Abstract
The steroid hormone ecdysone is the central regulator of insect developmental transitions. Recent new advances in our understanding of ecdysone action have relied heavily on the application of Drosophila melanogaster molecular genetic tools to study insect metamorphosis. In this review, we focus on three major aspects of Drosophila ecdysone biology: (a) factors that regulate the timing of ecdysone release, (b) molecular basis of stage- and tissue-specific responses to ecdysone, and (c) feedback regulation and coordination of ecdysone signaling.
Keywords: developmental timing, metamorphosis, prothoracicotropic hormone, Ashburner model, critical weight
INTRODUCTION
Insect model systems are ideal for studying the molecular mechanisms that regulate developmental transitions in multicellular organisms, largely because their individual developmental stages are clearly punctuated by molting and metamorphosis. These transition events are under strict endocrine control and have been of interest to entomologists for close to a century, since the classical study of Stefan Kopec (62) on the metamorphosis of the gypsy moth, Lymantria dispar. This pioneering work led to novel concepts such as neurosecretory cells (63, 137), contributing greatly to the development of modern endocrinology.
Several key hormones and neuropeptides are involved in the control of insect developmental transitions, but the steroid hormone ecdysone (E) is the master regulator. During larval stages, E is produced primarily in the prothoracic gland (PG), an endocrine tissue that expresses genes encoding E biosynthetic enzymes and intracellular substrate-trafficking molecules (45, 95). Once released into the hemolymph, E is converted to an active form, 20-hydroxyecdysone (20E), by a P450 monooxygenase that is expressed in many nonendocrine tissues (90). 20E is the primary molting hormone that binds to a nuclear receptor and initiates various gene expression cascades, which ultimately lead to the physiological, morphological, and behavioral changes associated with molting and metamorphosis. Traditionally, the term “ecdysone” has often been used as a generic name for both E and 20E. For simplicity, we follow this convention here.
Regulation of gene expression is clearly the central molecular process underlying ecdysone control of insect molting and metamorphosis (125), although many downstream effector genes still remain to be identified. Therefore, it is no exaggeration that all studies on insect developmental transitions eventually converge on understanding how the production, release, uptake, reception, downstream signaling, and metabolism of ecdysone are conducted and regulated. Answering these questions not only deepens our understanding of the mechanistic aspects of developmental transitions, but may also provide valuable models for human diseases. For example, studies on ecdysone biosynthesis (ecdysteroidogenesis) in the PG can provide insights into conserved physiological and pathological processes related to sterol trafficking and steroidogenesis (37, 44). Likewise, developmental defects that disrupt the coordination of ecdysone-controlled proliferation and differentiation in various tissues have long served as excellent models for cancer development (14, 37). Therefore, ecdysone research has had significant impact in areas beyond just insect pest control, disease vector management, or beneficial insect rearing such as apiculture.
During the past decade, the application of Drosophila melanogaster molecular genetic tools has provided new perspectives and uncovered new strategies for studying ecdysone signaling (30, 37). Here we review some of these recent advances and reflect on how they have altered our understanding of key traditional concepts of insect development, such as the classical scheme of insect endocrinology, Ashburner model, and critical weight checkpoint.
TIMING OF ECDYSONE RELEASE
Beyond the Classical Scheme: Prothoracic Gland as a Decision-Making Center
The classical scheme of insect endocrinology (25) describes how insect developmental transitions are regulated by three primary circulating factors: prothoracicotropic hormone (PTTH), juvenile hormone (JH), and ecdysone. The function and mode-of-action of JH are beyond the scope of this review; only the relationship between PTTH and ecdysone is considered here.
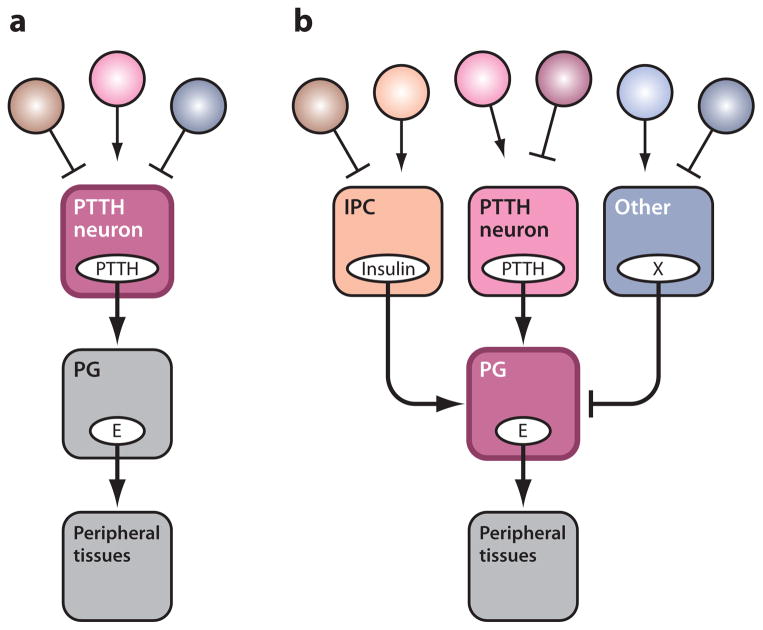
PTTH is a neuropeptide produced by two pairs of lateral neurosecretory cells in the insect brain (55, 71, 106, 115) and is the primary tropic factor for the ecdysteroidogenic activity of the PG. As illustrated in Figure 1a, the classical scheme posits that the release of PTTH from the brain is the key event that determines the timing of developmental transitions. According to this scheme, PTTH-producing neurons integrate and evaluate various environmental and developmental cues in order to determine when to progress to the next developmental stage. On the basis of both physiological and molecular genetic studies (71, 128), this assumption appears to be valid for at least one environmental input, the photoperiod. In recent years, however, evidence has accumulated that multiple factors, in addition to PTTH, act on the PG to control ecdysteroidogenesis. These observations suggest that the PG itself is the decision-making center for orchestrating developmental transitions (Figure 1b). According to this revised view, PTTH is a major (but not the only) developmental signal that triggers the onset of ecdysteroidogenesis. Additional tropic signals, especially nutritional signals reflecting the general metabolic status of the larvae, act directly on the PG to influence the timing of transition. Moreover, multiple static regulators likely suppress the ecdysteroidogenic activity of the gland, presumably until certain physiological conditions are met. By integrating all these signals, the PG decides when to proceed to the next developmental stage, and the ecdysteroidogenesis in the PG is upregulated. In this section we summarize recent findings of such regulators of PG activity and discuss their possible significance. For detailed descriptions of the molecular mechanisms involved in ecdysteroidogenesis, as well as a more complete list of PG regulators, readers are directed to other reviews (45, 70, 95).
Figure 1.
Schematic illustration of (a) the classical scheme and (b) the revised scheme of insect endocrinology. In the classical scheme, all the environmental as well as internal regulatory cues (circles) affect the function of the PTTH-producing neurons. In the revised scheme, such signals converge on the PG, which in turn decides the timing of developmental transition. Abbreviations: E, ecdysone; PTTH, prothoracicotropic hormone; PG, prothoracic gland; IPC, insulin-producing cell; X, unknown prothoracicostatic factor(s).
Prothoracicotropic Hormone: A Brain-Derived Regulator of Developmental Timing
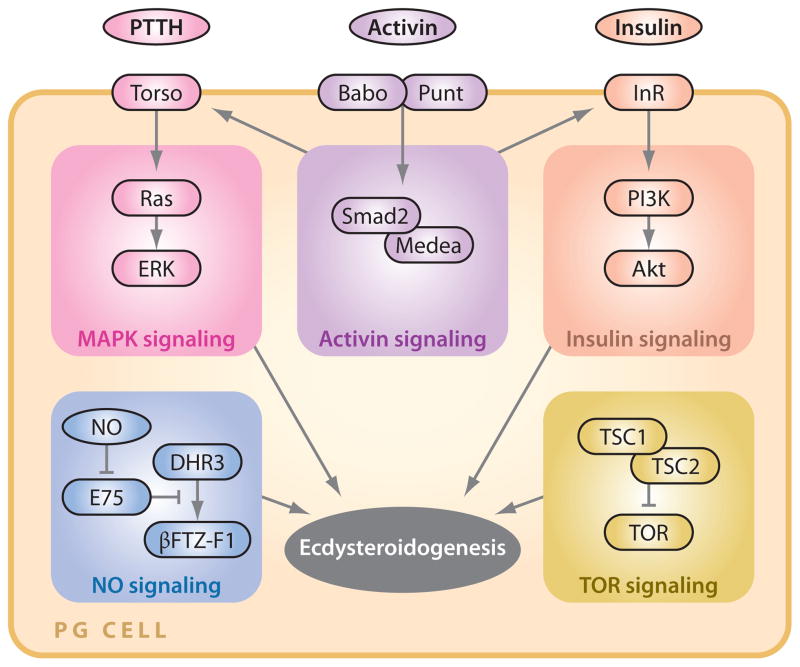
Although the existence of a brain-derived ecdysteroidogenic factor (originally called the brain hormone) has been known for 90 years (63) and the neuropeptide was characterized more than two decades ago (54, 55), it was only recently that the first loss-of-function study of PTTH was conducted in D. melanogaster (71). Although genetic ablation of the PTTH-producing neurons (also called the PG neurons in D. melanogaster) significantly delayed the onset of metamorphosis, larvae lacking PTTH eventually pupated and eclosed with bigger body sizes owing to the prolonged duration of the larval feeding stage. Therefore, PTTH is not absolutely required for metamorphosis, but rather it regulates the timing of metamorphosis and thereby controls the final body size. The loss of PTTH modified the timing of larval-larval molts only slightly, suggesting it plays a minor role, if any, in these earlier developmental transitions in D. melanogaster. The identification of Torso as the PTTH receptor (96) further confirmed the above findings and also elucidated that the MAPK signaling cassette is the major downstream effector of the PTTH signal (Figure 2), as has been extensively studied in lepidopteran model insects (45, 94, 106). PTTH up-regulates the transcript levels of certain ecdysteroidogenic enzymes in the PG through the MAPK signaling pathway to stimulate the activity of the PG (36, 71, 86), although the acute effect of PTTH is clearly independent of transcriptional regulation at least in a lepidopteran species (142) and is likely mediated by increased translation and/or phosphorylation of some key molecules (94), as in the case of acute regulation of steroidogenesis in mammals (121).
