Abstract
Herpes simplex virus type 1 infection of the mouse eye results in an impressive inflammatory response culminating in the death of the animal or the establishment of a “latent” infection depending on a number of ill-defined variables that include components of the innate and adaptive immune system. The application of type I interferon transgenes has been found to antagonize viral replication and spread from the eye to the nervous system. Associated with the in situ transfection of the cornea is the up-regulation of two inflammatory molecules, IL-6 and CXCL10. In this report, we will further examine the contribution these molecules may have in the host response to ocular infection with herpes simplex virus type 1.
Keywords: HSV-1, chemokine, CXCL10, type I interferon, interleukin-6
Corneal Infection with Herpes Simplex Virus Type 1
The general approach to infection of the murine eye with herpes simplex virus type 1 (HSV-1) includes placing a volume of a solution (typically an isotonic saline-based solution) ranging between 3 – 5 μl of an isotonic solution containing HSV-1 onto a scarified or in some protocols, a non-scarified cornea. Since the cornea’s outer layer is composed of dead epithelium, the removal of this layer via scarification facilitates the establishment of a successful infection. Upon infection, the virus replicates initially in the epithelial layer and into the stroma of the cornea (1) (Fig. 1). In approximately 25–50% of infected mice, the virus can be detected in the retina including cells within the layers consistent with ganglion cells, astrocytes, and microglia (Fig. 1). In the human host, reactivation of latent virus or severe acute infection has also been reported to infect the retina leading to acute retinal necrosis (2,3). As a result of infection, the inflammatory response is rapid and robust which can lead to unwarranted manifestations ultimately modifying visual acuity (e.g., retinal necrosis or herpetic keratitis). Thus, preservation of the visual axis and clearance of the noxious pathogen can be viewed as opposing forces relative to ocular HSV-1 infection. In recent times there has been significant research activity developing strategies that limit viral replication while at the same time reducing the inflammatory response. Some of the most important advances are presented in this review.
Figure 1. HSV-1 infection of the eye.
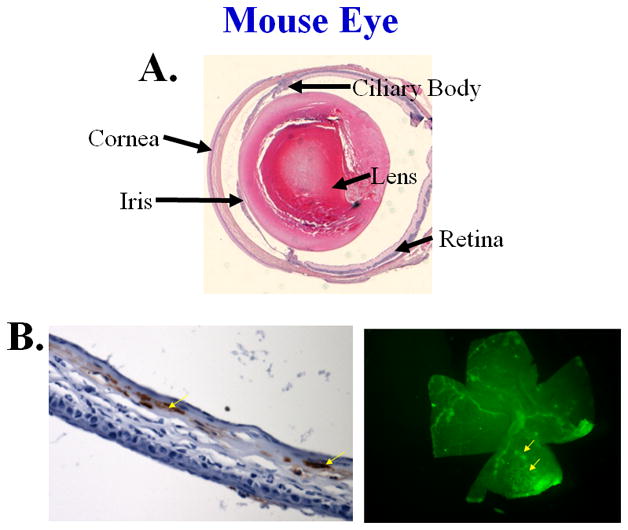
Panel A: Structure of the eye illustrating the major features, 40X. Panel B: Immunohistochemical staining of HSV-1 antigen of a corneal section (left panel, mag. at 200X) or the retina whole mount (right panel, mag at 40X) day 5–7 post infection. Viral antigen appears red (left panel) or green (right panel).
Corneal transfection with type I interferon transgenes
The transfection of corneal epithelium with the interferon (IFN)-α1 transgene has been found to have therapeutic application to ocular HSV-1 infection compared with recombinant IFN-α that appears to be limited to prophylactic application (4). While it is known the anti-viral activity of the IFN-α1 transgene depends on the presence of αβ T cell receptor-positive T lymphocytes and IFN-γ (5), other factors may also play a role. Along these lines, a number of immune-related genes were screened for induction or up-regulation in the cornea following in situ transfection with the IFN-α1 transgene including IL-2, IL-4, IL-6, IL-10, IL-12 (p35 and p40), IFN-γ, IFN-γ induced nitric oxide synthase (Nos-2), CCL2 (MCP-1), CCL4 (MIP-1β), CCL5 (RANTES), and CXCL10 (IP-10). Only two, IL-6 and CXCL10, appeared to be elevated within 24 hr post-transfection while the other genes were undetectable (6). As a result of this observation, both IL-6 and CXCL10 were the target of further investigation as a means to define the events associated with the establishment of an anti-viral environment by type I IFN transgenes.
