Abstract
Objective
T cells, particularly CD8+ T cells, are major participants in obesity-linked adipose tissue (AT) inflammation. We examined the mechanisms of CD8+ T-cell accumulation and activation in AT and the role of CD11a, a β2 integrin.
Approach and Results
CD8+ T cells in AT of obese mice showed activated phenotypes with increased proliferation and interferon-γ expression. In vitro, CD8+ T cells from mouse AT displayed increased interferon-γ expression and proliferation to stimulation with interleukin-12 and interleukin-18, which were increased in obese AT. CD11a was upregulated in CD8+ T cells in obese mice. Ablation of CD11a in obese mice dramatically reduced T-cell accumulation, activation, and proliferation in AT. Adoptive transfer showed that CD8+ T cells from wild-type mice, but not from CD11a-deficient mice, infiltrated into AT of recipient obese wild-type mice. CD11a deficiency also reduced tumor necrosis factor-α–producing and interleukin-12–producing macrophages in AT and improved insulin resistance.
Conclusions
Combined action of cytokines in obese AT induces proliferative response of CD8+ T cells locally, which, along with increased infiltration, contributes to CD8+ T-cell accumulation and activation in AT. CD11a plays a crucial role in AT inflammation by participating in T-cell infiltration and activation.
Keywords: adipose tissue, inflammation, insulin resistance, obesity
Obesity increases risk for type 2 diabetes mellitus and cardiovascular disease. Adipose tissue (AT) inflammation occurs in obesity and may link obesity and the related diseases.1–4 Initial studies indicated that macrophages were responsible for most inflammatory events in AT.5–8 More recent studies showed that T lymphocytes, especially CD8+ T cells, also accumulate in AT with activated phenotypes in obesity and participate in AT inflammation.9–14 The most recent studies showed a role of major histocompatibility complex class II on macrophages/dendritic cells (DCs) or adipocytes in the activation of CD4+ T cells in AT.15,16 However, it remains incompletely understood how CD8+ T cells, which showed a greater increase in AT in obesity,9,15 accumulate in AT and become activated.
During adaptive immunity, T cells become activated and proliferate in lymphoid organs when encountering antigens presented by antigen-presenting cells. After activation, T cells arrive at the site of inflammation and exert active roles in peripheral tissues.17,18 AT CD4+ T cells may become activated and proliferate in major histocompatibility complex class II–dependent and antigen-dependent manners.15,16 However, little information is available about obesity-related/specific antigens.16 In addition to their role in adaptive immunity, memory T cells, CD8+ T cells in particular, are also involved in innate immunity, becoming activated and proliferating under cytokine stimulation in the absence of antigens.19–21
T-cell recruitment, which is controlled by the combination of adhesion molecules and chemokines/receptors, is crucial for T-cell circulation among lymphoid organs and peripheral tissues.22 Lymphocyte function antigen-1 (LFA-1) is a β2-integrin expressed on T cells and other leukocytes and composed of a distinct α chain (CD11a) and a shared β chain with other β2 integrins.22 LFA-1 plays crucial roles in lymphocyte transendothelial migration and in immunologic synapse and T-cell activation through interaction with intercellular adhesion molecule-1 on endothelial cells or antigen-presenting cells.23 Because of its multiple roles in T-cell functions, LFA-1 has been an attractive target for immunosuppressive therapy, including prevention of inflammation and organ graft rejection.24,25 Nevertheless, a potential role of LFA-1 in obesity-linked AT inflammation has never been reported.
In the present study, we confirmed CD8+ T-cell accumulation with activated phenotypes in AT of obese mice. Further studies revealed that AT CD8+ T cells, most of which are memory T cells, can be activated and proliferate in vitro under stimulation of T-helper 1/T-cytotoxic 1 (Th1/Tc1)–polarizing cytokines that are increased in AT of obese mice. The proportions of CD11ahigh/CD8+ T cells were increased in obese mice. CD11a deficiency in obese mice markedly reduced T-cell accumulation and activation in AT. We further found that CD8+ T cells from wild-type (WT) mice, but not from CD11adeficient mice, infiltrated into AT of recipient obese WT mice. Finally, CD11a-deficient mice were protected from obesity-induced insulin resistance. Thus, our study provided more supporting data for the potential mechanisms of CD8+ T-cell accumulation and activation in AT in obesity and showed a crucial role of LFA-1 in CD8+ T-cell–related AT inflammation and metabolic dysfunctions associated with obesity.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Obese Mice Show Increased Activated CD8+ T Cells in AT
Fluorescence-activated cell sorter analysis of stromal/vascular cells (S/Vs) indicated that, compared with lean mice, obese mice had increased numbers of total T cells, CD4+ T cells, and CD8+ T cells in AT, with a greater increase in CD8+ T cells (Figure IA in the online-only Data Supplement), consistent with previous studies.9–12 Next, we focused on studying CD8+ T cells in AT. Compared with those in lean mice, the proportions of CD44+/CD62L− effector memory (TEM)/effector T (TE) cells and of CD44+/CD69+ activated T cells in the AT CD8+ T-cell population were higher in obese mice (Figure 1A and 1B). Consistently, the proportion of interferon-γ (IFN-γ)–producing CD8+ T cells and mRNA levels of IFN-γ, granzyme B, and interleukin-2 (IL-2), molecules expressed by activated T cells, were increased in AT of obese mice (Figure 1C and 1D). These results indicate that CD8+ T cells not only increased in number but also displayed activated phenotypes with polarization to Tc1 in AT of obese mice. Tc1/Th1-polarizing cytokines including IL-12, IL-18, and IL-15, produced primarily by macrophages/DCs,20 were also elevated in AT of obese mice (Figure 1D and Figure IB and IC in the online-only Data Supplement).
Figure 1.
T cells and T-cytotoxic 1 (Tc1)/T-helper 1 (Th1)– associated genes in adipose tissue (AT) of obese and lean mice. A, Proportions of CD44+/CD62L− effector memory T (TEM)/effector T (TE) cells in total CD8+ T cells from AT of lean and obese mice (n=10/group). B, Proportions of CD44+/CD69+ activated T cells in total CD8+ T cells from AT of lean and obese mice (n=4–9/group). C, Interferon γ (IFN-γ)–producing CD8+ T cells in AT of lean and obese mice (n=3–5/group). D, mRNA levels of Tc1/Th1-associated genes in AT of obese and lean mice measured by quantitative reverse transcriptase-polymerase chain reaction (n=5–8/group). E, Effect of coculture with AT from lean or obese mice (without or with neutralization of interleukin-12 [IL-12] and IL-18) on CD69 expression in CD8+ T cells from AT of lean mice (n=3/group). F, Effect of cytokine stimulation on IFN-γ production by CD8+ T cells from AT of lean mice (n=3–5/group). *P<0.05, **P<0.01, ***P<0.001 vs lean controls.