Figure 2.
Signaling pathways that positively regulate ecdysteroidogenesis in the PG in Drosophila melanogaster. Only the key components of each signaling pathway are depicted. Abbreviations: PG, prothoracic gland; PTTH, prothoracicotropic hormone; MAPK, mitogen-activated protein kinase; NO, nitric oxide; InR, insulin receptor; TOR, target of rapamycin.
What regulates the function of the PTTH-producing neurons? This central question needs to be addressed in order to understand how developmental timing and final body size are determined in insects. As discussed above, the light/dark cycle is definitely one of the cues controlling PG neuron function. These neurons are innervated by the pacemaker neurons that produce the principal circadian transmitter called pigment-dispersing factor (PDF) (71, 93, 115), and pdf mutants show altered ptth transcript levels (71). JH has also long been considered a negative regulator of PTTH release during the last larval instar of lepidopteran species (30, 81, 103), although conflicting conclusions have been reached in some other studies (76, 112) and the effect of JH signaling on developmental timing and PTTH release in D. melanogaster is less well understood (100, 101). However, considering the recent advances in our understanding of JH signaling, especially the identification of its receptor (19, 49), it seems prudent to investigate further the effect of JH on the PG neuron function by using D. melanogaster molecular genetic tools.
Halme et al. (41) demonstrated that ptth expression is downregulated when larval imaginal tissues are either physically or genetically damaged. Their work provided the first evidence that PTTH is involved in the shift of developmental timing triggered by damaged discs, a phenomenon studied for many years (47, 87, 116) as a model of interorgan communication. Because ptth expression fluctuates dramatically during development (71) and both photoperiod and disc damage affect the transcript levels of ptth, investigating the transcriptional regulators of ptth expression (e.g., 114) might provide a deeper understanding of the regulation of the PG neuron function.
INSULIN/IGF AND TOR SIGNALING IN THE PROTHORACIC GLAND
Insulin/insulin-like growth factor (IGF) signaling (IIS) has been studied extensively in D. melanogaster as a regulator of tissue growth (for reviews see 32, 42, 84). In 2005, three independent studies elucidated the importance of IIS in the PG (17, 21, 72), consistent with the historical fact that the first “prothoracicotropic hormone” purified from a lepidopteran species was an insulin family peptide (later renamed bombyxin) (48, 78). These findings led to a hypothesis that the growth of the PG acts as a sensor for the metabolic status of the whole organism (73). This makes intuitive sense and provides a simple model that the PG needs to be mature when commitment to metamorphosis happens. However, the meaning of “PG growth” in this context should be carefully considered. Because bombyxin is capable of stimulating ecdysteroidogenesis within several hours (59), cell size increase is clearly not the only reason for the tropic effect of IIS. This is also consistent with the observations that the PG cell overgrowth triggered by other pathways does not accelerate the timing of development (16, 21). It is possible that at least part of the ecdysteroidogenic effect of IIS is explained by potential crosstalk with the MAPK signaling pathway (56, 64). As discussed by Walsh & Smith (134), IIS might instead provide the competence of the PG to respond to other developmental cues like PTTH when sufficient nutrients have been acquired.
More recently, a report on the role of TOR (target of rapamycin) signaling in the PG provided another layer of putative interaction between nutritional signals and development (66). Detailed time-course analysis of TOR signaling activity in the PG in D. melanogaster, however, revealed that it is required after critical weight is attained (66), when even starvation cannot delay the timing of metamorphosis initiation (the concept of critical weight is discussed below). It is therefore unlikely that TOR signaling in the PG is the primary mediator of nutrition sensing by this endocrine gland, but it may interact with the PTTH pathway (38, 118, 119). Because the MAPK and TOR pathways share some common targets (105, 113), it would be interesting to examine in D. melanogaster how these two signaling pathways regulate each other.
Additional PG Regulatory Factors
TGFβ/Activin
A recent study revealed that TGFβ/Activin signaling in the PG is critical for ecdysteroidogenesis (36) (Figure 2). This requirement of TGFβ/Activin signaling can be explained by its transcriptional upregulation of torso and insulin receptor (InR) in PG cells. TGFβ/Activin thus seems to work as a competence signal that endows the PG with its responsiveness to developmental (PTTH) and nutritional (insulin) signals, thereby ensuring coordination between these two types of timing cues. The TGFβ pathway upregulates the production of Caenorhabditis elegans bile acid-like hormone called dafachronic acid, a potential functional counterpart to ecdysone in C. elegans (138). TGFβ-mediated sterol/steroid hormone production might therefore be an evolutionarily conserved mechanism for the regulation of developmental timing in metazoans.
Nitric oxide
Certain D. melanogaster nuclear receptors (e.g., E75 and βFTZ-F1) are expressed in the PG and are required so that proper ecdysteroidogenesis can take place (12, 88). A recent report (16) clearly demonstrated that these nuclear receptors in the PG are components of nitric oxide (NO) sensing machinery, where NO binds to its receptor E75 (92) to induce the expression of βFTZ-F1, which in turn upregulates expression of enzymes that regulate ecdysteroidogenesis (88) (Figure 2). This report provided the first direct evidence for the involvement of NO in the regulation of developmental transitions. Although the physiological information conveyed by this small diffusible second messenger remains unknown, it is interesting to speculate on its involvement in the sensing of oxygen supply (as discussed in Reference 144), which has been shown in other insects to act as a key factor in determining developmental timing and body size (18, 52).
Prothoracicostatic factors
JH acts as a static regulator of the PG (prothoracicostatic factor) in some insects including D. melanogaster (98, 107, 135), but its molecular machinery is poorly understood except in the silkworm, Bombyx mori, where it suppresses the expression of one of the ecdysteroidogenic enzymes in the PG (142). In B. mori, some neuropeptides also exert static activities in the PG through G-protein-coupled receptors (GPCRs) (143, 145), which likely couple with Gαi/o to suppress cyclic AMP (cAMP) levels in the PG cells. The importance of cAMP signaling in the PG of D. melanogaster, however, is not clear. Extracellular adenosine also exerts a static effect on the D. melanogaster PG through a GPCR, allowing its metabolizing enzymes (adenosine deaminase-related growth factors) to regulate developmental transitions (26). These prothoracicostatic factors can function as permissive checkpoint signals, the removal/clearance of which informs the PG that certain physiological conditions have been fulfilled to further proceed with developmental transitions. Detailed analyses of these prothoracicostatic factors, therefore, will likely help us understand what sort of physiological checkpoints must be satisfied to promote proper developmental transitions.
MOLECULAR BASIS OF STAGE- AND TISSUE-SPECIFIC RESPONSES TO ECDYSONE
Expanding the Ashburner Model: Diversity of Ecdysone Response over Space and Time
A number of classical studies on the ecdysone-induced puffing patterns of polytene chromosomes (3, 20) led to the now-established paradigm of the mode-of-action of ecdysone, which bridged the visible effects of this steroid hormone to the molecular events behind them. The model proposed by Michael Ashburner and colleagues, now called the Ashburner model, explains how the gene expression cascade is triggered upon binding of ecdysone to its receptor (3, 4, 99, 125). According to this model, ecdysone binding to its receptor initially activates the expression of genes in the early puff regions, but represses the expression of those in the late puffs. As the proteins encoded by the early puff genes become abundant, they repress their own promoters while activating the expression of late puff genes. This model has been confirmed over the years by D. melanogaster molecular genetic methods, although multiple ecdysone primary-response genes were also found outside the early puff loci, suggesting that ecdysone triggers much broader transcriptional responses than originally thought (46). Consequently, now the survey for ecdysone-inducible genes is conducted at the genomic scale (10, 35, 69).
While the Ashburner model nicely explains the temporal gene expression cascade triggered by a single ecdysone pulse, it does not necessarily provide an explanation for how individual tissues respond differently to the same ecdysone pulse (as represented by E63-1; 2) or how a certain tissue responds differently to multiple ecdysone pulses during development (as exemplified by E93; 67, 77). Although such diversity of ecdysone response over space and time can be explained in part by differential sensitivities of ecdysone-inducible genes to the ecdysone concentration (53) or differential expression patterns of the ecdysone receptor (EcR) isoforms (124, 129) as well as those of primary ecdysone-inducible transcription factors (15, 126), these mechanisms still seem insufficient to provide a full explanation of the diverse effects of ecdysone during development.