Type I IFN transgenes and IL-6
IL-6 is a pleiotropic cytokine produced in response to a number of viruses including lymphocytic choriomeningitis virus (7), Theiler’s murine encephalomyelitis virus (8), vesicular stomatitis virus (7), and herpes simplex virus (9, 10). The production of IL-6 has been linked to increased resistance to ocular HSV-1 infection (11, 12) perhaps acting as a chemoattractant for neutrophils (13) which are known to be involved in clearing virus from the eye (14,15). Previously, the transfection of mouse cornea with the IFN-α1 transgene was found to increase IL-6 mRNA levels as measured by RT-PCR (6). We interpret these results to suggest that IL-6 may be a contributing factor in establishing an anti-viral environment following type I IFN exposure. To address this hypothesis, mice deficient in IL-6 (IL-6 KO) were transfected with the IFN-α1 transgene and then characterized for resistance to ocular HSV-1 infection as measured by viral titer in the tear film and cumulative survival of infected mice. In the presence of the IFN-α1 transgene, wild type mice showed a reduction in the viral load recovered in the tear film within the time frame of detectable virus during the acute infection (i.e., days 1–5 post infection) compared to the wild type group (C57BL/6) transfected with the plasmid construct alone (Fig. 2). The absence of IL-6 did not affect the outcome of viral yield in the tear film. Specifically, similar to the wild type mice, IL-6 KO mice transfected with the IFN-α1 transgene also showed a reduction in the viral titer recovered in the tear film as well (Fig. 2). Therefore, from a local standpoint, the absence of IL-6 does not impact on the anti-viral efficacy of the IFN-α1 transgene.
Figure 2. HSV-1 titer in the tear film at times post infection.
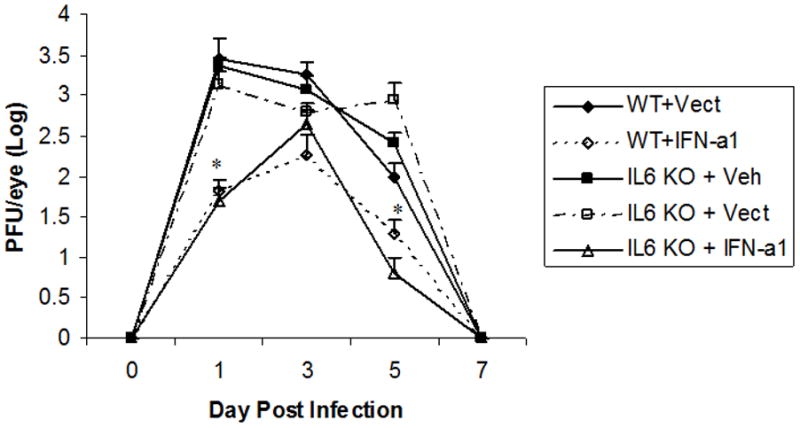
Wild type (C57BL/6, WT) and IL-6 deficient (IL6 KO) mice (n=10/group) were transfected with plasmid vector alone (Vect, 100 μg/eye) or plasmid containing the IFN-α1 transgene (IFN-a1, 100 μg/eye) and infected with HSV-1 (1000 plaque forming units/eye, strain McKrae) 24 hr post transfection. At the time indicated immediately before (day 0) or after infection (day 1–7), mouse eyes were swabbed, and the swab was placed in media. The viral content within the media was determined by plaque assay using Vero cells. *p<.05 comparing the IFN-α1 transgene wild type (WT) or IL-6 deficient (IL6 KO) transfected groups to the control vector (Vect) transfected or non-transfected, vehicle (Veh)-treated group as determined by ANOVA and Tukey’s post hoc t-test.
