Abstract
The transition from juvenile to adult is a fundamental process that allows animals to allocate resource toward reproduction after completing a certain amount of growth. In insects, growth to a species-specific target size induces pulses of the steroid hormone ecdysone that triggers metamorphosis and reproductive maturation. The past few years have seen significant progress in understanding the interplay of mechanisms that coordinate timing of ecdysone production and release. These studies show that the neuroendocrine system monitors complex size-related and nutritional signals, as well as external cues, to time production and release of ecdysone. Based on results discussed here, we suggest that developmental progression to adulthood is controlled by checkpoints that regulate the genetic timing program enabling it to adapt to different environmental conditions. These checkpoints utilize a number of signaling pathways to modulate ecdysone production in the prothoracic gland. Release of ecdysone activates an autonomous cascade of both feedforward and feedback signals that determine the duration of the ecdysone pulse at each developmental transitions. Conservation of the genetic mechanisms that coordinate the juvenile-adult transition suggests that insights from the fruit fly Drosophila will provide a framework for future investigation of developmental timing in metazoans.
1. INTRODUCTION
The timing of insect metamorphosis is not a simple function of chronological age, but instead adjusts itself depending on environmental conditions. Holometabolous insects develop through a series of larval stages called instars, each punctuated by a molt in which the old cuticle is shed and replaced by a new larger one. After a series of molts, the number of which is species-specific, a terminal larval phase is reached during which extensive growth occurs until a predetermined target size is reached. At this point, the larva enters the pupal stage and undergoes metamorphosis to form the reproductively mature adult (Mirth & Riddiford, 2007). The processes of metamorphosis has fascinated classical entomologist for almost a century in part because it touches upon a fundamental biological question: how are developmental transitions controlled? Answering this question has implications beyond just understanding developmental timing mechanisms in insects. It also will help us understand how growth and maturation processes are coordinated such that reproductively mature organisms are reliably produced despite potentially different environmental conditions encountered by each individual during development.
In insects growth and maturation are distinct processes with growth being restricted primarily to the larval stages (although some growth occurs during metamorphosis: Okamoto et al., 2009; Slaidina, Delanoue, Gronke, Partridge, & Leopold, 2009), which means that the size of the larva at the onset of metamorphosis largely determines the final size of the adult (Edgar, 2006; Mirth & Riddiford, 2007). Final body size is determined by the rate of mass accumulation and the duration of growth. Interestingly, recent studies show that members of the evolutionarily conserved insulin-like growth factor family coordinate both growth rate and duration by impinging on the endocrine system that controls onset of metamorphosis (Caldwell, Walkiewicz, & Stern, 2005; Colombani, Andersen, & Leopold, 2012; Colombani et al., 2005; Garelli, Gontijo, Miguela, Caparros, & Dominguez, 2012; Mirth, Truman, & Riddiford, 2005; Ou, Magico, & King-Jones, 2011; Walkiewicz & Stern, 2009). At the cellular level, two things determine body dimensions: the number of cells and their size. It is clear that the great size diversity among animals is largely determined by differences in cell number rather than cell volume. However, insects utilize both strategies to grow as many larval tissues sustain their rapid growth and metabolic activity by endoreplication cycles, a process of chromosomal replications without cell division (Edgar & Orr-Weaver, 2001; Lee, Davidson, & Duronio, 2009; Smith & Orr-Weaver, 1991). Increasing the DNA content allows cells to dramatically increase in volume without dividing and is responsible for most of the size increase during larval growth. During metamorphosis, the breakdown of obsolete endoreplicated larval tissues provides nutrients for growth and differentiation of mitotic neuroblasts and imaginal disk tissues that form the adult body.
Unlike the well-characterized genetic control of embryonic development, we are only now beginning to understand the developmental timing system that ensures unidirectional progression of developmental transitions. Recent genetic studies in the fruit fly Drosophila have provided new insight into how the developmental timing system evaluates larval growth and energy stores and integrates external cues such as photoperiod to time the onset of metamorphosis (Caldwell et al., 2005; Colombani et al., 2005, 2012; Garelli et al., 2012; Layalle, Arquier, & Leopold, 2008; McBrayer et al., 2007; Mirth et al., 2005). All of these signals are eventually communicated to the endocrine system responsible for producing the pulses of the steroid hormone 20-hydroxyecdysone (for simplicity, hereafter referred to as ecdysone) that triggers molting and metamorphosis (Gilbert, Rybczynski, & Warren, 2002). In Drosophila, a single pulse of ecdysone triggers each of the first two larval molts (Warren et al., 2006). During the terminal third instar stage, three low-level pulses followed by a high-level peak of ecdysone initiate the physiological and behavioral changes necessary to transform a food seeking larva into a nonfeeding immobile pupa. Although the roles of the initial low-level ecdysone peaks are not completely understood, the final major high-level peak triggers pupariation and the onset of metamorphosis.
Ecdysone is produced in the prothoracic gland (PG) by a series of reactions mediated by a Rieske oxygenase and enzymes encoded by the Halloween family of genes that include several cytochrome P450s and one dehydrogenase/reductase (Niwa et al., 2010; Ono et al., 2006; Petryk et al., 2003; Warren et al., 2002, 2004; Yoshiyama, Namiki, Mita, Kataoka, & Niwa, 2006; Yoshiyama-Yanagawa et al., 2011). Once released into circulation, ecdysone is taken up by several peripheral tissues including the gut, fat body and Malphigian tubules where it is converted to the active hormone 20-hydroxyecdysone by the P450 enzyme product of the shade gene (Petryk et al., 2003). Circulating 20-hydroxyecdysone then induces a systemic genetic response in multiple tissues by binding to a complex of the ecdysone receptor (EcR) and ultraspiracle, both members of the nuclear receptor family (King-Jones & Thummel, 2005). Peripheral tissues interpret the ecdysone signal in a cell-type specific manner, determined by the combination of specific EcR isoforms and cofactors expressed in the target tissue (Yamanaka, Rewitz, & O’Connor, 2013). The response to ecdysone includes the destruction of obsolete larval tissues by induction of cell death activators and the morphogenesis and differentiation of the imaginal disk tissues to adult structures (Baehrecke, 2000; D’Avino & Thummel, 2000; Siaussat, Porcheron, & Debernard, 2009).
The mechanisms timing the production and release of ecdysone are key to understanding developmental timing of metamorphosis. These are intimately linked to size cues because growth beyond a characteristic size triggers ecdysone release (Edgar, 2006; Mirth & Riddiford, 2007; Mirth & Shingleton, 2012). Here, we discuss progress from Drosophila studies that examine how the rates of ecdysone synthesis and inactivation/removal are regulated and illustrate how these studies provide insight into the mechanisms that time ecdysone pulses and their duration (Caceres et al., 2011; Caldwell et al., 2005; Colombani et al., 2005; Gibbens, Warren, Gilbert, & O’Connor, 2011; Layalle et al., 2008; McBrayer et al., 2007; Mirth et al., 2005; Ou et al., 2011; Rewitz, Larsen, et al., 2009; Rewitz, Yamanaka, Gilbert, & O’Connor, 2009; Rewitz, Yamanaka, & O’Connor, 2010).
2. CHECKPOINT CONTROLS FOR PROGRESSION OF DEVELOPMENT
2.1. Increasing complexity for control of ecdysone production and release
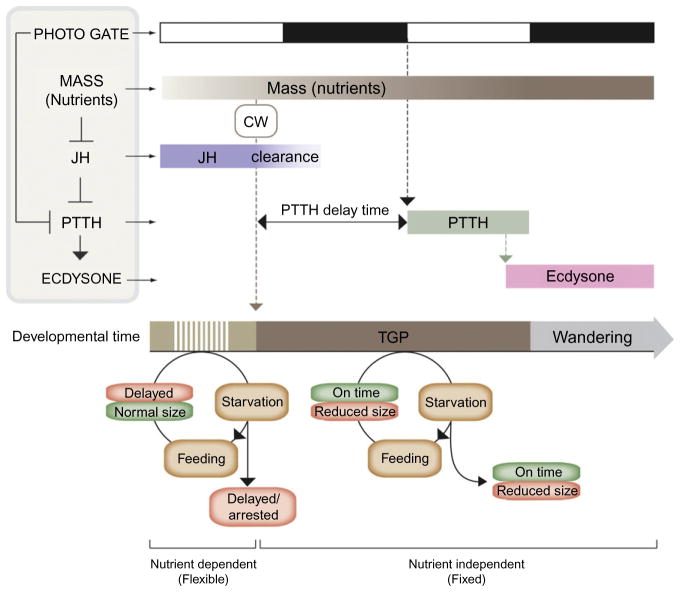
Early studies in Lepidoptera led to the development of a key concept known as “critical weight” that appears to switch larvae between a nutrition-dependent growth phase and a nutrition-independent timing program (Beadle, Tatum, & Glancy, 1938; Nijhout & Williams, 1974a, 1974b). Starvation before larvae reach critical weight suspends growth and delays developmental progression (Fig. 1.1). However, in larvae that have attained the correct target size, starvation prevents further growth but does not delay pupariation. Before attaining critical weight, larvae must pass another checkpoint called minimal viable weight, which is the minimal size at which sufficient nutrient storage has occurred to enable the larva to survive through metamorphosis without further feeding (Mirth & Riddiford, 2007; Mirth & Shingleton, 2012). Starvation before minimal viable weight results in larval death without an attempt to undergo metamorphosis. Minimal viable weight should not be mistaken for critical weight, which is the size where starvation no longer delays metamorphosis. In Drosophila minimal viable weight and critical weight are attained almost simultaneously, making the distinction difficult.
Figure 1.1.
Hormonal regulation of body size and timing of metamorphosis. According to classical work the larval accumulation of mass corresponding to the critical weight results in the breakdown of JH that inhibits PTTH release. Above critical weight, PTTH is release after a delay period determined by the time of JH clearance and the photo-period gating of PTTH. Starvation before critical weight results in a developmental delay of pupariation. If feeding is resumed critical weight is attained and normal body size achieved. In contrast, larvae starved above critical weight, when the metamorphic program is activated, pupariate on time but with a reduced size. As critical weight is independent of nutrition, final size is determined by the amount of growth in the interval between critical weight and cessation of feeding (wandering) called the terminal growth period (TGP). Photo gate, a PTTH gating mechanism imposed by the photo-period; CW, critical weight.