An important conceptual advance that has helped fill this void came from the investigation of the response to a pair of sequential ecdysone pulses during early metamorphosis in D. melanogaster, where a nuclear receptor, βFTZ-F1, expressed transiently during the interval provides the unique responsiveness to the second ecdysone peak. As such, it acts as a competence factor for stage-specific responses to ecdysone (13, 140, 141). The idea of a competence factor, whose presence endows the cells with the capacity to respond to ecdysone in a specific manner, can also be applied to tissue-specific ecdysone responses. Identification of such competence factors as well as a detailed investigation into their mode of action therefore is critical for expanding the Ashburner model to understand orchestrated responses to ecdysone at the systemic level.
As discussed by Rosenfeld et al. (102), coactivators and corepressors of nuclear receptors can provide a molecular basis for tissue- and/or stage-specific cues to be integrated into a hormonal response. In this section we review a series of recent reports concerning multiple cofactors that interact and cooperate with EcR, and discuss how such molecules can produce diversity in the ecdysone response in different physiological contexts.
Multiple Cofactors Involved in the Reception of Ecdysone
The core complex working to transduce the ecdysone signal is a heterodimer of two nuclear receptors, EcR (61) and Ultraspiracle (USP) (85). Although the dispensability of USP for the ecdysone-induced expression of glue genes during the mid-third-instar stage in D. melanogaster (22, 40) suggests an exciting possibility that USP itself can act as a competence factor in some situations, the expression of most other known ecdysone-dependent genes is mediated by this core heterodimer. In addition, molecular genetic studies of the past decade identified a plethora of cofactors that interact and cooperate with the EcR/USP complex, as described below.
Chromatin remodelers
Chromatin remodeling is a major epigenetic modification that uses ATP hydrolysis to alter histone-DNA contacts and thereby affect gene transcription (8, 79). In D. melanogaster, the nucleosome remodeling factor (NURF) complex (5) and the Brahma complex (150) are involved in ecdysone-mediated regulation of gene expression. In particular, NURF binds EcR in the presence of ecdysone, suggesting its role as an EcR coactivator during metamorphosis (5). The Brahma complex participates in gene transcription mediated by an ecdysone-inducible nuclear receptor, DHR3 (133), which might in part explain the involvement of this complex in ecdysone action. Detailed analyses of the epigenetic factor’s functions in a class of multidendritic neurons during ecdysone-triggered dendritic pruning (60) revealed an important requirement for the Brahma complex, whereas the NURF complex, whose importance has been shown in ecdysone-mediated control of oogenesis (1), is dispensable for the neuronal remodeling. Together, these studies suggest that epigenetic factors can provide tissue specificity to ecdysone response.
Histone modifiers
Histone modifiers are another class of epigenetic factors that covalently modify histone proteins (79, 122). Many types of histone modifiers are involved in ecdysone-mediated gene transcription in D. melanogaster: the acetyltransferases p160/SRC/Taiman (6) and p300/CBP (60), the lysine methyltransferase TRR (111), the poly(ADP-ribose) polymerase PARP (109, 131), and the arginine methyltransferase DART1 (58). All these histone modifiers interact with EcR in a ligand-dependent manner, thereby working as coactivators (except DART1, which is a corepressor) of ecdysone signaling. Moreover, such coupling of ecdysone signaling with histone modifications provides a molecular basis for repressors of ecdysone signaling, such as the corepressor SMRTER, which can recruit histone deacetylases (130).
Histone chaperones
This class of nucleosomal regulators facilitates transcription by associating with histones during processes such as the eviction of histone octamers at actively transcribed loci (24). One such histone chaperone, DEK, interacts with EcR in a ligand-independent manner and functions as an EcR coactivator in D. melanogaster (110).
Insulator-binding factors/cofactors
Insulators are DNA sequence elements that recruit DNA-binding proteins and their cofactors to control gene expression by mediating inter- and intrachromosomal interactions (33). A recent report has demonstrated that a common cofactor for insulator-binding proteins, centrosomal protein 190 (CP190), is required for stabilizing specific chromatin loops to properly promote ecdysone-mediated gene transcription (139). This finding suggests that the recruitment of insulator-binding factors and cofactors is another layer of ecdysone response modification that may be deployed in different physiological contexts. Because CP190-binding sites overlap with those of cohesin in the D. melanogaster genome (7), the direct role of cohesin in ecdysone-mediated gene transcription (89) might be explained by the same mechanism.
Other coactivators/corepressors
Other EcR cofactors identified in D. melanogaster include Alien (27), Bonus (9), Rigor mortis (34), and DOR (31). All these factors are unique in terms of their loss-of-function phenotypes as well as their interactions with other nuclear receptors, again consistent with the idea that EcR coactivators/corepressors can provide specificity to the ecdysone responsiveness of different tissues at particular times during development.
βFTZ-F1 and Histone Acetylation: A Case Study for Competence Factor Action
As listed above, a variety of EcR cofactors can modify ecdysone responses over space and time. How then do the known competence factors like βFTZ-F1 cooperate with these molecules to provide competency? An important insight came from a study on the mosquito Aedes aegypti, where it was found that βFTZ-F1 physically associates with the histone acetyltransferase p160/SRC (called Taiman in D. melanogaster and FISC in A. aegypti), recruiting it to the EcR/USP complex in a ligand-dependent manner (147). βFTZ-F1 also promotes EcR/USP/FISC loading onto target promoters, where local histone acetylation facilitates target gene transcription (147). This report thus reveals the molecular basis of how a competence factor can potentiate the ecdysone effect by interacting with EcR cofactors (Figure 3).
Figure 3.
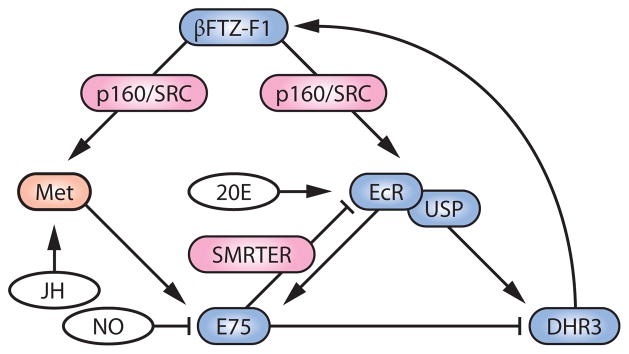
An integrative model of how βFTZ-F1 regulates ecdysone signaling in conjunction with other factors. Nuclear receptors are colored blue, the EcR coactivator/corepressor is colored red, the JH receptor Met is colored orange, and the ligands for the receptors are colored white. Abbreviations: 20E, 20-hydroxyecdysone; EcR, ecdysone receptor; Met, Methoprene-tolerant; JH, juvenile hormone; NO, nitric oxide; USP, Ultraspiracle.
As mentioned above, the EcR corepressor SMRTER can recruit histone deacetylases and thereby suppress coactivator effects of histone acetyltransferases. A recent report (50) nicely demonstrated that SMRTER recruitment to ecdysone-inducible gene loci is mediated by E75, whose binding site overlaps with EcR/USP. Therefore, E75 likely suppresses the function of βFTZ-F1 in two ways: The first is through the traditional inhibitory interaction with the direct βFTZ-F1 inducer DHR3 (51, 65, 136), and the second is through the indirect counteracting effects on histone acetylation. Being an ecdysone-inducible gene itself, E75 thus contributes to the transient nature of βFTZ-F1 as a temporal competence factor, by forming negative-feedback loops (Figure 3). Both of the above inhibitory effects of E75 on βFTZ-F1 function can be blocked by NO (16, 50), indicating critical involvement of this diffusible molecule in controlling competence of the cells to ecdysone.
Another interesting report describes βFTZ-F1 as a competence factor for JH activity (29), adding yet another layer of complexity to this regulatory network. E75 is activated in S2 cells as a primary response gene to JH (28), and this JH function requires βFTZ-F1 (29). This novel function of βFTZ-F1 is likely explained in the context of recent findings that p160/SRC dimerizes with the JH receptor Methoprene-tolerant (Met) in a ligand-dependent manner and thus likely functions as a Met coactivator (19, 68, 146). It is therefore conceivable that βFTZ-F1 can function as a competence factor for both ecdysone and JH signaling, through the recruitment of p160/SRC to their receptors (Figure 3). As discussed in Reference 49, however, further study is warranted to determine whether βFTZ-F1 plays any role in JH action in vivo.
As these examples demonstrate, EcR cofactors can provide machinery for competence factors to modulate ecdysone signaling over space and time. Moreover, such a modulatory network can provide a molecular basis for other signaling molecules such as NO and JH to further regulate ecdysone responses. Detailed analyses of each of the cofactors in different developmental processes should eventually enable us to determine how ecdysone signaling is refined to produce specific outputs in different contexts and how it is influenced by other signaling molecules.
