Abstract
The neural damage accompanying the hypoxia, reduced perfusion, and other consequences of sleep-disordered breathing found in obstructive sleep apnea, heart failure (HF), and congenital central hypoventilation syndrome (CCHS), appears in areas that serve multiple functions, including emotional drives to breathe, and involve systems that serve affective, cardiovascular, and breathing roles. The damage, assessed with structural magnetic resonance imaging (MRI) procedures, shows tissue loss or water content and diffusion changes indicative of injury, and impaired axonal integrity between structures; damage is preferentially unilateral. Functional MRI responses in affected areas also are time- or amplitude- distorted to ventilatory or autonomic challenges. Among the structures injured are the insular, cingulate, and ventral medial prefrontal cortices, as well as cerebellar deep nuclei and cortex, anterior hypothalamus, raphé, ventrolateral medulla, basal ganglia and, in CCHS, the locus coeruleus. Raphé and locus coeruleus injury may modify serotonergic and adrenergic modulation of upper airway and arousal characteristics. Since both axons and gray matter show injury, the consequences to function, especially to autonomic, cognitive, and mood regulation, are major. Several affected rostral sites, including the insular and cingulate cortices and hippocampus, mediate aspects of dyspnea, especially in CCHS, while others, including the anterior cingulate and thalamus, participate in initiation of inspiration after central breathing pauses, and the medullary injury can impair baroreflex and breathing control. The ancillary injury associated with sleep-disordered breathing to central structures can elicit multiple other distortions in cardiovascular, cognitive, and emotional functions in addition to effects on breathing regulation.
Keywords: Obstructive Sleep Apnea, Congenital Central Hypoventilation Syndrome, Heart Failure, Hypothalamus, Medulla, Brainstem, Magnetic Resonance Imaging, Dyspnea
Introduction
As is the case for many biological processes, insights into mechanisms of breathing can be revealed by pathology, and sleep-disordered breathing has been especially useful in providing such insights into respiratory regulation. Sleep normally exerts profound effects on breathing patterning, altering both rate, variability of rate, volume of efforts, and chemosensitivity, with differing states within sleep greatly modifying respiratory characteristics (Douglas et al. 1982; Skatrud & Dempsey 1983). Normal variations in breathing during sleep have done much to reveal contributions of neural structures leading to control of respiration, and disordered breathing induced by sleep offers even further insights.
Sleep disordered breathing
Several of the pathologies of breathing during sleep result from exaggerations of normal physiological changes that occur with routine transitions in sleep states, adding to even modest alterations in airway morphology or other condition that can exacerbate a breathing condition. An example of such a circumstance gives rise to perhaps the most common of sleep-disordered breathing, obstructive sleep apnea (OSA). Obstructive sleep apnea is characterized by collapse of the upper airway from atonia of the upper airway muscles, especially the genioglossal fibers of the tongue, in the presence of continued diaphragmatic efforts. The origins of the condition may arise from a combination of the normal loss of tone of all of the respiratory muscles, except for portions of the diaphragm, during rapid eye movement (REM) sleep, which leads to an enhanced potential for airway collapse (Sauerland & Harper 1976). Any added circumstance, such as airway restriction by enlarged tonsillar tissue, fat infiltration in the oropharynx, micrognathia, or deviated nasal septum, increases airflow speed with inspiratory efforts, leading to collapse from the Bernoulli principle. In the disorder, the loss of phasic inspiratory bursts in the upper airway muscles continues, inappropriately, even during quiet sleep to block airflow. The processes underlying that loss of upper airway muscle activation during quiet sleep likely arise from initial injury to respiratory patterning circuitry damaged with early obstruction, which presumably include cerebellar circuitry responsible for coordinating upper airway and diaphragmatic action. In some cases, it appears that initial central nervous system injury, especially in cerebellar coordination areas, resulting from developmental injury or stroke, provides the originating circumstances for OSA (Chokroverty, Sachdeo & Masdeu 1984). The repeated airway blockade in OSA leads to successive intermittent periods of hypoxia, with perhaps even more damaging rapid reperfusion with O2 after release of obstruction. Additional contributions to the injurious processes can develop from the excessive transient blood pressure elevations and release with the thoracic pressure changes accompanying OSA.
One general principle of breathing influences during sleep is the concept of the “wakefulness” stimulus (Orem 1990). The influence of waking is considered facilitatory to breathing, and is especially shown in a pathological condition, congenital central hypoventilation syndrome (CCHS), a condition accompanied by mutations of the paired homeobox (PHOX2B) gene which targets autonomic nervous system development (Patwari et al. 2010). The condition is characterized by a loss of drive to breathe during sleep (and, with more-severe mutations, even during waking), and a loss of ventilatory sensitivity to CO2 and O2. Survival in this syndrome requires nocturnal ventilatory support, with inevitable lapses in that support leading to multiple exposures to hypoxia. That exposure continues to induce further injury, as demonstrated by structural magnetic resonance imaging (MRI) studies collected at different times in the development of the condition (Kumar et al. 2012b).
A variation of central apnea is periodic, or Cheyne-Stokes breathing, common in heart failure (HF) patients (Francis et al. 2000), and often found together with OSA. This pattern of breathing, with incremental respiratory efforts, followed by periods of cessation of both upper airway and diaphragmatic activation, appears to result from multiple physiological factors, including enhanced chemoreflex gain and aberrant timing of central vs. peripheral chemoreception, perhaps compounded by prolonged circulation time in conditions with low cardiac output or chronically high sympathetic tone, such as OSA or HF; both conditions modify vascular tone, and thus, circulation time. The consequences of breathing cessation for sustained periods, followed by resumption of breathing lead to epochs of intermittent hypoxia, followed by rapid O2 uptake, as in OSA, although without the extreme thoracic and associated cardiovascular pressure changes of obstructed events. The intermittent hypoxia sequences, however, have the potential to induce serious brain injury.
Injury in sleep disordered breathing
Procedures for assessing injury and function
Magnetic resonance imaging (MRI) techniques have been useful for assessing central nervous system injury in sleep-disordered breathing; the procedures incorporate many variations for demonstrating tissue changes that accompany damage, generically called “structural imaging,” or alterations in blood flow to tissue which provide indications of local neural activity, called “functional magnetic resonance imaging (fMRI). In addition, determination of spectral signatures of metabolic components associated with cellular function, which differ by health of tissue, called magnetic resonance spectroscopy, can be used.
The earliest procedures to evaluate injury in sleep-disordered breathing were variations of volume changes in tissue. Those procedures involve collecting high resolution anatomical images (“T1-weighted”) of the entire brain, removing extraneous material outside the brain, separating gray and white matter (based on intensity differentiation of white and gray matter), evaluating mean values of tissue across a group of healthy controls, and comparing those values at each measurement point in a brain volume (“voxel”) against average values of the breathing-disordered group. The technique is termed “voxel-based morphometry,” and has been used extensively despite multiple difficulties in variation of tissue morphology across even healthy subjects, and concerns of multiple comparisons with the very large number of voxels in the brain. Nevertheless, the procedures have been invaluable in demonstrating the first indications of injury in OSA (Macey et al. 2002). The technique has been expanded to assess other aspects of tissue, including density. More precise morphometry analyses have been used for subcortical structures, and include manual tracing of high resolution T1-weighted images, incorporating those tracings into mathematical maps that can be averaged across healthy subjects, and statistical comparisons of surface maps with the patient groups (Narr et al. 2004; Thompson, Schwartz & Toga 1996). We have used such procedures for hippocampal surface mapping (Macey et al. 2009; Harper et al. 2012) and basal ganglia structures (Kumar et al. 2009a; Kumar et al. 2011b). Other procedures involve simple manual measurements to assess volumes of small structures, such as the mammillary bodies and fornix fibers (Kumar et al. 2008a; Kumar et al. 2009b; Kumar et al. 2009e).
Determining tissue changes from high-resolution T1 images and automated or manual measures provides an intuitive advantage for inferring injury, but cannot readily assess white matter damage, and is not useful where injury results in little change in tissue volume, but leaves tissue with membrane or other changes in which ionic flow is greatly disturbed. For those reasons, T2 relaxometry, which can assess free water content, and diffusion imaging procedures, which assess diffusion of water in tissue, have provided significant insights.
Among the structural MRI techniques, a focus has been on the presence and movement or diffusion of water molecules. Neurodegenerative processes typically show loss of neurons or myelin, leaving increased free water; measurement of this water content with a procedure called T2 relaxometry has been useful in assessment of pediatric sleep disordered breathing, HF, and other neurological disorders (Woo et al. 2009; Kumar et al. 2005; Mueller et al. 2007). Water movement is especially valuable for defining integrity of nervous tissue, since such diffusion is normally constrained by membrane boundaries, and when those membranes are damaged by injury, more water movement results. Assessment of the ease of diffusion in any direction, or “mean diffusivity” thus can provide an index of injury. Diffusion imaging has other implementations; if the direction of movement is assessed using a procedure called “diffusion tensor” imaging, procedures for displaying direction, tracking, and integrity of nerve fibers can be evaluated. Nerve fibers are typically wrapped in myelin, constraining fluid movement in parallel with the fiber; assessing such restrained movement can display fiber direction, and can enable tracking of nerve fibers through defined regions of interest. Moreover, since water movement within myelinated axons is constrained principally along the path of the fiber, breakdowns in myelin will allow ionic movement outside of those constraints, and can be measured using a technique called fractional anisotropy (FA). Derivatives of diffusion tensor imaging procedures can be used to evaluate that movement perpendicular or along the path of the fiber; those procedures are called radial and axial diffusion procedures, respectively, and can assess myelin or axonal integrity.