Several classical studies using lepidopteran insects suggest that attainment of critical weight is followed by a drop in juvenile hormone (JH) levels, which is permissive for prothoracicotropic hormone (PTTH) release at a certain time defined by the photoperiod. According to this scheme, the release of PTTH from the brain is the principal event committing the PG to ramp up ecdysone production and release to trigger metamorphosis (Nijhout & Williams, 1974a, 1974b). Although the decline in circulating JH levels following attainment of critical weight is believed to be the key determinant for PTTH release in lepidopterans, a role for JH titer drop in triggering PTTH release from the PG neurons in Drosophila is less clear since ablation of the corpus allatum, the organ responsible for JH synthesis, does not appear to alter critical weight or accelerate the timing of pupariation in this species (Riddiford, 2011; Riddiford, Truman, Mirth, & Shen, 2010). Instead, a body of recent evidence shows that, although PTTH release is an important event, other factors converge on the PG to coordinate ecdysone synthesis and release. For example, recent studies have demonstrated the importance of insulin/TOR signaling in the PG for critical weight assessment and final body size determination (Caldwell et al., 2005; Colombani et al., 2005; Gibbens et al., 2011; Layalle et al., 2008; Mirth et al., 2005), while other factors such as myosuppression appear to act negatively to reduce PG activity (Yamanaka et al., 2005; Yamanaka et al., 2010; Yamanaka et al., 2006; see Marchal et al., 2010 for a review of factors regulating PG synthesis of ecdysone). Together these studies place the PG in a more central position compared to the classical scheme that focuses on the PTTH-producing neurons.
Starvation does not change critical size, showing that it is genetically determined and unaffected by environmental factors (Beadle et al., 1938). For example, genetic reduction of insulin signaling before critical weight slows larval growth rate and delays the attainment of critical weight and pupariation (Shingleton, Das, Vinicius, & Stern, 2005). However, it does not change the critical weight, which means that it does not alter final adult body size. On the other end, reducing insulin signaling after attainment of the critical weight reduces final adult size but does not delay pupariation. This defines another concept important for attainment of final body size which is the “terminal growth period” (also known as the interval to cessation of growth). The terminal growth period is the time interval between attainment of critical weight and the cessation of feeding that occurs when larvae transition to the wandering stage (Edgar, 2006; Warren et al., 2006). It is important to note that after critical weight is achieved, development proceeds on a fixed temporal schedule that is independent of nutritional status. However, nutrients can still have a strong influence on final body size during this time. If nutrient levels are high for instance, a normal body size is produced, while if they are low a small adult is formed.
At least two size-related checkpoints, one mediated by growth and energy status of the larval-specific tissues and the other mediated by the maturation and patterning of adult precursor tissues, known as the imaginal disks, regulate timing of metamorphosis (Edgar, 2006; Poodry & Woods, 1990). Starvation delays development in animals that lack imaginal disks, suggesting that the mechanism which coordinates growth and ecdysone release with the nutritional status resides in the nonmitotic larval cells.
2.2. Linking nutrition to developmental timing
Insects undergo a tremendous amount of growth from the embryo to the final larval stage that is ready to initiate metamorphosis (Britton & Edgar, 1998). Because the environment affects growth rate, but not critical weight, the larva must coordinate cellular growth and nutrient storage with the timing of metamorphosis. Nutrients absorbed by the gut are not the only signals that trigger cell growth in most organs. Instead, systemic growth in Drosophila is regulated by seven insulin-like peptides known as Dilp1–7 that signal through a single conserved insulin receptor (InR) (Rulifson, Kim, & Nusse, 2002; Wu & Brown, 2006). The insulin-producing cells (IPCs) in the brain are major sites for Dilp production and release. However, insulin signaling must be coordinated with nutrient availability which is sensed by the fat body (analogous to adipose and liver tissues of vertebrates). In a growth permissive, nutrient-rich environment, the fat body non-cell-autonomously regulates growth through the release of fat body-derived signals (FDSs) (Colombani et al., 2003; Geminard, Rulifson, & Leopold, 2009; Rideout, Marshall, & Grewal, 2012). In the fat body, TOR acts as a sensor of nutrients from the gut and responds cell-autonomously to amino acids and ATP levels. Amino acids are crucial dietary components for growth and developmental progression. Although growth of some tissues is largely decoupled from nutrients, the fat body responds rapidly to changes in the internal milieu (Britton & Edgar, 1998). Inhibiting amino acid transport, and thus, TOR signaling in the fat body is sufficient to delay metamorphosis and nonautonomously reduce insulin signaling and systemic growth (Colombani et al., 2003). Recently, it was found that nutrient mediated inhibition of Maf1, a repressor of RNA polymerase III-dependent tRNA transcription, in the fat body nonautonomously increases growth and accelerates pupariation (Rideout et al., 2012). Inhibition of Maf1 is TOR dependent and promotes transcription of tRNAMet which is a limiting factor for protein synthesis in the fat body that controls organismal growth and timing of pupariation. Together, these results suggest that normal timing of metamorphosis is dependent on a positive signal from that fat body which is a central tissue for coordinating nutrient availability with organismal growth and timing of maturation.
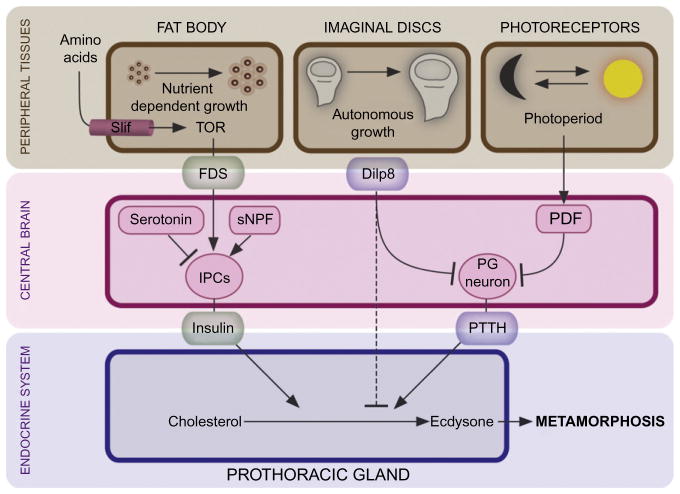
The FDS appears to be a humoral factor(s) that conveys the fat body amino acid status to the IPCs in the brain to regulate Dilp release (Geminard et al., 2009; Rideout et al., 2012). Combined with observations that insulin regulates ecdysone release from the PG (Caldwell et al., 2005; Colombani et al., 2005; Mirth et al., 2005), it provides a mechanism for coupling nutrient status information with ecdysone release (Fig. 1.2). Consistent with this, increasing insulin signaling from the IPCs is sufficient to induce premature ecdysone release and pupariation (Walkiewicz & Stern, 2009). These studies agree with the fact that bombyxin, an insulin-like peptide from the silkworm Bombyx mori, was originally characterized for its ability to stimulate ecdysone synthesis (Kiriishi, Nagasawa, Kataoka, Suzuki, & Sakurai, 1992; Nagasawa et al., 1986; Rybczynski, 2005). Although insulin stimulates PG cell growth, the effect on ecdysone synthesis appears to be specific as growth inducers such as dMyc and cyclin D/Cdk4 increase PG cell size, but not ecdysone synthesis (Colombani et al., 2005). Ecdysone produced in the PG also feeds back on the fat body to regulate organismal growth (Colombani et al., 2005; Delanoue, Slaidina, & Leopold, 2010).
Figure 1.2.
The developmental timing system monitors environmental (nutrient status and photoperiod) and developmental (disks maturation) cues. The fat body acts as a nutrient sensor that coordinates nutrient uptake with systemic growth and developmental timing. In growth permissive environments, the fat body secretes an unknown fat body-derived signal (FDS), in response to dietary amino acids, that stimulates release of insulin from the insulin producing cells (IPCs) of the brain which acts on the PG and stimulates ecdysone release. Serotonin and short neuropeptide F (sNPF) also impinge on the IPCs and regulate insulin release. In addition to insulin, the developmental timing program checks the status of the imaginal disks. Disk growth and maturation is controlled by a tissue-autonomous program that via Dilp8 crosstalks with the neuroendocrine system. Disks secrete Dilp8 which suppresses ecdysone release presumably by inhibition of PTTH release from the PG neurons until they have completed a certain amount of growth or regenerated from tissue damage. Superimposed on this, the clock neurons producing the pigment dispersing factor (PDF) impinge on the PG neurons and regulate PTTH release according to the photoperiod.
The TOR-dependent amino acid sensor that resides in the larval fat body cells may ensure that after each molt a certain amount of nutrient-dependent growth is required to reset the “developmental timer.” This checkpoint ensures a minimal period of feeding before the PG becomes competent to produce an ecdysone pulse. Considering that insulin probably plays a central role in relaying the critical weight signal, the fat body sensor may be a key parameter in critical weight assessment. Accumulating evidence suggests that the fat body with its metabolic and endocrine activities (Britton & Edgar, 1998; Colombani et al., 2003; Geminard et al., 2009; Kamakura, 2011) will be the key to understanding how physiological parameters associated with mass and energy balance are integrated with the endocrine system to time maturation. In addition to relaying a signal via insulin, the FDSs may also act directly on the PG itself or indirectly through stimulation of PTTH release that then acts on the PG. It is also intriguing to note that recent evidence implicates oxygen limitation during growth of the tobacco hornworm Manduca sexta as a key determinator for timing of molting and metamorphosis (Callier & Nijhout, 2011), and hypoxia affects TOR activity (Wullschleger, Loewith, & Hall, 2006).
Another interesting possibility for how fat cells might sense size is that they may monitor endoreplication number. Endoreplicative tissues such as the fat body rely on polytene replication to increase the size of cells that are fully differentiated at the end of embryonic development. In the fat body, dietary amino acids are intimately linked to the endoreplicative machinery, which means that starvation results in rapid arrest of DNA replication (Britton & Edgar, 1998). However, changing fat body cell size and endoreplication number via insulin signaling does not affect developmental timing or systemic growth (Colombani et al., 2003), arguing against the possibility that endoreplication is the key for measuring cell size.
Although previous work shows that insulin regulates ecdysone biosynthesis (Caldwell et al., 2005; Colombani et al., 2005; Gibbens et al., 2011; Mirth et al., 2005; Walkiewicz & Stern, 2009), it is unlikely that insulin signaling alone generates the series of ecdysone pulses that are produced during the third instar (Warren et al., 2006). More likely, insulin is part of the size-sensing system involved in producing the first of three low-level ecdysone peaks in the third instar that coincides with the critical weight checkpoint (Rewitz & O’Connor, 2011). Passing this first checkpoint, corresponding to the accumulation of a certain amount of mass and nutrients, could allow the integration of other signals leading to production of subsequent peaks. Among other checkpoints, one that likely needs to be satisfied is that the imaginal disks must have developed sufficiently so that differentiation can take place during metamorphosis.