FEEDBACK REGULATION AND COORDINATION OF ECDYSONE SIGNALING
The Critical Weight Checkpoint and the Molecular Basis of Commitment
The idea of critical weight in insects was defined and established by physiological studies conducted mainly in the tobacco hornworm, Manduca sexta (80, 82). Experimentally, critical weight is defined as the weight after attainment of which the time course to metamorphosis initiation can no longer be delayed by starvation. In other words, when last-instar larvae attain a certain body size after a period of feeding and growth, the switch to metamorphosis that fixes the schedule of later transition events is turned on (for a recent review, see 73). The traditional model proposed on the basis of physiological experiments has now been reinforced by D. melanogaster genetic studies, which made clear that the activation of ecdysteroidogenesis in the PG is the molecular event that corresponds to the attainment of critical weight (17, 21, 72, 74).
Presumably, the attainment of critical weight provides a checkpoint that ensures that larvae have stored enough nutrients to make it through the nonfeeding period that accompanies metamorphosis. Moreover, it is likely that critical weight is not the only checkpoint that needs to be satisfied before ecdysteroidogenesis is activated, considering the existence of multiple prothoracicostatic factors that can function as permissive checkpoint signals. Although the nature of such checkpoint signals and their sensing mechanisms is in need of further investigation, there is another aspect to this concept that is important to recognize: Once ecdysteroidogenesis in the PG is upregulated, all the downstream processes become intrinsically driven and refractory to further changes in physiological status. Molecularly, such commitment is likely explained by multiple layers of positive- and negative-feedback circuits involved in ecdysone signaling, as well as by a highly systematic response to ecdysone that drives the process forward in a coordinated manner across all tissues. In this final section we summarize various examples of this self-adjusting nature of ecdysone signaling and discuss how these observations, along with future studies, should enable us to eventually describe how ecdysone signaling functions at the organismal scale to drive developmental transitions.
Feedback Regulation of Ecdysone Signaling
If all the events that occur after the commitment of the PG to ecdysteroidogenesis are predetermined, it is reasonable to assume that PTTH production and release are influenced by feedback control from the PG. This possibility, however, has not been studied extensively at the molecular level. Both positive- and negative-feedback effects of ecdysone on the PG have been demonstrated by physiological studies (11, 108, 123), but key molecules involved in these processes have not yet been elucidated either. Although some ecdysone-inducible nuclear receptors expressed in the PG have been speculated to be the candidates for such feedback regulation on the PG (12), they are involved in the NO signaling pathway as discussed above (16), and their importance in feedback regulation on PG function has not been clarified. Accordingly, much about the self-sustaining nature of ecdysone production remains to be elucidated at the molecular level, although the existence of such a mechanism is almost certain given the ample physiological evidence.
On the other hand, feedback regulation of ecdysone signaling in peripheral tissues has been more extensively studied in D. melanogaster. This includes both positive and negative feedback, because the increase and also the decrease of ecdysone signaling (which together form a pulse of ecdysone) are important for unidirectional progression of the developmental transition (97). Below we summarize examples of feedback regulation of ecdysone signaling in peripheral tissues.
Positive-feedback regulation
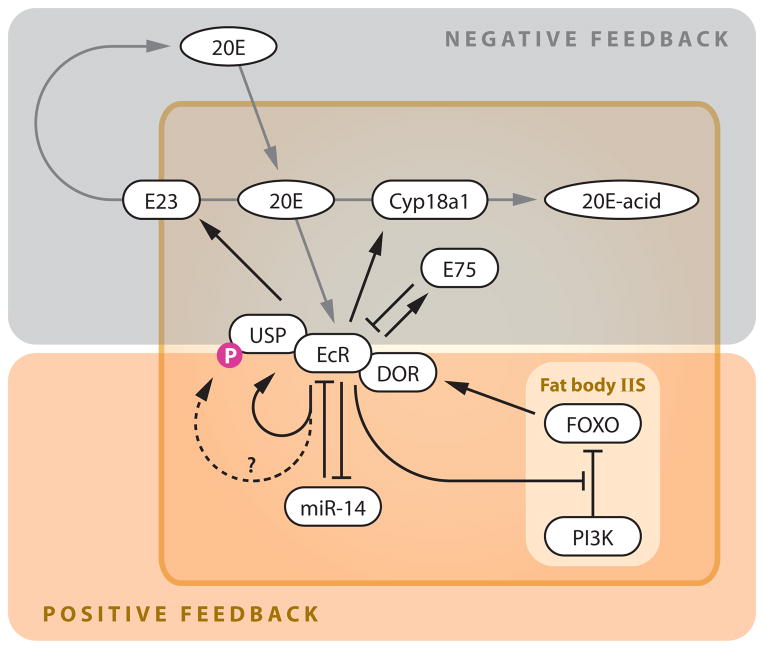
The most obvious positive feedback is the transcriptional regulation of EcR by ecdysone. This not only includes direct induction of EcR expression by ecdysone (53), but also involves an indirect regulation mediated by the microRNA miR-14 (132). miR-14 suppresses EcR expression through binding to its target sites on the 3′ UTRs of EcR isoforms, whereas ecdysone acts via EcR to downregulate miR-14 expression, thus forming a feedback loop (132) (Figure 4). The EcR partner USP is also regulated by ecdysone signaling, not only through its expression but also through its phosphorylation (120). Because phosphorylated USP seems to be the primary nuclear form (50), ecdysone might be regulating USP subcellular localization to form another positive-feedback loop (Figure 4). The direct involvement of EcR in this process, however, has yet to be demonstrated. The EcR coactivator DOR has a unique feature in that its expression is suppressed by IIS in the fat body (31). Because ecdysone signaling in the fat body antagonizes IIS at the levels of both PI3K activity (104) and FOXO localization (21), DOR is located at the core of a positive-feedback loop formed by ecdysone/IIS crosstalk (Figure 4).
Figure 4.
Feedback regulation of ecdysone signaling in peripheral tissues. See text for details. Abbreviations: 20E, 20-hydroxyecdysone; EcR, ecdysone receptor; IIS, insulin/IGF signaling; USP, Ultraspiracle.
Negative-feedback regulation
Except for the regulation of histone acetylation via E75 (Figure 3), the known ecdysone negative-feedback mechanisms in peripheral tissues adjust the intracellular concentration of ecdysone (Figure 4). One mechanism involves the ecdysone-inducible ATP-binding cassette transporter E23, which has been suggested to pump ecdysone out of the cell to lower its cytoplasmic concentration (43). Another example is Cyp18a1, which is an ecdysone-metabolizing cytochrome P450 whose expression is induced by ecdysone (39, 46, 97). Because both of these molecules eventually lower the effective concentrations of ecdysone, each one provides a mechanism for producing a transient, self-limiting pulse of ecdysone signaling that appears to be required for unidirectional developmental transitions to occur (97).
Coordinated Responses to Ecdysone at the Systemic Level: Case Studies
Probably the most elegant aspect of the autonomous response to ecdysone is the interplay between different tissues and organs. Insect tissues respond to ecdysone not only differently but coordinately, so that physiological as well as behavioral events triggered by ecdysone are conducted in harmony. Being a suitable model for interorgan communication studies (91), D. melanogaster has provided valuable insights into how individual tissue responses to ecdysone are coordinated at the whole-organism level, as documented by the two following examples (Figure 5).
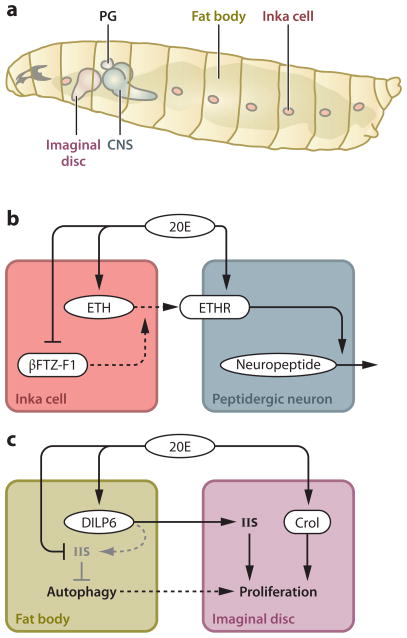
Figure 5.
Interorgan communication upon ecdysone response. (a) Fly larval organs involved in the interplays described in the text. (b) Coordinated ETH/ETHR signaling that triggers ecdysis behavior. (c) Communication between the fat body and imaginal discs promotes the development of adult structures during metamorphosis. Abbreviations: 20E, 20-hydroxyecdysone; PG, prothoracic gland; CNS, central nervous system; ETH, ecdysis-triggering hormone; ETHR, ETH receptor; IIS, insulin/IGF signaling; Crol, crooked legs.
Ecdysis behavior
Insect molting is a series of events that begins with apolysis, the separation of the cuticle from the epidermis, and ends with ecdysis, the shedding of the old cuticle. Ecdysis is an excellent model for an innate behavior that is orchestrated by ecdysone (127, 148). Ecdysone initiates a neuropeptide-signaling cascade beginning with the release of ecdysis-triggering hormone (ETH) from peritracheal endocrine cells called Inka cells (Figure 5a). ETH is received by a GPCR (ETH receptor, or ETHR) expressed in central peptidergic neurons, which are activated sequentially in response to ETH to shape the behavioral sequence observed during ecdysis (57). In Inka cells, ecdysone induces eth expression through EcR, leading to massive accumulation of ETH (Figure 5b). At the same time, ETHR expression in peptidergic neurons is upregulated by ecdysone in the central nervous system, providing the nervous system with the competence to respond to ETH (148) (Figure 5b). High ecdysone titers block ETH release and ecdysis onset (149), giving enough time for other tissues to respond to ecdysone before ecdysis is eventually initiated by ETH release in response to the decline of the ecdysone titer. This low-ecdysone-triggered ETH release is mediated most likely by βFTZ-F1, whose expression is induced only when the ecdysone titer drops (97, 148) (Figure 5b). In this way, peripheral and central peptidergic cells coordinate their response to ecdysone, so that this complex behavioral sequence happens in the right order and in a highly integrated manner.