CD8+ T Cells From AT of Lean Mice Can Be Activated With Cytokines Alone In Vitro
Most of the AT CD8+ T cells including those in lean mice were CD44+ memory T cells (Figure 1A). Previous studies showed innate immune response of memory T cells to cytokine stimulation.19–21 Increased IL-12, IL-18, and IL-15 levels in AT of obese mice suggested a Tc1/Th1-polarizing milieu. Coculture with AT of obese mice increased CD69 expression on CD8+ T cells from AT of lean mice, whereas adding anti–IL-12– and anti–IL-18–neutralizing antibodies inhibited obese AT-induced CD69 expression (Figure 1E). When cultured with IL-2 alone, few CD8+ T cells from AT of lean mice expressed IFN-γ. In contrast, combining IL-12 and IL-18 significantly increased IFN-γ expression, and adding IL-2 to IL-12 and IL-18 further increased IFN-γ expression in CD8+ T cells from AT of lean mice (Figure 1F). These data indicate that in obesity, the combined action of cytokines secreted by AT can activate CD8+ T cells locally in AT.
CD8+ TEM/TE Cells Proliferate in AT in Obesity
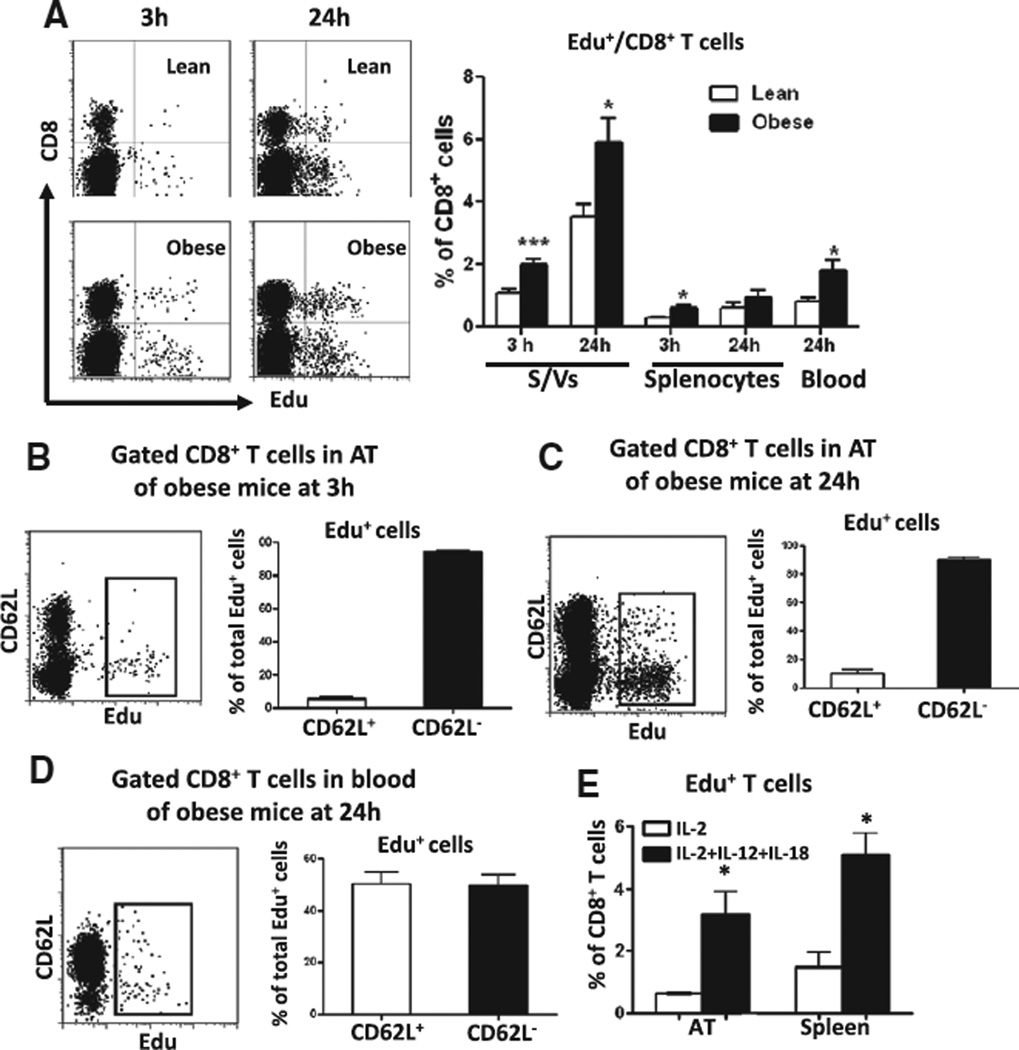
T-cell activation requires cell cycle entry and proliferation. At 3 hours after Edu injection, Edu+/CD8+ T cells appeared in spleen but not in blood (Figure II in the online-only Data Supplement). At the same time, we observed Edu+/CD8+ T cells in S/Vs of AT (Figure 2A), indicating that CD8+ T cells proliferated in AT (and spleen). The proportion of proliferating CD8+ T cells was significantly higher in AT of obese mice than of lean mice and also higher in AT than in splenocytes (Figure 2A).
Figure 2.
T-cell proliferation in mouse adipose tissue (AT). A, Left, Representative fluorescence-activated cell sorter (FACS) dot plots showing Edu+ T cells (in gated CD3+ cells) in stromal/vascular cells (S/Vs) of lean and obese mice at 3 and 24 hours after Edu injection. Right, Quantification of the proportions of Edu+/CD8+ T cells in total CD8+ T cells in S/Vs, splenocytes, and blood (n=3–9/group). FACS analysis of CD62L on gated CD8+ T cells in S/Vs of obese mice at 3 hours (B) and 24 hours (C) after Edu injection (n=3/group). D, CD62L expression on gated CD8+ T cells in blood of obese mice at 24 hours after Edu injection (n=3/group). E, Effect of cytokine stimulation on proliferation of AT or splenic CD8+ T cells in vitro. *P≤0.05, ***P<0.001 vs lean controls or interleukin-2 (IL-2) alone.