The capacity to control viral replication within the cornea does not necessarily dictate the outcome of the infection. As the virus replicates within the cornea, it is also transported back into the ganglion via retrograde transport by innervated sensory processes that extend into the cornea (16). Within the ganglion (i.e., trigeminal ganglion, TG), the virus replicates and establishes a latent infection as well as travels to the central nervous system (CNS) where it continues to replicate. Although it is not entirely clear, it is thought that if the virus goes unchecked within the CNS the host (mouse) will succumb to the infection as a result of encephalitis. Therefore, it was necessary to determine if the lack of IL-6 could modify the survival of mice receiving the IFN-α1 transgene against ocular HSV-1 infection. Consistent with previous observations using ICR outbred mice, C57BL/6 mice (wild type) transfected in the cornea with the IFN-α1 transgene showed a significant enhancement in survival compared to the wild type recipients of the plasmid vector alone (Fig. 3). However, in the absence of IL-6, the efficacy of the IFN-α1 transgene was lost. Specifically, IL-6 KO mice transfected with the IFN-α1 transgene showed a similar level of survival compared to IL-6 KO mice transfected with the plasmid vector alone or IL-6 KO animals treated with the vehicle (saline) alone (Fig. 3). These results suggest that while IL-6 is not involved in controlling HSV-1 in the eye, it is involved in preventing encephalitis in the CNS. Further experiments are needed in order to delineate at what level IL-6 is involved in the IFN-α1-mediated, anti-viral efficacy against HSV-1 infection. For example, the absence of IL-6 may impact on viral replication in the TG or within the CNS through changes in the development of CTLs (17), antibody production (18), or immune-enhancing adjuvant activity (19).
Figure 3. IL-6 is required for anti-viral efficacy against ocular HSV-1 following IFN-α1 transgene transfection.
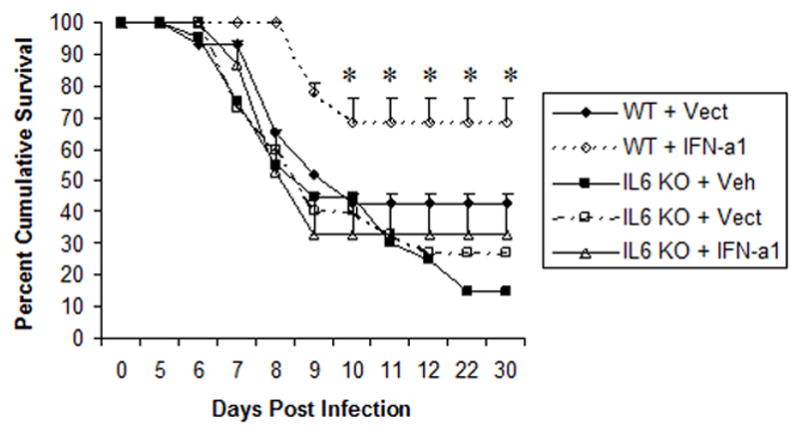
Wild type (C57BL/6, WT) and IL-6 deficient mice (IL6 KO) (n=15–20/group) were transfected with plasmid vector alone (Vect, 100 μg/eye) or plasmid containing the IFN-α1 transgene (IFN-a1, 100 μg/eye) and infected with HSV-1 (1000 plaque forming units/eye, strain McKrae) 24 hr post transfection. The mice were monitored for survival out to the establishment of latency, day 30 post infection. The results are shown as percent survival. *p<.02 comparing the WT + IFN-α1 group to all other groups as determined by the Mann-Whitney rank order test.
Type I IFN and CXCL10
The extravasation of leukocytes from the blood into tissue is mediated, in part, by a group of molecules collectively known as chemokines. The chemokines are divided into four families on the basis of the arrangement of conserved cysteines or lack thereof (for lymphotactin) including the CXC, CX3C, and CC families. Within the cornea, the constitutive expression of chemokine genes was initially noted by RT-PCR (Table I) (20). However, only two of the seven chemokines measured by RT-PCR were detectable at the protein level by ELISA including CCL2 (21) and CXCL10 (1). Whereas CCL2 influences the migration of monocytes, memory T cells, basophils, and NK cells (22), CXCL10 targets the migration of neutrophils, monocytes, and activated T cells and NK cells (23–26). Since CXCL10 reportedly has anti-viral activity (27) and is locally up-regulated by the IFN-α1 transgene following corneal transfection (6), it was possible that CXCL10 directly contributes to the resistance to HSV-1 infection by the type I IFN transgene. In order to follow-up on this notion, neutralizing antibody to CXCL10 was administered to mice ocularly infected with HSV-1. The antibody was found to significantly reduce the expression of CXCL10 in the eye during the first three days post infection compared to control IgG-treated, HSV-1 infected mice (1). More importantly, the suppression in CXCL10 expression resulted in a transient rise in the HSV-1 titer recovered in the cornea of treated mice. However, CXCL10 was not found to have a direct effect on HSV-1 replication as measured by adding recombinant CXCL10 to a fibroblast cell line or primary murine splenic lymphocytes infected with HSV-1 (1). Consequently, the action of CXCL10 was indirect possibly through the recruitment of inflammatory cells. In fact, HSV-1-infected mice treated with the anti-CXCL10 antibody showed a significant reduction in corneal inflammation during acute infection based on edema, reduction in infiltrating cells, and a reduction in the expression of other chemokines including CXCL1, CCL3, and CCL5 (1). A reduction in the inflammatory process following neutralization with the anti-CXCL10 antibody may be due to the suppression in CCL3 expression which has been linked to the development of herpetic keratitis (28). Alternatively, CXCL10 through its receptor CXCR3 promotes the development and recruitment of TH1 cells and neutrophils into inflammatory sites (29–31). Therefore, preventing TH1 development or attraction of leukocytes into the diseased tissue by preventing the interaction of CXCL10 with CXCR3 may also contribute towards a reduction in the host response to infection. Taken together, it seems likely that a combination of antagonizing cytokine and chemokine expression along with a reduction of infiltrating cells into the infected cornea ultimately diminishes the host inflammatory process to HSV-1.