Imaginal disc growth
External structures of adult flies, such as wings and legs, develop from imaginal discs that undergo extensive patterning during larval development. Pulses of ecdysone during metamorphosis then promote additional cell proliferation and differentiation, such that the appropriate adult structures are formed at the right time. Ecdysone induces expression of an insulin-like peptide gene named dilp6 in the fat body to promote nutrient-independent growth during metamorphosis (83, 117). Wings of dilp6 mutant adults have normal cell size but reduced cell number (83), demonstrating that DILP6 indirectly mediates the effect of ecdysone on cell proliferation in imaginal discs (Figure 5c). Ecdysone antagonizes IIS in the fat body (21, 104), which probably blocks the autocrine effect of DILP6 (Figure 5c). This inhibition of IIS by ecdysone in the fat body promotes autophagy within this tissue (104), which might further support imaginal disc proliferation indirectly by providing energy and nutrients. Moreover, ecdysone promotes imaginal disc proliferation and differentiation more directly, at least in part via an ecdysone-inducible transcription factor named Crooked legs (Crol) (23) (Figure 5c). Crol affects the Wingless signaling pathway in the discs, thereby promoting cell cycle progression (75). In this way, different responses to ecdysone signaling by the fat body and imaginal discs elegantly shape the dynamic, highly coordinated interaction between these tissues. Although this is still a single case study, additional examples should eventually lead to a more global understanding of ecdysone signaling at the organismal scale.
SUMMARY POINTS.
Recent studies have revealed multiple signaling pathways that regulate ecdysteroidogenesis in the PG. These studies suggest a modification of the classical scheme of insect endocrinology, in which PTTH was the only regulator of ecdysone synthesis and release. In the modified scheme, the PG, rather than the brain, is the decision-making entity that regulates the timing of insect development.
D. melanogaster molecular genetic studies have expanded the Ashburner model by revealing multiple EcR cofactors that are necessary for proper ecdysone response in different contexts. Competence factors such as βFTZ-F1 most likely cooperate with these cofactors to diversify ecdysone response over space and time.
The classical concept of critical weight indicates a highly autonomous, self-integrated nature of ecdysone signaling. Molecularly, this can be explained by multiple layers of positive- and negative-feedback regulation built into the process, as well as by coordinated responses of multiple tissues to ecdysone.
Acknowledgments
We thank Kirst King-Jones, Dusan Zitnan, Aidan Peterson, and Mary Jane Shimell for a critical reading of the manuscript. N.Y. was supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science in the writing of this review and is supported by NIH grant K99 HD073239. K.F.R. is supported by the Danish Council for Independent Research, Natural Sciences, and M.B.O. is supported by NIH grant R01 GM093301.
Glossary
- E
ecdysone
- PG
prothoracic gland
- Classical scheme
a framework developed by early insect endocrinologists of how PTTH, JH, and ecdysone direct and characterize insect molting and metamorphosis
- Ashburner model
an established classical model of how ecdysone binding to its receptor triggers gene expression cascades in chronological order
- Critical weight
weight specific to each insect species after which no further feeding is required for metamorphosis to be initiated on schedule
- PTTH
prothoracicotropic hormone
- Juvenile hormone (JH)
a pleiotropic sesquiterpenoid hormone whose classical function is to determine the nature of developmental transitions triggered by ecdysone
- Insulin/IGF signaling (IIS)
a signaling pathway composed of multiple ligands and one receptor in Drosophila and involved in many physiological processes
- Competence
the preset capacity of the target cells to respond to a hormone in a specific manner
- E75
an ecdysone-inducible nuclear receptor that contains heme and is responsive to nitric oxide
- Nitric oxide (NO)
a diffusible second messenger considered an important regulator of developmental transitions through its interaction with E75
- GPCR
G-protein-coupled receptor
- EcR
ecdysone receptor
- USP
Ultraspiracle
- βFTZ-F1
an orphan nuclear receptor with critical functions in both ecdysone production in the PG and ecdysone reception in peripheral tissues
- Met
Methoprene-tolerant
- ETH
ecdysis-triggering hormone
- Imaginal discs
parts of holometabolous insect larvae that eventually form external adult structures such as eyes, wings, and legs
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Naoki Yamanaka, Email: yamanaka_naoki@hotmail.com.
Kim F. Rewitz, Email: rewitz@ruc.dk.
Michael B. O’Connor, Email: moconnor@umn.edu.
LITERATURE CITED
- 1.Ables ET, Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell. 2010;7:581–92. doi: 10.1016/j.stem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andres AJ, Thummel CS. The Drosophila 63F early puff contains E63-1, an ecdysone-inducible gene that encodes a novel Ca2+-binding protein. Development. 1995;121:2667–79. doi: 10.1242/dev.121.8.2667. [DOI] [PubMed] [Google Scholar]
- 3.Ashburner M, Chihara C, Meltzer P, Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–62. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- 4.Ashburner M, Richards G. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster III Consequences of ecdysone withdrawal. Dev Biol. 1976;54:241–55. doi: 10.1016/0012-1606(76)90302-x. [DOI] [PubMed] [Google Scholar]
- 5.Badenhorst P, Xiao H, Cherbas L, Kwon SY, Voas M, et al. The Drosophila nucleosome remodeling factor NURF is required for ecdysteroid signaling and metamorphosis. Genes Dev. 2005;19:2540–45. doi: 10.1101/gad.1342605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by Taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–58. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 7.Bartkuhn M, Straub T, Herold M, Herrmann M, Rathke C, et al. Active promoters and insulators are marked by the centrosomal protein 190. EMBO J. 2009;28:877–88. doi: 10.1038/emboj.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker PB, Horz W. ATP-dependent nucleosome remodeling. Annu Rev Biochem. 2002;71:247–73. doi: 10.1146/annurev.biochem.71.110601.135400. [DOI] [PubMed] [Google Scholar]
- 9.Beckstead R, Ortiz JA, Sanchez C, Prokopenko SN, Chambon P, et al. Bonus, a Drosophila homolog of TIF1 proteins, interacts with nuclear receptors and can inhibit βFTZ-F1-dependent transcription. Mol Cell. 2001;7:753–65. doi: 10.1016/s1097-2765(01)00220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckstead RB, Lam G, Thummel CS. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 2005;6:R99. doi: 10.1186/gb-2005-6-12-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beydon P, Lafont R. Feedback inhibition of ecdysone production by 20-hydroxyecdysone in Pieris brassicae pupae. J Insect Physiol. 1983;29:529–33. [Google Scholar]
- 12.Bialecki M, Shilton A, Fichtenberg C, Segraves WA, Thummel CS. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev Cell. 2002;3:209–20. doi: 10.1016/s1534-5807(02)00204-6. [DOI] [PubMed] [Google Scholar]
- 13.Broadus J, McCabe JR, Endrizzi B, Thummel CS, Woodard CT. The Drosophila βFTZ-F1 orphan nuclear receptor provides competence for stage-specific responses to the steroid hormone ecdysone. Mol Cell. 1999;3:143–49. doi: 10.1016/s1097-2765(00)80305-6. [DOI] [PubMed] [Google Scholar]
- 14.Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–39. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- 15.Burtis KC, Thummel CS, Jones CW, Karim FD, Hogness DS. The Drosophila 74EF early puff contains E74, a complex ecdysone-inducible gene that encodes two ets-related proteins. Cell. 1990;61:85–99. doi: 10.1016/0092-8674(90)90217-3. [DOI] [PubMed] [Google Scholar]
- 16.Caceres L, Necakov AS, Schwartz C, Kimber S, Roberts IJ, Krause HM. Nitric oxide coordinates metabolism, growth, and development via the nuclear receptor E75. Genes Dev. 2011;25:1476–85. doi: 10.1101/gad.2064111. Reveals the critical function of NO in ecdysone signaling; see also Reference 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol. 2005;15:1785–95. doi: 10.1016/j.cub.2005.09.011. Highlights the importance of IIS in the PG; see also References 21 and 72. [DOI] [PubMed] [Google Scholar]
- 18.Callier V, Nijhout HF. Control of body size by oxygen supply reveals size-dependent and size-independent mechanisms of molting and metamorphosis. Proc Natl Acad Sci USA. 2011;108:14664–69. doi: 10.1073/pnas.1106556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles JP, Iwema T, Epa VC, Takaki K, Rynes J, Jindra M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108:21128–33. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clever U. Actinomycin and puromycin: effects on sequential gene activation by ecdysone. Science. 1964;146:794–95. doi: 10.1126/science.146.3645.794. [DOI] [PubMed] [Google Scholar]