2.3. Signaling between the neuroendocrine timing system and a tissue-autonomous size assessment program
It is well known that lesions to imaginal disks, induced by either physical damage, radiation or genetic manipulation, also delay metamorphosis (Bryant & Levinson, 1985; Hussey, Thompson, & Calhoun, 1927; Poodry & Woods, 1990; Sehnal & Bryant, 1993; Simpson, Berreur, & Berreur-Bonnenfant, 1980; Simpson & Scheinderman, 1975; Stieper, Kupershtok, Driscoll, & Shingleton, 2008). The fact that transplantation of damaged disks delays metamorphosis suggests that they produce a secreted signal that acts on the endocrine system to suppress ecdysone release (Dewes, 1973). This developmental delay allows extra time for damaged or growth perturbed disks to regenerate and reach their target size before metamorphosis. Since these processes require cell proliferation, dividing disk cells presumably produce a signal that suppresses ecdysone release. Consistent with this view, pupariation is delayed in disk overgrowth mutants (Sehnal & Bryant, 1993). Importantly, disk growth is determined by an autonomous genetic program that allows them to stop their growth at a specific size even in culture conditions (Bryant & Levinson, 1985). The preprogrammed target size thus functions as a checkpoint that verifies that sufficient growth of disk tissue has occurred before development can progress (Fig. 1.2).
Recently, two elegant genetic studies identified the elusive disk signal as Dilp8, a molecule evolutionarily related to the insulin-like peptides (Colombani et al., 2012; Garelli et al., 2012). Genetic manipulations that (a) prolong the growth period before disks reach their correct target size, (b) give rise to neoplastic disk growth, or (c) damage tissue all result in upregulation of Dilp8 expression. Reducing the expression of Dilp8 does not lead to premature pupariation which agrees with previous studies showing that the disk signal is a permissive factor for developmental progression. However, it is not clear how the Dilp8 signal converges on the endocrine system to regulate ecdysone production or release. Potentially, it could work either directly on the PG, through PTTH, or both to suppress ecdysone production. Genetic disruption of disk growth mainly prolongs the duration of the third instar (L3) up to 5 days (Colombani et al., 2012), similar to the phenotype observed in larvae lacking either PTTH or its receptor Torso (McBrayer et al., 2007; Rewitz, Yamanaka, et al. 2009). Like larvae with reduced PTTH signaling, the second (L2) to L3 transition is only a few hours delayed under conditions that affect disk growth (Colombani et al., 2012). These observations suggest that Dilp8 prevents ecdysone production by inhibition of PTTH signaling. This also agrees with the fact that disk damage, like loss of PTTH, delays but does not prevent pupariation (Colombani et al., 2012; Garelli et al., 2012; Halme, Cheng, & Hariharan, 2010; McBrayer et al., 2007; Rewitz, Yamanaka, et al. 2009). Dilp8 may act at one or more levels in the PTTH signaling pathway, including production of PTTH, release of PTTH from varicosities on the PG, or by limiting processing of PTTH to its active form. An alternative but not mutually exclusive model is that Dilp8 may inhibit the capacity of the PG to respond to PTTH, perhaps by affecting Torso production or activity. Based on an earlier study which showed that disk damage reduces PTTH expression in the PG neurons (Halme et al., 2010), it seems likely that Dilp8 acts at least in part to suppress ecdysone production by downregulation of PTTH synthesis in the PG neurons.
Determining the precise temporal timing of Dilp8 activity and whether it correlates with one of the other small ecdysone peaks is an important area for future investigation. Interestingly, normal Dilp8 expression peaks early during the third instar and drops from 80 to 96 h AEL (after egg laying) (Colombani et al., 2012). The drop in Dilp8 expression coincides with the time when larvae reach critical weight at about 80 h AEL. This may suggest that the endocrine system is reading the Dilp8 drop as the critical weight checkpoint is reached. However, disk damage mainly delays pupariation by increasing the terminal growth period (Stieper et al., 2008), which indicates that the disks exert their suppressive influence postcritical weight, whereas nutritional restriction has no effect beyond this point. Moreover, tissue damage in the imaginal disks 96 h AEL, well after passing the critical weight checkpoint, delays pupariation (Wells & Johnston, 2012). Thus, maturation of larval-specific and adult precursor tissues may not coincide, and critical weight and imaginal disk maturation are likely different checkpoints, each of which may independently regulate the small fluctuations in the ecdysone titer prior to the larger molting peak. Although passing the critical weight checkpoint leads to nutrient-independent patterning of the disks (Mirth, Truman, & Riddiford, 2009), overexpression of Dilp8, which delays pupariation, does not influence disk patterning (Colombani et al., 2012), suggesting that Dilp8 decrease is the result, and not the cause, of disk maturation. The fact that Dilp8 mutants pupariate almost on time further supports the view that two independent timing mechanisms exist, one in the adult progenitor tissues and one in the larval-specific tissues. A detailed analysis of the exact time for the drop in Dilp8 expression and the ecdysone profile may help unraveling if Dilp8 is responsible for the critical weight peak or one of the subsequent low-level ecdysone peaks. Taken together, these data together with the fact that manipulating insulin signaling in the disks does not affect timing (Stieper et al., 2008) show that ecdysone production is controlled by a developmental checkpoint run by an intrinsic genetic program that monitors disk maturation and patterning largely independent of nutrients.
2.4. The brain relays internal and external cues to the endocrine system
Some checkpoint signals from peripheral tissues such as fat body and disks are likely communicated through the central brain to the endocrine system. For example, nutritional assessment must ensure that shutdown of feeding only occurs when enough mass has been accumulated to survive the nonfeeding metamorphic stage. The brain regulates insulin release from the IPCs in response to the fat body-derived nutritional signal. If this signal directly regulates the IPCs or other classes of neurons that control the activity of the IPCs is not known. Cell-autonomous nutrient sensing that controls the release of adipokinetic hormone, the functional equivalent of the mammalian glucagon, does not seem to operate in the insulin release control (Kim & Rulifson, 2004). Instead, insulin secretion is controlled by PKA/CREB and ERK activity stimulated by short neuropeptide F (sNPF) or other neuropeptides and neurotransmitters such as serotonin and GABA (Birse, Soderberg, Luo, Winther, & Nassel, 2011; Enell, Kapan, Soderberg, Kahsai, & Nassel, 2010; Kaplan, Zimmermann, Suyama, Meyer, & Scott, 2008; Lee et al., 2008; Luo, Becnel, Nichols, & Nassel, 2012; Walkiewicz & Stern, 2009). Inhibition of PKA/CREB in the IPCs increases insulin signaling and results in premature ecdysone production and metamorphosis. Conversely, animals with ablated IPCs and mutants with increased serotonin levels that inhibit insulin release from the IPCs have reduced growth rates and delayed pupariation (Kaplan et al., 2008; Rulifson et al., 2002). This shows that neural circuits as well as endocrine mechanisms, such as the FDS, that regulate insulin release from the IPCs are important for the control of ecdysone production. Insulin likely acts directly on the PG (see below); in fact, the axons of the IPCs exit the brain and release insulin from terminals on the anterior aorta and corpus cardiacum close to the PG portion of the ring gland. It is also possible, however, that systemic insulin acts on the nerve endings of the PG neurons to send a retrograde signal that affects PTTH production. In general, the identity and mechanisms by which both internal and environmental inputs control PTTH activity is poorly understood, with perhaps the exception of photoperiodic inputs.
As found for lepidopterans, PTTH release in Drosophila appears to be controlled by the photoperiod (Fig. 1.2), since the circadian clock neurons, producing the pigment dispersing factor (PDF), impinge on the PG neurons (McBrayer et al., 2007; Siegmund & Korge, 2001). The photoperiodic gating of PTTH recurs daily but is presumably not “read” by the system until passage through other checkpoints have verified that the larva has completed enough growth (Nijhout, 1981). In pdf mutant larvae the periodicity of PTTH transcription is disrupted and clock mutations alter developmental timing (Kyriacou, Oldroyd, Wood, Sharp, & Hill, 1990; McBrayer et al., 2007), suggesting a circadian checkpoint regulating PTTH release. In lepidopterans, the PTTH release event is downstream of critical weight and the period between critical weight and PTTH secretion (determined by the time required for JH clearance) is called the PTTH delay period (Nijhout & Williams, 1974a, 1974b). Interestingly, rearing Drosophila larvae with increased insulin signaling in the PG under constant light decreases time to pupariation (Mirth et al., 2005). One possible interpretation is that the PG of these larvae is prematurely estimating that critical weight has been reached because of the enhanced insulin signaling and the constant light condition then reduces the PTTH delay period.
Future studies should examine the mechanism(s) regulating PG neuron activity including the importance of transcriptional control, as well as processing and release of PTTH. Presently, the mechanism controlling PTTH release is only known for some insects in the group Hemiptera, where activation of abdominal stretch receptors in response to the attainment of a certain size results in PTTH release (Nijhout, 1981, 2003). Although distension of stretch receptors is not sufficient for PTTH release and initiation of molting in insects outside this order, peripheral sensory neurons tiling the larval body wall have been linked with changes in feeding behavior associated with the progression of development from the feeding to wandering stage in Drosophila (Ainsley, Kim, Wegman, Pettus, & Johnson, 2008; Wegman, Ainsley, & Johnson, 2010).
As discussed above, timing of pupariation involves checkpoints that ensure coordination of growth between larval and adult precursor tissues. However, it is less clear how each of these signals contributes to each of the discrete pulses of ecdysone seen during the third instar (Warren et al., 2006). One possibility is that clearance of all checkpoints is required to produce the first low-level, critical weight associated ecdysone peak and the successive subsequent peaks are generated autonomously by feedback circuits. However, the ecdysone peaks do not occur with a regular ultradian rhythm; the time between peaks being 8, 12, or 16 h (Warren et al., 2006). Another, and perhaps more likely, explanation is that the endocrine system monitors growth, energy status, and photoperiod checkpoints successively, each of which results in a low-level ecdysone peak that drives a subsequent part of the developmental program, ultimately resulting in pupariation and the initiation of metamorphosis.