Evidence of neural injury accompanying sleep-disordered breathing includes impaired physiological responses during spontaneous changes in behavior or sleep and waking states, and evoked responses to autonomic or ventilatory challenges that are mediated through central nervous processes. These chronic state-related changes are substantial in affected individuals, and include sustained hypertension, marked sympathetically-mediated sweating, and altered hormonal and glucose levels in OSA, HF, and CCHS (Harsch et al. 2003; Pallayova et al. 2006; Tamura et al. 2012; Agapitou et al. 2013). The dysfunctional steady-state physiological patterns extend to dynamic actions as well, with heart rate and breathing responses altered in magnitude and timing, especially during recovery periods, to blood pressure challenges in OSA (Harper et al. 2003; Henderson et al. 2003).
Insights into the effects of the structural changes in tissue can be obtained by examining central nervous system responses to challenges using MRI techniques by inferring such responses to changes in oxygenation of the vasculature to the tissue (Ogawa et al. 1990). Other means of inferring such changes are available or under investigation, such as inferring fluid changes across cellular membranes with neural activity (Le Bihan 2003), but the most common means is determination of blood oxygen level dependent (BOLD) contrast, and its coupling to neural activity. The procedure depends on assessment of changes in blood volume and magnetization between oxygen-rich and relatively deoxygenated blood during changes in oxygenation in the course of perfusing activated tissue. The technique is widely used to assess brain function, to show interconnectivity between brain areas, and to indicate disturbances in brain area activity. The BOLD technique also provides indications of cerebral hemodynamics, and has been used to evaluate large changes in whole-brain cerebral vasculature and blood oxygenation, such as those that occur in CCHS to hypercapnia, hyperoxia, or hypoxia (Macey et al. 2003a).
An evolving technology, termed “arterial spin labeling” allows non-invasive quantification of cerebral blood flow by using magnetization of blood as contrast. While this procedure shows altered cerebral blood flow in CCHS (Macey et al. 2010a), the technique is still being refined.
The consequences to brain tissue of repeated intermittent hypoxia and breathing resumption, or sustained hypoxia in other forms of sleep disordered breathing, are severe, with injury possibly exacerbated by perfusion and blood pressure changes that accompany apnea. The injury appears as loss of tissue in essential central nervous system areas for cardiovascular and breathing control, affective regulation, and cognitive processes. In addition, injury is expressed as changes in the extent of water content or diffusion in tissue, and in the number and integrity of fibers (axonal and myelin integrity) between essential regulatory areas. Hypoxia and reduced perfusion influences associated with sleep-disordered breathing presumably mediates the damage, with hypoxic effects on glial support tissue likely participating in that injury (Alix et al. 2012). Excitotoxic injury from hypoxia may contribute to damage in structures receiving long-axon projections, such as cerebellar Purkinje neurons receiving input from climbing fibers of the inferior olive, and CA1 neurons of the hippocampus receiving Schaffer collaterals from CA3 neurons. The Purkinje cells often see injury from excitoxic injury (Welsh et al. 2002), and excessive excitation of Schaffer collaterals in temporal lobe epilepsy may underlie the sclerosis found in the fascia dentata, CA1 and CA4 hippocampal fields (Babb & Brown 1987). Attempts to model the OSA experience by exposing rodent models to intermittent hypoxia, a principal feature of OSA, results in injury patterns that remarkably parallel sites of injury in humans, with cerebellar (Pae, Chien & Harper 2005), and limbic, including hippocampal, injuries especially noted (Veasey et al. 2004); the cognitive deficits shared between the animal and human conditions are also particularly noteworthy (Row et al. 2003). The impact is not confined to structural changes; diminished or exaggerated, and time-shifted functional magnetic resonance imaging (fMRI) responses appear to pressor and ventilatory challenges in all three types of sleep-disordered breathing (Figure 8) (Woo et al. 2007; Henderson et al. 2003; Macey et al. 2006; Woo et al. 2005a; Macey et al. 2005b; Macey et al. 2005a; Harper et al. 2005; Macey et al. 2004; Macey et al. 2003b; Harper et al. 2003; Ogren et al. 2010; Ogren et al. 2012).
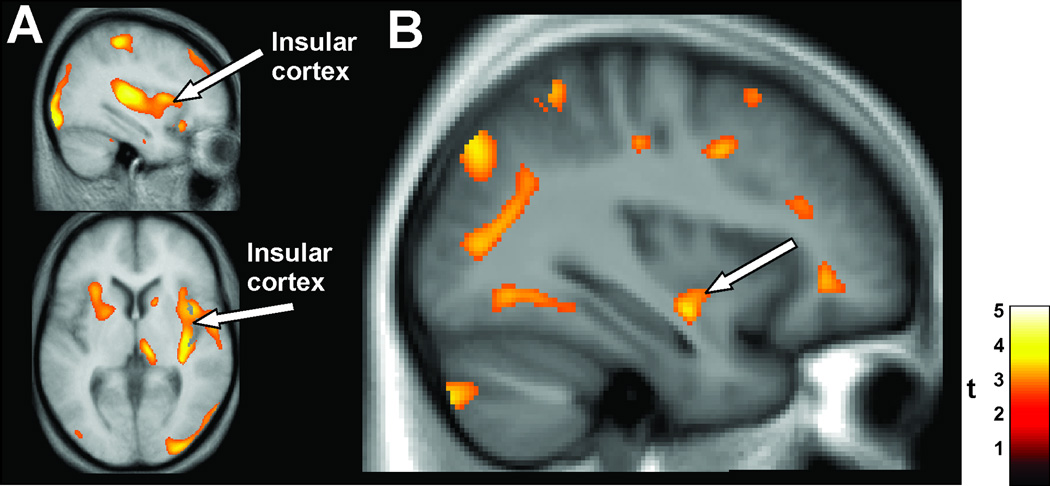
Figure 8.

A: BOLD functional MRI responses in the fastigial nucleus of the cerebellum and responsive portions of the insular cortices (arrows) in OSA (n = 8) and control (n = 15) subjects to three sequential Valsalva pressor challenges, relative to baseline values. B: Left (yellow highlight) and right (yellow highlight) insular cortex responses over the entire insular cortical regions during three averaged Valsalva maneuvers in CCHS (n = 9) and control (n = 25) subjects. CCHS subjects show muted responses relative to marked drops during the Valsalva challenge and recovery periods shown by controls. SI= signal intensity. % Change = Change in signal from mean baseline values. (A, from Henderson et al. 2003; B, from Ogren et al. 2010).
The affected brain areas are widespread, ranging from the medulla and cerebellum to cortical and limbic sites, and especially involve regions mediating affect and sympathetic drive. Remarkably, the extent of injury varies with sex, with females showing significantly more damage in selected areas over males (Macey et al. 2012a); the mechanisms underlying the gender-based differences in injury remain unclear.
Damage in affective areas
A remarkable aspect of HF, OSA, and CCHS patients is that all three groups show significant injury in central areas that mediate affect, assist in cardiovascular regulation, or contribute to integration of breathing and cardiovascular control. These structures include limbic cortical areas classically associated with cardiovascular regulation in addition to affect, such as the insular and cingulate cortices, the ventral medial prefrontal cortex, and the hippocampus and closely-related fornix and mammillary bodies (Figures 1, 2, and 6). In addition, cerebellar cortex and deep nuclei also were injured (Figure 3); although traditionally ignored in consideration of respiratory control despite well-known roles in muscle coordination, these cerebellar areas now are known to mediate cardiovascular, chemoreceptor, and respiratory somatic stimuli (Moruzzi 1940; Xu & Frazier 2002; Xu & Frazier 1997; Reis & Golanov 1997), The injury is expressed as loss of regional tissue volume, or changes in water content or diffusion within a region (Kumar et al. 2012a; Macey et al. 2002).
Figure 1.
A: Insular cortex injury in 9 HF vs 27 healthy control subjects, expressed as tissue loss, occurring preferentially on the right side; B: insular cortex (arrow) injury, expressed as mean diffusivity changes, in 23 OSA vs 23 healthy control subjects (Derived from Woo et al. 2003, and Kumar et al. 2012a).
Figure 2.
Increased axial and radial diffusivity in 12 CCHS vs. 30 control subjects; rostral areas. Injury appears in anterior and sub-genu cingulate, ventral medial prefrontal cortex (a,b,c), insular cortex (f), and basal ganglia (e). (From Kumar et al. 2010).
Figure 6.
A: Left, Mammillary bodies from a male control subject and Right, an age- and gender-matched HF patient. B: Left, One control male and Right, an age- and gender-matched CCHS patient. (C) Scattergram of mammillary body volumes in 43 OSA and 66 control subjects showing substantially reduced volumes in OSA. (A from Kumar et al. 2009e, B from Kumar et al. 2009b, C from Kumar et al. 2008a).