- 21.Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, et al. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–70. doi: 10.1126/science.1119432. Describes IIS in the PG and antagonism between ecdysone and IIS in the fat body. [DOI] [PubMed] [Google Scholar]
- 22.Costantino BF, Bricker DK, Alexandre K, Shen K, Merriam JR, et al. A novel ecdysone receptor mediates steroid-regulated developmental events during the mid-third instar of Drosophila. PLoS Genet. 2008;4:e1000102. doi: 10.1371/journal.pgen.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Avino PP, Thummel CS. crooked legs encodes a family of zinc finger proteins required for leg morphogenesis and ecdysone-regulated gene expression during Drosophila metamorphosis. Development. 1998;125:1733–45. doi: 10.1242/dev.125.9.1733. [DOI] [PubMed] [Google Scholar]
- 24.De Koning L, Corpet A, Haber JE, Almouzni G. Histone chaperones: an escort network regulating histone traffic. Nat Struct Mol Biol. 2007;14:997–1007. doi: 10.1038/nsmb1318. [DOI] [PubMed] [Google Scholar]
- 25.Doane WW. Role of hormones in insect development. In: Counce SJ, Waddington CH, editors. Developmental Systems: Insects. New York: Academic; 1973. pp. 291–497. [Google Scholar]
- 26.Dolezal T, Dolezelova E, Zurovec M, Bryant PJ. A role for adenosine deaminase in Drosophila larval development. PLoS Biol. 2005;3:e201. doi: 10.1371/journal.pbio.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dressel U, Thormeyer D, Altincicek B, Paululat A, Eggert M, et al. Alien, a highly conserved protein with characteristics of a corepressor for members of the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:3383–94. doi: 10.1128/mcb.19.5.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubrovsky EB, Dubrovskaya VA, Berger EM. Hormonal regulation and functional role of Drosophila E75A orphan nuclear receptor in the juvenile hormone signaling pathway. Dev Biol. 2004;268:258–70. doi: 10.1016/j.ydbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Dubrovsky EB, Dubrovskaya VA, Bernardo T, Otte V, DiFilippo R, Bryan H. The Drosophila FTZ-F1 nuclear receptor mediates juvenile hormone activation of E75A gene expression through an intracellular pathway. J Biol Chem. 2011;286:33689–700. doi: 10.1074/jbc.M111.273458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar BA. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–16. doi: 10.1038/nrg1989. Far-sighted review that preceded the current surge of developmental timing studies in Drosophila. [DOI] [PubMed] [Google Scholar]
- 31.Francis VA, Zorzano A, Teleman AA. dDOR is an EcR coactivator that forms a feed-forward loop connecting insulin and ecdysone signaling. Curr Biol. 2010;20:1799–808. doi: 10.1016/j.cub.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 32.Garofalo RS. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol Metab. 2002;13:156–62. doi: 10.1016/s1043-2760(01)00548-3. [DOI] [PubMed] [Google Scholar]
- 33.Gaszner M, Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet. 2006;7:703–13. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- 34.Gates J, Lam G, Ortiz JA, Losson R, Thummel CS. rigor mortis encodes a novel nuclear receptor interacting protein required for ecdysone signaling during Drosophila larval development. Development. 2004;131:25–36. doi: 10.1242/dev.00920. [DOI] [PubMed] [Google Scholar]
- 35.Gauhar Z, Sun LV, Hua S, Mason CE, Fuchs F, et al. Genomic mapping of binding regions for the ecdysone receptor protein complex. Genome Res. 2009;19:1006–13. doi: 10.1101/gr.081349.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbens YY, Warren JT, Gilbert LI, O’Connor MB. Neuroendocrine regulation of Drosophila metamorphosis requires TGFβ/Activin signaling. Development. 2011;138:2693–703. doi: 10.1242/dev.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert LI. Drosophila is an inclusive model for human diseases, growth and development. Mol Cell Endocrinol. 2008;293:25–31. doi: 10.1016/j.mce.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Gu SH, Yeh WL, Young SC, Lin PL, Li S. TOR signaling is involved in PTTH-stimulated ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2012;42:296–303. doi: 10.1016/j.ibmb.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Guittard E, Blais C, Maria A, Parvy JP, Pasricha S, et al. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev Biol. 2011;349:35–45. doi: 10.1016/j.ydbio.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Hall BL, Thummel CS. The RXR homolog Ultraspiracle is an essential component of the Drosophila ecdysone receptor. Development. 1998;125:4709–17. doi: 10.1242/dev.125.23.4709. [DOI] [PubMed] [Google Scholar]
- 41.Halme A, Cheng M, Hariharan IK. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr Biol. 2010;20:458–63. doi: 10.1016/j.cub.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hietakangas V, Cohen SM. Regulation of tissue growth through nutrient sensing. Annu Rev Genet. 2009;43:389–410. doi: 10.1146/annurev-genet-102108-134815. [DOI] [PubMed] [Google Scholar]
- 43.Hock T, Cottrill T, Keegan J, Garza D. The E23 early gene of Drosophila encodes an ecdysone-inducible ATP-binding cassette transporter capable of repressing ecdysone-mediated gene activation. Proc Natl Acad Sci USA. 2000;97:9519–24. doi: 10.1073/pnas.160271797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang X, Suyama K, Buchanan J, Zhu AJ, Scott MP. A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development. 2005;132:5115–24. doi: 10.1242/dev.02079. [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Warren JT, Gilbert LI. New players in the regulation of ecdysone biosynthesis. J Genet Genomics. 2008;35:1–10. doi: 10.1016/S1673-8527(08)60001-6. [DOI] [PubMed] [Google Scholar]
- 46.Hurban P, Thummel CS. Isolation and characterization of fifteen ecdysone-inducible Drosophila genes reveal unexpected complexities in ecdysone regulation. Mol Cell Biol. 1993;13:7101–11. doi: 10.1128/mcb.13.11.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussey RG, Thompson WR, Calhoun ET. The influence of X-rays on the development of Drosophila larvae. Science. 1927;66:65–66. doi: 10.1126/science.66.1698.65. [DOI] [PubMed] [Google Scholar]
- 48.Ishizaki H, Suzuki A. The brain secretory peptides that control moulting and metamorphosis of the silkmoth, Bombyx mori. Int J Dev Biol. 1994;38:301–10. [PubMed] [Google Scholar]
- 49.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 50.Johnston DM, Sedkov Y, Petruk S, Riley KM, Fujioka M, et al. Ecdysone- and NO-mediated gene regulation by competing EcR/USP and E75A nuclear receptors during Drosophila development. Mol Cell. 2011;44:51–61. doi: 10.1016/j.molcel.2011.07.033. One of two important papers about NO function in ecdysone signaling published in 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kageyama Y, Masuda S, Hirose S, Ueda H. Temporal regulation of the mid-prepupal gene FTZ-F1: DHR3 early late gene product is one of the plural positive regulators. Genes Cells. 1997;2:559–69. doi: 10.1046/j.1365-2443.1997.1460344.x. [DOI] [PubMed] [Google Scholar]
- 52.Kaiser A, Klok CJ, Socha JJ, Lee WK, Quinlan MC, Harrison JF. Increase in tracheal investment with beetle size supports hypothesis of oxygen limitation on insect gigantism. Proc Natl Acad Sci USA. 2007;104:13198–203. doi: 10.1073/pnas.0611544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karim FD, Thummel CS. Temporal coordination of regulatory gene expression by the steroid hormone ecdysone. EMBO J. 1992;11:4083–93. doi: 10.1002/j.1460-2075.1992.tb05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kataoka H, Nagasawa H, Isogai A, Ishizaki H, Suzuki A. Prothoracicotropic hormone of the silkworm, Bombyx mori: amino acid sequence and dimeric structure. Agric Biol Chem. 1991;55:73–86. [PubMed] [Google Scholar]
- 55.Kawakami A, Kataoka H, Oka T, Mizoguchi A, Kimura-Kawakami M, et al. Molecular cloning of the Bombyx mori prothoracicotropic hormone. Science. 1990;247:1333–35. doi: 10.1126/science.2315701. [DOI] [PubMed] [Google Scholar]
- 56.Kim SE, Cho JY, Kim KS, Lee SJ, Lee KH, Choi KY. Drosophila PI3 kinase and Akt involved in insulin-stimulated proliferation and ERK pathway activation in Schneider cells. Cell Signal. 2004;16:1309–17. doi: 10.1016/j.cellsig.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 58.Kimura S, Sawatsubashi S, Ito S, Kouzmenko A, Suzuki E, et al. Drosophila arginine methyltransferase 1 (DART1) is an ecdysone receptor co-repressor. Biochem Biophys Res Commun. 2008;371:889–93. doi: 10.1016/j.bbrc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Kiriishi S, Nagasawa H, Kataoka H, Suzuki A, Sakurai S. Comparison of the in vivo and in vitro effects of bombyxin and prothoracicotropic hormone on prothoracic glands of the silkworm, Bombyx mori. Zool Sci. 1992;9:149–55. [Google Scholar]
- 60.Kirilly D, Wong JJ, Lim EK, Wang Y, Zhang H, et al. Intrinsic epigenetic factors cooperate with the steroid hormone ecdysone to govern dendrite pruning in Drosophila. Neuron. 2011;72:86–100. doi: 10.1016/j.neuron.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- 62.Kopec S. Experiments on metamorphosis of insects. Bull Int Acad Cracov. 1917;B:57–60. [Google Scholar]