3. SIGNALS CONVERGING ON THE PG
3.1. PTTH and insulin/TOR coordinate PG cell growth and ecdysone production
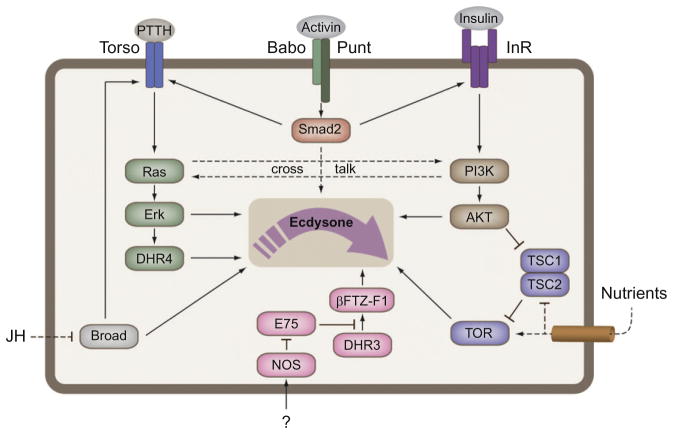
Like in peripheral tissues, the insulin/TOR pathway promotes growth of PG cells and therefore can have an indirect effect on total levels of ecdysone simply by controlling the PG cell size. However, several studies have shown that insulin/TOR also has a direct role in regulating ecdysone synthesis in the PG (Caldwell et al., 2005; Colombani et al., 2005; Gibbens et al., 2011; Layalle et al., 2008; Mirth et al., 2005). Under this view, the developmental timer in the PG is informed of the nutritional status via insulin/TOR to adjust ecdysone synthesis (Fig. 1.3). Although the role insulin plays in the production of the ecdysone peaks is not clear, as described insulin is likely involved in setting the critical weight parameter that commits the PG to ecdysone biosynthesis. Assessment of the temporal requirement for insulin during development has demonstrated that reduced insulin signaling delays timing of pupariation before, but not after, the critical weight checkpoint (Shingleton et al., 2005). This implies that insulin signaling is not important for stimulation of ecdysone biosynthesis in the PG during the postcritical weight period, and so may not influence the two low-level ecdysone peaks at 20 and 28 h after ecdysis to the third instar nor the subsequent high-level peak at 44 h that is thought to trigger pupariation (Warren et al., 2006). In fact, starvation after reaching critical weight, which is assumed to reduce insulin signaling in the PG, actually slightly accelerates pupariation in Drosophila (Mirth & Riddiford, 2007; Stieper et al., 2008). Thus, insulin signaling seems to be required for the first critical weight peak approximately 8 h after the L2/L3 transition. Increase in insulin signaling possibly informs the endocrine system of the nutritional status of the larva. But how does the system sense critical size? One possibility is that some threshold level of insulin stimulates the biosynthetic pathway directly to produce the critical weight ecdysone peak. Another possibility is that insulin makes the PG commit to ecdysone synthesis by providing competence to other signals such as PTTH which then dictates the timing of some, or perhaps all, of the subsequent ecdysone peaks during the third instar. Under this view, insulin signaling would not directly produce the critical weight peak. This is consistent with the observation that insulin does not stimulate ecdysone biosynthesis in Manduca (Walsh & Smith, 2011).
Figure 1.3.
Mechanisms converging on the PG time ecdysone production. TGFβ/Activin is required for normal expression of the insulin receptor (InR) and torso, which provides glandular competence to PTTH (developmental) and insulin (nutrient) cues. Furthermore, the PG harbors a TOR-dependent nutrients sensor that presumably allows compensation for poor nutrient environments. Under such conditions, ecdysone release is delayed which prolongs the growth period. Broad is required for normal expression of the Halloween genes encoding the enzymes mediating ecdysone synthesis, although it is not clear if JH regulates broad in the PG. Nitric oxide (NO) generated by the nitric oxide synthase (NOS) regulates nuclear receptor signaling in the PG. In turn, NO inhibits E75, a repressor of DHR3 which then activates expression of βFTZ-F1, a nuclear receptor required for expression of at least two key ecdysone biosynthetic enzymes, Phantom and Disembodied.
From one perspective, it seems more intuitive that pulses of PTTH generate the precisely timed ecdysone peaks, instead of insulin. For example, PTTH is a more potent inducer of ecdysone synthesis than insulin and is released from neurons that directly innervate the PG (McBrayer et al., 2007; Rybczynski, 2005). Transcription of PTTH exhibits ultradian periodicity during the third instar (McBrayer et al., 2007), also making PTTH a likely candidate for a periodic ecdysone pulse generator. Recently, nucleocytoplasmic trafficking of the nuclear receptor DHR4 in the PG was shown to be PTTH dependent (Ou et al., 2011). Lack of PTTH or torso disrupts the first nucleocytoplasmic transition 4 h after the L2/L3 transition. This implies that PTTH signaling indeed occurs in advance to the critical weight peak. A second low-level ecdysone peak 90 h AEL is associated with a change in glue gene expression that allows the puparium to adhere to its substrate. The third low-level ecdysone peak 98 h AEL is associated with wandering behavior and the cessation of feeding. Consistent with this, reduced ecdysone levels results in a prolonged feeding period, delayed wandering and asynchrony of the ecdysone gene response (McBrayer et al., 2007; Rewitz, Yamanaka, et al. 2009).
If the role of insulin is to endow the PG with competence to produce ecdysone in response to PTTH, it would have to interact with a central component in the PTTH signaling machinery or ecdysone biosynthetic pathway. Interestingly, nutrient-dependent torso expression has been observed in Bombyx (Young, Yeh, & Gu, 2012), perhaps involving insulin. Moreover, the transcription factor Broad is required for expression of torso (Xiang, Liu, & Huang, 2010) and insulin induces expression of broad in the wing disks of Manduca, overcoming the inhibition by JH (Koyama, Syropyatova, & Riddiford, 2008). Assuming a similar scenario in the PG, insulin might increase broad levels to upregulate torso expression, providing competence to PTTH that in turn leads to commitment for metamorphosis. In agreement with a role in regulating ecdysone synthesis, broad null mutants fail to pupariate and broad is expressed in the PG after the L2/L3 transition with a rapid increase during wandering as the ecdysone titer rises (Kiss, Beaton, Tardiff, Fristrom, & Fristrom, 1988; Zhou, Zhou, Truman, & Riddiford, 2004).
In the previous section, we considered how TOR in the fat body might help generate FDSs that act either on the PG or PTTH activity. However TOR signaling in the PG itself is also important for determining timing since recent evidence suggests that TOR plays a role in the PG in the regulation of ecdysone synthesis (Layalle et al., 2008). In contrast to moderate changes in insulin signaling, reducing TOR activity in the PG prolongs larval development after the critical weight checkpoint (Colombani et al., 2005; Layalle et al., 2008). Although reducing TOR signaling delays pupariation, activation of TOR in the PG under normal food conditions does not accelerate development (Layalle et al., 2008). This only occurs under conditions with restricted nutrition, suggesting an upper limit for nutrient activation of ecdysone signaling. Like TOR, increasing insulin signaling in the PG only accelerates metamorphosis in larvae reared without yeast supplement (Mirth et al., 2005). This again supports the idea that nutrients provide a positive signal that is limited by the maximum growth rate. In contrast, increasing Ras signaling in the PG dramatically accelerates metamorphosis even under standard food conditions (Rewitz, Yamanaka, et al. 2009). The MAPK pathway is the major pathway relaying the signal downstream of the PTTH receptor Torso (Fig. 1.3). Like insulin signaling, activating the MAPK pathway in the PG results in increased PG cell growth, and the larvae pupariate at a smaller size than normal. On the other hand, decreasing MAPK signaling in the PG results in developmental delay and overgrowth similar to larvae lacking PTTH or Torso. Together with insulin, the importance of TOR signaling in the PG may help explain why overgrown larvae lacking PTTH eventually pupariate (McBrayer et al., 2007).
In addition to activating the MAPK pathway, studies in lepidopterans have shown that PTTH also activates a complex network of pathways that include PKA/cAMP, calcium and PKC (Rybczynski, 2005; Rybczynski & Gilbert, 2006). Whether Torso is required for activation of these alternative pathways and whether their activation is conserved in Drosophila and how they contribute to either the production and/or release of ecdysone from the PG requires additional study.
The finding that under low nutrient conditions TOR informs the PG to extend the growth period (Layalle et al., 2008) is difficult to reconcile with the fact that starvation after critical weight accelerates metamorphosis in Drosophila (Mirth & Riddiford, 2007; Stieper et al., 2008). One explanation might be a cross talk between the PTTH and the TOR pathway in the PG. Previous work has shown that rapamycin inhibits PTTH-stimulated ecdysone synthesis (Rybczynski, 2005). Indeed, PTTH has been shown to regulate 4E-BP and S6 kinase (S6K), a major target of TOR (Gu, Yeh, Young, Lin, & Li, 2012; Rybczynski, 2005). In addition to its stimulatory effect on ecdysone synthesis, PTTH has a non-steroidogenic trophic effect on PG growth and protein synthesis (Rybczynski, 2005; Rybczynski & Gilbert, 1994) and lack of PTTH reduces PG cell size (Ghosh, McBrayer, & O’Connor, 2010). PTTH-induced TOR signaling seems to rely on PI3K (Gu et al., 2012), suggesting that PTTH and insulin converge on PI3K to regulate PG cell growth and ecdysone biosynthesis. On the other hand, insulin stimulation of ecdysone synthesis may, at least in part, involve cross talk with the MAPK (Rewitz, Yamanaka, et al. 2009).
How do PTTH and insulin increase activity of the ecdysone biosynthetic pathway? PTTH-stimulated ecdysone synthesis occurs within minutes and requires translation and likely posttranslational protein modifications (Rewitz, Larsen, et al., 2009; Rybczynski, 2005). The rapid increase in the flux through the biosynthetic pathway that converts cholesterol to ecdysone possibly involves regulation of a rate-limiting molecule(s) that acts in, the so-called Black Box reaction(s), an early step in the biosynthetic pathway which is not completely understood (Gilbert et al., 2002). Consistent with this view, in lepidopterans PTTH stimulates the rapid increase in translation and phosphorylation of Spook, a cytochrome P450 enzyme involved in the Black Box (Ono, Morita, Asakura, & Nishida, 2012; Ono et al., 2006; Rewitz, Larsen, et al., 2009). However, it is not clear if the Black Box is the rate-limiting reaction in all insects.