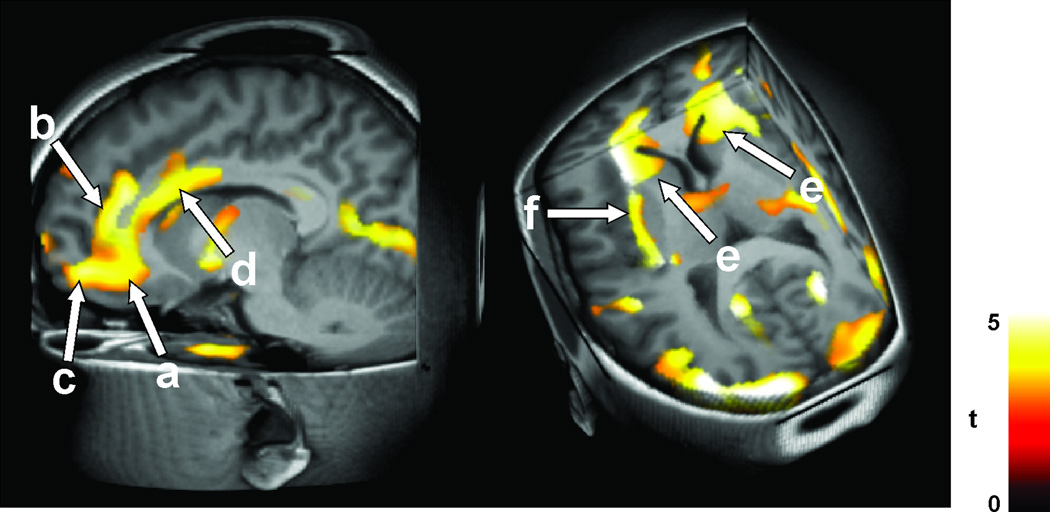
Figure 3.
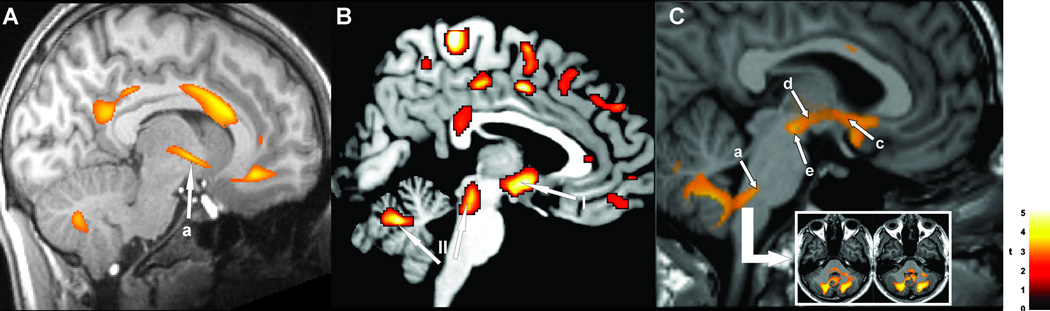
Injury in A: 12 CCHS vs 28 age-and gender- matched healthy control subjects, shown by T2-relaxometry in hypothalamus (a); B: injury in OSA shown by fractional anisotropy, n=3 OSA vs 3 control subjects, I=hypothalamus, II= cerebellum and midbrain; and C: injury in 16 HF vs 26 control subjects, with axial diffusivity, showing damage from the midline raphé to the cerebellum (a), within the hypothalamus (c), and along the medial forebrain bundle (d), and midbrain (e). (A from Kumar et al. 2005, C from Kumar et al. 2011c).
The contributions of affect to breathing are frequently overlooked in respiratory control, despite the obvious inputs revealed by the shortness of breath experienced during extreme excitement, gasps occurring during sudden fright, and the difficulty found in voice pitch and volume control during grief. All of those characteristics indicate descending influences from negative affective perceptions onto pontine respiratory phase switching and medullary sites, and are mediated by limbic structures following recognition of the threat or condition, as interpreted by cortical areas.
Affective roles are often more easily revealed in pathologies accompanying disease, and are especially illustrated by dyspnea, the sensation of air hunger with high CO2 exposure or extreme exertion at maximal cardiac output. The insula, as well as the cingulate cortex and hippocampus, plays a significant role in mediating the perception of dyspnea, as indicated by multiple fMRI studies (Peiffer et al. 2001; Banzett et al. 2000). The perception of air hunger provides a significant drive to breathe, triggering gasping or major inspiratory efforts in anyone subjected to high CO2 or to extreme exercise. Loss of the perception of air hunger, which occurs in individuals with CCHS, contributes to a breathing failure beyond mere loss of CO2 or O2 sensitivity in the syndrome. The loss is often accompanied with even more widespread lack of affect, which leads to risk-taking respiratory behaviors, such as challenging each other or unaffected playmates to underwater breath-holding games, sometimes with fatal consequences. The absence of sense of dyspnea and loss of chemosensitivity places extreme demands on caregivers who must be aware when their charges fail to breathe when placed in quiet circumstances, such as TV watching. The lack of affect which contributes to high-risk behaviors has sometimes been compared with adolescents with Type 1 diabetes who also show injury in similar limbic regions; such children will often compromise survival with insulin use to modify body weight for esthetic reasons rather than maintenance of appropriate glucose levels. The lack of affect in CCHS becomes a special concern in teenage years, where affected children engage in high-risk behavior, and begin disregarding nocturnal ventilation without parental oversight; those circumstances are the principal cause of death in affected adolescents.
Dyspnea is a serious accompaniment of HF and chronic obstructive pulmonary disease, to the extent that even very mild exercise of short walks is avoided because of the dread of an inability to obtain sufficient air for the task, in contrast to CCHS children who have no sensitivity to even extraordinary high levels of CO2 and do not perceive breathlessness to excessive exercise. HF, OSA, and CCHS are all accompanied by insular and cingulate cortex and hippocampal injury, principal structures involved in mediating dyspnea, although the precise subregions vary with condition (Macey et al. 2012b; Kumar et al. 2012a; Kumar et al. 2011c; Kumar et al. 2011b; Kumar et al. 2011a; Kumar et al. 2010; Woo et al. 2009; Macey et al. 2009; Kumar et al. 2009c; Macey et al. 2008; Kumar et al. 2006; Kumar et al. 2005; Woo et al. 2003; Macey et al. 2002; Kumar et al. 2008a; Kumar et al. 2008b). Damage in those areas apparently contributes to enhancing or inhibiting that sensation. The extent of injury in the insula can be seen in Figures 1 and 2, which show loss of tissue, preferentially in the right insula in HF, increased mean diffusivity, an index of injury, in OSA, and axial and radial diffusivity changes in the insula, anterior cingulate, and ventral medial prefrontal cortex in CCHS. The injury in brain areas involved in dyspnea is widespread in all three sleep-disordered breathing conditions studied here, and the MRI procedures used to describe the damage do not have the spatial resolution to discriminate sub-areas that specifically underlie enhancing or inhibiting the sensation of dyspnea, which likely lie in close proximity. A demonstration of the proximity of differing fMRI responses in the insula can be found to autonomic challenges (Macey et al. 2012c). In addition to differential responses of subregions within a damaged area, projections to other areas mediating dyspnea may be separately affected in CCHS vs HF, thus resulting in a failure of dyspnea to develop, or, conversely, very enhanced perceptions.
The laterality of the injury is significant for roles in sympathetic regulation. HF, OSA, and CCHS are all characterized by high sympathetic tone (Narkiewicz & Somers 2003; Patwari et al. 2010; Burch 1978). The right anterior insula plays a significant role in baroreflex regulation, as demonstrated by a range of stimulation and stroke evidence (Oppenheimer et al. 1992; Zhang, Dougherty & Oppenheimer 1998; Cechetto & Hachinski 1997; Oppenheimer & Hachinski 1992). The insula is topographically organized for particular afferents (Macey et al. 2012c; Ture et al. 1999), with functional interactions with the hypothalamus modified in OSA (Wu et al. 2010). The range of afferent projections covers a vast array of inputs; pain is represented in a major fashion on the insula (Henderson, Rubin & Macefield 2011), but the sensory input also includes kinesthetic and other intereoceptive sensations, with that cortical structure serving a major integrative role in modifying autonomic and other activity (Craig 2011). Injury to the insula, as appears in all three sleep-disordered breathing conditions, would interfere with sensory integration in that cortical region, influences to the hypothalamus and other limbic areas; those insular-related njuries have the potential to modify contributions to breathing.
Rostral affective, thermal and hormonal sites
Although the circuitry for respiratory oscillatory timing lies primarily in medullary sites, with lung and other peripheral afferent influences acting on pontine phase-switching areas to modify that timing, much of the integrative emotional influences that regulate breathing patterning are sited in more-rostral areas. These rostral sites are principally limbic regions, and neural circuitry within those areas is closely integrated with cardiovascular and other autonomic roles. The respiratory influences of some of those sites have been known for decades; the central nucleus of the amygdala, for example, which shows state-dependent discharge with the respiratory cycle (Zhang, Harper & Frysinger 1986; Frysinger & Harper 1986), has major projections to parabrachial timing regions (Hopkins & Holstege 1978; Holstege, Bandler & Saper 1996). The structure paces inspiratory onset to single pulse electrical stimulation, a relationship which disappears with functional dissolution of that projection by onset of sleep (Harper et al. 1984). The amygdala is damaged in all three sleep-disordered breathing conditions (Ogren et al. 2012; Macey et al. 2012a; Ogren et al. 2010; Woo et al. 2007; Woo et al. 2005b; Woo et al. 2005a; Macey et al. 2005b; Harper et al. 2005; Macey et al. 2003b), and a major projection path, the median forebrain bundle, is injured in HF (Kumar et al. 2011c); Figure 3).
