]