In addition to the acute regulation, PTTH elicits a long-term transcriptional effect that involves upregulation of the Halloween genes encoding the ecdysone biosynthetic enzymes (Keightley, Lou, & Smith, 1990; Niwa et al., 2005; Yamanaka et al., 2007). High expression of the ecdysone biosynthetic enzymes coincides with the ecdysone peaks, suggesting that upregulation of the components in the pathway is required to increase the steroidogenic capacity of the gland (Rewitz, Rybczynski, Warren, & Gilbert, 2006; Yamanaka et al., 2007). The importance of the transcriptional regulation is highlighted by evidence suggesting that low expression of these genes, including spook, presumably prevents PTTH-stimulated ecdysone synthesis despite PTTH being able to induce a glandular phosphorylation response in the early fifth instar Bombyx (Gu & Chow, 2005; Lin & Gu, 2007; Yamanaka et al., 2007). Reduced expression of the ecdysone biosynthetic enzymes in PTTH-ablated larvae indicates that PTTH plays a role in the transcriptional up-regulation of these genes in Drosophila (McBrayer et al., 2007). However, the moderate effect of PTTH does not fully explain the dramatic increase in expression of these genes during development (Yamanaka et al., 2007). Thus, other mechanisms must be involved in the transcriptional regulation of the Halloween genes. Consistent with this notion, the transcriptional repressor DHR4, a key target of PTTH signaling, has only minor effect on Halloween gene expression (Ou et al., 2011). Other mechanisms might involve feedforward regulation by the action of ecdysone itself, believed to autonomously activate the PG in culture, or perhaps involve input from TGFβ/Activin and/or nitric oxide signaling (see discussion below: Caceres et al., 2011; Gibbens et al., 2011; Yamanaka et al., 2007).
In addition to the enzymes involved in ecdysone biosynthesis, npc1 is also expressed specifically in the PG where it is thought to be involved in supplying cholesterol for steroidogenesis (Huang, Suyama, Buchanan, Zhu, & Scott, 2005). Normal expression of npc1 and the Halloween genes requires Broad, which is interesting considering that JH, which suppresses broad expression, also inhibits spook expression in Bombyx (Xiang et al., 2010; Yamanaka et al., 2007).
3.2. TGFβ/Activin and nitric oxide signaling are essential for steroidogenesis
Although several factors have been shown to regulate PG activity (see Marchal et al., 2010 for a recent review), recent reports have highlighted the essential role of nitric oxide (NO) and TGFβ/Activin (Caceres et al., 2011; Gibbens et al., 2011). In peripheral tissues, ecdysone elicits a genetic response through a set of primary ecdysone-inducible nuclear receptors (King-Jones & Thummel, 2005). However, some of these nuclear receptors, such as E75, DHR3, and βFTZ-F1, are also involved in the production of ecdysone in the PG (Caceres et al., 2011; Parvy et al., 2005). Although little is known about the factors involved in the transcriptional regulation of the Halloween genes, βFTZ-F1 is required for expression of the genes phantom and disembodied (Parvy et al., 2005). Expression of βFTZ-F1 requires activation by DHR3, which is repressed by E75. Activity of the NO synthase (NOS), catalyzing the production of NO, is required for NO-mediated inhibition of E75 activity in the PG (Caceres et al., 2011). Reducing expression of NOS in the PG results in a failure to produce ecdysone and an inability to undergo metamorphosis. These larvae have enlarged PG cells with increased endoreplication number, consistent with the view that increased PG cell size alone is not sufficient for high level ecdysone production (Colombani et al., 2005). Together, these results show that NO signaling has an essential role in the regulation of ecdysone production that controls developmental transitions, although the mechanism regulating NO signaling in the PG remains unknown.
In addition to NO, recent work in Drosophila has shown that TGFβ/Activin signaling is essential for ecdysone synthesis and developmental transitions (Gibbens et al., 2011). Interestingly, TGFβ and insulin signaling also converge in Caenorhabditis elegans on the synthesis of the steroid hormone dafachronic acid (DA), which interacts with the nuclear receptor DAF-12 to determine whether larvae undergo reproductive development or arrest in a dauer state (Tennessen & Thummel, 2011). In Drosophila, reduced TGFβ/Activin signaling in the PG, mediated by knockdown of dSmad2, the major downstream mediator of TGFβ signals, causes developmental arrest in the third instar (Gibbens et al., 2011). Disrupting TGFβ/Activin reduces expression of torso and the InR in the PG, presumably blocking the ability to synthesize ecdysone in response to PTTH and insulin. Moreover, restoring Torso/MAPK or InR/Akt in the PG rescues the phenotype of dSmad2-RNAi knockdown. Interestingly, resupplying the insulin receptor in the PG of larvae with reduced PG expression of dSmad2 restores protein, but not transcript levels, of key ecdysone biosynthetic enzymes. Conversely, activating Torso/Ras signaling in the PG of these larvae rescues the reduced expression of these genes. Thus, potentially insulin and PTTH regulate the ecdysone biosynthetic machinery by distinct mechanisms one via transcription and the other via translational control. Together, these observations indicate that TGFβ/Activin signaling is important for the PG to develop competence to respond to additional developmental (PTTH) and nutritional (insulin) cues. Presently, it is not known whether TGFβ/Activin signaling is developmentally regulated to provide stage-specific competence or is required for constitutive expression of torso and InR. Another interesting possibility is that TGFβ/Activin signaling is linked to the effect of the SUMOylation pathway, since it has also been demonstrated that knockdown of the SUMO gene, smt3, in the PG arrests development in the L3 (Talamillo et al., 2008). In this regard, it is interesting to note that Medea, the co-Smad that forms a complex with dSmad2, is a primary target of SUMOylation (Miles et al., 2008).
4. SHAPING DISCRETE ECDYSONE PULSES
4.1. Feedback control of ecdysone production
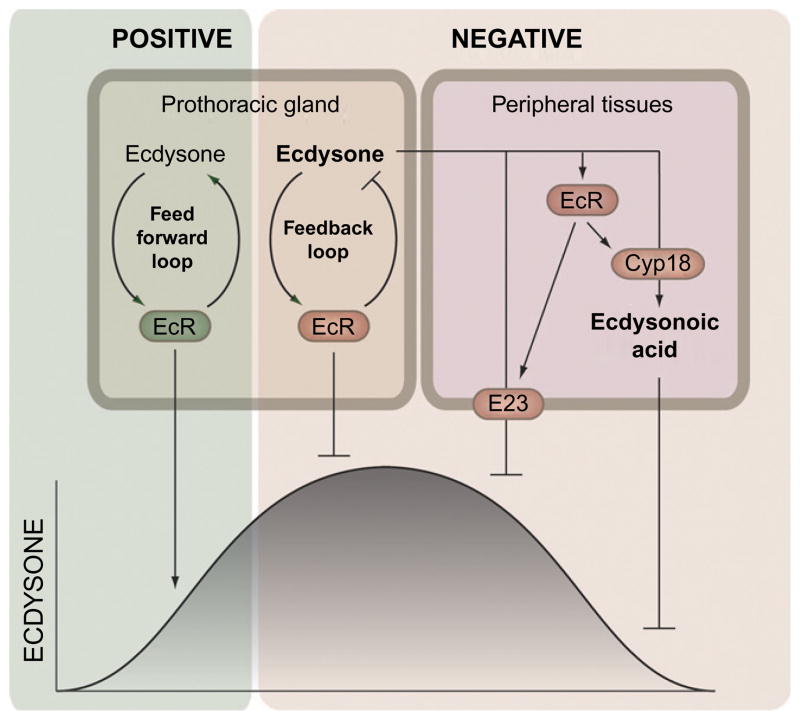
In addition to the mechanisms that increase ecdysone synthesis, there must be ways to shut down PG activity as well as inactivate and remove ecdysone from circulation in order to produce the temporally defined pulses of ecdysone. Thus, the endocrine system must be under feedback regulation and receive inputs from its own target tissues (Fig. 1.4). This idea is consistent with studies showing that ecdysone inhibits ecdysone synthesis in the PG of lepidopterans (Beydon & Lafont, 1983; Gilbert et al., 2002; Sakurai & Williams, 1989). Furthermore, EcR is expressed in the PG of Drosophila during the time when the larvae initiate pupariation (Talbot, Swyryd, & Hogness, 1993). Long-term incubation of the PG in the presence of ecdysone reduces the sensitivity to PTTH (Gilbert, Song, & Rybczynski, 1997; Song & Gilbert, 1998), which indicates that ecdysone likely down-regulates torso or other components mediating PTTH signaling or ecdysteroidogenesis.
Figure 1.4.
Feedback control shapes the ecdysone pulses. A short positive and a long negative feedback loop are believed to operate in the PG to control synthesis of ecdysone. The short positive feedforward loop presumably amplifies the ecdysone signal causing a fast increase of the titer. On the other hand, a long negative feedback loop shuts off the PG which allows peripheral mechanisms to clear ecdysone from the system. Ecdysone induces two feedback mechanisms, in tissues peripheral to the PG, which are eventually responsible for lowering cellular levels (E23) and the decline of the titer (Cyp18). Ecdysone induces Cyp18 that eliminates ecdysone by converting it into the inactive ecdysonoic acid. The ecdysone-inducible E23 encodes an ABC transporter believed to pump ecdysone out of the cells. Together these feedback mechanisms determine the duration of the ecdysone pulses.
Conversely, when the activity of the PG is low, ecdysone appears to stimulate its own synthesis, a mechanism that can increase and amplify the signal. Further amplification of the signal may occur through the EcR autoregulatory loop, through which EcR induces its own expression (Koelle et al., 1991). These observations suggest that ecdysone synthesis in the PG is controlled by feedforward and feedback loops that rapidly modulate the ecdysone titer and determine the temporal boundaries for the pulses. Interestingly, torso expression is induced by ecdysone, indicating a potential feedforward regulatory mechanism (Young et al., 2012). On the other hand, PTTH stimulation decreases torso mRNA levels, suggesting a desensitizing mechanism similar to that observed for InR (Puig, Marr, Ruhf, & Tjian, 2003). Although some controversies exist about the role of ecdysone in the PG (Kozlova & Thummel, 2002; Yamanaka & O’Connor, 2011), such feedback regulation makes intuitive sense and the physiological significance awaits further investigations.
Feedback control of PG activity through EcR may involve other ecdysone-inducible genes required for the ecdysone response in peripheral tissues. It is interesting to note that in a recent study DHR4, previously characterized as an ecdysone-inducible gene, was shown to be necessary to control the duration of the ecdysone pulses (King-Jones, Charles, Lam, & Thummel, 2005; Ou et al., 2011; Rewitz & O’Connor, 2011). When located in the nucleus, DHR4 represses ecdysone production, which helps establish the duration of the ecdysone pulses. Although PTTH-regulated nucleocytoplasmic shuttling of the DHR4 protein could account for this, the rapid effect of DHR4 RNAi argues that there is more to this than simple shuttling of a stable DHR4 protein. Cycling of DHR4 transcript in concert with PTTH-stimulated ecdysone peaks would presumably be a way to help terminate ecdysone production and regulate the duration of the pulse.