A significant drive to breathe is provided by thermal sources, with the anterior hypothalamus underlying that influence on ventilation. The extent of that influence varies with sleep state, with REM sleep largely removing that drive, as demonstrated by warming of the hypothalamus during waking, quiet, or REM sleep (Ni et al. 1994). Although, strictly speaking, temperature is not an “emotional” drive, the anterior hypothalamus contains substantial circuitry that contributes to regulation of autonomic and endocrine action associated with affect.
All three diseases with compromised breathing during sleep show significant hypothalamic injury. In CCHS, the injury is substantial, extending from the anterior hypothalamus caudally through posterior regions (Figure 3A-a); in OSA, the injury is primarily in anterior regions (Figure 3B-I), but in HF, damage also extends throughout the entire rostral-caudal extent (Figure 3C-c). In all three sleep disordered conditions of OSA, HF, and CCHS, injury includes the raphé system (Woo et al. 2009; Kumar et al. 2011c; Kumar et al. 2008b), which contains serotonergic neurons that play a significant role in pain and modulation of upper airway musculature (Brink et al. 2006; Kubin et al. 1992). The consequences to raphé injury in CCHS are notable in the vasculature, with exceptionally dilated basilar arteries (Kumar et al. 2009d).
The implications for temperature regulation, hormone release, and autonomic control with hypothalamic injury are significant, and almost certainly contribute to the exaggerated sympathetic tone and symptoms characteristic of impaired hormone release in all three conditions. The loss of temperature regulation is especially apparent in CCHS; such children have a great deal of trouble remaining warm at night, even during summer months, show cool extremities, and have difficulty with breathing at high temperatures (Vanderlaan et al. 2004).
Hormonal regulation is especially a concern with the hypothalamic injury in sleep-disordered breathing conditions. Over 86% of obese male diabetic patients show moderate to severe levels of OSA (Tasali, Mokhlesi & Van Cauter 2008; Foster et al. 2009), suggesting significant links between the two conditions. The hypothalamic injury in OSA likely contributes to the impaired glucagon and sympathetic influences on pancreatic hormones found in diabetic patients. CCHS children are often hypoglycemic (Vanderlaan et al. 2004), and HF patients show reduced growth hormone and insulin-like growth factor 1 levels, among other hormones, including testosterone (Kontoleon et al. 2003), and increased atrial natriuretic peptide and brain natriuretic peptide. Although the release sites of some of these hormones may rest with peripheral structures, hypothalamic influences exert influences on those sites. In some of these conditions, the presence of diabetes in addition to the primary breathing disordered condition, such as OSA, gives rise to even further injury in limbic and other areas typically affected in the breathing disorder (Harper et al. 2009).
Affective disorders; depression and anxiety
Among the brain areas affected by sleep-disordered breathing are sites within the insula, anterior cingulate, and medial frontal cortices, hippocampus, fornix, mammillary bodies, amygdala and cerebellum. All of those structures are altered in individuals with depressive signs, even without sleep disordered breathing (Sprengelmeyer et al. 2011; Neumeister, Charney & Drevets 2005; Cross et al. 2008; Akashiba et al. 2002; Saunamaki & Jehkonen 2007). The structural findings are a concern, because OSA and HF are both accompanied by very high levels of depressive signs, with nearly half of each patient group showing higher than normal symptoms (Macey et al. 2010b; Akashiba et al. 2002; Murberg et al. 1998; Vaccarino et al. 2001). In addition, a slightly smaller proportion of OSA patients show high levels of anxiety symptoms (Saunamaki & Jehkonen 2007). OSA patients with depression and anxiety show significant structural injury, compared to OSA patients without those affective symptoms, with very prominent injury in the anterior cingulate (Cross et al. 2008; Kumar et al. 2009c) (Figure 4).
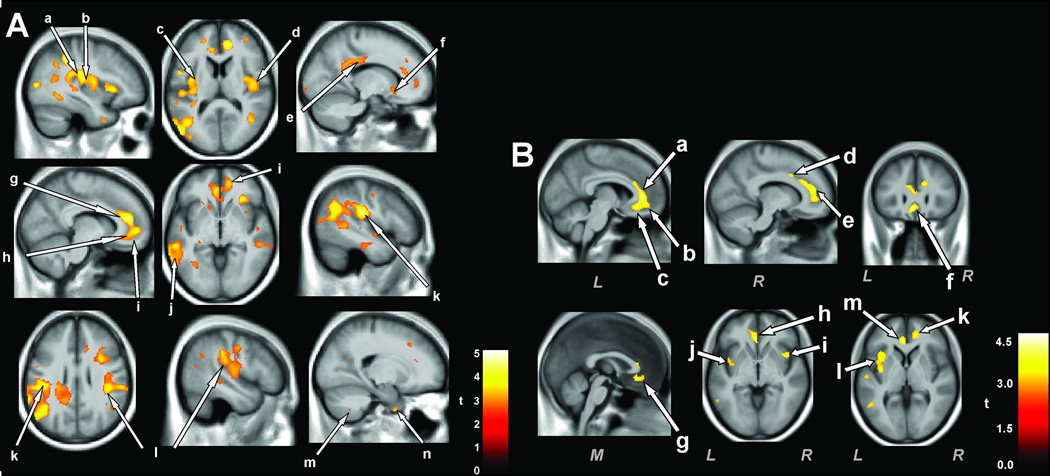
Figure 4.
A: Areas of injury, assessed with T2-relaxometry techniques in OSA patients with depressive signs (Beck Depression Inventory II >10) (n = 13) vs. OSA patients without depression (n = 27). B: Injured areas of anxious OSA patients (Beck Anxiety Inventory > 9, n = 16) vs. non-anxious OSA (n = 30). The insula A-a,b,c,d; B-i,j,l, and anterior and sub genu cingulate Ag,h,; Ba,b,c are consistently affected in both depression and anxiety. Other regions are also affected, and include the prefrontal cortex Ai, lateral temporal cortex, Aj, lateral parietal cortex (Al) and basal forebrain, Bg. (A from Cross et al. 2008; B, from Kumar et al. 2009c).
The cingulate cortex, especially the anterior cingulate portion, has also been implicated in both cardiovascular and breathing roles by a range of stimulation and recording evidence. Single neuron discharge in humans recorded for reasons related to epileptic seizure focus localization shows relationships with respiratory and cardiovascular patterning (Frysinger & Harper 1986). Several structures, including cerebellar cortical regions and the anterior cingulate are synchronized to onset of inspiratory bursts in periodic breathing, as demonstrated by fMRI signals at onset of breathing bursts, Figure 5). Signals in the anterior cingulate cortex decline to onset of those inspiratory bursts. The anterior cingulate is severely injured in all three sleep disordered breathing conditions (Macey et al. 2012b; Macey et al. 2012a; Kumar et al. 2012b; Kumar et al. 2012a; Kumar et al. 2011c; Ogren et al. 2010; Kumar et al. 2010; Macey et al. 2008; Kumar et al. 2005; Woo et al. 2003; Macey et al. 2002), and some of the injury is shown in Figures 2 and 4; that injury may contribute to failed resumption of inspiration in periodic breathing, sustained central apnea, and perhaps failed onset of upper airway activation in OSA.
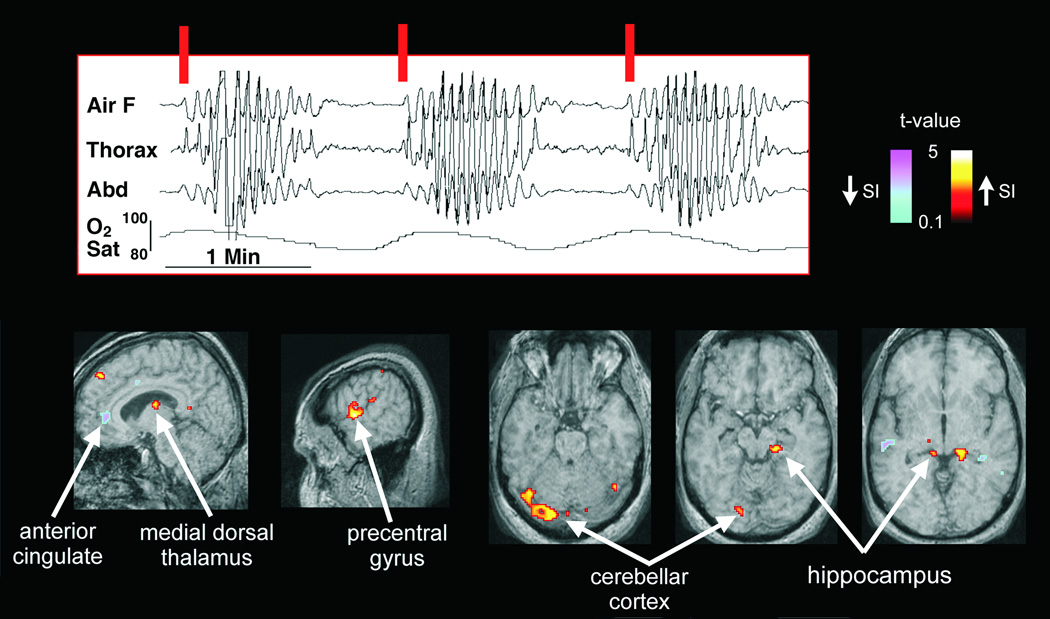
Figure 5.
