Abstract
Central oxytocin mediates the acquisition of a filial preference for maternal odour in rat pups, manifested by their huddling preferences. The present study was designed to examine whether maternal care modulates oxytocin concentrations in rat pups and, if so, how different types of maternal contact are associated with the pups’ oxytocin concentrations. Pairs of 14-day-old littermates were removed from their home cage for 1 h and then placed with a lactating foster mother for 2 h, or they remained isolated at room temperature. Enzyme immunoassays revealed that maternal care and maternal separation can differentially modulate pups’ oxytocin concentrations. Both hypothalamic and serum oxytocin increased during the 1-h separation. Pups placed with a foster mother after the separation maintained the same concentrations in the hypothalamus and serum through the fostering period. By contrast, pups placed with no mother showed a further increase in hypothalamic oxytocin but serum oxytocin decreased. Behavioural analyses revealed that skin-to-skin contact with the mother, but not simple physical contact or maternal licking / grooming, was positively correlated with the pups’ hypothalamic oxytocin concentrations. These neuroendocrine data match previous findings showing that skin-to-skin contact with mother facilitates the acquisition of the pups’ huddling preference for a maternally-associated odour. Taken together, the present study suggests that maternal skin-to-skin contact stimulates pups’ central oxytocin, at the same time as creating the conditions for inducing a preference for maternal odour and establishing a social affiliation in rat pups; the natural schedule of maternal separation and reunion may modulate pups’ oxytocin concentrations, providing scaffolding for the acquisition of their filial huddling preference.
Keywords: oxytocin, maternal care, maternal separation, skin-to-skin contact, rat pup, affiliative behaviour, attachment, associative learning
Oxytocin (OT) is a nonapeptide neurohormone synthesised primarily in the paraventricular nucleus (PVN) and supraoptic nucleus of the hypothalamus. OT is transported via magnocellular neurosecretory cells to the posterior pituitary for coordinated release into systemic circulation. Oxytocinergic cells in the PVN also project directly to central targets, including the median eminence, brainstem and spinal cord (1–3). Although its best-known functions may be in lactation and parturition, OT also acts as a neurochemical to regulate emotion as well as a variety of social behaviours, including social recognition (4–7), pair bonding (8, 9) and maternal behaviour (10–12) in rodents and ungulates. OT is also associated with love (13), trust (14) and fear (15) in humans. Moreover, OT is reputed to reduce physiological and behavioural responses to stress (16, 17).
OT appears to play an important role in neural and behavioural development. OT has been implicated in modulating neurodevelopmental disorders (18), such as autism (19–22) and schizophrenia (23–25). Manipulation of OT concentrations in early life induces variations in c-Fos immunoreactivity, OT and vasopressin immunoreactivity in the PVN and hypothalamic-pituitary-adrenal (HPA) axis activity, as well as social behaviours in adult rodents including parental behaviour, partner preferences and male–male aggression (26). Nevertheless, most studies involving the effects of OT on social behaviours have focused on adult animals, leaving unexplored the possible effects of OT on social behaviour in young, immature animals.
Recently, a vital role for OT in the early social development of rats was confirmed in a study reporting that oxytocinergic function is necessary for the acquisition of a filial huddling preference (27). Specifically, i.c.. administration of an oxytocin antagonist (OTA) blocked the formation of a huddling preference for a maternally-paired odour. Huddling is an affiliative behaviour observed throughout the life of Norway rats (28), a renowned ‘contact species’ (29), and is expressed soon after birth when it is controlled primarily by thermal stimuli (28, 30–33). By the end of the second postnatal week, the most salient stimuli that control huddling are olfactory and the behaviour is decidedly social (28, 34). It is well established that the pups’ olfactory-based, filial huddling preference is acquired through their experiences with the mother in the nest during the first two postnatal weeks (34–36). OT or OTA injection into i.c.v. also affected mother–pup interactions (27), thereby focusing attention on the potential roles of maternal care in modulating offspring OT.
To determine the significance of maternal care for the acquisition of pups’ huddling preference and its related physiology, Kojima and Alberts (37, 38) devised a single-session procedure with which rat pups form an affiliative preference for an arbitrary, novel odorant after a single, 2-h interaction with a maternally-active foster mother bearing that odorant on her fur. From studies using such procedures, they identified the maternal stimuli that are (and are not) sufficient to induce the preference for a maternally-paired odour that is manifested by filial huddling among rat pups. It is helpful to summarise pertinent knowledge about rat maternal care in relation to olfactory learning in pups because this sets the stage for the present neuroendcrinological study and its interpretation. During the acquisition of a huddling preference through interactions with a scented foster mother, pups can engage in nipple attachment and milk intake. Nevertheless, neither suckling, nor milk reward is necessary for the acquisition of huddling preference for the maternally-paired odour (35, 37, 38). Similarly, neither maternal licking, nor mechanotactile stimulation by stroking is associated with pups’ odour preferences in huddling (37, 38). Instead, maternal contact that provides warmth, such as maternal hovering over pups and skin-to-skin contact, is associated with the degree of the filial preference (37, 38).
The contribution of thermotactile stimulation from such warm maternal contact to the odour-guided huddling preference is reliable and robust, and this form of reinforcement has been separated from general warming of the pup. That is, an odour experienced when in contact with a warm, fur-wrapped cylinder in a room temperature cage, which simulates a pup making contact with a mother in the colony room, becomes a preferred odour in pups. By contrast, the same odour experienced in conjunction with a compartment that is either warmed to thermoneutrality or at colony temperature does not become preferred (37, 38). Thus, the tactile component of maternal care that delivers thermotactile warmth to large areas of the pups’ skin leads to an affiliative preference for odours associated with the mother (38). These behavioural findings provide evidence that different reinforcers (e.g. suckling stimulation, milk, stroking or licking, and warmth) affect different behavioural systems at different ages in young mammals and that we cannot make unqualified assertions that universal rewards or specific periods exist during which learning occurs (37). For example, stroking a pup’s body with a soft brush can produce approach behaviour to a conditioned odour in pups aged 9 days or younger, but not older (39, 40). We find that thermotactile stimuli are effective for rapid induction of a filial preference in such older pups (27, 37, 38), at a stage that conforms to the typical developmental onset of their social attractions (28, 36).