- 63.Kopec S. Studies on the necessity of the brain for the inception of insect metamorphosis. Biol Bull. 1922;42:323–42. [Google Scholar]
- 64.Kwon HB, Kim SH, Kim SE, Jang IH, Ahn Y, et al. Drosophila extracellular signal-regulated kinase involves the insulin-mediated proliferation of Schneider cells. J Biol Chem. 2002;277:14853–58. doi: 10.1074/jbc.M110366200. [DOI] [PubMed] [Google Scholar]
- 65.Lam GT, Jiang C, Thummel CS. Coordination of larval and prepupal gene expression by the DHR3 orphan receptor during Drosophila metamorphosis. Development. 1997;124:1757–69. doi: 10.1242/dev.124.9.1757. [DOI] [PubMed] [Google Scholar]
- 66.Layalle S, Arquier N, Leopold P. The TOR pathway couples nutrition and developmental timing in Drosophila. Dev Cell. 2008;15:568–77. doi: 10.1016/j.devcel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Lee CY, Wendel DP, Reid P, Lam G, Thummel CS, Baehrecke EH. E93 directs steroid-triggered programmed cell death in Drosophila. Mol Cell. 2000;6:433–43. doi: 10.1016/s1097-2765(00)00042-3. [DOI] [PubMed] [Google Scholar]
- 68.Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci USA. 2011;108:638–43. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li TR, White KP. Tissue-specific gene expression and ecdysone-regulated genomic networks in Drosophila. Dev Cell. 2003;5:59–72. doi: 10.1016/s1534-5807(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 70.Marchal E, Vandersmissen HP, Badisco L, Van de Velde S, Verlinden H, et al. Control of ecdysteroidogenesis in prothoracic glands of insects: a review. Peptides. 2010;31:506–19. doi: 10.1016/j.peptides.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 71.McBrayer Z, Ono H, Shimell M, Parvy JP, Beckstead RB, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell. 2007;13:857–71. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol. 2005;15:1796–807. doi: 10.1016/j.cub.2005.09.017. One of the milestone papers about IIS in the PG published in 2005. [DOI] [PubMed] [Google Scholar]
- 73.Mirth CK, Riddiford LM. Size assessment and growth control: how adult size is determined in insects. Bioessays. 2007;29:344–55. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- 74.Mirth CK, Truman JW, Riddiford LM. The ecdysone receptor controls the post-critical weight switch to nutrition-independent differentiation in Drosophila wing imaginal discs. Development. 2009;136:2345–53. doi: 10.1242/dev.032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell N, Cranna N, Richardson H, Quinn L. The Ecdysone-inducible zinc-finger transcription factor Crol regulates Wg transcription and cell cycle progression in Drosophila. Development. 2008;135:2707–16. doi: 10.1242/dev.021766. [DOI] [PubMed] [Google Scholar]
- 76.Mizoguchi A. Effects of juvenile hormone on the secretion of prothoracicotropic hormone in the last- and penultimate-instar larvae of the silkworm Bombyx mori. J Insect Physiol. 2001;47:767–75. doi: 10.1016/s0022-1910(01)00052-x. [DOI] [PubMed] [Google Scholar]
- 77.Mou X, Duncan DM, Baehrecke EH, Duncan I. Control of target gene specificity during metamorphosis by the steroid response gene E93. Proc Natl Acad Sci USA. 2012;109:2949–54. doi: 10.1073/pnas.1117559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagasawa H, Kataoka H, Isogai A, Tamura S, Suzuki A, et al. Amino acid sequence of a prothoracicotropic hormone of the silkworm Bombyx mori. Proc Natl Acad Sci USA. 1986;83:5840–43. doi: 10.1073/pnas.83.16.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–87. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 80.Nijhout HF. The control of body size in insects. Dev Biol. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 81.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol. 1974;61:493–501. doi: 10.1242/jeb.61.2.493. [DOI] [PubMed] [Google Scholar]
- 82.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): growth of the last-instar larva and the decision to pupate. J Exp Biol. 1974;61:481–91. doi: 10.1242/jeb.61.2.481. [DOI] [PubMed] [Google Scholar]
- 83.Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, et al. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell. 2009;17:885–91. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 85.Oro AE, McKeown M, Evans RM. Relationship between the product of the Drosophila ultraspiracle locus and the vertebrate retinoid X receptor. Nature. 1990;347:298–301. doi: 10.1038/347298a0. [DOI] [PubMed] [Google Scholar]
- 86.Ou Q, Magico A, King-Jones K. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biol. 2011;9:e1001160. doi: 10.1371/journal.pbio.1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parker NF, Shingleton AW. The coordination of growth among Drosophila organs in response to localized growth-perturbation. Dev Biol. 2011;357:318–25. doi: 10.1016/j.ydbio.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 88.Parvy JP, Blais C, Bernard F, Warren JT, Petryk A, et al. A role for βFTZ-F1 in regulating ecdysteroid titers during post-embryonic development in Drosophila melanogaster. Dev Biol. 2005;282:84–94. doi: 10.1016/j.ydbio.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 89.Pauli A, van Bemmel JG, Oliveira RA, Itoh T, Shirahige K, et al. A direct role for cohesin in gene regulation and ecdysone response in Drosophila salivary glands. Curr Biol. 2010;20:1787–98. doi: 10.1016/j.cub.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petryk A, Warren JT, Marques G, Jarcho MP, Gilbert LI, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci USA. 2003;100:13773–78. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajan A, Perrimon N. Drosophila as a model for interorgan communication: lessons from studies on energy homeostasis. Dev Cell. 2011;21:29–31. doi: 10.1016/j.devcel.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reinking J, Lam MM, Pardee K, Sampson HM, Liu S, et al. The Drosophila nuclear receptor e75 contains heme and is gas responsive. Cell. 2005;122:195–207. doi: 10.1016/j.cell.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 94.Rewitz KF, Larsen MR, Lobner-Olesen A, Rybczynski R, O’Connor MB, Gilbert LI. A phosphoproteomics approach to elucidate neuropeptide signal transduction controlling insect metamorphosis. Insect Biochem Mol Biol. 2009;39:475–83. doi: 10.1016/j.ibmb.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans. 2006;34:1256–60. doi: 10.1042/BST0341256. [DOI] [PubMed] [Google Scholar]
- 96.Rewitz KF, Yamanaka N, Gilbert LI, O’Connor MB. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science. 2009;326:1403–5. doi: 10.1126/science.1176450. [DOI] [PubMed] [Google Scholar]
- 97.Rewitz KF, Yamanaka N, O’Connor MB. Steroid hormone inactivation is required during the juvenile-adult transition in Drosophila. Dev Cell. 2010;19:895–902. doi: 10.1016/j.devcel.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richard DS, Gilbert LI. Reversible juvenile hormone inhibition of ecdysteroid and juvenile hormone synthesis by the ring gland of Drosophila melanogaster. Experientia. 1991;47:1063–66. doi: 10.1007/BF01923343. [DOI] [PubMed] [Google Scholar]
- 99.Richards G. The ecdysone regulatory cascades in Drosophila. In: Wassarman PM, editor. Advances in Developmental Biology. London: JAI Press; 1997. pp. 81–135. [Google Scholar]
- 100.Riddiford LM, Ashburner M. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol. 1991;82:172–83. doi: 10.1016/0016-6480(91)90181-5. [DOI] [PubMed] [Google Scholar]
- 101.Riddiford LM, Truman JW, Mirth CK, Shen YC. A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development. 2010;137:1117–26. doi: 10.1242/dev.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–28. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 103.Rountree DB, Bollenbacher WE. The release of the prothoracicotropic hormone in the tobacco hornworm, Manduca sexta, is controlled intrinsically by juvenile hormone. J Exp Biol. 1986;120:41–58. doi: 10.1242/jeb.120.1.41. [DOI] [PubMed] [Google Scholar]
- 104.Rusten TE, Lindmo K, Juhasz G, Sass M, Seglen PO, et al. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev Cell. 2004;7:179–92. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 105.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–48. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 106.Rybczynski R. Prothoracicotropic hormone. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Oxford, UK: Elsevier; 2005. pp. 61–123. [Google Scholar]
- 107.Sakurai S, Okuda M, Ohtaki T. Juvenile hormone inhibits ecdysone secretion and responsiveness to prothoracicotropic hormone in prothoracic glands of Bombyx mori. Gen Comp Endocrinol. 1989;75:222–30. doi: 10.1016/0016-6480(89)90074-9. [DOI] [PubMed] [Google Scholar]
- 108.Sakurai S, Williams CM. Short-loop negative and positive feedback on ecdysone secretion by prothoracic gland in the tobacco hornworm, Manduca sexta. Gen Comp Endocrinol. 1989;75:204–16. doi: 10.1016/0016-6480(89)90072-5. [DOI] [PubMed] [Google Scholar]
- 109.Sawatsubashi S, Maki A, Ito S, Shirode Y, Suzuki E, et al. Ecdysone receptor-dependent gene regulation mediates histone poly(ADP-ribosyl)ation. Biochem Biophys Res Commun. 2004;320:268–72. doi: 10.1016/j.bbrc.2004.05.157. [DOI] [PubMed] [Google Scholar]