Edu+ T cells started to appear in blood 6 hours after Edu injection, indicating T-cell release from lymphoid organs. At 24 hours, ≤2% of CD8+ T cells in blood were Edu+ (Figure 2A and Figure II in the online-only Data Supplement), and we observed a higher proportion of Edu+/CD8+ T cells in S/Vs (Figure 2A), which may be because of more CD8+ T cells undergoing proliferation in AT and also infiltration of blood (Edu+) CD8+ T cells as confirmed by our subsequent T-cell adoptive transfer study. Compared with lean mice, obese mice also had higher proportions of Edu+/CD8+ T cells in S/Vs at 24 hours after injection (Figure 2A).
At 3 hours after Edu injection, ≈95% of proliferating CD8+ cells in AT of obese mice were CD62L− (Figure 2B), consistent with TEM/TE phenotypes. At 24 hours after injection, the proportion of CD62L+ Edu+/CD8+ T cells increased (Figure 2C; 10.1±1.2% at 24 hours versus 5.7±0.7% at 3 hours; P<0.05), perhaps suggesting infiltration of blood CD62L+CD8+ T cells, which constituted ≈50% of blood Edu+/CD8+ T cells (Figure 2D). In vitro, compared with IL-2 alone, combined IL-2, IL-12, and IL-18 increased the proliferation rate of AT CD8+ T cells and also that of CD8+ T cells from splenocytes (Figure 2E).
Collectively, these data suggest that AT CD8+ T cells can be activated and proliferate locally under the stimulation of AT-secreted cytokines and that the local cytokine-induced activation and proliferation may contribute to accumulation of activated CD8+ T cells in AT in obesity.
Proportions of CD11ahigh/CD8+ T Cells Are Increased in Obese Mice
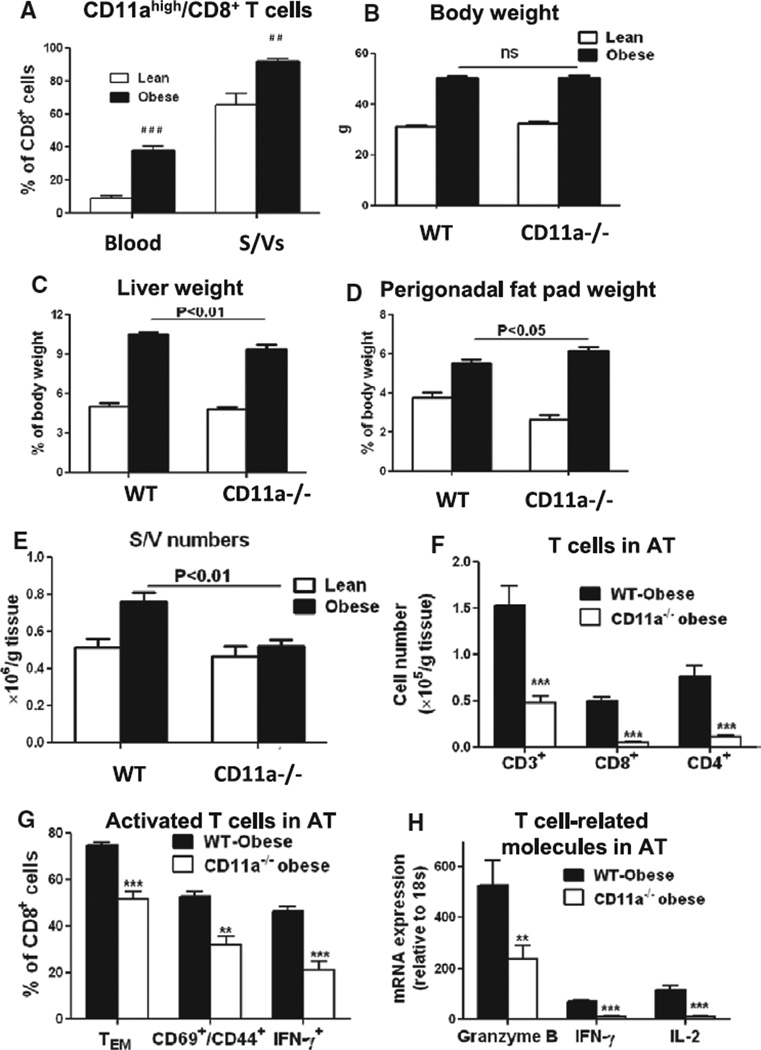
To examine further the mechanisms of T-cell accumulation and activation in AT, we next focused on CD11a. Based on CD11a levels, we found 2 major CD8+ T-cell populations, CD11ahigh and CD11aint, in both blood and S/Vs (Figure IIIA and IIIB in the online-only Data Supplement). Compared with leans, obese mice (on high-fat diet [HFD] for 16 weeks) showed significant increases in the proportion of CD11ahigh/CD8+ T cells in blood and S/Vs (Figure 3A). Furthermore, although CD11ahigh/CD8+ T cells in S/Vs of lean mice contained CD62L− and CD62L+ cells, CD62L− cells dominated the CD11ahigh/CD8+ T-cell population in S/Vs of obese mice (Figure IIIB in the online-only Data Supplement). All proliferating CD8+ T cells in AT of obese mice were also CD11ahigh (Figure IIIC in the online-only Data Supplement). A time course study indicated that proportions of CD11ahigh/CD8+ T cells in blood tended to increase at 8 weeks and increased significantly at 12 weeks after HFD (Figure IIID in the online-only Data Supplement).
Figure 3.
CD11a and T-cell–related inflammation in adipose tissue (AT) of obese mice. A, Quantification of the proportions of CD11ahigh/CD8+ T cells in blood and stromal/vascular cells (S/Vs) of lean and obese wild-type (WT) mice (n=4–6/group). # #P<0.01, # # #P<0.001 vs lean controls. B, Body weight and ratios of (C) liver and (D) perigonadal fat pad weights to body weight of lean and obese WT and CD11a−/− mice (on chow diet or high-fat diet for 16 weeks; n=15–20 mice/group). E, Numbers of S/Vs per gram AT of lean and obese WT and CD11a−/− mice (n=15–19 mice/group). F, Numbers of total CD3+ T cells, CD8+ cells, and CD4+ T cells per gram AT of obese WT and CD11a−/− mice examined by fluorescence-activated cell sorter analysis (n=6–8 mice/group). G, Proportions of CD44+/CD62L− effector T (TE) and effector memory T (TEM) cells, CD69+/CD44+ T cells, and interferon γ (IFN-γ)–expressing T cells in CD8+ T cells in S/Vs of obese WT and CD11a−/− mice (n=5–13/group). H, mRNA levels of T-cytotoxic 1 (Tc1)/T-helper 1 (Th1)– associated genes in AT of obese WT and CD11a−/− mice measured by quantitative reverse transcriptase-polymerase chain reaction (n=6–7/group). **P<0.01, ***P<0.001 vs obese WT.