The present study aimed to monitor the concentrations of central (hypothalamic) and peripheral (serum) concentrations of OT in pups at a series of time points comprising the acquisition process of a filial huddling preference. We applied a standardised method for preference learning, which is to provide a period of maternal care from a scented foster mother to a pair of pups, and we employed one of the standard control conditions that includes a similar sequence of handling and treatment but does not lead to a preference (37, 38). We used 14-day-old pups as subjects because they are at the age when filial learning is rapid, robust and requires central OT (27, 37, 38). In accordance with our previous standardised methods, foster mothers were used to provide a discrete period of maternal care in an observation cage. Foster mothers readily accept the pups and display maternal behaviour to them; a multitude of earlier studies confirm that foster mothers’ interactions are rewarding to the pups and sufficient to induce a preference for a maternally-paired odour (27, 35, 37, 38). From the video records of the mother–pup interactions, we extracted quantitative measures of skin-to-skin contact, as well as other, less complete forms of body contact, such as maternal licking and briefer physical contacts. In the present study, we examined whether these behavioural variables were related to OT concentrations in the same individual pups.
Materials and methods
Animals
Eighty, 14-day-old rat pups from ten litters were subjects. Moreover, ten lactating females, matched to postpartum age, served as foster mothers. All animals were derived from Sprague–Dawley stocks originally purchased from Taconic Farms (Germantown, NY, USA) and bred in the Animal Behaviour Laboratory colony at Indiana University. Litters were born and reared in standard maternity tubs (48 × 20 × 26 cm) with food and water available ad lib. The vivarium was maintained under a 12:12 h light / dark cycle (lights on 07:00 h) at 22.0 ± 2 °C and 40–70% humidity. Litters were reduced to eight pups (four males and four females) on postnatal day (PD) 1 (day of birth = PD 0). A needle-punch (30-gauge) implement (Ketchum Manufacturing Inc., Ottawa, ON, Canada) was used to tattoo the ventral aspect of the paw(s) by PD 7 or ear(s) after PD 7 for individual identification. Animal care and experimentation were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Bloomington Institutional Animal Care and Use Committee at Indiana University.
Procedures
To examine the effects of maternal care on pups’ OT concentrations during the acquisition of a huddling preference, we employed a procedure previously used for preference conditioning (37, 38). In the present study, however, experimental odours were not used, although we adhered to the same schedule of parings with a maternally-active foster mother. Thus, on PD 14, eight littermate pups (four males and four females) were removed from their home cages. Immediately after removal, a pair of male and female pups was sacrificed to measure basal OT concentrations (see below). The remaining six pups were placed as male–female pairs for 1 h in standard mouse cages (30 × 13 × 19 cm) with fresh bedding. A foster mother was also removed from her home cage 1 h before the treatment session and was placed for the same duration in the treatment arena, which was a standard maternity cage (48 × 20 × 26 cm) with fresh bedding and water. At the end of the 1-h separation, a second pair was sacrificed to measure OT concentrations before the treatment. The remaining two pairs were then each assigned to one of two treatment groups: (i) Maternal Condition, in which pups were placed in the maternity cage with a foster mother at room temperature (22.5 °C) for 2 h and (ii) Non-Maternal Condition, in which pups remained in the standard mouse cage at room temperature (22.5 °C) for 2 h. At the end of the treatment session, all remaining pups were sacrificed to measure post-treatment concentrations of OT. After the session, the foster mother was returned to her home cage. Body weights were not measured to avoid stimulation of peripheral nerves originating in the skin and / or muscle during voiding or handling that could affect OT concentrations (41). All experiments were conducted during the light phase, starting in the morning (09:00–10:00 h).
Behavioural observations
Behavioural interactions during the 2-h long ‘Maternal Condition’ in the treatment arena were recorded via an overhead camera. We applied the operational definitions of maternal contact used successfully to identify antecedents of the preference learning in individual rat pups (38). These definitions were focused on ‘maternal licking’, which provides mechanotactile stimulation to the pup, maternal ‘skin-to-skin contact’, which involved complete, or almost complete coverage of a pup’s body by the mother’s body, providing thermotactile stimulation to the pup, and ‘simple physical contact’, which was a general and inclusive category that comprised all forms of touching between the mother and a pup. Variations in maternal licking have been reported to be associated with a variety of neurological and behavioural phenotypes in adult offspring (42–45), suggesting the significance of tactile stimulation for offspring development (46). Although tactile stimulation from maternal care provided to pups includes mechanotactile (e.g. licking / grooming) and thermotactile (e.g. skin-to-skin contact) stimulation, rarely have mechanotactile and thermotactile components of maternal contact been examined separately. Nevertheless, we have found that they can be dissociated and found that the thermotactile component of maternal contact is essential for the pups’ filial huddling preference (37, 38).
Using the time display on the digitised video, we quantified the frequency and duration of these three categories of maternal contact, which were not mutually exclusive. (i) Maternal licking comprised all bouts lasting 10 s or more of oral contact directed to any part of a pup’s body. During such contact, pups reliably receive robust, mechanotactile stimulation; this may occur with or without a bout of skin-to-skin contact (38). The 10-s criterion helped to distinguish bouts of licking from other, potentially similar appearing, brief acts, such as sniffing that did not provide such mechanotactile stimulation. (ii) Simple physical contact was scored when a pup had any contact with the mother lasting 30 s or more. This category included all forms of maternal contact stimuli impinging on the pup and could involve minimal areas of body contact such as that derived from sniffing, grooming or incidental contact when resting nearby, or extensive regional contact that could occur when nursing underneath the mother’s body or huddling side by side. The 30-s criteria was used to define temporally significant bouts of mechanotactile and / or thermotactile stimulation and helped achieve measurement reliability. (iii) Skin-to-skin contact was scored when the mother’s body covered for 1 min or more either: (a) the front of a pup’s head including ears and forelegs, (b) pup hind legs and hip or (c) all of the pup’s legs with contact between pup ventral area and the mother’s body. This category was established to identify maternal contact bouts that reliably provided thermotactile stimulation to the pup and the 1-min criterion was intended to exclude bouts of contact that did not involve heat transfer across large areas of body contact. In rare cases involving the other pup hovering over the focal pup, it was unclear whether the focal pup was in one of these three positions. Those instances were counted as skin-to-skin contact as long as the focal pup had physical contact with the mother. All three categories of maternal contact emphasised the sensory stimulation received by individual pups; the data required observations of individual, pup–mother interactions. This emphasis distinguishes our perspective from other approaches that, for example, focus on the postures or activities of the mother displayed to the whole litter (47), which may reflect only indirectly and less precisely the pups’ experiences.