Brain areas recruited on resumption of breathing after periodic cessation (Cheyne-Stokes breathing). Whole-brain BOLD fMRI signals triggered by onset of inspiratory efforts at the burst of breathing patterns (vertical red lines) in two OSA patients who also experienced Cheyne-Stokes breathing were collected. Signals emerging above baseline included activity of the anterior cingulate, dorsal medial thalamus, and portions of the hippocampus and cerebellum which responded to the respiratory burst onsets; in some structures, the responses were an increase in signal (thalamus, precentral cortex, cerebellar cortex, hippocampus; warm colors), in others, a decrease (cingulate; cool colors) (From Henderson et al. 2006).
Among the areas showing changes in sleep-disordered breathing is the hippocampus-fornix-mammillary body system. The fornix projections from the hippocampus to the mammillary bodies, and the subsequent projections to the anterior thalamus and more-caudal projections form an essential system for short-term memory and for processing spatial orientation (Buckley et al. 2004; Paredes et al. 2000; Santin et al. 1999). The hippocampus shows injury in all three sleep conditions, reflected as hippocampal surface depressions or tissue loss; the fornix fibers are fewer in CCHS and HF, and the mammillary bodies have reduced volumes, preferentially on one side, in all three sleep-disordered breathing conditions; in HF, some of the mammillary bodies are nearly absent (Kumar et al. 2008a; Kumar et al. 2009b; Kumar et al. 2009e); Figure 6. Assessment of certain aspects of this system, including mammillary body volume and fornix fiber count, also directly relate to the severity of depression and anxiety symptoms (Cross et al. 2008; Kumar et al. 2009c).
Axonal Injury
The influences of rostral structures on brainstem respiratory patterning circuitry must be conveyed by axonal systems. All of the sleep-disordered breathing conditions are accompanied by significant axonal injury, and that injury especially appears in limbic structures that convey signals related to affective processes, but also appears in essential motor and blood pressure regulatory fibers, such as pontine projections to the cerebellum. The extent of axonal injury is also prominent in CCHS, which also shows extensive corpus callosum injury, particularly in areas mediating language and ocular control (Kumar et al. 2011a; Kumar et al. 2008b; Kumar et al. 2010). The integrity of the anterior cingulate cortex in mediating affective disorders, blood pressure, and in triggering onset of inspiration is important for function, but actions within those structures must be conveyed to other structures for ultimate influence on respiratory patterning. The integrity of the principal axons within the anterior cingulate cortex, the cingulum bundle, is reduced in OSA, CCHS, and HF (Macey et al. 2008; Kumar et al. 2010; Kumar et al. 2011c) (Figure 7). The fornix fibers, as noted earlier, are especially compromised in CCHS and HF. The fiber injury appears to be preferentially lateralized; for example, pontocerebellar fibers are injured, but are principally lost on one side (Figure 7). The injury in all three conditions extends to the midline raphé system, significant for serotonergic and thyrotropin-releasing hormone (TRH) contributions to function (both serotonin and TRH are generated within the raphé system (Barnes et al. 2010), and fiber loss in HF additionally occurs prominently in the medial forebrain bundle (Figure 3).
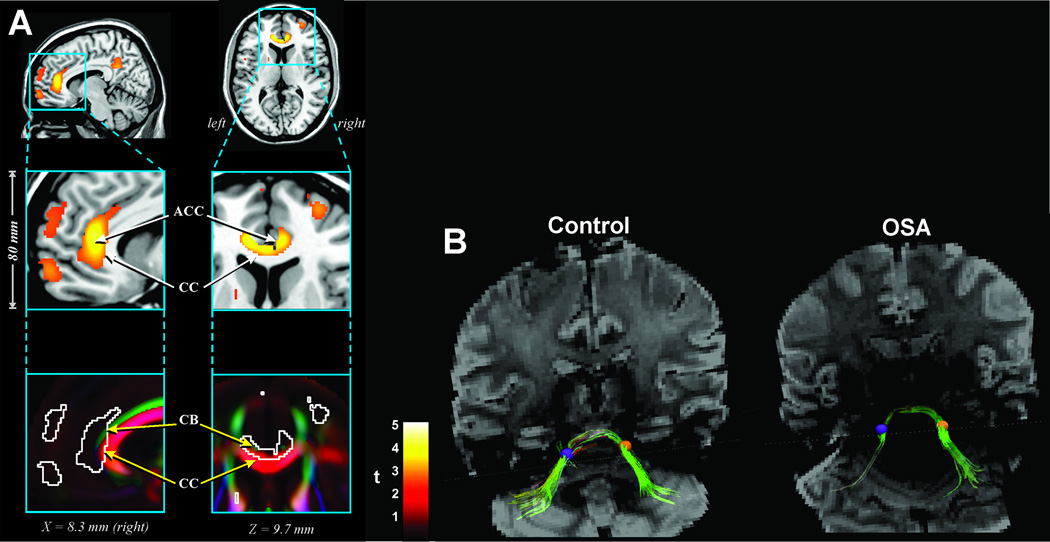
Figure 7.
A: Fractional anisotropy measures in 41 OSA vs 69 control subjects indicating injury in the cingulum bundle fibers and corpus callosum; B: Fiber tracking of pontocerebellar fibers in an OSA subject through identical regions of interest (ROI) (left; purple circle ROI; Right, orange circle ROI) on the left and right side showing the preferential loss of fibers on the left side. ACC= anterior cingulate cortex; CC= corpus callosum, CB= cingulum bundle (A, from Macey et al. 2008).
Any evaluation of axonal or neuronal cell body integrity which may result from hypoxia or from perfusion deficiencies must be concerned with the processes of injury, and mechanisms which interfere with glial function likely contribute to that damage. Assessment of processes that interfere with ATP production, the energy source for Na and K ion pumps, should be part of that examination, since the loss of tissue in structures such as the mammillary bodies has classically been associated with deficiencies in those energy sources for neurons within those structures. Loss of those energy sources most frequently stems from low thiamine and magnesium levels through nutritional deficiencies, interference with enzymatic processes in producing thiamine, or high fluid losses which flush these water-soluble nutrients (Thornalley 2005; Harper 2006; Read 1978). All three of the sleep-disordered breathing conditions considered here show the potential for interference with these processes. HF is accompanied by intestinal malabsorption and typically, very high fluid loss (through diuresis), and thiamine deficiency has been well-demonstrated in this condition (Seligmann et al. 1991; da Cunha et al. 2002; Hanninen et al. 2006). OSA is characterized by profuse sweating with accompanying fluid loss, and CCHS is accompanied by malabsorption and profuse sweating. Although outcomes need to be validated with larger samples, we found at least a subset of OSA adult patients and CCHS children show low thiamine and/or low magnesium levels. The protective effect of thiamine against cell death induced by hypoxia and reperfusion injury, a pattern expected by intermittent hypoxia followed by reperfusion with restoration of breathing following apnea, has been demonstrated in cell culture studies (Shin et al. 2004; Sheline & Wei 2006).
Functional impairment of central structures
The substantial plasticity and redundancy in neural circuitry have the potential to minimize the functional consequences of the structural injury found in sleep-disordered breathing. However, there is now an abundance of evidence that the injury to the central areas result in functionally distorted outcomes of central action in addition to the marked memory, cognitive, affective, and autonomic disturbances found in the condition. The evidence arises from functional magnetic resonance imaging (fMRI) responses to imposed autonomic or ventilatory challenges. FMRI responses to three sequential Valsalva maneuvers show muted and time-distorted responses in the insular cortex and cerebellum in OSA, CCHS, and HF (Henderson et al. 2003; Ogren et al. 2010; Ogren et al. 2012), Figure 8). Impaired cerebellar and limbic responses also appear to cold pressor, CO2, and hypoxic responses in CCHS or OSA subjects (Macey et al. 2005b; Harper et al. 2005; Harper et al. 2003).