- 110.Sawatsubashi S, Murata T, Lim J, Fujiki R, Ito S, et al. A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev. 2010;24:159–70. doi: 10.1101/gad.1857410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sedkov Y, Cho E, Petruk S, Cherbas L, Smith ST, et al. Methylation at lysine 4 of histone H3 in ecdysone-dependent development of Drosophila. Nature. 2003;426:78–83. doi: 10.1038/nature02080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sehnal F, Maroy P, Mala J. Regulation and significance of ecdysteroid titre fluctuations in lepidopterous larvae and pupae. J Insect Physiol. 1981;27:535–44. [Google Scholar]
- 113.Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, et al. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25:2781–91. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shiomi K, Fujiwara Y, Atsumi T, Kajiura Z, Nakagaki M, et al. Myocyte enhancer factor 2 (MEF2) is a key modulator of the expression of the prothoracicotropic hormone gene in the silkworm, Bombyx mori. FEBS J. 2005;272:3853–62. doi: 10.1111/j.1742-4658.2005.04799.x. [DOI] [PubMed] [Google Scholar]
- 115.Siegmund T, Korge G. Innervation of the ring gland of Drosophila melanogaster. J Comp Neurol. 2001;431:481–91. doi: 10.1002/1096-9861(20010319)431:4<481::aid-cne1084>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 116.Simpson P, Berreur P, Berreur-Bonnenfant J. The initiation of pupariation in Drosophila: dependence on growth of the imaginal discs. J Embryol Exp Morphol. 1980;57:155–65. [PubMed] [Google Scholar]
- 117.Slaidina M, Delanoue R, Gronke S, Partridge L, Leopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell. 2009;17:874–84. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song Q, Gilbert LI. S6 phosphorylation results from prothoracicotropic hormone stimulation of insect prothoracic glands: a role for S6 kinase. Dev Genet. 1994;15:332–38. doi: 10.1002/dvg.1020150404. [DOI] [PubMed] [Google Scholar]
- 119.Song Q, Gilbert LI. Multiple phosphorylation of ribosomal protein S6 and specific protein synthesis are required for prothoracicotropic hormone-stimulated ecdysteroid biosynthesis in the prothoracic glands of Manduca sexta. Insect Biochem Mol Biol. 1995;25:591–602. doi: 10.1016/0965-1748(94)00100-v. [DOI] [PubMed] [Google Scholar]
- 120.Song Q, Sun X, Jin XY. 20E-regulated USP expression and phosphorylation in Drosophila melanogaster. Insect Biochem Mol Biol. 2003;33:1211–18. doi: 10.1016/j.ibmb.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 121.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–44. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 122.Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annu Rev Biochem. 2011;80:473–99. doi: 10.1146/annurev-biochem-061809-175347. [DOI] [PubMed] [Google Scholar]
- 123.Takaki K, Sakurai S. Regulation of prothoracic gland ecdysteroidogenic activity leading to pupal metamorphosis. Insect Biochem Mol Biol. 2003;33:1189–99. doi: 10.1016/j.ibmb.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 124.Talbot WS, Swyryd EA, Hogness DS. Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell. 1993;73:1323–37. doi: 10.1016/0092-8674(93)90359-x. [DOI] [PubMed] [Google Scholar]
- 125.Thummel CS. Flies on steroids—Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996;12:306–10. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 126.Thummel CS, Burtis KC, Hogness DS. Spatial and temporal patterns of E74 transcription during Drosophila development. Cell. 1990;61:101–11. doi: 10.1016/0092-8674(90)90218-4. [DOI] [PubMed] [Google Scholar]
- 127.Truman JW. Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitam Horm. 2005;73:1–30. doi: 10.1016/S0083-6729(05)73001-6. [DOI] [PubMed] [Google Scholar]
- 128.Truman JW, Riddiford LM. Physiology of insect rhythms. 3 The temporal organization of the endocrine events underlying pupation of the tobacco hornworm. J Exp Biol. 1974;60:371–82. doi: 10.1242/jeb.60.2.371. [DOI] [PubMed] [Google Scholar]
- 129.Truman JW, Talbot WS, Fahrbach SE, Hogness DS. Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development. 1994;120:219–34. doi: 10.1242/dev.120.1.219. [DOI] [PubMed] [Google Scholar]
- 130.Tsai CC, Kao HY, Yao TP, McKeown M, Evans RM. SMRTER, a Drosophila nuclear receptor coregulator, reveals that EcR-mediated repression is critical for development. Mol Cell. 1999;4:175–86. doi: 10.1016/s1097-2765(00)80365-2. [DOI] [PubMed] [Google Scholar]
- 131.Tulin A, Spradling A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science. 2003;299:560–62. doi: 10.1126/science.1078764. [DOI] [PubMed] [Google Scholar]
- 132.Varghese J, Cohen SM. microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev. 2007;21:2277–82. doi: 10.1101/gad.439807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vorobyeva NE, Nikolenko JV, Krasnov AN, Kuzmina JL, Panov VV, et al. SAYP interacts with DHR3 nuclear receptor and participates in ecdysone-dependent transcription regulation. Cell Cycle. 2011;10:1821–27. doi: 10.4161/cc.10.11.15727. [DOI] [PubMed] [Google Scholar]
- 134.Walsh AL, Smith WA. Nutritional sensitivity of fifth instar prothoracic glands in the tobacco hornworm, Manduca sexta. J Insect Physiol. 2011;57:809–18. doi: 10.1016/j.jinsphys.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 135.Watson RD, Bollenbacher WE. Juvenile hormone regulates the steroidogenic competence of Manduca sexta prothoracic glands. Mol Cell Endocrinol. 1988;57:251–59. doi: 10.1016/0303-7207(88)90081-0. [DOI] [PubMed] [Google Scholar]
- 136.White KP, Hurban P, Watanabe T, Hogness DS. Coordination of Drosophila metamorphosis by two ecdysone-induced nuclear receptors. Science. 1997;276:114–17. doi: 10.1126/science.276.5309.114. [DOI] [PubMed] [Google Scholar]
- 137.Wigglesworth VB. The hormonal regulation of growth and reproduction in insects. In: Beament JW, editor. Advances in Insect Physiology. New York: Academic; 1964. pp. 247–336. [Google Scholar]
- 138.Wollam J, Antebi A. Sterol regulation of metabolism, homeostasis, and development. Annu Rev Biochem. 2011;80:885–916. doi: 10.1146/annurev-biochem-081308-165917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wood AM, Van Bortle K, Ramos E, Takenaka N, Rohrbaugh M, et al. Regulation of chromatin organization and inducible gene expression by a Drosophila insulator. Mol Cell. 2011;44:29–38. doi: 10.1016/j.molcel.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Woodard CT, Baehrecke EH, Thummel CS. A molecular mechanism for the stage specificity of the Drosophila prepupal genetic response to ecdysone. Cell. 1994;79:607–15. doi: 10.1016/0092-8674(94)90546-0. [DOI] [PubMed] [Google Scholar]
- 141.Yamada M, Murata T, Hirose S, Lavorgna G, Suzuki E, Ueda H. Temporally restricted expression of transcription factor βFTZ-F1: significance for embryogenesis, molting and metamorphosis in Drosophila melanogaster. Development. 2000;127:5083–92. doi: 10.1242/dev.127.23.5083. [DOI] [PubMed] [Google Scholar]
- 142.Yamanaka N, Honda N, Osato N, Niwa R, Mizoguchi A, Kataoka H. Differential regulation of ecdysteroidogenic P450 gene expression in the silkworm, Bombyx mori. Biosci Biotechnol Biochem. 2007;71:2808–14. doi: 10.1271/bbb.70420. [DOI] [PubMed] [Google Scholar]
- 143.Yamanaka N, Hua YJ, Roller L, Spalovska-Valachova I, Mizoguchi A, et al. Bombyx prothoracicostatic peptides activate the sex peptide receptor to regulate ecdysteroid biosynthesis. Proc Natl Acad Sci USA. 2010;107:2060–65. doi: 10.1073/pnas.0907471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamanaka N, O’Connor MB. Nitric oxide directly regulates gene expression during Drosophila development: Need some gas to drive into metamorphosis? Genes Dev. 2011;25:1459–63. doi: 10.1101/gad.2080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yamanaka N, Zitnan D, Kim YJ, Adams ME, Hua YJ, et al. Regulation of insect steroid hormone biosynthesis by innervating peptidergic neurons. Proc Natl Acad Sci USA. 2006;103:8622–27. doi: 10.1073/pnas.0511196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J Biol Chem. 2011;286:8437–47. doi: 10.1074/jbc.M110.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhu J, Chen L, Sun G, Raikhel AS. The competence factor βFtz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol Cell Biol. 2006;26:9402–12. doi: 10.1128/MCB.01318-06. Pioneering study on the molecular mechanisms of βFTZ-F1 function as a competence factor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zitnan D, Kim YJ, Zitnanova I, Roller L, Adams ME. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen Comp Endocrinol. 2007;153:88–96. doi: 10.1016/j.ygcen.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zitnan D, Ross LS, Zitnanova I, Hermesman JL, Gill SS, Adams ME. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron. 1999;23:523–35. doi: 10.1016/s0896-6273(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 150.Zraly CB, Middleton FA, Dingwall AK. Hormone-response genes are direct in vivo regulatory targets of Brahma (SWI/SNF) complex function. J Biol Chem. 2006;281:35305–15. doi: 10.1074/jbc.M607806200. [DOI] [PubMed] [Google Scholar]