Sample collection
At the time of sacrifice, pups were anaesthetised with an i.p. injection of a combination of ketamine (100 mg / kg; Henry Schein, Melville, NY, USA) and xylazine (10 mg / kg; Henry Schein). Once the pup was unresponsive, its heart was exposed by cutting through the diaphragm, and a blood sample was obtained from the left cardiac ventricle. Next, the brain was saline perfused via an infusion pump through the left cardiac ventricle. After perfusion, the brain was removed, immediately placed on dry ice, wrapped in aluminium foil, and stored at −80 °C until peptide extraction. Serum was collected after centrifugation of the blood sample (1600 g for 15 min at 4 °C) and aprotonin (500 KIU / ml; Sigma-Aldrich, St Louis, MO, USA) was added before storage at −80 °C.
Brain peptide extraction
On the day of extraction for the brain samples, each individual brain was transferred to a plastic weigh boat on a bed of dry ice and the frozen hypothalamus was manually dissected out and weighed. The hypothalamus was transferred to a glass test tube, and 500 μl of cold phosphate-buffered saline with 500 KIU / ml aprotonin was immediately added. Samples were sonicated in an ice bath until a uniform homogenate resulted. Five hundred microlitres of 0.5% acetic acid was added and then samples were briefly vortexed and placed in a 96 °C water bath for 10 min, in accordance with a modified OT extraction procedure (48). After centrifugation (3000 g for, 15 min at 4 °C), supernatants were decanted into polypropylene microcentrifuge tubes and evaporated in a refrigerated speed vacuum overnight. Dried extracts were stored at −20 °C until analysis.
Oxytocin enzyme immunoassay (EIA)
OT concentrations were measured using a commercial EIA kit (Catalog #900-153; Assay Designs, Ann Arbor, MI, USA) in accordance with the manufacturer’s instructions. The antiserum used in the kit is highly specific; cross-reactivity is 42.2% for Arg8-Vasotocin, 15.4% for Oxytocin-SH, 0.6% for Arg8-Vasopressin-SH, 0.6% for Lys8-Vasopressin-SH and < 0.2% for other similar neuropeptides. Dried hypothalamus extracts were reconstituted in 500 μl of assay buffer, vortexed and assayed undiluted in duplicate. Serum samples were diluted 1:2 in assay buffer and run in duplicate. The assay was validated for parallelism by comparing serial dilutions of pooled hypothalamic extract and unextracted serum to the standard curve (r2 = 0.96 and 0.99, respectively). Recovery of known amounts of OT standard added to pooled hypothalamic extract and unextracted serum was 97.6% (y = 0.99x − 0.90; r2 = 0.99) and 124.2% (y = 0.95x + 16.35; r2 = 0.99), respectively. Intra-assay variation averaged 5.0% (range 0.0–13.4%) in hypothalamus samples and 4.1% (range 0.0–15.2%) in serum samples. Inter-assay variation in hypothalamic and serum samples was 10.2% and 20.3%, respectively. The sensitivity of this assay is 11.7 pg / ml.
Statistical analysis
As noted earlier, OT concentrations were measured in pups at one of three predetermined time points: (i) immediately after removal from the home cage (basal); (ii) at the end of the 1-h separation; or (iii) at the end of the 2-h treatment with either a foster mother or no maternal treatment. A mixed model two-way ANOVA was used to assess differences in OT concentrations among these four measurements and between sexes, entering time / treatment and sex as a fixed factor and their interaction and litter as a random factor. Litter was entered in the model to control for possible litter effects (e.g. effects of pups’ biological mother on OT system) (43, 49) and the estimated mean ± SEM in the ANOVA model was reported. If a fixed factor was significant in the ANOVA model, post-hoc pairwise comparisons were made using least significant difference tests.
To examine the interaction between maternal contact and OT concentrations in pups, Pearson correlation (r) tests were performed using duration, frequency and bout duration of the three types of physical contact with the mother described above. Pearson correlation tests were also conducted to examine the association between hypothalamic and serum OT concentrations measured in the same individual pups. Five hypothalamic samples (two basal, one maternal treatment, two non-maternal treatment) were considered as outliers and excluded from the analyses; one sample outranged the mean ± 3 SD in data that excluded four extremes shown in a boxplot, which, by the definition, exceeded three times the interquartile range below the first quartile or above the third quartile. All analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Hypothalamic and serum oxytocin in pups
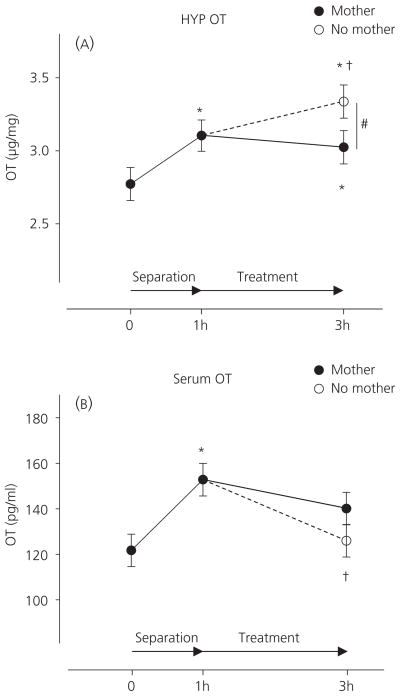
OT concentrations in the hypothalamus differed significantly across the four measurements (Fig. 1A; F3,58 = 4.20, P = 0.009) but not between females and males (F1,58 = 2.89, P = 0.094), and no interaction was found between treatment and sex (F3,58 = 1.13, P = 0.346). Compared to basal concentrations (n = 18, 2.8 ± 0.1 μg / mg), hypothalamic OT increased after the 1-h separation (n = 20, 3.1 ± 0.1 μg / mg, P = 0.014) and at the end of the 2-h treatment with either the Maternal Condition (n = 19, 3.0 ± 0.1 μg / mg, P = 0.041) or the Non-Maternal Condition (n = 18, 3.3 ± 0.1 μg / mg, P < 0.001). Compared to post-separation concentrations, OT increased in the Non-Maternal Condition group (P = 0.046) but not in the Maternal Condition group (P = 0.685). Consequently, hypothalamic OT concentrations in pups placed with a foster mother were significantly lower than in those placed with no mother (P = 0.019).