Pons and medulla
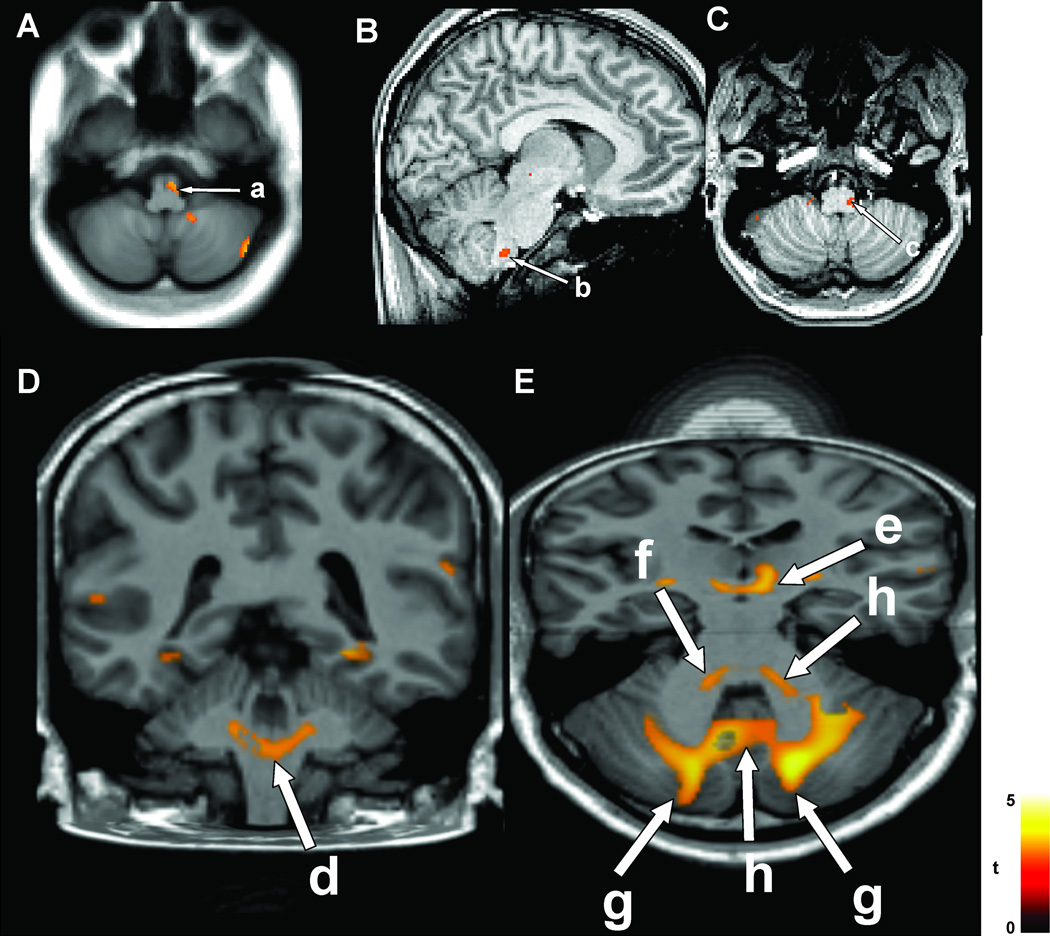
Although affective influences on breathing mainly stem from limbic and other rostral structures, pontine and medullary areas are also affected in all three sleep-disordered breathing conditions. The final common path for much of the control of breathing and blood pressure includes particular medullary nuclei, and especially includes the dorsal and ventrolateral medulla. The ventrolateral medulla in OSA and CCHS, a large extent of the raphé in CCHS, the locus coeruleus in CCHS (Kumar et al. 2008b); verified in CCHS autopsy material (Tomycz et al. 2010), and the dorsal medulla and raphé in HF show injury, as indicated by diffusion tensor imaging measures (Figure 9). The locus coeruleus provides adrenergic innervation and important components of arousal and attention selection (Aston-Jones & Cohen 2005), while the raphé, in addition to its mood and temperature roles, also assists in modulation of upper airway muscle tone (Kubin et al. 1992). The medullary injury in OSA and CCHS is principally unilateral, which poses unique concerns for conditions of high sympathetic tone, since asymmetric sympathetic drive predisposes to certain forms of cardiac arrhythmia (Oppenheimer 2006).
Figure 9.
A: Ventrolateral medulla injury (a) in 23 OSA vs 23 control subjects, based on mean diffusivity; cerebellar injury also appears on right side. B&C: Lateral medullary injury in 12 CCHS vs 26 controls, based on axial diffusivity (b, c). (A from Kumar et al. 2012a, B&C from Kumar et al. 2008b).
At least in developmental animal models, intermittent hypoxia injury can appear in peripheral neural structures, such as vagal afferent innervation of the aortic arch (Ai et al. 2009). The impact of such injury in human conditions has yet to be explored.
Asymmetry of injury
A remarkable aspect of both the functional and structural impairments found in sleep-disordered breathing was the asymmetrical nature of the injuries, an unexpected outcome, if one assumes that hypoxia/ischemia from sleep-disordered breathing conditions exerts effects uniformly across all neural regions. However, that assumption ignores evidence of structural asymmetry in the brain (for review and hypothesis, see: (Vallortigara & Rogers 2005), noted in several pathological conditions, e.g., autism (Escalante-Mead, Minshew & Sweeney 2003), Parkinson’s Disease (Toth, Rajput & Rajput 2004), and schizophrenia (Li et al. 2007), functional lateralization in such actions as sympathetic regulation in the right insular cortex (Oppenheimer 2006), or the well-known primary sensory, motor, and language lateralization in cortical sites, or lateralization of portions of the vasculature; the verterbral arteries, for example, often differ in size, a relationship independent of handedness (Cagnie et al. 2006). Structural insular and medial frontal cortex injuries were preferentially right-sided, mammillary body volume loss was principally left-sided, OSA ventrolateral medullary injury was right-sided, and cerebellar injury showed both unilateral and bilateral representation, depending on sleep-disordered breathing condition (e.g., bilateral in HF of Figure 3C inset, right sided in OSA patients in Figure 9A); an example of the unilaterally-injured axonal projection data in OSA is shown in Figure 7B. Left and right cerebellar responses to a Valsalva challenge were asymmetric in both CCHS and HF (Ogren et al. 2012; Ogren et al. 2010). However, CCHS insular responses to a Valsalva challenge were bilateral (Figure 8), despite the asymmetrical structural injury to that cortical area. The lateralization of responses may arise from several sources, since, as noted, components of the vascular supply to the brain are often asymmetric, with more-perfused sites perhaps being more affected in selected structures with extreme changes in perfusion accompanying apnea. The mechanisms underlying the asymmetry remain speculative, but the very high degree of damage to sympathetic areas, preferentially on the right side (right insula, right ventromedial prefrontal cortex) suggest that evaluation of that autonomic lateralization may provide insights into potential vascular contributions to processes of injury in the condition.
Conclusions
Sleep-disordered breathing is accompanied by significant central nervous system injury; the damaged sites are localized in both rostral and brainstem regions, and especially affected are limbic structures that mediate mood, cognition, and cardiovascular action. The injury appears in both neurons and fibers, and also involves damage to myelin support for fibers. The injury appears to be mediated by intermittent and chronic hypoxia and processes associated with restoration of oxygen to tissue, perhaps assisted by loss of essential nutrients responsible for maintaining ATP in conditions of excitotoxic damage. A commonality of injured structures appears across three sleep-disordered breathing conditions of OSA, CCHS, and HF; impaired functional responses with altered extent and timing of signals confirm the significance of structural damage. The injury likely underlies the impaired autonomic, memory, and executive processing, as well as dyspnea, depression, and anxiety behaviors found in the conditions.
Acknowledgments
This research was supported by R01 HL113251-01 and R01 NR013625-01. We thank Rebecca Harper, Paula Wu, and Bram Birrer for assistance with data collection and analyses.