Table I.
Chemokine family and Target Cell(s)
| Name | Family of Chemokine | Target cell(s) |
|---|---|---|
| CXCL1 | CXC | Neutrophils |
| CXCL10 | CXC | Neutrophils, activated T cells and NK cells |
| CXCL3 | CXC | Neutrophils, endothelial cells |
| CCL3 | CC | Monocytes, T cells, NK cells, dendritic cells |
| CCL4 | CC | Monocytes, T cells, NK cells, dendritic cells |
| CCL2 | CC | Monocytes, memory T cells, basophils, NK cells |
| CCL5 | CC | Memory T cells, eosinophils, basophils, NK cells, dendritic cells |
Although only a representative list, these chemokines are expressed in the cornea following ocular HSV-1 infection (20).
Based on previous observations in which it was found that targeted disruption of the CXCL10 gene or neutralization of CXCL10 expression exacerbated viral infections of the central nervous system (CNS) (32–34), it was predicted that hindering CXCL10 expression in mice ocularly infected with HSV-1 would likewise lead to dire consequences for the host. In fact, blocking CXCL10 expression led to a significant rise in infectious virus recovered in the ganglion whose processes innervate the eye in HSV-1-infected mice (1). However, the survival of HSV-1-infected mice treated with the anti-CXCL10 antibody was enhanced compared to IgG-treated controls (Fig. 4). Therefore, it is likely that the inflammatory process initiated through the expression of CXCL10 in response to the viral infection also manifests unwarranted effects within the CNS leading to encephalitis and ultimately death of the host. A balance between the inflammatory process and clearance of the insulting pathogen must be realized for the survival of the host. This idea seems particularly true within the CNS where it has been found over-expression of cytokine genes has devastating effects on the host (35–37). Whether this scenario holds true relative to HSV-1 infection and neutralization of CXCL10 within the CNS has yet to be addressed.
Figure 4. Anti-CXCL10 antibody treatment of HSV-1-infected mice prolongs survival.
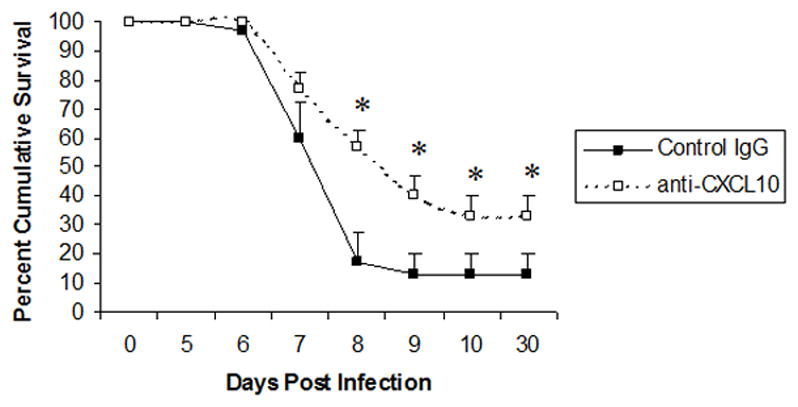
ICR mice (n=30/group) were infected with HSV-1 (300 pfu/eye) and inoculated with 100 μg of anti-CXCL10 IgG (open square) or control IgG (black square) at time 0 and 2 and 5 days post infection. Mice were then recorded for survival. *, p<.05 comparing the control to the anti-CXCL10-treated, HSV-1-infected mice as determined by the Mann-Whitney U test. This figure is a summary of 6 experiments with 5 mice/group/experiment.