Fig. 1.
Hypothalamic (HYP: A) and serum (B) oxytocin (OT) concentrations (mean ± SEM) at the beginning of 1-h separation (0 h), at the end of 1-h separation (1 h), and at the end of treatment (3 h) with either a foster mother (closed circle) or no mother (open circle). Significant differences from basal concentration and from the concentration at the end of 1-h separation are indicated by an asterisk (*) and dagger (†), respectively. A significant difference between the two treatments is indicated by a hash symbol (#).
There were also significant differences in serum OT concentrations across the four measurements (Fig. 1B; F3,63 = 3.92, P = 0.012) but not between females and males (F1,63 = 0.088, P = 0.768), and no interaction was found between treatment and sex (F3,58 = 0.06, P = 0.982). Maternal separation for 1 h increased serum OT (152.8 ± 7.1 pg / ml, P = 0.003) compared to basal concentrations (121.7 ± 7.1 pg / ml). After the separation, pups exposed to the Maternal Condition maintained similar concentrations (140.1 ± 7.1 pg / ml) compared to the end of the 1-h separation (P = 0.213) with a trend towards significant difference from basal concentrations (P = 0.074). By contrast, pups exposed to the Non-Maternal Condition displayed decreased serum OT concentrations (126.0 ± 7.1 pg / ml) compared to the end of the 1-h separation (P = 0.010), although these concentrations were not significantly lower than that in pups placed with a foster mother (P = 0.167).
Correlations between maternal contact and oxytocin concentrations in pups
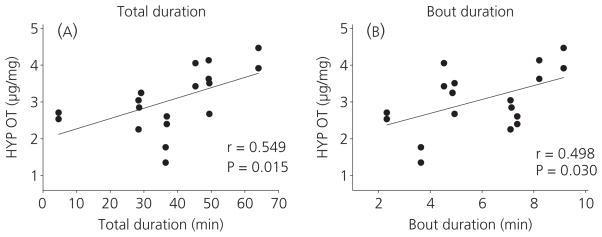
Table 1 displays averages of total duration, frequency and bout duration of physical contact with the foster mother during the 2-h treatment session. The correlation analysis indicated that only total duration and bout duration of skin-to-skin contact were positively associated with OT in the hypothalamus (r = 0.549, P = 0.015; r = 0.498, P = 0.030, respectively), as shown in Fig. 2. There was no association between serum OT and any types of maternal contact.
Table 1.
Total Duration, Frequency, and Bout Duration (Mean ± SEM) of Simple Physical Contact, Licking / Grooming, Skin-to-Skin Contact Received by Pups in Maternal Condition Group.
| Types of physical contact with mother | Total duration (min) | Frequency | Bout duration (min) |
|---|---|---|---|
| Simple physical contact | 103.0 ± 1.3 | 13.6 ± 0.9 | 8.4 ± 0.7 |
| Licking / grooming | 9.3 ± 1.1 | 13.0 ± 1.4 | 0.7 ± 0.0 |
| Skin-to-skin contact | 37.6 ± 3.7a | 6.5 ± 0.6 | 5.9 ± 0.5a |
Measure of maternal contact associated with hypothalamic oxytocin concentrations in pups.
Fig. 2.
Correlation of oxytocin (OT) concentrations in the hypothalamus (HYP) with total duration (A) and bout duration (B) of skin-to-skin contact between mother and pup.
Correlations between central and peripheral OT in pups
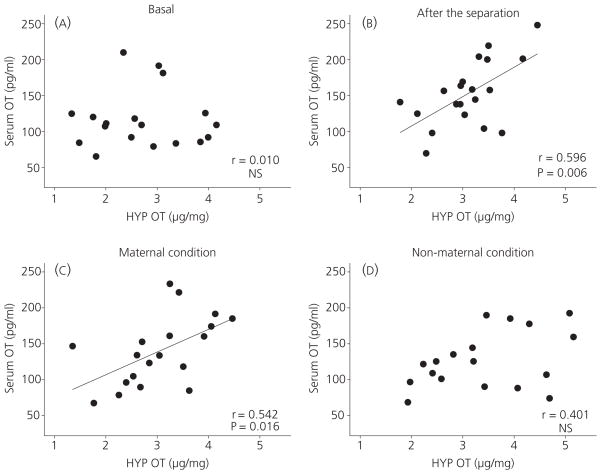
Figure 3 illustrates the correlation between central and peripheral OT at baseline, at the end of the separation, and at the end of the 2-h treatment with either a foster mother or no mother. Initially (Fig. 3A), there was no correlation between central and peripheral OT (r = 0.010, P = 0.967). A significant correlation was identified, however, at the end of the 1-h separation (Fig. 3B; r = 0.596, P = 0.006). The positive correlation was maintained in pups placed for 2 h with a mother after the separation (Fig. 3C; r = 0.542, P = 0.016). By contrast pups exposed to no mother after the initial separation displayed no correlation between central and peripheral OT (Fig. 3D; r = 0.410, P = 0.091).
Fig. 3.
Correlation between central and peripheral oxytocin (OT) concentrations at baseline (A), after the 1-h separation (B), in pups being with mother for 2 h after the separation (C), and in pups being with no mother for 2 h after the separation (D). Significant correlation is indicated by a line. HYP, hypothalamus; NS, not significant.