CD11a−/− Mice on HFD Show Normal Weight Gain But Decreased T-Cell Content and Activation in AT
Compared with WT mice, CD11a−/− mice had similar body weight on chow diet and showed normal weight gain after HFD (Figure IVA in the online-only Data Supplement and Figure 3B). However, obese CD11a−/− mice had smaller livers but larger perigonadal fat pads than obese WT mice (Figure 3C and 3D).
Total S/V numbers per gram AT were increased in obese WT, but not in obese CD11a−/− mice, compared with their lean counterparts and were significantly lower in obese CD11a−/− than in obese WT (Figure 3E). Concomitantly, numbers of crown-like structures, which consisted of macrophages/DCs and T cells,5,9 per field of AT section and average cell numbers per crown-like structure were lower in obese CD11a−/− mice than in obese WT mice (Figure IVB and IVC in the online-only Data Supplement). Fluorescence-activated cell sorter analysis indicated that the relative ratio of T cells, but not macrophages/DCs, in total S/Vs was decreased in obese CD11a−/− mice compared with obese WT mice (Figure IVD in the online-only Data Supplement).
Compared with obese WT, obese CD11a−/− mice showed dramatic reductions in the numbers of CD4+, CD8+, and total T cells per gram AT (Figure 3F). Furthermore, the proportions of activated T cells, including CD62L−/CD44+ TE/TEM cells, CD44+/CD69+ cells, and IFN-γ–producing cells, in AT CD8+ T cells and mRNA levels of T-cell activation markers, including granzyme B, IFN-γ, and IL-2, in AT were also lower in obese CD11a−/− mice than in obese WT (Figure 3G and 3H). These results suggest that CD11a deficiency in obese mice not only decreased T-cell numbers but also suppressed T-cell (CD8+) activation in AT.
In contrast, γδT cell numbers in AT and numbers of total T cells and CD8+ T cells in blood were similar between CD11a−/− and WT mice (Figure VA and VB in the online-only Data Supplement). The proportions of CD8+ CD62L−/CD44+ TE/TEM cells in blood and spleen were low and were not affected by CD11a deficiency (Figure VI in the online-only Data Supplement).
CD11a Is Critical for CD8+ T-Cell Infiltration and Proliferation in AT of Obese Mice
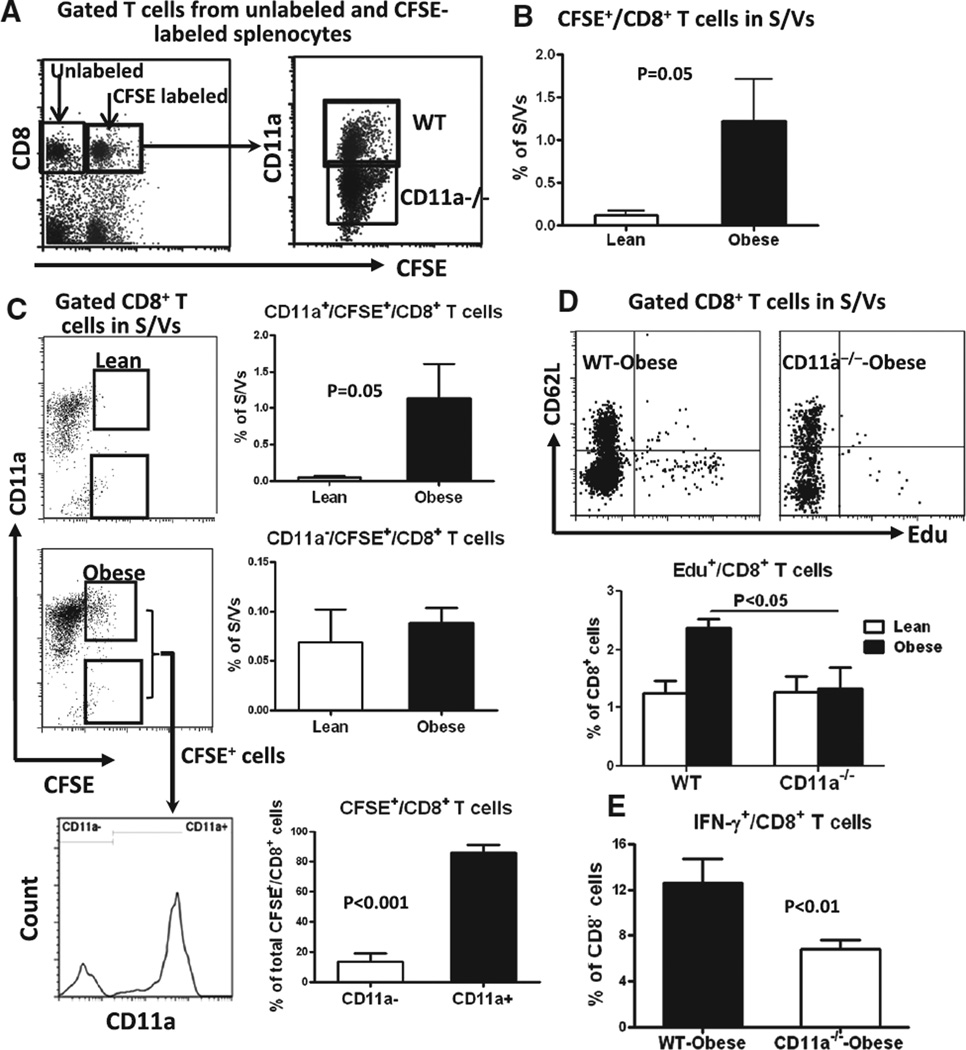
Competitive homing assay showed that at 6 hours after injection of mixed carboxyfluorescein succinimidyl ester (CFSE)-labeled splenocytes from CD11a−/− and WT mice (Figure 4A) into recipient WT mice, CFSE+/CD8+ T cells appeared in AT of recipient mice, with more CFSE+/CD8+ T cells observed in AT of obese recipients than of lean recipients (Figure 4B and 4C). The increases in CFSE+/CD8+ T cells in AT of obese recipients were because of increases in CD11a+/CFSE+ T cells (from WT donors) but not CD11a−/CFSE+ T cells (from CD11a−/− donors; Figure 4C). Of note, ≈90% of total CD8+/CFSE+ T cells in AT of obese recipients were CD11a+, and only ≈10% were CD11a− (Figure 4C). These results indicated that CD8+ T cells infiltrate into AT in obesity and that CD11a is crucial for this process.