References
- Agapitou V, Dimopoulos S, Kapelios C, Karatzanos E, Manetos C, Georgantas A, Ntalianis A, Terrovitis J, Karga H, Nanas S. Hormonal imbalance in relation to exercise intolerance and ventilatory inefficiency in chronic heart failure. J Heart Lung Transplant. 2013;32:431–436. doi: 10.1016/j.healun.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Ai J, Wurster RD, Harden SW, Cheng ZJ. Vagal afferent innervation and remodeling in the aortic arch of young-adult fischer 344 rats following chronic intermittent hypoxia. Neuroscience. 2009;164:658–666. doi: 10.1016/j.neuroscience.2009.06.066. [DOI] [PubMed] [Google Scholar]
- Akashiba T, Kawahara S, Akahoshi T, Omori C, Saito O, Majima T, Horie T. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122:861–865. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- Alix JJ, Zammit C, Riddle A, Meshul CK, Back SA, Valentino M, Fern R. Central axons preparing to myelinate are highly sensitivity to ischemic injury. Ann Neurol. 2012;72:936–951. doi: 10.1002/ana.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Babb TL, Brown WJ. In: Surgical treatment of the epilepsies. Engel J Jr, editor. New York: Raven Press; 1987. pp. 511–540. [Google Scholar]
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11:2117–2120. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- Barnes MJ, Rogers RC, Van Meter MJ, Hermann GE. Co-localization of TRHR1 and LepRb receptors on neurons in the hindbrain of the rat. Brain Res. 2010;1355:70–85. doi: 10.1016/j.brainres.2010.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink TS, Hellman KM, Lambert AM, Mason P. Raphe magnus neurons help protect reactions to visceral pain from interruption by cutaneous pain. J Neurophysiol. 2006;96:3423–3432. doi: 10.1152/jn.00793.2006. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Charles DP, Browning PG, Gaffan D. Learning and retrieval of concurrently presented spatial discrimination tasks: role of the fornix. Behav Neurosci. 2004;118:138–149. doi: 10.1037/0735-7044.118.1.138. [DOI] [PubMed] [Google Scholar]
- Burch GE. The role of the central nervous system in chronic congestive heart failure. Am Heart J. 1978;95:255–261. doi: 10.1016/0002-8703(78)90471-4. [DOI] [PubMed] [Google Scholar]
- Cagnie B, Petrovic M, Voet D, Barbaix E, Cambier D. Vertebral artery dominance and hand preference: is there a correlation? Man Ther. 2006;11:153–156. doi: 10.1016/j.math.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Hachinski V. Cardiovascular consequence of experimental stroke. Baillieres Clin Neurol. 1997;6:297–308. [PubMed] [Google Scholar]
- Chokroverty S, Sachdeo R, Masdeu J. Autonomic dysfunction and sleep apnea in olivopontocerebellar degeneration. Arch Neurol. 1984;41:926–931. doi: 10.1001/archneur.1984.04050200032014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Cross RL, Kumar R, Macey PM, Doering LV, Alger JR, Yan-Go FL, Harper RM. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31:1103–1109. [PMC free article] [PubMed] [Google Scholar]
- da Cunha S, Albanesi Filho FM, da Cunha Bastos VL, Antelo DS, Souza MM. Thiamin, selenium, and copper levels in patients with idiopathic dilated cardiomyopathy taking diuretics. Arq Bras Cardiol. 2002;79:454–465. doi: 10.1590/s0066-782x2002001400003. [DOI] [PubMed] [Google Scholar]
- Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax. 1982;37:840–844. doi: 10.1136/thx.37.11.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Mead PR, Minshew NJ, Sweeney JA. Abnormal brain lateralization in high-functioning autism. J Autism Dev Disord. 2003;33:539–543. doi: 10.1023/a:1025887713788. [DOI] [PubMed] [Google Scholar]
- Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, Darcey V, Kuna ST, Sleep ARG. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DP, Willson K, Davies LC, Coats AJ, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102:2214–2221. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- Frysinger RC, Harper RM. Cardiac and respiratory relationships with neural discharge in the anterior cingulate cortex during sleep-walking states. Exp Neurol. 1986;94:247–263. doi: 10.1016/0014-4886(86)90100-7. [DOI] [PubMed] [Google Scholar]
- Hanninen SA, Darling PB, Sole MJ, Barr A, Keith ME. The prevalence of thiamin deficiency in hospitalized patients with congestive heart failure. J Am Coll Cardiol. 2006;47:354–361. doi: 10.1016/j.jacc.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Harper C. Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur J Neurol. 2006;13:1078–1082. doi: 10.1111/j.1468-1331.2006.01530.x. [DOI] [PubMed] [Google Scholar]
- Harper RM, Frysinger RC, Trelease RB, Marks JD. State-dependent alteration of respiratory cycle timing by stimulation of the central nucleus of the amygdala. Brain Res. 1984;306:1–8. doi: 10.1016/0006-8993(84)90350-0. [DOI] [PubMed] [Google Scholar]
- Harper RM, Kumar R, Macey PM, Ogren JA, Richardson HL. Functional neuroanatomy and sleep-disordered breathing: implications for autonomic regulation. Anat Rec. 2012;295:1385–1395. doi: 10.1002/ar.22514. [DOI] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Henderson LA, Woo MA, Macey KE, Frysinger RC, Alger JR, Nguyen KP, Yan-Go FL. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol. 2003;94:1583–1595. doi: 10.1152/japplphysiol.00881.2002. [DOI] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Kumar R, Woo MA. Neural Injury in Diabetic Versus Non-Diabetic Obstructive Sleep Apnea Patients: A Pilot Study. Sleep. 2009;32:A341–A341. [Google Scholar]
- Harper RM, Macey PM, Woo MA, Macey KE, Keens TG, Gozal D, Alger JR. Hypercapnic exposure in congenital central hypoventilation syndrome reveals CNS respiratory control mechanisms. J Neurophysiol. 2005;93:1647–1658. doi: 10.1152/jn.00863.2004. [DOI] [PubMed] [Google Scholar]
- Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Macey KE, Macey PM, Woo MA, Yan-Go FL, Harper RM. Regional brain response patterns to Cheyne-Stokes breathing. Respir Physiol Neurobiol. 2006;150:87–93. doi: 10.1016/j.resp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Rubin TK, Macefield VG. Within-limb somatotopic representation of acute muscle pain in the human contralateral dorsal posterior insula. Hum Brain Mapp. 2011;32:1592–1601. doi: 10.1002/hbm.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Woo MA, Macey PM, Macey KE, Frysinger RC, Alger JR, Yan-Go F, Harper RM. Neural responses during Valsalva maneuvers in obstructive sleep apnea syndrome. J Appl Physiol. 2003;94:1063–1074. doi: 10.1152/japplphysiol.00702.2002. [DOI] [PubMed] [Google Scholar]
- Holstege G, Bandler R, Saper CB. The emotional motor system. Prog Brain Res. 1996;107:3–6. doi: 10.1016/s0079-6123(08)61855-5. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Kontoleon PE, Anastasiou-Nana MI, Papapetrou PD, Alexopoulos G, Ktenas V, Rapti AC, Tsagalou EP, Nanas JN. Hormonal profile in patients with congestive heart failure. Int J Cardiol. 2003;87:179–183. doi: 10.1016/s0167-5273(02)00212-7. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Kumar R, Ahdout R, Macey PM, Woo MA, Avedissian C, Thompson PM, Harper RM. Reduced caudate nuclei volumes in patients with congenital central hypoventilation syndrome. Neuroscience. 2009a;163:1373–1379. doi: 10.1016/j.neuroscience.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, Harper RM. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett. 2008a;438:330–334. doi: 10.1016/j.neulet.2008.04.071. [DOI] [PubMed] [Google Scholar]
- Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012a;90:2043–2052. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Lee K, Macey PM, Woo MA, Harper RM. Mammillary body and fornix injury in congenital central hypoventilation syndrome. Pediatr Res. 2009b;66:429–434. doi: 10.1203/PDR.0b013e3181b3b363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Cross RL, Woo MA, Yan-Go FL, Harper RM. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety. 2009c;26:480–491. doi: 10.1002/da.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome. J Magn Reson Imaging. 2006;24:1252–1258. doi: 10.1002/jmri.20759. [DOI] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr Res. 2008b;64:275–280. doi: 10.1203/PDR.0b013e31817da10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Keens TG, Harper RM. Neuroanatomic deficits in congenital central hypoventilation syndrome. J Comp Neurol. 2005;487:361–371. doi: 10.1002/cne.20565. [DOI] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Harper RM. Rostral brain axonal injury in congenital central hypoventilation syndrome. J Neurosci Res. 2010;88:2146–2154. doi: 10.1002/jnr.22385. [DOI] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Harper RM. Selectively diminished corpus callosum fibers in congenital central hypoventilation syndrome. Neuroscience. 2011a;178:261–269. doi: 10.1016/j.neuroscience.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM. Dilated basilar arteries in patients with congenital central hypoventilation syndrome. Neurosci Lett. 2009d;467:139–143. doi: 10.1016/j.neulet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Nguyen HD, Ogren JA, Macey PM, Thompson PM, Fonarow GC, Hamilton MA, Harper RM, Woo MA. Global and regional putamen volume loss in patients with heart failure. Eur J Heart Fail. 2011b;13:651–655. doi: 10.1093/eurjhf/hfr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Woo MA, Birrer BV, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Mammillary bodies and fornix fibers are injured in heart failure. Neurobiol Dis. 2009e;33:236–242. doi: 10.1016/j.nbd.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011c;307:106–113. doi: 10.1016/j.jns.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Woo MS, Macey PM, Woo MA, Harper RM. Progressive gray matter changes in patients with congenital central hypoventilation syndrome. Pediatr Res. 2012b;71:701–706. doi: 10.1038/pr.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Li X, Branch CA, Ardekani BA, Bertisch H, Hicks C, DeLisi LE. fMRI study of language activation in schizophrenia, schizoaffective disorder and in individuals genetically at high risk. Schizophr Res. 2007;96:14–24. doi: 10.1016/j.schres.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey KE, Macey PM, Woo MA, Harper RK, Alger JR, Keens TG, Harper RM. fMRI signal changes in response to forced expiratory loading in congenital central hypoventilation syndrome. J Appl Physiol. 2004;97:1897–1907. doi: 10.1152/japplphysiol.00359.2004. [DOI] [PubMed] [Google Scholar]