Discussion
The present study was designed to track OT concentrations in the brain (hypothalamus) and periphery (blood serum) at selected times during a 3-h long regimen used to induce a filial huddling preference in preweanling rats. We focused on OT because this neuropeptide is necessary for the acquisition of the affiliative preference (27). In addition, maternal contact, particularly maternal contact that provides thermotactile stimulation to a pup, is critical for the acquisition of pups’ filial huddling preference (37, 38). The results of the present study suggest, as predicted, that the presence of a mother differentially modulated pups’ OT concentrations, particularly centrally, compared to the Non-Maternal Condition. Moreover, pups’ OT concentrations, particularly in the hypothalamus, varied as a function of the amount of maternal skin-to-skin contact received by pups, whereas serum OT concentrations did not show the same pattern. Therefore, the present study suggests that maternal care, perhaps through maternal skin-to-skin contact, can modulate pups’ OT concentration, particularly centrally.
The positive association between pups’ OT and skin-to-skin contact corresponds well with the finding that maternal provision of cutaneous warmth is positively associated with the magnitude of the pups’ preference for a maternally-associated odour (37, 38). By contrast, warmth provided by the heated compartment, without a localised source of heat with which to contact, failed to induce a preference for the associated odour (38). Thus, it appears that pups require a bodily zone of warmth coupled with tactile stimulation, and perhaps with the accompanying profile of neuropeptide concentrations defined by the present study, to form a social odour preference. Although cutaneous warmth, such as that derived from maternal skin-to-skin contact, is reported to increase OT concentrations in adult rat cerebrospinal fluid (50), it cannot be concluded at this point whether cutaneous warmth, general warmth, or both, can modulate pups’ OT concentrations. Nevertheless, the present study, combined with previous studies (27, 37, 38), provides evidence that maternal contact reliably providing cutaneous warmth to pups may potentiate OT concentrations and facilitate the pups’ acquisition of a filial huddling preference.
The present study provides novel and noteworthy findings because, to our knowledge, there are no other studies in which central and peripheral OT have been measured concurrently in preweanling animals, particularly during exposure to different social environments (e.g. with or without maternal care). It is instructive to summarise the results more fully because the changes in both central and peripheral OT that were observed over time, as well as with different experiences, indicate a picture of complex, but orderly relations between experience and OT concentrations that likely involve other neurochemical systems significant for early olfactory learning. The regimen that we use for single-session, maternal conditioning of a filial preference (37, 38) begins with a 1-h separation during which 14-day-old littermate pairs are removed from the nest and maintained together in a cage with clean bedding at room temperature. Here, one pair of pups was sacrificed at the time of separation to establish baseline concentrations of central and peripheral OT. When we compared baseline hypothalamic and serum OT within individuals, concentrations were notably noncorrelated (Fig. 3a).
At the end of the 1-h separation, both central and peripheral OT increased significantly (114.8% and 125.6%, respectively) from their baselines (Fig. 1), possibly reflecting a response to maternal separation similar to a stress-induced increase in peripheral OT described in adults (51). Consequently, at the end of the 1-h separation, we saw a striking, positive correlation between serum and hypothalamic OT (Fig. 3B). Increased OT in the pups after the 1-h separation may reflect a stress reaction. Maternal separation can activate the HPA axis and the sympathetic nervous system, in immature rodents potentiating concentrations of adrenocorticotrophin (ACTH), corticotrophin- releasing hormone, corticosterone (CORT) and norepinephrine (52–56). Hypothalamic OT can function to attenuate HPA axis activity (17), lowering the basal and / or stress-induced concentrations of ACTH and CORT, as a coping reaction to stress (57, 58).
In the last phase of the regimen, some pup pairs spent 2 h with a maternally-active foster mother, whereas sibling controls spent those 2 h in undisturbed pairs without maternal stimulation. Measurements taken at the end of this 2-h treatment period revealed that the different social conditions differentially modulated the pups’ OT concentrations. In pups placed with a mother, central OT increased no further and remained at the same concentration (Fig. 1A, solid line) as that seen before the 2-h interactions with the mother. By contrast, the sibling controls that continued in isolation sustained further increases in central OT (Fig. 1A, broken line). The picture was different for serum OT in the same pups. Here, pups exposed to no maternal care showed decreased concentrations of OT in the periphery, whereas pups exposed to maternal stimulation maintained the peripheral concentrations (Fig. 1B). Central and peripheral OT concentrations continued to correlate positively in the pups united with a mother (Fig. 3C). Nevertheless, this central–peripheral correlation deteriorated in the pups that remained isolated from maternal care (Fig. 3D), suggesting that maternal separation can initially stimulate both central and peripheral OT but, with continued separation (in the 1–3 h timeframe used here), an attenuation of peripheral release can develop, whereas central OT concentrations increase. Loss of the positive correlation between central and peripheral OT may be related to dissociated regulation between dendritical and axonal release (59).
Video recordings of the 2-h session with the foster mother were analysed for a variety of maternal activities. Of these behavioural parameters, maternal skin-to-skin contact with individual pups was positively related to hypothalamic OT in the same individuals (Fig. 2). By contrast, serum OT did not correlate with any of the maternal contact parameters measured in the present study. Although maternal care, particularly warmth and touch, has been hypothesised to be integral to activation of the maternal OT system and be important for positive social interactions between mothers and infants (60), there have been few studies examining how different types of maternal contact affects infant’s OT concentrations. The present study provides the first evidence that skin-to-skin contact with mother may stimulate OT concentrations in preweanling rats.
Interestingly, despite our attempts to find systematic relationships between maternal licking / grooming (whole body as well as anogenital) and OT concentrations in 14-day-old rats, such results were not obtained. These negative findings parallel a lack of detectable effects of maternal licking / grooming in the formation of the OT-dependent, filial preference in pups (27, 35, 37, 38). By contrast, mechanotactile stimulation provided by vibration can induce an increased central and peripheral OT in adult rats (50), and anogenital stroking stimulates PVN oxytocinergic neurones in 7-day-old rabbits (61) and OT concentrations of the lumbosacral spinal cord in 10-day-old rats (62). It should be recognised, however, that these important observations were not based on direct measures in natural settings or on manipulations of maternal licking but, instead, came from forms of mechanical stimulation that differ, both qualitatively and quantitatively, from the natural, species-typical stimuli. Moreover, there are often differences in the age(s) at which licking, ‘licking-like’ or stroking stimulation is applied, and we are becoming increasingly aware of the importance of age, reinforcement modality and task demands in early learning (39, 40, 63), as well as the dramatic neurodevelopmental organisation of the OT system during the preweanling period (64, 65).