Figure 4.
Migration, proliferation, and interferon γ (IFN-γ) expression of T cells from CD11a−/− mice. A, Left, Representative fluorescence-activated cell sorter (FACS) analysis of gated T cells in unlabeled and carboxyfluorescein succinimidyl ester (CFSE)-labeled splenocytes used for adoptive transfer. Right, CD11a expression on gated CD8+ T cells in CFSEl-abeled splenocytes mixed from wild-type (WT) and CD11a−/− mice. B, FACS analysis of stromal/vascular cells (S/Vs) of lean and obese recipient WT mice at 6 hours after adoptive transfer showing more CD8+ T-cell infiltration (CFSE+) into adipose tissue (AT) of obese recipient mice (n=3/group). C, CD11a expression on (CFSE+) CD8+ T cells infiltrated into AT (n=3/group). Lean and obese in B and C indicate lean and obese mice as recipients. D, CD8+ T-cell proliferation in AT in vivo determined at 3 hours after Edu injection. Top, Representative FACS analysis of gated CD8+ T cells in S/Vs. Bottom, Quantification of the proportions of Edu+ CD8+ T cells in AT of WT and CD11a−/− mice (n=3–4/group). E, IFN-γ expression in WT and CD11a−/− mouse AT CD8+ T cells stimulated with interleukin-2 (IL-2), IL-12, and IL-18 in vitro (n=4/group).
T-cell proliferation assay indicated that at 3 hours after Edu injection, obese CD11a−/− mice did not show increased proportions of Edu+/CD8+ T cells in AT compared with lean CD11a−/− mice and had a lower proportion of AT Edu+/CD8+ T cells than obese WT (Figure 4D), indicating reduced CD8+ T-cell proliferation in AT of obese CD11a−/− mice. Tissue culture showed that after stimulation with IL-2, IL-12, and IL-18, the proportion of IFN-γ–producing cells was significantly lower in CD8+ T cells from AT of obese CD11a−/− mice than of obese WT mice (Figure 4E). These results suggest that CD11a plays a crucial role in T-cell–related AT inflammation by participating in T-cell infiltration, activation, and proliferation in AT.
Obese CD11a−/− Mice Have Reduced Numbers of M1-Type Macrophages/DCs in AT
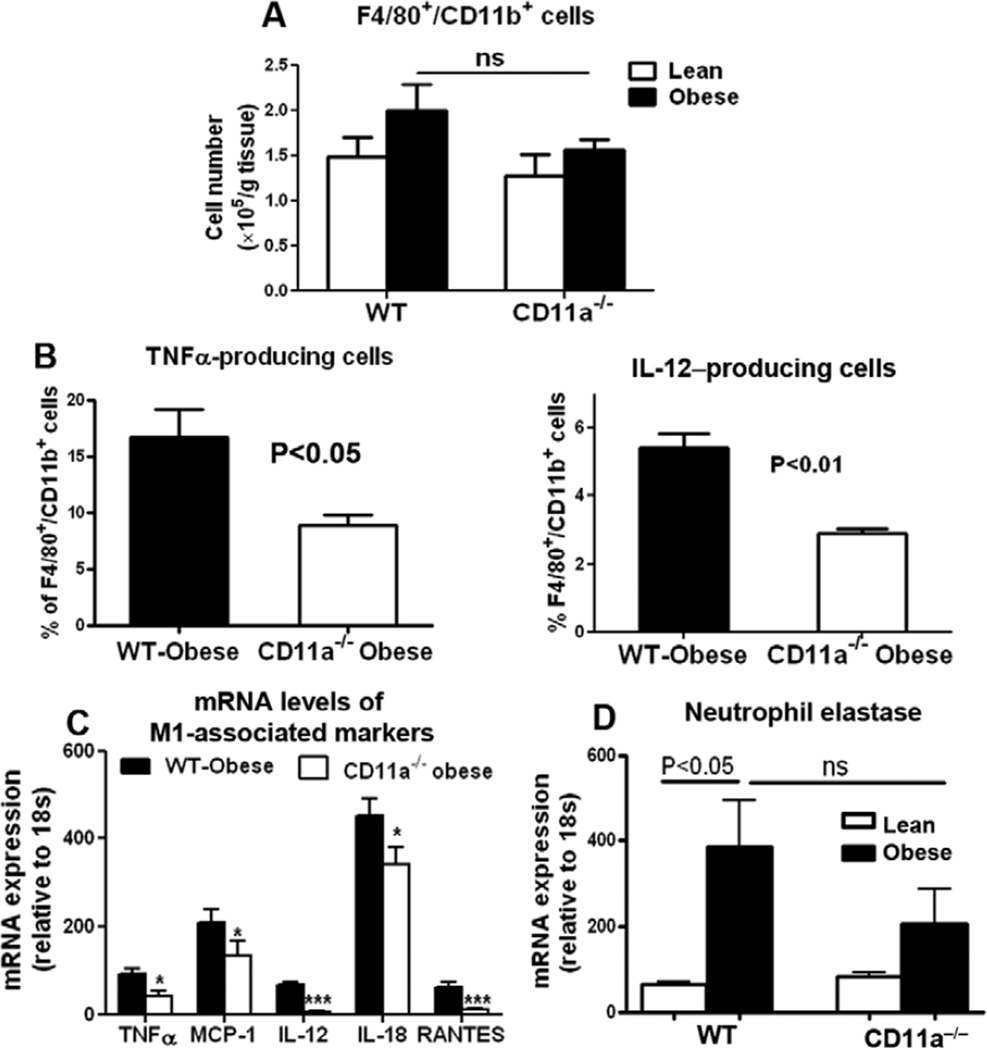
Obesity in WT mice increased macrophages/DCs in AT.5–8 Compared with obese WT, obese CD11a−/− mice tended to have lower numbers of F4/80+/CD11b+ macrophages/DCs in AT (Figure 5A). The relative ratio of CD11c+/macrophage galactose-type C-type lectin 1− cells8,26 in total F4/80+/CD11b+ macrophages/DCs increased and that of CD11c−/macrophage galactose-type C-type lectin 1+ cells decreased in both obese WT and CD11a−/− mice compared with their lean counterparts, with no significant differences between obese CD11a−/− and obese WT mice (Figure VIIA in the online-only Data Supplement). However, compared with obese WT mice, obese CD11a−/− mice showed significant reductions in the proportions of tumor necrosis factor-α–producing and IL-12–producing M1 macrophages/DCs8 (Figure 5B) and levels of M1 markers, including tumor necrosis factor-α, monocyte chemoattractant protein-1, IL-12, IL-18, and regulated on activation, normal T cell expressed and secreted in AT (Figure 5C and Figure VIIB in the online-only Data Supplement), suggesting that CD11a deficiency protects obese mice against M1 polarization of AT macrophages/DCs.