4.2. Termination of ecdysone pulses by feedback regulated degradation
Although ecdysone production in the PG must be turned off to bring levels back to basal, circulating ecdysone must also be removed to terminate the pulse. Two elegant feedback mechanisms have evolved to rapidly decrease circulating levels of ecdysone following a peak (Fig. 1.4). The first mechanism involves Cyp18a1, a cytochrome P450 enzyme required for the metabolic inactivation of ecdysone (Guittard et al., 2011; Rewitz et al., 2010). Cyp18a1 is required for the rapid decline of the ecdysone titer after the peak that triggers pupariation. Loss of Cyp18a1 results in elevated ecdysone levels that disrupt the mid-prepupal expression of βFTZ-F1, which is necessary for the genetic response to ecdysone that drives unidirectional progression of development (Rewitz et al., 2010). Cy p18a1 expression is induced by ecdysone, providing a mechanism for generating a pulse, where elevated ecdysone levels are responsible for its eventual decline. In addition to metabolic inactivation, another mechanism ensures that cellular levels of ecdysone are reduced following a peak. One of the early ecdysone-inducible genes, E23, encodes an ABC transporter protein (Hock, Cottrill, Keegan, & Garza, 2000). E23 is one of the last of the early genes to be induced which makes physiological sense because the function of E23 is to pump ecdysone out of the cells and reduce cellular concentration. Together with feedback regulation of the PG activity, these peripheral mechanisms provide an autonomous regulatory system that determines the duration of the ecdysone pulses.
5. SUMMARY AND PERSPECTIVES
Steroids synthesized in response to signaling pathways including insulin-like peptides, TGFβ and other neuropeptides control the developmental transition leading to maturation in worms, insects and mammals. Recent progress from Drosophila research shows that nutrient sensitive insulin signaling and tissue-autonomous size determination are part of the underlying size-monitoring mechanisms that activate the endocrine system and initiate maturation once a characteristic size is achieved. An autonomous genetic size determining program that is dependent on the number of cell divisions and relatively insensitive to nutrient uptake controls disk growth. In contrast, growth of endoreplicative larval tissues is tightly coupled with nutrient availability and relies on the increase in cell size rather than number. Because larvae pupariate even in the absence of disks, Dilp8 is a permissive signal, rather than a positive “trigger” of pupariation, which provides a checkpoint to ensure that the adult precursor tissues are ready for metamorphosis. Conversely, nutrient sensitive growth influenced by the fat body likely provides positive signals to the endocrine system.
Based on recent insights discussed here, we propose that developmental timing is regulated by a series of checkpoints that verify a certain amount of nutrient stores and disk growth before a circadian input allows developmental progression (Fig. 1.5). The first checkpoint, which probably corresponds to critical weight, is the key decision whether to enter or delay pupariation if nutrient becomes limited. Another checkpoint verifies that the imaginal disks are ready, and finally the photoperiod is monitored to impose a circadian gate on the developmental transition. Although the mechanisms responsible for generating each peak of ecdysone are poorly understood, these peaks likely coordinate developmental and behavioral transitions during the third instar. Potentially each checkpoint control mechanism may be translated into one of the low-level ecdysone peaks which ensure that the molecular events are correctly ordered for unidirectional developmental progression. Alternatively, passing all checkpoints may activate a neuroendocrine switch to an autonomous program that produces the successive pulses of ecdysone. While it is obvious that the onset of metamorphosis is regulated by a number of cues from both the external and internal environment, further studies are needed to decipher how these cues are integrated to produce the sequential pulses of ecdysone.
Figure 1.5.
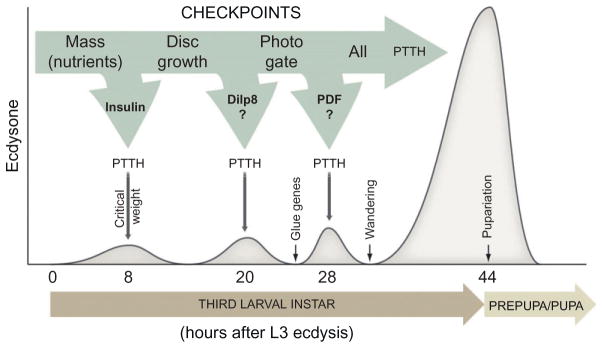
Checkpoint controls of developmental timing of metamorphosis. The figure shows a proposed model for checkpoint control of ecdysone release and timing of pupariation. Accumulation of nutrients corresponding to the critical weight presumably results in the insulin dependent production of the critical weight ecdysone peak 8 h after L3 ecdysis. Maturation and patterning of the imaginal disks and the photoperiod are also verified by the endocrine system before development can proceed. These checkpoints may potentially translate into to the other low-level ecdysone peaks 20 and 28 h after L3 ecdysis. When all checkpoints are cleared, developmental transition to the metamorphic stage can take place.
Despite recent advances, the secreted FDSs have not been identified. The identification of the FDSs is the key to a more comprehensive understanding of how peripheral nutritional signals coordinate timing of ecdysone production. In this regard, the increasing prevalence of childhood obesity in humans has been associated with premature onset of puberty and reproductive dysfunction (Ahmed, Ong, & Dunger, 2009; Kaplowitz, 2008). Like developmental timing in insects, nutritional regulation of human pubertal timing is influenced by peripheral nutritional signals from adipose tissues similar to the fat body. We note a study by Norbert Perrimon’s group identifying Unpaired 2 as a secreted FDS, functionally equivalent to human leptin, that regulates systemic growth presumably through its effect on insulin release from the IPCs (note added in proof).
The insulin-like molecules seem to be part of a conserved genetic mechanism that controls timing of maturation. In C. elegans, mutations disrupting insulin signaling result in dauer formation and arrest before the sexual maturation independent of the environmental conditions (Kimura, Tissenbaum, Liu, & Ruvkun, 1997). Moreover, puberty is delayed by mutations affecting insulin-like growth factor (IGF) signaling and in children diagnosed with certain types of diabetes (Divall et al., 2010; Domene et al., 2009; Kjaer, Hagen, Sando, & Eshoj, 1992; Messina et al., 2011). The discovery that Dilp8 coordinates tissue growth with developmental timing further highlights the central role for these conserved molecules in the control of body size, maturation, and metabolism.
How insulin/TOR and PTTH signaling interact in the PG to coordinate the timing of ecdysone pulses remain poorly understood. Insulin may work upstream of PTTH either by producing the critical weight ecdysone peak that allows subsequent PTTH release or by mediating glandular growth that provides competence to respond to PTTH. Because the PG neurons are anatomically developed earlier, regulating the PG competence might be important to prevent a premature response to the PTTH. Alternatively, these two pathways may work together to control ecdysone production in the PG. Perhaps signaling through both pathways is necessary to fully activate ecdysone biosynthesis. Investigating the role of transcriptional and posttranscriptional regulation of components in the ecdysone biosynthetic pathway as well as the underlying mechanisms will be the key to understanding the changes in the PG activity required to produce pulses of ecdysone.
In most systems, mechanisms responsible for timing the juvenile-adult transition are poorly understood, in part, because it involves complex inter-organ communication to monitor numerous internal and external cues such as organ size, nutritional status, and photoperiod. As illustrated here, the number of factors that converge on the neuroendocrine system to control such transition in insects has expanded substantially during the recent years. However, these studies have also indicated, that as found for many other aspects of development, the overall architecture of the system that coordinates juvenile-adult transitions is conserved. Therefore, future insights from Drosophila and other insects will continue to serve as a general paradigm for understanding how metazoans coordinate growth and developmental timing.
Acknowledgments
K.F.R. is supported by grant 11-105446 from the Danish Council for Independent Research, Natural Sciences. N.Y. is supported by NIH grant K99 HD073239 and M.B.O by NIH R01 GM093301.
References
- Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends in Endocrinology and Metabolism. 2009;20:237–242. doi: 10.1016/j.tem.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Ainsley JA, Kim MJ, Wegman LJ, Pettus JM, Johnson WA. Sensory mechanisms controlling the timing of larval developmental and behavioral transitions require the Drosophila DEG/ENaC subunit, Pickpocket1. Developmental Biology. 2008;322:46–55. doi: 10.1016/j.ydbio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Baehrecke EH. Steroid regulation of programmed cell death during Drosophila development. Cell Death and Differentiation. 2000;7:1057–1062. doi: 10.1038/sj.cdd.4400753. [DOI] [PubMed] [Google Scholar]
- Beadle G, Tatum E, Glancy C. Food level in relation to rate of development and eye pigmentation in Drosophila melanogaster. The Biological Bulletin. 1938;75:447–462. [Google Scholar]
- Beydon P, Lafont R. Feedback inhibition of ecdysone production by 20-hydroxyecdysone in Pieris brassicae pupae. Journal of Insect Physiology. 1983;29:529–533. [Google Scholar]
- Birse RT, Soderberg JA, Luo J, Winther AM, Nassel DR. Regulation of insulin-producing cells in the adult Drosophila brain via the tachykinin peptide receptor DTKR. The Journal of Experimental Biology. 2011;214:4201–4208. doi: 10.1242/jeb.062091. [DOI] [PubMed] [Google Scholar]
- Britton JS, Edgar BA. Environmental control of the cell cycle in Drosophila: Nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125:2149–2158. doi: 10.1242/dev.125.11.2149. [DOI] [PubMed] [Google Scholar]
- Bryant PJ, Levinson P. Intrinsic growth control in the imaginal primordia of Drosophila, and the autonomous action of a lethal mutation causing overgrowth. Developmental Biology. 1985;107:355–363. doi: 10.1016/0012-1606(85)90317-3. [DOI] [PubMed] [Google Scholar]
- Caceres L, Necakov AS, Schwartz C, Kimber S, Roberts IJ, Krause HM. Nitric oxide coordinates metabolism, growth, and development via the nuclear receptor E75. Genes & Development. 2011;25:1476–1485. doi: 10.1101/gad.2064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Current Biology. 2005;15:1785–1795. doi: 10.1016/j.cub.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Callier V, Nijhout HF. Control of body size by oxygen supply reveals size-dependent and size-independent mechanisms of molting and metamorphosis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14664–14669. doi: 10.1073/pnas.1106556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J, Andersen DS, Leopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336:582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, et al. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- D’Avino PP, Thummel CS. The ecdysone regulatory pathway controls wing morphogenesis and integrin expression during Drosophila metamorphosis. Developmental Biology. 2000;220:211–224. doi: 10.1006/dbio.2000.9650. [DOI] [PubMed] [Google Scholar]