The difference in pups’ hypothalamic OT concentrations between different social conditions shown in Fig. 1 suggests that maternal care suspends the continuing increase in central OT seen in pups that received no maternal care. Instead, higher-than-baseline concentrations were maintained in pups reunited with a mother. There are studies showing that moderately high concentrations of OT potentiate social recognition (66, 67), whereas higher doses attenuate it (68, 69), suggesting the effects of OT on social recognition are dose-dependent (66, 67). It is possible that the acquisition of a huddling preference may benefit from modulated but higher than basal concentrations of OT, whereas pups in the Non-Maternal Condition did not acquire a huddling preference (37, 38) as a result of their high concentrations of central OT.
When we first standardised our single-session conditioning regimen (37), the initial, 1-h separation period was used as a period to complete experiment-related chores, take initial measures, and equate the pups’ initial state before odour conditioning. The present finding showing that the separation itself affected pup’s OT, however, leads us to suspect that this 1-h period of separation from the mother and the nest may be integral to the pups’ learning. Trials in our laboratory for which the 1-h separation period was not used did not induce filial preference in huddling (J. R. Alberts and J. Novak, unpublished data), and we note with interest a recent study concerning olfactory learning in infant mice (70) in which the learning was absent without a pre-training separation period. Perhaps the normal rhythms of maternal contact bouts with offspring may constitute a natural schedule of separation and reunion that modulate OT concentrations and, in this way, serve as scaffolding for the acquisition of the pups’ filial huddling preference.
Whatever the details of possible preconditions for the acquisition of an odour-guided filial preference in pups, we have assembled a body of data that show the importance of maternal contact for reliably providing conductive warmth to pups (i.e. hovering over pup and skin-to-skin contact) as a necessary and sufficient element in the pup’s learning for odour preference (37, 38), and also that such pup’s learning can be prevented by pharmacologically blocking the action of central OT (27). The present study fortifies these analyses with data showing a direct, positive link between maternal skin-to-skin contact and pup’s hypothalamic OT. Whether this maternal modulation of OT was a result of alterations in central synthesis, storage or release, or even a peripheral effect, was not an objective of the present study. Our questions concerned how the OT system in rat pups responds to the presence of the mother, and how its response is associated with the mother’s contact. Now that we have evidence of socially-induced changes in the pups’ OT system, we can consider possible mechanisms by which maternal contact could stimulate OT release in the offspring.
When in skin-to-skin contact with the mother, pups may engage in nutritive or non-nutritive suckling. Indeed, we noted (but did not measure) nipple attachments that occurred during maternal skin-to-skin contact in the present study. Milk intake can stimulate OT concentrations and activate oxytocinergic neurones in rat and rabbit pups (61, 71). Nevertheless, milk transfer does not reliably occur during the 2-h interaction between a mother and two pups in the present study, as we have shown previously in an experimental setting identical to that employed here (37, 38). As additional corroboration, we monitored but observed no stretch response, which, in pups, is an invariant, reflex-based index of milk intake (72). Although the absence of milk transfer during such a mother–pup reunion might be unexpected, it has been noted that nipple stimulation from a small number of pups (< 4) does not efficiently promote milk ejection, elevated levels of hormonal measures related to milk production / ejection or nursing behaviours in dams (73–80). Thus, we posit that milk intake does not contribute to the positive relationship between skin-to-skin contact and pup’s hypothalamic OT during a relatively brief (e.g. 2 h), mother–pup interaction after reunion. Perhaps, non-nutritive suckling can affect pups’ OT concentrations via cholecystokinin (CCK). CCK activates oxytocinergic neurones and increases OT concentrations (81, 82), and non-nutritive suckling induces elevations of CCK in calves (83).
Although the present study cannot exclude non-nutritive suckling effects, neither milk nor nipple contact is necessary for the acquisition of the filial preference mediated by central OT in 2-week-old pups (35,37,38). Because thermotactile stimulation is the most dominant stimulus received by pups in the present context (38), we speculate that thermotactile stimulation received during maternal skin-to-skin contact may activate the pups’ OT system, perhaps via the vagus nerve, which becomes part of a functional axis with the hypothalamus by PD 10 (71). It is well established that stimulation of vagal afferent nerves through mechanoreceptors and chemoreceptors (84) can induce OT release [e.g. by gastric distension (71,81), by CCK (81,82,85) and by electrical stimulation (41)]. It was recently reported that the vagus nerve is also activated by hyperthermia through its thermoreceptors on organ tissues (86,87). Note, however, that the vagus nerve apparently does not convey thermal information directly from peripheral, cutaneous stimulation (88). Instead, the implication is that thermotactile stimulation gradually warms by conduction the deeper tissues and organs, stimulating heat-sensitive vagal fibres in the viscera that project to hypothalamic OT neurones. Concordant with deep tissue warming as a source of OT activation are the data indicating that frequency of skin-to-skin contact with mother had no association with pup OT concentrations, whereas bout duration parameters (i.e. both average episode and total duration) were positively associated with the pups’ hypothalamic OT. Moreover, this hypothesis is consistent with the finding that warm ambience does not induce a filial huddling (38); warm air may not easily affect deeper tissues innervated by vagus nerves.
To our knowledge, the present study provides the first evidence that maternal care may modulate OT concentrations in rat pups. Considering that the OT system develops dramatically during the postnatal period (64,65) and that the level and types of maternal care changes as pups grow (89), the present findings should be applied carefully to pups at different developmental stages. For example, there are age-related changes in the efficacy of stroking and electric shock on early olfactory learning, which have been related to the developmental changes in the norepinephrine and CORT systems (39). It is possible that different maternal stimuli may differentially affect the pups’ learning in relation to the state of the OT system during development. Further studies using developmental approaches are needed to understand how different kinds of stimulation from maternal care affect pups’ OT responses, such as the mechanisms that mediate the association between pups’ OT concentration and skin-to-skin contact with the mother.