Figure 5.
Obese CD11a−/− mice have reduced numbers of M1-type macrophages/dendritic cells (DCs) in adipose tissue (AT) compared with wildtype (WT) controls. A, Numbers of F4/80+/CD11b+ macrophages/DCs per gram AT and (B) proportions of tumor necrosis factor (TNF) α–producing and interleukin-12 (IL-12)–producing F4/80+/CD11b+ macrophages/DCs in stromal/vascular cells (S/Vs) of WT and CD11a−/− mice examined by fluorescence-activated cell sorter (n=3–6 mice/group). C, mRNA levels of M1-associated genes in AT of obese WT and CD11a−/− mice examined by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR; n=7–9/group). *P<0.05, ***P<0.001 vs obese WT. D, mRNA levels of neutrophil elastase in AT of lean and obese WT mice and CD11a−/− mice examined by quantitative RT-PCR (n=5–9/group).
Consistent with a previous study,27 neutrophil elastase was increased in AT of obese WT compared with lean WT mice. Compared with obese WT, obese CD11a−/− mice tended to have lower levels of neutrophil elastase in AT (Figure 5D).
Obese CD11a−/− Mice Show Improved Metabolic Functions
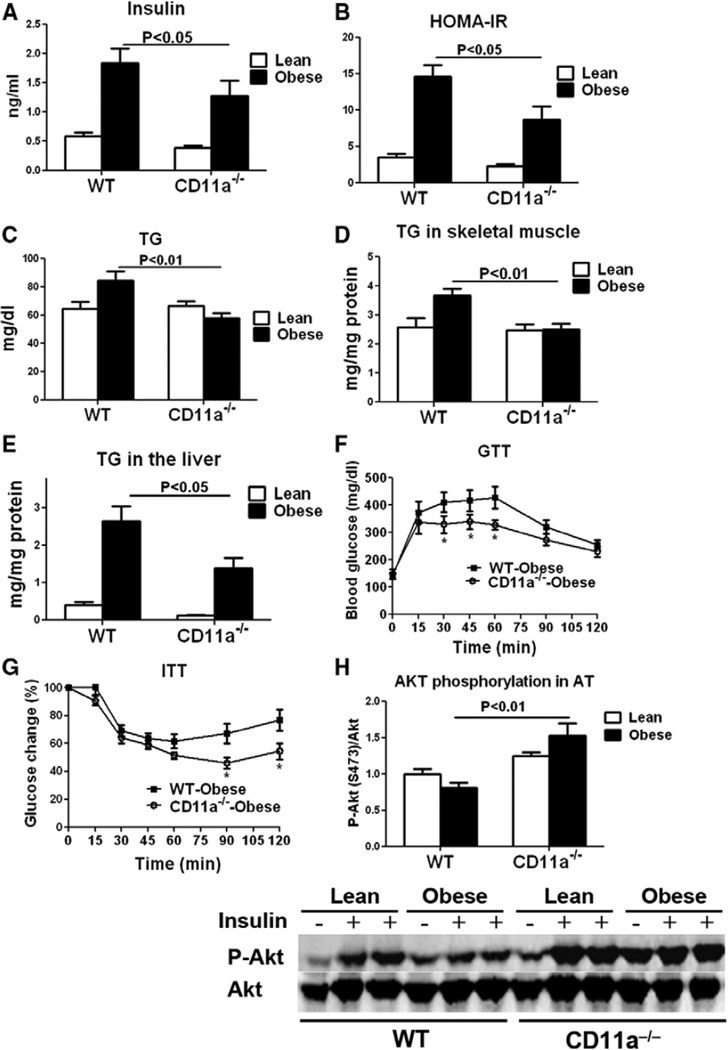
Compared with lean WT, obese WT mice showed insulin resistance as indicated by higher plasma levels of insulin and glucose (Figure 6A and Figure VIIIA in the online-only Data Supplement) and higher homeostasis model assessment of insulin resistance (Figure 6B). Obese WT mice also had higher plasma levels of triglyceride (TG) and cholesterol and greater TG content in skeletal muscle and the liver than in lean WT mice (Figure 6C–6E and Figure VIIIB in the online-only Data Supplement). Compared with obese WT, obese CD11a−/− mice had significantly lower plasma insulin levels and lower homeostasis model assessment of insulin resistance (Figure 6A and 6B). Plasma TG levels and TG content in skeletal muscle and the liver were also lower in obese CD11a−/− mice than in WT mice (Figure 6C–6E). Compared with obese WT mice, obese CD11a−/− mice showed improved glucose tolerance in glucose tolerance test (Figure 6F) and ameliorated insulin resistance in insulin tolerance test (Figure 6G). Examination of insulin sensitivity in various tissues indicated that, compared with lean WT, obese WT mice showed blunted insulin-stimulated Akt Ser473 phosphorylation in AT, skeletal muscle, and liver, indicating insulin resistance in all these tissues (Figure 6H and Figure VIIIC and VIIID in the online-only Data Supplement). Compared with obese WT, obese CD11a−/− mice showed higher levels of Ser473-phosphorylated Akt in AT (Figure 6H) but not in skeletal muscle or the liver (Figure VIIIC and VIIID in the online-only Data Supplement). All these results indicate that deficiency of CD11a protects mice against obesity-induced metabolic dysfunctions.
Figure 6.