Summary
In situ transfection of scarified cornea with the murine IFN-α1 transgene leads to efficient suppression of HSV-1 replication and reduced pathology associated with the infection. Two inflammatory molecules are selectively induced by the transgene including IL-6 and CXCL10. We found that the absence of IL-6 had no detrimental effect on the anti-viral efficacy by type I IFN within the area proximal to the initial infection. Yet, the absence of IL-6 did have an impact on HSV-1 pathogenesis negating the efficacious effect of the type I IFN transgene in enhancing survival of infected mice. In contrast to the results with IL-6, neutralization of CXCL10 enhanced the survival of HSV-1-infected mice. In addition, suppressing CXCL10 expression was found to facilitate a transient increase in viral replication in the cornea. Correlating with the suppression of CXCL10 expression was a reduction in ocular inflammation with lower levels of additional chemokines including CCL2, CCL3, and CCL5. Based on these observations, we propose CXCL10 to be a sentinel chemokine within the cornea that upon up-regulation leads to the induction of other chemokines secreted by cells proximal to the infection or via cells infiltrating the inflamed site (Fig. 5). It is the combination of local chemokine expression and recruitment of leukocytes that ultimately clears the virus, a process that may be modified by type I IFN enhancement of CXCL10 expression. Nevertheless, the relationship between the anti-viral effects elicited by the IFN-α1 transgene and CXCL10 have not been firmly established. IFN-α induction of CXCL10 appears to be at the level of dendritic cells and monocytes (38,39) both of which are reportedly found in the anterior segment of the eye (40,41). Therefore, is the expression of CXCL10 localized to these cells or are additional cells involved? During ocular infection with HSV-1, which infiltrating cells express the receptor for CXCR3 (receptor for CXCL10) and when do they begin to populate the stroma? Does the absence of CXCL10 modify the development of TH1 cells during ocular HSV-1 infection? How does neutralizing CXCL10 change the trafficking of leukocytes from the regional lymph nodes to the site of infection as it progresses from the eye to the nervous system? These and other questions are tantamount to understanding the predicted central role of CXCL10 in ocular inflammation as a result of infection with or without IFN-α1 transfection.
Figure 5. Proposed scenario in the initiation of corneal inflammation following ocular HSV-1 infection.
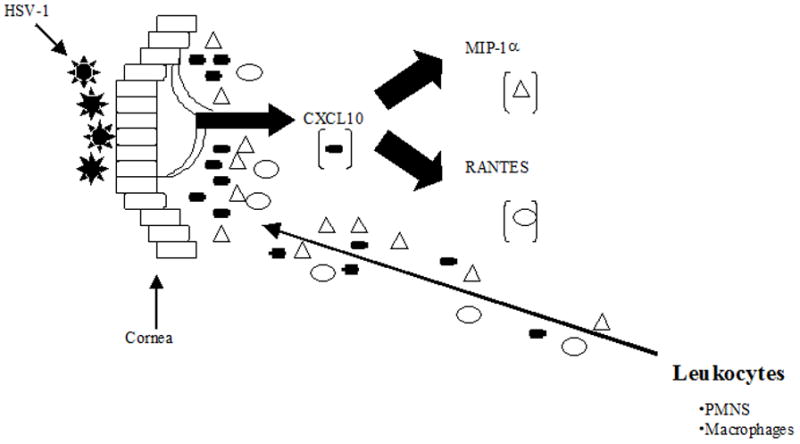
Following infection of the cornea with HSV-1, local cells (could include Langerhan’s cells and dendritic cells or macrophages peripheral to the central cornea) increase expression of CXCL10. Either by direct or indirect means, CXCL10 promotes the expression of additional chemokines including MIP-1α and RANTES (it is our hypothesis that recruiting PMNs and macrophages greatly enhances the production of these chemokines). Collectively, the expression of these chemokines recruit leukocytes (initially NK cells and PMNs) into the site of infection. Central to this proposed model is the expression of MIP-1α (initially) by CXCL10 as it elicits the infiltration of PMNs. However, subsequent infiltration of T cells and NK cells and macrophages will be influenced by RANTES and CXCL10. In the absence of CXCL10, a reduced level of MIP-1α is produced significantly reducing the initial infiltration. In the figure, the thin arrow depicts the chemical gradient of chemokines as chemoattractants for leukocytes from the bloodstream.