Acknowledgments
The authors are grateful for the support from National Institutes of Health grants MH082019 and MH28355 and Indiana University Faculty Research Support Program to J.R.A., and a Grant-in-Aid of Research from the Graduate School, Indiana University to S.K. We thank David Kersey for helpful technical advice during validation of the oxytocin assays.
References
- 1.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 2.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brain-stem to the paraventricular and supraoptic nuclei in the rat. Brain Res Brain Res Rev. 1982;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 3.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;62:69–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 4.Engelmann M, Ebner K, Wotjak CT, Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res. 1998;90:89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 7.Popik P, van Ree JM. Neurohypophyseal peptides and social recognition in rats. Prog Brain Res. 1998;119:415–436. doi: 10.1016/s0079-6123(08)61585-x. [DOI] [PubMed] [Google Scholar]
- 8.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 9.Young LJ, Wang ZX. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 10.Keverne EB, Kendrick KM. Oxytocin facilitation of maternal behavior in sheep. Ann NY Acad Sci. 1992;652:83–101. doi: 10.1111/j.1749-6632.1992.tb34348.x. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen CA. Oxytocin control of maternal behavior – regulation by sex steroids and offspring stimuli. Ann NY Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiol Behav. 2003;80:233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 15.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 18.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- 21.Insel TR, O’Brien DJ, Leckman JF. Oxytocin, vasopressin, and autism: is there a connection? Biol Psychiatry. 1999;45:145–157. doi: 10.1016/s0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- 22.Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: potential rodent models of autism. Int J Dev Neurosci. 2005;23:235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 24.Goldman M, Marlow-O’Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res. 2008;98:247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res. 2010;124:13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 27.Kojima S, Alberts JR. Oxytocin mediates the acquisition of filial, odor-guided huddling for maternally-associated odor in preweanling rats. Horm Behav. 2011;60:549–558. doi: 10.1016/j.yhbeh.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberts JR, Brunjes PC. Ontogeny of thermal and olfactory determinants of huddling in the rat. J Comp Physiol Psychol. 1978;92:897–906. doi: 10.1037/h0077533. [DOI] [PubMed] [Google Scholar]
- 29.Barnett SA. The Rat: A Study in Behaviour. London: Methuen; 1963. [Google Scholar]
- 30.Alberts JR. Huddling by rat pups: multisensory control of contact behavior. J Comp Physiol Psychol. 1978;92:220–230. doi: 10.1037/h0077458. [DOI] [PubMed] [Google Scholar]
- 31.Alberts JR. Huddling by rat pups: group behavioral mechanisms of temperature regulation and energy-conservation. J Comp Physiol Psychol. 1978;92:231–245. doi: 10.1037/h0077459. [DOI] [PubMed] [Google Scholar]
- 32.Sokoloff G, Blumberg MS. Competition and cooperation among huddling infant rats. Dev Psychobiol. 2001;39:65–75. doi: 10.1002/dev.1030. [DOI] [PubMed] [Google Scholar]
- 33.Sokoloff G, Blumberg MS, Adams MM. A comparative analysis of huddling in infant Norway rats and Syrian golden hamsters: does endothermy modulate behavior? Behav Neurosci. 2000;114:585–593. doi: 10.1037//0735-7044.114.3.585. [DOI] [PubMed] [Google Scholar]
- 34.Brunjes PC, Alberts JR. Olfactory stimulation induces filial preferences for huddling in rat pups. J Comp Physiol Psychol. 1979;93:548–555. doi: 10.1037/h0077571. [DOI] [PubMed] [Google Scholar]
- 35.Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Dev Psychobiol. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- 36.Alberts JR. Huddling by rat pups: ontogeny of individual and group behavior. Dev Psychobiol. 2007;49:22–32. doi: 10.1002/dev.20190. [DOI] [PubMed] [Google Scholar]
- 37.Kojima S, Alberts JR. Maternal care can rapidly induce an odor-guided huddling preference in rat pups. Dev Psychobiol. 2009;51:95–105. doi: 10.1002/dev.20349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojima S, Alberts JR. Warmth from skin-to-skin contact with mother is essential for the acquisition of filial huddling preference in preweanling rats. Dev Psychobiol. 2011;53:813–827. doi: 10.1002/dev.20565. [DOI] [PubMed] [Google Scholar]
- 39.Moriceau S, Sullivan RM. Neurobiology of infant attachment. Dev Psychobiol. 2005;47:230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan RM, Wilson DA. The locus-coeruleus, norepinephrine, and memory in newborns. Brain Res Bull. 1994;35:467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 41.Stock S, Uvnas-Moberg K. Increased plasma-levels of oxytocin in response to afferent electrical-stimulation of the sciatic and vagal nerves and in response to touch and pinch in anesthetized rats. Acta Physiol Scand. 1988;132:29–34. doi: 10.1111/j.1748-1716.1988.tb08294.x. [DOI] [PubMed] [Google Scholar]
- 42.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 45.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 46.Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 48.Vacher CM, Fretier P, Creminon C, Calas A, Hardin-Pouzet H. Activation by serotonin and noradrenaline of vasopressin and oxytocin expression in the mouse paraventricular and supraoptic nuclei. J Neurosci. 2002;22:1513–1522. doi: 10.1523/JNEUROSCI.22-05-01513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- 50.Uvnas-Moberg K, Bruzelius G, Alster P, Lundeberg T. The antinociceptive effect of nonnoxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand. 1993;149:199–204. doi: 10.1111/j.1748-1716.1993.tb09612.x. [DOI] [PubMed] [Google Scholar]
- 51.Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. J Neuroendocrinol. 2004;16:308–312. doi: 10.1111/j.0953-8194.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- 52.Harvey AT, Moore H, Lucot JB, Hennessy MB. Monoamine activity in anterior hypothalamus of guinea-pig pups separated from their mothers. Behav Neurosci. 1994;108:171–176. doi: 10.1037//0735-7044.108.1.171. [DOI] [PubMed] [Google Scholar]