CD11a−/− mice were protected against obesity-induced metabolic dysfunctions. A, Fasting plasma insulin levels, (B) calculated homeostasis model assessment of insulin resistance (HOMAIR), and (C) fasting plasma triglyceride (TG) levels of lean and obese wild-type (WT) and CD11a−/− mice (n=8/group). TG content in (D) skeletal muscle and (E) liver of WT and CD11a−/− mice (n=6–8/group). Intraperitoneal (F) glucose and (G) insulin tolerance tests in WT and CD11a−/− mice (n=7/group). *P<0.05 vs obese WT. H, Insulin-stimulated Ser473 phosphorylation of Akt in adipose tissue of lean and obese WT and CD11a−/− mice determined by Western blot (n=4 mice/group)
Neutralization of CD11a in Obese Mice Improves Glucose Tolerance and Reduces the Proportion of CD8+ T Cells in AT
We next neutralized CD11a by injecting KBA, a neutralizing anti-mouse CD11a antibody,22,28 in WT mice, with established obesity every other day for 9 times. Compared with controls, neutralization of CD11a significantly improved glucose tolerance in obese mice (Figure IXA in the online-only Data Supplement). CD11a neutralization also significantly reduced the ratio of CD8+ T cells in total AT T cells and tended to reduce the ratio of CD11c+/macrophage galactose-type C-type lectin 1− cells in total AT macrophages/DCs without significantly changing the proportions of total T cells and macrophages/DCs in S/Vs (Figure IXB and IXC in the online-only Data Supplement). Blockade of CD11a did not significantly alter the proportions of total T cells and CD8+ and CD4+ T cells in splenocytes (data not shown).
Discussion
Recent studies addressed previously unrecognized roles of T cells, particularly CD8+ T cells, in obesity-induced AT inflammation and insulin resistance.9–12,14,29 In adaptive immunity, T cells become activated and proliferate mainly in lymphoid organs in the presence of antigens and antigen-presenting cells.18 Recently, Morris et al16 and Deng et al15 reported a potential role of adaptive immunity in CD4+ T-cell–related AT inflammation. However, how CD8+ T cells accumulate and become activated in AT remains largely unknown.
In the current study, we demonstrated that CD8+ T cells (most of which were memory T cells) from AT of lean mice showed activation and proliferation in vitro on stimulation with Th1/Tc1-polarizing cytokines, which were increased in AT of obese mice. We also observed CD8+ T-cell infiltration into AT with increased infiltration in obesity. Therefore, increased infiltration and local activation and proliferation may contribute to the increased accumulation and activation of CD8+ T cells in AT in obesity. We also found that CD11a is crucial for AT inflammation by participating in T-cell infiltration and activation.
A role of memory CD8+ T cells from lymphoid organs in innate immunity has been reported.19–21 Here, we report that CD8+ T cells from AT showed innate immune response as indicated by our in vitro study, which, combined with reports showing a polyclonal T cell receptor repertoire of CD8+ T cells in AT of obese humans and mice,9,30 suggested a potential of AT CD8+ T cells in innate immunity in obesity. Occurrence of T-cell activation in nonlymphoid tissues has been reported.31 Here, we provide the evidence for potential CD8+ T-cell activation in AT. First, AT included higher proportions of activated CD8+ T cells and TEM/TE cells than blood; second, cytokine profile showed Th1/Tc1-polarizing milieu in AT of obese mice; third, in vitro, AT CD8+ T cells were activated by AT from obese mice or by cytokines elevated in AT of obese mice; fourth, CD8+ T cells proliferated in AT in obesity. The higher proportions of proliferating CD8+ T cells in AT of obese mice than in AT of lean mice and higher proportions of proliferating CD8+ T cells in AT than in splenocytes in vivo also support our hypothesis that the local AT milieu, with increased levels of Tc1/Th1-polarizing cytokines in obesity, activates CD8+ T cells and induces CD8+ T-cell proliferation locally in AT. T-cell proliferation has recently been reported in peripheral blood of obese humans,30 and CD4+ T-cell proliferation has just been reported in mouse AT.16 Here, we report CD8+ T-cell proliferation in AT in obesity.
The presence of memory T cells, which may be generated in response to environmental organisms, in blood and infiltration of these T cells into AT before or during obesity may be crucial for AT inflammation. When obesity occurs, the major pathogenic events of CD8+ T-cell–mediated AT inflammation may start with activation of memory CD8+ T cells in the T-cell–activating milieu of AT as indicated in our current study and a previous report.9 Upregulation of IL-12 and IL-18, which play important roles in innate immune response of memory T cells,19,20 in AT of obese mice points to the potential role of these cytokines in AT CD8+ T-cell activation and proliferation, which was confirmed by our in vitro studies. In addition to activation and proliferation of the resident memory T cells, obesity increased T-cell infiltration into AT. It is conceivable that the infiltrated T cells would also respond to the Th1/Tc1-polarizing milieu in AT, leading to inflammation amplification in AT.
Based on the above discussion, 1 strategy that may inhibit T-cell–related AT inflammation is to limit T-cell infiltration and suppress T-cell activation in AT. LFA-1 plays active roles in T-cell trafficking18,32 and activation.23 Therefore, we focused on LFA-1 and revealed a crucial role of LFA-1 in obesity-induced T-cell–related AT inflammation. Based on the demonstrated role of LFA-118,32 and our study, decreased infiltration of T cells may largely explain the reduction of T cells in AT of CD11a−/− mice. Compared with obese WT, obese CD11a−/− mice also had lower levels of Th1/Tc1-polarizing cytokines in AT, possibly caused by a feedback loop of T-cell effects on macrophages/DCs (see the following discussion). Given the effects of Th1/Tc1-polarizing cytokines on AT CD8+ T cells, the reductions in these cytokines may explain the decreased activation and proliferation of CD8+ T cells in AT of obese CD11a−/− mice. In addition, LFA-1 on T cells is involved in T-cell activation through interaction with intercellular adhesion molecule-1 on antigen-presenting cells.23 Therefore, LFA-1–intercellular adhesion molecule-1 interaction may also contribute to T-cell activation in AT.33 However, this pathway requires antigen presentation. Without information on obesity-specific antigens, we were unable to test the relevance of this potential to obesity.
Macrophages/DCs with polarization to M1 play crucial roles in AT inflammation.5,6,34,35 Our data showing no significant reductions in total macrophages/DCs and unchanged CD11c+ cell proportions in AT of obese CD11a−/− mice suggest that LFA-1 may not play a direct role in monocyte infiltration in AT. This was not surprising given that very late activation antigen-4 and CD11c play a dominant role in monocyte trafficking.18,36 Th1/Tc1 cells are crucial for macrophage/DC M1 polarization.37 Therefore, we postulate that decreases in Th1/Tc1 cells may have caused the reduced proportions of M1 macrophages/DCs and decreased levels of M1 markers, which in turn further lower T-cell activation, in AT of obese CD11a−/− mice. The trend toward lower neutrophil elastase in AT of obese CD11a−/− mice indicates that LFA-1 may also play a role in neutrophil-related AT inflammation.