- Delanoue R, Slaidina M, Leopold P. The steroid hormone ecdysone controls systemic growth by repressing dMyc function in Drosophila fat cells. Developmental Cell. 2010;18:1012–1021. doi: 10.1016/j.devcel.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Dewes E. Regeneration in transplanted halves of male genital disks and its influence upon duration of development in Ephestia kühniella Z. Rouxs Archives of Developmental Biology. 1973;172:349–354. doi: 10.1007/BF00577885. [DOI] [PubMed] [Google Scholar]
- Divall SA, Williams TR, Carver SE, Koch L, Bruning JC, Kahn CR, et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. The Journal of Clinical Investigation. 2010;120:2900–2909. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domene HM, Hwa V, Argente J, Wit JM, Camacho-Hubner C, Jasper HG, et al. Human acid-labile subunit deficiency: Clinical, endocrine and metabolic consequences. Hormone Research. 2009;72:129–141. doi: 10.1159/000232486. [DOI] [PubMed] [Google Scholar]
- Edgar BA. How flies get their size: Genetics meets physiology. Nature Reviews Genetics. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: More for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- Enell LE, Kapan N, Soderberg JA, Kahsai L, Nassel DR. Insulin signaling, lifespan and stress resistance are modulated by metabotropic GABA receptors on insulin producing cells in the brain of Drosophila. PLoS One. 2010;5:e15780. doi: 10.1371/journal.pone.0015780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336:579–582. doi: 10.1126/science.1216735. [DOI] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metabolism. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Ghosh A, McBrayer Z, O’Connor MB. The Drosophila gap gene giant regulates ecdysone production through specification of the PTTH-producing neurons. Developmental Biology. 2010;347:271–278. doi: 10.1016/j.ydbio.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbens YY, Warren JT, Gilbert LI, O’Connor MB. Neuroendocrine regulation of Drosophila metamorphosis requires TGFbeta/Activin signaling. Development. 2011;138:2693–2703. doi: 10.1242/dev.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annual Review of Entomology. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- Gilbert LI, Song Q, Rybczynski R. Control of ecdysteroidogenesis: Activation and inhibition of prothoracic gland activity. Invertebrate Neuroscience. 1997;3:205–216. doi: 10.1007/BF02480376. [DOI] [PubMed] [Google Scholar]
- Gu SH, Chow YS. Analysis of ecdysteroidogenic activity of the prothoracic glands during the last larval instar of the silkworm, Bombyx mori. Archives of Insect Biochemistry and Physiology. 2005;58:17–26. doi: 10.1002/arch.20029. [DOI] [PubMed] [Google Scholar]
- Gu SH, Yeh WL, Young SC, Lin PL, Li S. TOR signaling is involved in PTTH-stimulated ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochemistry and Molecular Biology. 2012;42:296–303. doi: 10.1016/j.ibmb.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Guittard E, Blais C, Maria A, Parvy JP, Pasricha S, Lumb C, et al. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Developmental Biology. 2011;349:35–45. doi: 10.1016/j.ydbio.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Halme A, Cheng M, Hariharan IK. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Current Biology. 2010;20:458–463. doi: 10.1016/j.cub.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock T, Cottrill T, Keegan J, Garza D. The E23 early gene of Drosophila encodes an ecdysone-inducible ATP-binding cassette transporter capable of repressing ecdysone-mediated gene activation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9519–9524. doi: 10.1073/pnas.160271797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Suyama K, Buchanan J, Zhu AJ, Scott MP. A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development. 2005;132:5115–5124. doi: 10.1242/dev.02079. [DOI] [PubMed] [Google Scholar]
- Hussey RG, Thompson WR, Calhoun ET. The influence of X-rays on the development of Drosophila larvae. Science. 1927;66:65–66. doi: 10.1126/science.66.1698.65. [DOI] [PubMed] [Google Scholar]
- Kamakura M. Royalactin induces queen differentiation in honeybees. Nature. 2011;473:478–483. doi: 10.1038/nature10093. [DOI] [PubMed] [Google Scholar]
- Kaplan DD, Zimmermann G, Suyama K, Meyer T, Scott MP. A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. Genes & Development. 2008;22:1877–1893. doi: 10.1101/gad.1670508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(Suppl 3):S208–S217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- Keightley DA, Lou KJ, Smith WA. Involvement of translation and transcription in insect steroidogenesis. Molecular and Cellular Endocrinology. 1990;74:229–237. doi: 10.1016/0303-7207(90)90228-z. [DOI] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Charles JP, Lam G, Thummel CS. The ecdysone-induced DHR4 orphan nuclear receptor coordinates growth and maturation in Drosophila. Cell. 2005;121:773–784. doi: 10.1016/j.cell.2005.03.030. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. Nuclear receptors—A perspective from Drosophila. Nature Reviews Genetics. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- Kiriishi S, Nagasawa H, Kataoka H, Suzuki A, Sakurai S. Comparison of in vivo and in vitro effects of Bombyxin and prothoracicotropic hormone on the prothoracic glands of the silkworm, Bombyx mori. Zoological Science. 1992;9:149–155. [Google Scholar]
- Kiss I, Beaton AH, Tardiff J, Fristrom D, Fristrom JW. Interactions and developmental effects of mutations in the Broad-Complex of Drosophila melanogaster. Genetics. 1988;118:247–259. doi: 10.1093/genetics/118.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer K, Hagen C, Sando SH, Eshoj O. Epidemiology of menarche and menstrual disturbances in an unselected group of women with insulin-dependent diabetes mellitus compared to controls. The Journal of Clinical Endocrinology and Metabolism. 1992;75:524–529. doi: 10.1210/jcem.75.2.1639955. [DOI] [PubMed] [Google Scholar]
- Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- Koyama T, Syropyatova MO, Riddiford LM. Insulin/IGF signaling regulates the change in commitment in imaginal discs and primordia by overriding the effect of juvenile hormone. Developmental Biology. 2008;324:258–265. doi: 10.1016/j.ydbio.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Kozlova T, Thummel CS. Spatial patterns of ecdysteroid receptor activation during the onset of Drosophila metamorphosis. Development. 2002;129:1739–1750. doi: 10.1242/dev.129.7.1739. [DOI] [PubMed] [Google Scholar]
- Kyriacou CP, Oldroyd M, Wood J, Sharp M, Hill M. Clock mutations alter developmental timing in Drosophila. Heredity (Edinb) 1990;64(Pt 3):395–401. doi: 10.1038/hdy.1990.50. [DOI] [PubMed] [Google Scholar]
- Layalle S, Arquier N, Leopold P. The TOR pathway couples nutrition and developmental timing in Drosophila. Developmental Cell. 2008;15:568–577. doi: 10.1016/j.devcel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Lee HO, Davidson JM, Duronio RJ. Endoreplication: Polyploidy with purpose. Genes & Development. 2009;23:2461–2477. doi: 10.1101/gad.1829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, et al. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nature Cell Biology. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Lin JL, Gu SH. In vitro and in vivo stimulation of extracellular signal-regulated kinase (ERK) by the prothoracicotropic hormone in prothoracic gland cells and its developmental regulation in the silkworm, Bombyx mori. Journal of Insect Physiology. 2007;53:622–631. doi: 10.1016/j.jinsphys.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Luo J, Becnel J, Nichols CD, Nassel DR. Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT1A receptor. Cellular and Molecular Life Sciences. 2012;69:471–484. doi: 10.1007/s00018-011-0789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal E, Vandersmissen HP, Badisco L, Van de Velde S, Verlinden H, Iga M, et al. Control of ecdysteroidogenesis in prothoracic glands of insects: A review. Peptides. 2010;31:506–519. doi: 10.1016/j.peptides.2009.08.020. [DOI] [PubMed] [Google Scholar]
- McBrayer Z, Ono H, Shimell M, Parvy JP, Beckstead RB, Warren JT, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Developmental Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina MF, Arrigo T, Valenzise M, Ghizzoni L, Caruso-Nicoletti M, Zucchini S, et al. Long-term auxological and pubertal outcome of patients with hereditary insulin-like growth factor-I deficiency (Laron and growth hormone-gene deletion syndrome) treated with recombinant human insulin-like growth factor-I. Journal of Endocrinological Investigation. 2011;34:292–295. doi: 10.1007/BF03347088. [DOI] [PubMed] [Google Scholar]
- Miles WO, Jaffray E, Campbell SG, Takeda S, Bayston LJ, Basu SP, et al. Medea SUMOylation restricts the signaling range of the Dpp morphogen in the Drosophila embryo. Genes & Development. 2008;22:2578–2590. doi: 10.1101/gad.494808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirth C, Shingleton AW. Integrating body and organ size in Drosophila: Recent advances and outstanding problems. Frontiers in Endocrinology. 2012;3:49. doi: 10.3389/fendo.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Current Biology. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Mirth CK, Riddiford LM. Size assessment and growth control: How adult size is determined in insects. Bioessays. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- Mirth CK, Truman JW, Riddiford LM. The ecdysone receptor controls the post-critical weight switch to nutrition-independent differentiation in Drosophila wing imaginal discs. Development. 2009;136:2345–2353. doi: 10.1242/dev.032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H, Kataoka H, Isogai A, Tamura S, Suzuki A, Mizoguchi A, et al. Amino acid sequence of a prothoracicotropic hormone of the silkworm Bombyx mori. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5840–5843. doi: 10.1073/pnas.83.16.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF. Physiological control of molting in insects. American Zoologist. 1981;21:631–640. [Google Scholar]
- Nijhout HF. The control of body size in insects. Developmental Biology. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): Cessation of juvenile hormone secretion as a trigger for pupation. The Journal of Experimental Biology. 1974a;61:493–501. doi: 10.1242/jeb.61.2.493. [DOI] [PubMed] [Google Scholar]
- Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): Growth of the last-instar larva and the decision to pupate. The Journal of Experimental Biology. 1974b;61:481–491. doi: 10.1242/jeb.61.2.481. [DOI] [PubMed] [Google Scholar]
- Niwa R, Namiki T, Ito K, Shimada-Niwa Y, Kiuchi M, Kawaoka S, et al. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the ‘Black Box’ of the ecdysteroid biosynthesis pathway. Development. 2010;137:1991–1999. doi: 10.1242/dev.045641. [DOI] [PubMed] [Google Scholar]