Acknowledgments
This work was supported by USPHS grant AI053108, NS41249, NEI core grant P30 EY12190, and a Research to Prevent Blindness Stein Professorship (to DJJC).
References
- 1.Carr DJJ, Chodosh J, Ash J, Lane TE. Effect of anti-CXCL10 monoclonal antibody on herpes simplex virus type 1 keratitis and retinal infection. J Virol. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavesio CE, Conrad DK, McCluskey PJ, Mitchell SM, Towler HM, Lightman S. Delayed acute retinal necrosis after herpetic encephalitis. Br J Ophthalmol. 1997;81:415–420. doi: 10.1136/bjo.81.5.415a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levinson RD, Reidy R, Chiu MT. Acute retinal necrosis after neonatal herpes encephalitis. Br J Ophthalmol. 1999;83:123–124. doi: 10.1136/bjo.83.1.123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noisakran S, Carr DJJ. Topical application of the cornea post-infection with plasmid DNA encoding interferon-α1 but not recombinant interferon-αA reduces herpes simplex virus type 1-induced mortality in mice. J Neuroimmunol. 2001;121:49–58. doi: 10.1016/s0165-5728(01)00442-8. [DOI] [PubMed] [Google Scholar]
- 5.Carr DJJ, Noisakran S. The antiviral efficacy of the murine alpha-1 interferon transgene against ocular herpes simplex virus type 1 requires the presence of CD4+, αβ T-cell receptor-positive T lymphocytes with the capacity to produce gamma interferon. J Virol. 2002;76:9398–9406. doi: 10.1128/JVI.76.18.9398-9406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noisakran S, Carr DJJ. Plasmid DNA encoding IFN-α1 antagonizes herpes simplex virus type 1 ocular infection through CD4+ and CD8+ T lymphocytes. J Immunol. 2000;164:6435–6443. doi: 10.4049/jimmunol.164.12.6435. [DOI] [PubMed] [Google Scholar]
- 7.Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin-6 produced in the central nervous system in viral disease. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- 8.Rubor N, Sierra A. Interleukin-6 production by brain tissue and cultured astrocytes infected with Theiler’s murine encephalomyelitis virus. Glia. 1993;9:41–47. doi: 10.1002/glia.440090106. [DOI] [PubMed] [Google Scholar]
- 9.Aurelius E, Andersson B, Forsgren M, Skoldenberg B, Strannegard O. Cytokines and other markers of intrathecal immune response in patients with herpes simplex encephalititis. J Infect Dis. 1994;170:678–681. doi: 10.1093/infdis/170.3.678. [DOI] [PubMed] [Google Scholar]
- 10.Oshima M, Azuma H, Suzutani T, Ikeda H, Okuno A. Direct and mononuclear cell mediated effects on interleukin 6 production by glioma cells in infection with herpes simplex virus type 1. J Med Virol. 2001;63:252–258. doi: 10.1002/1096-9071(200103)63:3<252::aid-jmv1009>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Carr DJJ, Campbell IL. Transgenic expression of interleukin-6 in the central nervous system confers protection against acute herpes simplex virus type-1 infection. J Neurovirol. 1999;5:449–457. doi: 10.3109/13550289909045373. [DOI] [PubMed] [Google Scholar]
- 12.LeBlanc RA, Pesnicak L, Cabral ES, Godleski M, Straus SE. Lack of interleukin-6 (IL-6) enhances susceptibility to infection but does not alter latency or reactivation of herpes simplex virus type 1 in IL-6 knockout mice. J Virol. 1999;73:8145–8151. doi: 10.1128/jvi.73.10.8145-8151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002;43:737–743. [PubMed] [Google Scholar]
- 14.Tumpey TM, Chen SH, Kunkel SL, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease. Herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 16.Shimeld C, Efstathiou S, Hill T. Tracking the spread of a lacZ-tagged herpes simplex virus type 1 between the eye and the nervous system of the mouse: Comparison of primary and recurrent infection. J Virol. 2001;75:5252–5262. doi: 10.1128/JVI.75.11.5252-5262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renauld J-C, Vink A, Van Snick J. Accessory signals in murine cytolytic T cell responses. J Immunol. 1989;143:1894–1898. [PubMed] [Google Scholar]