Improvements of metabolic functions in obese CD11a−/− mice consistently support crucial roles of inflammation in metabolic abnormalities in obesity. In addition to their contribution to AT macrophage/DC activation,9,12 activated T cells and the major Th1/Tc1 cytokine, IFN-γ, dysregulate preadipocyte/adipocyte functions, causing insulin resistance and lipid storage impairment in adipocytes.11,12,38 Impaired lipid storage in adipocytes would enhance lipid transfer from adipocytes to other tissues such as skeletal muscle and liver, causing ectopic lipid deposition and insulin resistance in these tissues. Decreases in Th1/Tc1 cells in AT of obese CD11a−/− mice are, therefore, expected to improve adipocyte function with greater capacity to store lipid and less lipid transfer to other tissues. Consistent with this notion are our data showing larger fat pads, lower blood TG, less TG in skeletal muscle, and smaller livers in obese CD11a−/− mice. In addition to decreased Tc1/Th1 cells, reductions in AT M1 macrophages/DCs and neutrophil elastase may also contribute to the improvements in metabolic functions of obese CD11a−/− mice. Improvements in glucose tolerance and reductions in the ratios of CD8+ T cells in AT T cells with neutralization of CD11a in obese WT mice further support a role of CD11a in obesity-related AT inflammation and metabolic dysfunctions. The lesser reductions in AT CD8+ T cells and unchanged total T cells and CD4+ T cells in AT with CD11a neutralization compared with obese CD11a−/− mice may be because of the short period of treatment and potentially relatively slow turnover of T cells in AT with established obesity.
Although our in vitro studies revealed obesity-related antigen-independent response of AT CD8+ T cells induced by cytokines secreted by obese AT, we do not exclude the possibility that AT CD8+ T cells are activated in an obesity-related antigen-dependent manner. Identification of obesity-specific antigens, if there are any, would help to refine the investigation.
Taken together, our results support a potential model for CD8+ T-cell–related AT inflammation. HFD induces local AT inflammation with release of proinflammatory mediators such as IL-12 and IL-18, which activate AT resident CD8+ memory T cells and induce Tc1 polarization and proliferation, leading to CD8+ TEM/TE cell expansion in AT. When obesity progresses, CD8+ T-cell infiltration into AT increases, and the infiltrated T cells are also activated by the local Tc1-polarizing milieu, becoming TEM/TE cells with increased production of IFN-γ, which, in turn, activates macrophages/DCs and induces M1 polarization, thereby constituting an inflammatory loop leading to inflammation amplification in AT. CD11a deficiency inhibits T-cell infiltration and activation in AT, resulting in decreased TEM/TE cell accumulation in AT and disruption of the vicious circle of AT inflammation, which decrease macrophage/DC activation and ameliorate AT inflammation.
Supplementary Material
Significance.
T cells, particularly CD8+ T cells, play important roles in adipose tissue (AT) inflammation and the development of metabolic dysfunctions in obesity. However, the mechanisms of CD8+ T-cell–related AT inflammation remain incompletely understood. We currently provide evidence for proliferation of CD8+ T cells in AT with increased proliferation in obesity and also in vivo evidence for increased infiltration of CD8+ T cells into AT in obesity. We demonstrated that CD11a, a β2 integrin, was upregulated on CD8+ T cells in obese mice and played a crucial role in obesity-linked CD8+ T-cell–related AT inflammation and metabolic dysfunctions. Therefore, our study provides novel mechanisms for CD8+ T-cell–related AT inflammation and identifies CD11a as a crucial molecule for obesity-linked AT inflammation and metabolic dysfunctions. These results may provide direction for the development of novel therapeutic strategies for obesity-linked metabolic disorders.
Acknowledgments
We thank Kerrie Jara for editorial assistance.
Sources of Funding
This work was supported by National Institutes of Health grants HL098839 (to H. Wu) and DK078847 (to C.M. Ballantyne) and United States Department of Agriculture/Agricultural Research Service (USDA/ARS) grant 6250-51000-055-30 (to C.W. Smith).
Nonstandard Abbreviations and Acronyms
- AT
adipose tissue
- DC
dendritic cell
- HFD
high-fat diet
- S/V
stromal/vascular cell
- Tc1
T-cytotoxic 1 cell
- TE
effector T cell
- TEM
effector memory T cell
- TG
triglyceride
- Th1
T-helper 1 cell
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.302077/-/DC1.
Disclosures
None.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, 3rd, Kirk EA, O’Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremer AA, Devaraj S, Afify A, Jialal I. Adipose tissue dysregulation in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E1782–E1788. doi: 10.1210/jc.2011-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH, Smith CW, Ballantyne CM. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2010;30:186–192. doi: 10.1161/ATVBAHA.109.198044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 12.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng T, Lyon CJ, Minze LJ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, Lumeng CN. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 18.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 19.Berg RE, Cordes CJ, Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Tamang DL, Redelman D, Alves BN, Vollger L, Bethley C, Hudig D. Induction of granzyme B and T cell cytotoxic capacity by IL-2 or IL-15 without antigens: multiclonal responses that are extremely lytic if triggered and short-lived after cytokine withdrawal. Cytokine. 2006;36:148–159. doi: 10.1016/j.cyto.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Rodgers JR, Perrard XY, Perrard JL, Prince JE, Abe Y, Davis BK, Dietsch G, Smith CW, Ballantyne CM. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol. 2004;173:297–306. doi: 10.4049/jimmunol.173.1.297. [DOI] [PubMed] [Google Scholar]
- 24.Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, Walicke P, Dummer W, Wang X, Garovoy MR, Pariser D Efalizumab Study Group. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003;349:2004–2013. doi: 10.1056/NEJMoa030002. [DOI] [PubMed] [Google Scholar]
- 25.Badell IR, Russell MC, Thompson PW, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120:4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 29.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Weerd K, Dik WA, Schrijver B, Schweitzer DH, Langerak AW, Drexhage HA, Kiewiet RM, van Aken MO, van Huisstede A, van Dongen JJ, van der Lelij AJ, Staal FJ, van Hagen PM. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2012;61:401–408. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamann A, Jablonski-Westrich D, Duijvestijn A, Butcher EC, Baisch H, Harder R, Thiele HG. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988;140:693–699. [PubMed] [Google Scholar]
- 33.Brake DK, Smith EO, Mersmann H, Smith CW, Robker RL. ICAM-1 expression in adipose tissue: effects of diet-induced obesity in mice. Am J Physiol Cell Physiol. 2006;291:C1232–C1239. doi: 10.1152/ajpcell.00008.2006. [DOI] [PubMed] [Google Scholar]
- 34.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 38.McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, Smyth EM, Reilly MP. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem. 2009;284:31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.