- Macey KE, Macey PM, Woo MA, Henderson LA, Frysinger RC, Harper RK, Alger JR, Yan-Go F, Harper RM. Inspiratory loading elicits aberrant fMRI signal changes in obstructive sleep apnea. Respir Physiol Neurobiol. 2006;151:44–60. doi: 10.1016/j.resp.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Macey PM, Alger JR, Kumar R, Macey KE, Woo MA, Harper RM. Global BOLD MRI changes to ventilatory challenges in congenital central hypoventilation syndrome. Respir Physiol Neurobiol. 2003a;139:41–50. doi: 10.1016/j.resp.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Ogren JA, Woo MA, Harper RM. Images in sleep medicine. Altered cerebral blood flow in a patient with congenital central hypoventilation syndrome. Sleep Med. 2010a;11:589–590. doi: 10.1016/j.sleep.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–977. [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Yan-Go FL, Woo MA, Harper RM. Sex differences in white matter alterations accompanying obstructive sleep apnea. Sleep. 2012a;35:1603–1613. doi: 10.5665/sleep.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Henderson LA, Alger JR, Frysinger RC, Woo MA, Yan-Go F, Harper RM. Functional magnetic resonance imaging responses to expiratory loading in obstructive sleep apnea. Respir Physiol Neurobiol. 2003b;138:275–290. doi: 10.1016/j.resp.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Woo MA, Keens TG, Harper RM. Aberrant neural responses to cold pressor challenges in congenital central hypoventilation syndrome. Pediatr Res. 2005a;57:500–509. doi: 10.1203/01.PDR.0000155757.98389.53. [DOI] [PubMed] [Google Scholar]
- Macey PM, Moiyadi AS, Kumar R, Woo MA, Harper RM. Decreased cortical thickness in central hypoventilation syndrome. Cereb Cortex. 2012b;22:1728–1737. doi: 10.1093/cercor/bhr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Richard CA, Kumar R, Woo MA, Ogren JA, Avedissian C, Thompson PM, Harper RM. Hippocampal volume reduction in congenital central hypoventilation syndrome. PLoS One. 2009;4:e6436. doi: 10.1371/journal.pone.0006436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Woo MA, Kumar R, Cross RL, Harper RM. Relationship between obstructive sleep apnea severity and sleep, depression and anxiety symptoms in newly-diagnosed patients. PLoS One. 2010b;5:e10211. doi: 10.1371/journal.pone.0010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, Harper RM. Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic deficits in congenital central hypoventilation syndrome. J Appl Physiol. 2005b;98:958–969. doi: 10.1152/japplphysiol.00969.2004. [DOI] [PubMed] [Google Scholar]
- Macey PM, Wu P, Kumar R, Ogren JA, Richardson HL, Woo MA, Harper RM. Differential responses of the insular cortex gyri to autonomic challenges. Auton Neurosci. 2012c;168:72–81. doi: 10.1016/j.autneu.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G. Paleocerebellar inhibition of vasomotor and respiratory carotid sinus reflexes. J Neurophysiol. 1940;3:20–32. [Google Scholar]
- Mueller SG, Laxer KD, Schuff N, Weiner MW. Voxel-based T2 relaxation rate measurements in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2007;48:220–228. doi: 10.1111/j.1528-1167.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murberg TA, Bru E, Aarsland T, Svebak S. Functional status and depression among men and women with congestive heart failure. Int J Psychiatry Med. 1998;28:273–291. doi: 10.2190/8TRC-PX8R-N498-7BTP. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Charney DS, Drevets WC. Hippocampus, VI. Depression and the Hippocampus. Am J Psychiatry. 2005;162:1057. doi: 10.1176/appi.ajp.162.6.1057. [DOI] [PubMed] [Google Scholar]
- Ni H, Zhang J, Glotzbach SF, Schechtman VL, Harper RM. Dynamic respiratory responses to preoptic/anterior hypothalamic warming in the sleeping cat. Sleep. 1994;17:657–664. doi: 10.1093/sleep/17.8.657. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Macey PM, Kumar R, Fonarow GC, Hamilton MA, Harper RM, Woo MA. Impaired cerebellar and limbic responses to the valsalva maneuver in heart failure. Cerebellum. 2012;11:931–938. doi: 10.1007/s12311-012-0361-y. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Macey PM, Kumar R, Woo MA, Harper RM. Central autonomic regulation in congenital central hypoventilation syndrome. Neuroscience. 2010;167:1249–1256. doi: 10.1016/j.neuroscience.2010.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S. Cerebrogenic cardiac arrhythmias: cortical lateralization and clinical significance. Clin Auton Res. 2006;16:6–11. doi: 10.1007/s10286-006-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Hachinski VC. The cardiac consequences of stroke. Neurol Clin. 1992;10:167–176. [PubMed] [Google Scholar]
- Orem J. The nature of the wakefulness stimulus for breathing. Prog Clin Biol Res. 1990;345:23–30. discussion 31. [PubMed] [Google Scholar]
- Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375:123–128. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- Pallayova M, Donic V, Donicova V, Tomori Z, Gresova S. The effect of continuous positive airway pressure on glucose excursions in diabetics with sleep-disordered breathing: the results of continuous glucose monitoring. Eur Respir Rev. 2006;15:218–220. [Google Scholar]
- Paredes J, Winters RW, Schneiderman N, McCabe PM. Afferents to the central nucleus of the amygdala and functional subdivisions of the periaqueductal gray: neuroanatomical substrates for affective behavior. Brain Res. 2000;887:157–173. doi: 10.1016/s0006-8993(00)02972-3. [DOI] [PubMed] [Google Scholar]
- Patwari PP, Carroll MS, Rand CM, Kumar R, Harper R, Weese-Mayer DE. Congenital central hypoventilation syndrome and the PHOX2B gene: a model of respiratory and autonomic dysregulation. Respir Physiol Neurobiol. 2010;173:322–335. doi: 10.1016/j.resp.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med. 2001;163:951–957. doi: 10.1164/ajrccm.163.4.2005057. [DOI] [PubMed] [Google Scholar]
- Read DJ. The aetiology of the sudden infant death syndrome: current ideas on breathing and sleep and possible links to deranged thiamine neurochemistry. Aust N Z J Med. 1978;8:322–336. doi: 10.1111/j.1445-5994.1978.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Golanov EV. Autonomic and vasomotor regulation. Int Rev Neurobiol. 1997;41:121–149. doi: 10.1016/s0074-7742(08)60350-5. [DOI] [PubMed] [Google Scholar]
- Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- Santin LJ, Rubio S, Begega A, Arias JL. Effects of mammillary body lesions on spatial reference and working memory tasks. Behav Brain Res. 1999;102:137–150. doi: 10.1016/s0166-4328(99)00011-x. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Saunamaki T, Jehkonen M. Depression and anxiety in obstructive sleep apnea syndrome: a review. Acta Neurol Scand. 2007;116:277–288. doi: 10.1111/j.1600-0404.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- Seligmann H, Halkin H, Rauchfleisch S, Kaufmann N, Motro M, Vered Z, Ezra D. Thiamine deficiency in patients with congestive heart failure receiving long-term furosemide therapy: a pilot study. Am J Med. 1991;91:151–155. doi: 10.1016/0002-9343(91)90007-k. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Wei L. Free radical-mediated neurotoxicity may be caused by inhibition of mitochondrial dehydrogenases in vitro and in vivo. Neuroscience. 2006;140:235–246. doi: 10.1016/j.neuroscience.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Shin BH, Choi SH, Cho EY, Shin MJ, Hwang KC, Cho HK, Chung JH, Jang Y. Thiamine attenuates hypoxia-induced cell death in cultured neonatal rat cardiomyocytes. Mol Cells. 2004;18:133–140. [PubMed] [Google Scholar]
- Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol. 1983;55:813–822. doi: 10.1152/jappl.1983.55.3.813. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, Matthews K. The insular cortex and the neuroanatomy of major depression. J Affect Disord. 2011;133:120–127. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Tamura A, Kawano Y, Watanabe T, Kadota J. Obstructive sleep apnea increases hemoglobin A1c levels regardless of glucose tolerance status. Sleep Med. 2012;13:1050–1055. doi: 10.1016/j.sleep.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev. 2005;1:287–298. doi: 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- Tomycz ND, Haynes RL, Schmidt EF, Ackerson K, Kinney HC. Novel neuropathologic findings in the Haddad syndrome. Acta Neuropathol. 2010;119:261–269. doi: 10.1007/s00401-009-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C, Rajput M, Rajput AH. Anomalies of asymmetry of clinical signs in parkinsonism. Mov Disord. 2004;19:151–157. doi: 10.1002/mds.10685. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil DC, Al-Mefty O, Yasargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–733. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38:199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. discussion 589–633. [DOI] [PubMed] [Google Scholar]
- Vanderlaan M, Holbrook CR, Wang M, Tuell A, Gozal D. Epidemiologic survey of 196 patients with congenital central hypoventilation syndrome. Pediatr Pulmonol. 2004;37:217–229. doi: 10.1002/ppul.10438. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O'Hearn E, Molliver ME, Aicher SA. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–359. [PubMed] [Google Scholar]
- Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15:214–223. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, Harper RM. Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail. 2005a;11:437–446. doi: 10.1016/j.cardfail.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, Harper RM. Aberrant central nervous system responses to the Valsalva maneuver in heart failure. Congest Heart Fail. 2007;13:29–35. doi: 10.1111/j.1527-5299.2007.05856.x. [DOI] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Macey KE, Keens TG, Woo MS, Harper RK, Harper RM. FMRI responses to hyperoxia in congenital central hypoventilation syndrome. Pediatr Res. 2005b;57:510–518. doi: 10.1203/01.PDR.0000155763.93819.46. [DOI] [PubMed] [Google Scholar]
- Wu P, Macey PM, Kumar R, Harper RM. Axonal projections between insular gyri and the hypothalamus. Soc Neurosci Abs. 2010:694.7. [Google Scholar]
- Xu F, Frazier DT. Respiratory-related neurons of the fastigial nucleus in response to chemical and mechanical challenges. J Appl Physiol. 1997;82:1177–1184. doi: 10.1152/jappl.1997.82.4.1177. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum. 2002;1:35–40. doi: 10.1080/147342202753203078. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Harper RM, Frysinger RC. Respiratory modulation of neuronal discharge in the central nucleus of the amygdala during sleep and waking states. Exp Neurol. 1986;91:193–207. doi: 10.1016/0014-4886(86)90037-3. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Dougherty PM, Oppenheimer SM. Characterization of baroreceptor-related neurons in the monkey insular cortex. Brain Res. 1998;796:303–306. doi: 10.1016/s0006-8993(98)00268-6. [DOI] [PubMed] [Google Scholar]