- 53.Hennessy MB, Tamborski A, Schiml P, Lucot J. The influence of maternal separation on plasma-concentrations of ACTH, epinephrine, and norepinephrine in guinea-pig pups. Physiol Behav. 1989;45:1147–1152. doi: 10.1016/0031-9384(89)90101-7. [DOI] [PubMed] [Google Scholar]
- 54.Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal-deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol. 1991;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 56.Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic- pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Brain Res Dev Brain Res. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- 57.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 58.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitaryadrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol. 1999;11:867–872. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 60.Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 61.Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Brain Res Dev Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- 62.Lenz KM, Sengelaub DR. Maternal care effects on the development of a sexually dimorphic motor system: the role of spinal oxytocin. Horm Behav. 2010;58:575–581. doi: 10.1016/j.yhbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woo CC, Leon M. Sensitive period for neural and behavioral response development to learned odors. Brain Res Dev Brain Res. 1987;36:309–313. doi: 10.1016/0165-3806(87)90038-1. [DOI] [PubMed] [Google Scholar]
- 64.Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiol Behav. 2003;79:65–70. doi: 10.1016/s0031-9384(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 65.Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R. Poly-modal dose–response curve for oxytocin in the social recognition test. Neuropeptides. 1995;28:251–255. doi: 10.1016/0143-4179(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 67.Popik P, Vetulani J, Vanree JM. Low-doses of oxytocin facilitate social recognition in rats. Psychopharmacology (Berl) 1992;106:71–74. doi: 10.1007/BF02253591. [DOI] [PubMed] [Google Scholar]
- 68.Dantzer R, Bluthe RM, Koob GF, Lemoal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 69.Popik P, Vetulani J. Opposite action of oxytocin and its peptide antagonists on social memory in rats. Neuropeptides. 1991;18:23–27. doi: 10.1016/0143-4179(91)90159-g. [DOI] [PubMed] [Google Scholar]
- 70.Armstrong CM, DeVito LM, Cleland TA. One-trial associative odor learning in neonatal mice. Chem Senses. 2006;31:343–349. doi: 10.1093/chemse/bjj038. [DOI] [PubMed] [Google Scholar]
- 71.Nelson EE, Alberts JR, Tian Y, Verbalis JG. Oxytocin is elevated in plasma of 10-day-old rats following gastric distension. Brain Res Dev Brain Res. 1998;111:301–303. doi: 10.1016/s0165-3806(98)00147-3. [DOI] [PubMed] [Google Scholar]
- 72.Lincoln DW, Hill A, Wakerley JB. Milk-ejection reflex of rat: intermittent function not abolished by surgical levels of anesthesia. J Endocrinol. 1973;57:459–476. doi: 10.1677/joe.0.0570459. [DOI] [PubMed] [Google Scholar]
- 73.Cramer CP, Thiels E, Alberts JR. Weaning in rats: 1. Maternal behavior. Dev Psychobiol. 1990;23:479–493. doi: 10.1002/dev.420230604. [DOI] [PubMed] [Google Scholar]
- 74.Lincoln DW, Wakerley JB. Factors governing periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in rat. J Physiol (Lond) 1975;250:443–461. doi: 10.1113/jphysiol.1975.sp011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mattheij JAM, Gruisen EFM, Swarts JJM. Suckling-induced rise of plasma prolactin in lactating rats: its dependence on stage of lactation and litter size. Horm Res. 1979;11:325–336. doi: 10.1159/000179070. [DOI] [PubMed] [Google Scholar]
- 76.Mena F, Grosvenor CE. Effect of number of pups upon suckling-induced fall in pituitary prolactin concentration and milk ejection in rat. Endocrinology. 1968;82:623–626. doi: 10.1210/endo-82-3-623. [DOI] [PubMed] [Google Scholar]
- 77.Morag M, Popliker F, Yagil R. Effect of litter size on milk yield in the rat. Lab Anim. 1975;9:43–47. doi: 10.1258/002367775780994844. [DOI] [PubMed] [Google Scholar]
- 78.Russell JA. Milk-yield, suckling behavior and milk ejection in the lactating rat nursing litters of different sizes. J Physiol (Lond) 1980;303:403–415. doi: 10.1113/jphysiol.1980.sp013295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in norway rats. 1. Effects of variations in the quality and quantity of pup stimuli. Physiol Behav. 1990;47:993–1011. doi: 10.1016/0031-9384(90)90026-z. [DOI] [PubMed] [Google Scholar]
- 80.Yagil R, Etzion Z, Berlyne GM. Changes in rat milk quantity and quality due to variations in litter size and high ambient temperature. Lab Anim Sci. 1976;26:33–37. [PubMed] [Google Scholar]
- 81.Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987;253:R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- 82.Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–1419. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- 83.de Passille AMB, Christopherson R, Rushen J. Nonnutritive sucking by the calf and postprandial secretion of insulin, CCK, and gastrin. Physiol Behav. 1993;54:1069–1073. doi: 10.1016/0031-9384(93)90326-b. [DOI] [PubMed] [Google Scholar]
- 84.Verberne AJM, Saita M, Sartor DM. Chemical stimulation of vagal afferent neurons and sympathetic vasomotor tone. Brain Res Brain Res Rev. 2003;41:288–305. doi: 10.1016/s0165-0173(02)00269-2. [DOI] [PubMed] [Google Scholar]
- 85.Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 86.Ruan T, Gu QH, Kou YR, Lee LY. Hyperthermia increases, sensitivity of pulmonary C-fibre afferents in rats. J Physiol (Lond) 2005;565:295–308. doi: 10.1113/jphysiol.2005.084319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang L, Jones S, Brody K, Costa M, Brookes SJH. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol. 2004;286:983–991. doi: 10.1152/ajpgi.00441.2003. [DOI] [PubMed] [Google Scholar]
- 88.Szekely M. The vagus nerve in thermoregulation and energy metabolism. Auton Neurosci. 2000;85:26–38. doi: 10.1016/S1566-0702(00)00217-4. [DOI] [PubMed] [Google Scholar]
- 89.Rosenblatt JS, Lehrman DS. Maternal behavior of the laboratory rat. In: Rheingold L, editor. Maternal Behavior in Mammals. New York, NY: Wiley; 1963. pp. 8–57. [Google Scholar]