- Niwa R, Sakudoh T, Namiki T, Saida K, Fujimoto Y, Kataoka H. The ecdysteroidogenic P450 Cyp302a1/disembodied from the silkworm, Bombyx mori, is transcriptionally regulated by prothoracicotropic hormone. Insect Molecular Biology. 2005;14:563–571. doi: 10.1111/j.1365-2583.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, O’Connor MB, et al. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Developmental Cell. 2009;17:885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Morita S, Asakura I, Nishida R. Conversion of 3-oxo steroids into ecdysteroids triggers molting and expression of 20E-inducible genes in Drosophila melanogaster. Biochemical and Biophysical Research Communications. 2012;421:561–566. doi: 10.1016/j.bbrc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Ono H, Rewitz KF, Shinoda T, Itoyama K, Petryk A, Rybczynski R, et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Developmental Biology. 2006;298:555–570. doi: 10.1016/j.ydbio.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Ou Q, Magico A, King-Jones K. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biology. 2011;9:e1001160. doi: 10.1371/journal.pbio.1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvy JP, Blais C, Bernard F, Warren JT, Petryk A, Gilbert LI, et al. A role for betaFTZ-F1 in regulating ecdysteroid titers during post-embryonic development in Drosophila melanogaster. Developmental Biology. 2005;282:84–94. doi: 10.1016/j.ydbio.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Petryk A, Warren JT, Marques G, Jarcho MP, Gilbert LI, Kahler J, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13773–13778. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poodry CA, Woods DF. Control of the developmental timer for Drosophila pupariation. Roux’s Archives of Developmental Biology. 1990;199:219–227. doi: 10.1007/BF01682081. [DOI] [PubMed] [Google Scholar]
- Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: Downstream and feedback regulation of the insulin receptor pathway. Genes & Development. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz K, O’Connor MB. Timing is everything: PTTH mediated DHR4 nucleocytoplasmic trafficking sets the tempo of Drosophila steroid production. Frontiers in Endocrinology. 2011;2:108. doi: 10.3389/fendo.2011.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz KF, Larsen MR, Lobner-Olesen A, Rybczynski R, O’Connor MB, Gilbert LI. A phosphoproteomics approach to elucidate neuropeptide signal transduction controlling insect metamorphosis. Insect Biochemistry and Molecular Biology. 2009;39:475–483. doi: 10.1016/j.ibmb.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochemical Society Transactions. 2006;34:1256–1260. doi: 10.1042/BST0341256. [DOI] [PubMed] [Google Scholar]
- Rewitz KF, Yamanaka N, Gilbert LI, O’Connor MB. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science. 2009;326:1403–1405. doi: 10.1126/science.1176450. [DOI] [PubMed] [Google Scholar]
- Rewitz KF, Yamanaka N, O’Connor MB. Steroid hormone inactivation is required during the juvenile-adult transition in Drosophila. Developmental Cell. 2010;19:895–902. doi: 10.1016/j.devcel.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford LM. When is weight critical? The Journal of Experimental Biology. 2011;214:1613–1615. doi: 10.1242/jeb.049098. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Truman JW, Mirth CK, Shen YC. A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development. 2010;137:1117–1126. doi: 10.1242/dev.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout EJ, Marshall L, Grewal SS. Drosophila RNA polymerase III repressor Maf1 controls body size and developmental timing by modulating tRNAiMet synthesis and systemic insulin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1139–1144. doi: 10.1073/pnas.1113311109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Rybczynski R. The prothoracicotropic hormone. In: Gilbert LI, Iatrou K, Gill S, editors. Comprehensive molecular insect science. Oxford: Elsevier; 2005. pp. 61–123. [Google Scholar]
- Rybczynski R, Gilbert LI. Changes in general and specific protein synthesis that accompany ecdysteroid synthesis in stimulated prothoracic glands of Manduca sexta. Insect Biochemistry and Molecular Biology. 1994;24:175–189. doi: 10.1016/0965-1748(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Rybczynski R, Gilbert LI. Protein kinase C modulates ecdysteroidogenesis in the prothoracic gland of the tobacco hornworm, Manduca sexta. Molecular and Cellular Endocrinology. 2006;251:78–87. doi: 10.1016/j.mce.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Williams CM. Short-loop negative and positive feedback on ecdysone secretion by prothoracic gland in the tobacco hornworm, Manduca sexta. General and Comparative Endocrinology. 1989;75:204–216. doi: 10.1016/0016-6480(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Sehnal F, Bryant PJ. Delayed pupariation in Drosophila imaginal disc overgrowth mutants is associated with reduced ecdysteroid titer. Journal of Insect Physiology. 1993;39:1051–1059. [Google Scholar]
- Shingleton AW, Das J, Vinicius L, Stern DL. The temporal requirements for insulin signaling during development in Drosophila. PLoS Biology. 2005;3:e289. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siaussat D, Porcheron P, Debernard S. The Ecdysteroids’ effects in the control of cell proliferation and differentiation. In: Smagghe G, editor. Ecdysone: Structures and functions. Netherlands: Springer; 2009. pp. 185–204. [Google Scholar]
- Siegmund T, Korge G. Innervation of the ring gland of Drosophila melanogaster. The Journal of Comparative Neurology. 2001;431:481–491. doi: 10.1002/1096-9861(20010319)431:4<481::aid-cne1084>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Simpson P, Berreur P, Berreur-Bonnenfant J. The initiation of pupariation in Drosophila: Dependence on growth of the imaginal discs. Journal of Embryology and Experimental Morphology. 1980;57:155–165. [PubMed] [Google Scholar]
- Simpson P, Scheinderman HA. Isolation of temperature sensitive mutations blocking clone development in Drosophila melanogaster, and effects of a temperature sensitive cell lethal mutation on pattern formation in imaginal disks. Wilhelm Rouxs Archives of Developmental Biology. 1975;178:247–275. doi: 10.1007/BF00848432. [DOI] [PubMed] [Google Scholar]
- Slaidina M, Delanoue R, Gronke S, Partridge L, Leopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Developmental Cell. 2009;17:874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AV, Orr-Weaver TL. The regulation of the cell cycle during Drosophila embryogenesis: The transition to polyteny. Development. 1991;112:997–1008. doi: 10.1242/dev.112.4.997. [DOI] [PubMed] [Google Scholar]
- Song Q, Gilbert LI. Alterations in ultraspiracle (USP) content and phosphorylation state accompany feedback regulation of ecdysone synthesis in the insect prothoracic gland. Insect Biochemistry and Molecular Biology. 1998;28:849–860. doi: 10.1016/s0965-1748(98)00075-7. [DOI] [PubMed] [Google Scholar]
- Stieper BC, Kupershtok M, Driscoll MV, Shingleton AW. Imaginal discs regulate developmental timing in Drosophila melanogaster. Developmental Biology. 2008;321:18–26. doi: 10.1016/j.ydbio.2008.05.556. [DOI] [PubMed] [Google Scholar]
- Talamillo A, Sanchez J, Cantera R, Perez C, Martin D, Caminero E, et al. Smt3 is required for Drosophila melanogaster metamorphosis. Development. 2008;135:1659–1668. doi: 10.1242/dev.020685. [DOI] [PubMed] [Google Scholar]
- Talbot WS, Swyryd EA, Hogness DS. Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell. 1993;73:1323–1337. doi: 10.1016/0092-8674(93)90359-x. [DOI] [PubMed] [Google Scholar]
- Tennessen JM, Thummel CS. Coordinating growth and maturation - insights from Drosophila. Current Biology. 2011;21:R750–R757. doi: 10.1016/j.cub.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkiewicz MA, Stern M. Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS One. 2009;4:e5072. doi: 10.1371/journal.pone.0005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh AL, Smith WA. Nutritional sensitivity of fifth instar prothoracic glands in the tobacco hornworm, Manduca sexta. Journal of Insect Physiology. 2011;57:809–818. doi: 10.1016/j.jinsphys.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Warren JT, Petryk A, Marques G, Jarcho M, Parvy JP, Dauphin-Villemant C, et al. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11043–11048. doi: 10.1073/pnas.162375799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JT, Petryk A, Marques G, Parvy JP, Shinoda T, Itoyama K, et al. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: A P450 enzyme critical in ecdysone biosynthesis. Insect Biochemistry and Molecular Biology. 2004;34:991–1010. doi: 10.1016/j.ibmb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Warren JT, Yerushalmi Y, Shimell MJ, O’Connor MB, Restifo LL, Gilbert LI. Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: Correlations with changes in gene activity. Developmental Dynamics. 2006;235:315–326. doi: 10.1002/dvdy.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman LJ, Ainsley JA, Johnson WA. Developmental timing of a sensory-mediated larval surfacing behavior correlates with cessation of feeding and determination of final adult size. Developmental Biology. 2010;345:170–179. doi: 10.1016/j.ydbio.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells BS, Johnston LA. Maintenance of imaginal disc plasticity and regenerative potential in Drosophila by p53. Developmental Biology. 2012;361:263–276. doi: 10.1016/j.ydbio.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annual Review of Entomology. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Liu Z, Huang X. br regulates the expression of the ecdysone biosynthesis gene npc1. Developmental Biology. 2010;344:800–808. doi: 10.1016/j.ydbio.2010.05.510. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Honda N, Osato N, Niwa R, Mizoguchi A, Kataoka H. Differential regulation of ecdysteroidogenic P450 gene expression in the silkworm, Bombyx mori. Bioscience, Biotechnology, and Biochemistry. 2007;71:2808–2814. doi: 10.1271/bbb.70420. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Hua YJ, Mizoguchi A, Watanabe K, Niwa R, Tanaka Y, et al. Identification of a novel prothoracicostatic hormone and its receptor in the silkworm Bombyx mori. The Journal of Biological Chemistry. 2005;280:14684–14690. doi: 10.1074/jbc.M500308200. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Hua YJ, Roller L, Spalovska-Valachova I, Mizoguchi A, Kataoka H, et al. Bombyx prothoracicostatic peptides activate the sex peptide receptor to regulate ecdysteroid biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2060–2065. doi: 10.1073/pnas.0907471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, O’Connor MB. Nitric oxide directly regulates gene expression during Drosophila development: Need some gas to drive into metamorphosis? Genes & Development. 2011;25:1459–1463. doi: 10.1101/gad.2080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Rewitz K, O’Connor MB. Ecdysone control of developmental transitions: Lesson from Drosophila research. Annual Review of Entomology. 2013;58:497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Zitnan D, Kim YJ, Adams ME, Hua YJ, Suzuki Y, et al. Regulation of insect steroid hormone biosynthesis by innervating peptidergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8622–8627. doi: 10.1073/pnas.0511196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama-Yanagawa T, Enya S, Shimada-Niwa Y, Yaguchi S, Haramoto Y, Matsuya T, et al. The conserved Rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. The Journal of Biological Chemistry. 2011;286:25756–25762. doi: 10.1074/jbc.M111.244384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama T, Namiki T, Mita K, Kataoka H, Niwa R. Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development. 2006;133:2565–2574. doi: 10.1242/dev.02428. [DOI] [PubMed] [Google Scholar]
- Young SC, Yeh WL, Gu SH. Transcriptional regulation of the PTTH receptor in prothoracic glands of the silkworm, Bombyx mori. Journal of Insect Physiology. 2012;58:102–109. doi: 10.1016/j.jinsphys.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhou B, Truman JW, Riddiford LM. Overexpression of broad: A new insight into its role in the Drosophila prothoracic gland cells. The Journal of Experimental Biology. 2004;207:1151–1161. doi: 10.1242/jeb.00855. [DOI] [PubMed] [Google Scholar]