- 18.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Takahara Y, Taniguchi T, Kishimoto T. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce Ig. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 19.Larsen DL, Dybdahl-Sissoko N, McGregor MW, Drape R, Neumann V, Swain WF, Lunn DP, Olsen CW. Coadministration of DNA encoding interleukin-6 and hemagglutinin confers protection from influenza virus challenge in mice. J Virol. 1998;72:1704–1708. doi: 10.1128/jvi.72.2.1704-1708.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y-H, Yan X-T, Oakes JE, Lausch RN. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J Virol. 1996;70:1277–1281. doi: 10.1128/jvi.70.2.1277-1281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumpey TM, Cheng H, Yan X-T, Oakes JE, Lausch RN. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J Leukoc Biol. 1998;63:486–492. doi: 10.1002/jlb.63.4.486. [DOI] [PubMed] [Google Scholar]
- 22.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 23.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human-interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farber JM. Mig and IP-10. CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 25.Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, Moser B. The chemokine receptor CXCR3 mediates rapid and shearing resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol. 1998;28:961–972. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Michalec L, Choudhury BK, Postlewait E, Wild JS, Alam R, Lett-Brown M, Sur S. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol. 2002;168:846–852. doi: 10.4049/jimmunol.168.2.846. [DOI] [PubMed] [Google Scholar]
- 27.Mahalingam S, Farber JM, Karupiah G. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity in vivo. J Virol. 1999;73:1479–1491. doi: 10.1128/jvi.73.2.1479-1491.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumpey TM, Cheng H, Cook DN, Smithies O, Oakes JE, Lausch RN. Absence of macrophage inflammatory protein-1α prevents the development of blinding herpes stromal keratits. J Virol. 1998;72:3705–3710. doi: 10.1128/jvi.72.5.3705-3710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabin RL, Alston MA, Sircus JC, Knollmann-Ritschel B, Moratz C, Ngo D, Farber JM. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J Immunol. 2003;171:2812–2824. doi: 10.4049/jimmunol.171.6.2812. [DOI] [PubMed] [Google Scholar]
- 30.Xie JH, Nomura N, Lu M, chen S-L, Koch GE, Weng Y, Rosa R, Di Salvo J, Mudgett J, Peterson LB, Wicker LS, DeMartino JA. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. 2003;73:771–780. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- 31.Boztug K, Carson MJ, Pham-Mitchell N, Asensio VC, DeMartino J, Campbell IL. Leukocyte infiltration, but not neurodegeneration, in the CNS of transgenic mice with astrocyte production of the CXC chemokine ligand 10. J Immunol. 2002;169:1505–1515. doi: 10.4049/jimmunol.169.3.1505. [DOI] [PubMed] [Google Scholar]
- 32.Liu MT, Chen BP, Oertel P, Buchmeier MJ, Armstrong D, Hamilton TA, Lane TE. Cutting Edge: The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- 33.Liu MT, Keirstead HS, Lane TE. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- 34.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2003;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 35.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MBA, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell IL, Krucker T, Stefensen S, Akwa Y, Powell HC, Lane T, Carr DJ, Gold LH, Henricksen SJ, Siggins GR. Structural and functional neuropathology in transgenic mice with CNS expression of IFN-α. Brain Res. 1999;835:46–61. doi: 10.1016/s0006-8993(99)01328-1. [DOI] [PubMed] [Google Scholar]
- 37.Pagenstecher A, Lassmann S, Carson MJ, Kincaid CL, Stalder AK, Campbell IL. Atrocyte-targeted expression of IL-12 induces active cellular immune responses in the central nervous system and modulates experimental allergic encephalomyelitis. J Immunol. 2000;164:4481–4492. doi: 10.4049/jimmunol.164.9.4481. [DOI] [PubMed] [Google Scholar]
- 38.Padovan E, Spagnoli GC, Ferrantini M, Heberer M. IFN-alpha2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J Leukoc Biol. 2002;71:669–676. [PubMed] [Google Scholar]
- 39.Blackwell SE, Krieg AM. CpG-A-induced monocyte IFN-γ-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-α. J Immunol. 2003;170:4061–4068. doi: 10.4049/jimmunol.170.8.4061. [DOI] [PubMed] [Google Scholar]
- 40.Hendricks RL, Janowicz M, Tumpey TM. Critical role of corneal Langerhan’s cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse cornea. J Immunol. 1992;148:2522–2529. [PubMed] [Google Scholar]
- 41.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]


