Abstract
Background
Yoga is a popular mind-body therapy that has demonstrated beneficial effects on psychological, behavioral, and functional outcomes. However, few studies have investigated effects on inflammatory processes. This study tested the hypothesis that an Iyengar yoga intervention specifically designed for fatigued breast cancer survivors would lead to decreases in inflammation-related gene expression and circulating markers of proinflammatory cytokine activity.
Methods
Breast cancer survivors with persistent cancer-related fatigue were randomized to a 12-week Iyengar yoga intervention (n = 16) or a 12-week health education control condition (n = 15). Blood samples were collected at baseline, post-intervention, and at a 3-month follow-up for genome-wide transcriptional profiling and bioinformatic analyses. Plasma inflammatory markers and salivary cortisol were also assessed.
Results
In promoter-based bioinformatics analyses, the yoga group showed reduced activity of the pro-inflammatory transcription factor nuclear factor kappa B (NF-κB), increased activity of the anti-inflammatory glucocorticoid receptor, and reduced activity of cAMP response element-binding protein (CREB) family transcription factors relative to controls (all ps < .05). There was also a significant intervention effect on the soluble tumor necrosis factor receptor type II (sTNF-RII), a marker of TNF activity; plasma levels of sTNF-RII remained stable in the yoga group, whereas levels of this marker increased in the health education group (p = .028). A similar, non-significant trend was observed for the interleukin 1 receptor antagonist (p = .16). No significant changes in C reactive protein (CRP), interleukin 6 (IL-6), or diurnal cortisol measures were observed.
Conclusions
A 12-week restorative Iyengar yoga intervention reduced inflammation-related gene expression in breast cancer survivors with persistent fatigue. These findings suggest that a targeted yoga program may have beneficial effects on inflammatory activity in this patient population, with potential relevance for behavioral and physical health. ClinicalTrials.gov identifier: NCT00727662.
Keywords: yoga, inflammation, gene expression, fatigue, cancer, cortisol
INTRODUCTION
Yoga is an ancient mind-body practice that has become increasingly popular in Western culture. In Western countries, yoga typically refers to postures (asanas), breath control (pranayama), and/or meditation. Yoga is traditionally believed to have beneficial effects on physical and mental health, and research conducted over the past several decades has subjected those beliefs to empirical scrutiny. Indeed, randomized controlled trials of yoga interventions conducted with a wide range of healthy and clinical populations suggest that yoga has beneficial effects on stress, anxiety, depressive symptoms, pain, and aspects of physical function, although conclusions are limited by poor trial quality in many cases (Raub, 2002; Bower et al., 2005; Bussing et al., 2012a; Bussing et al., 2012b).
There is growing interest in the effects of yoga on physiological processes that may underlie effects on health. Inflammation plays a key role in the onset and progression of a number of physical conditions, including cancer, cardiovascular disease, and diabetes (Libby and Theroux, 2005; Pierce et al., 2009), and is also linked to depression, pain, fatigue, and other behavioral disturbances (Irwin and Cole, 2011). However, there have been surprisingly few studies examining effects of yoga on inflammatory processes. Two randomized trials conducted with heart failure patients found that an 8-week Hatha yoga program led to significant reductions in serum concentrations of IL-6 and CRP relative to standard medical treatment (Pullen et al., 2008; Pullen et al., 2010). Another study examined the impact of a single session of Iyengar-based restorative yoga on inflammatory markers in experienced and novice yoga practitioners (Kiecolt-Glaser et al., 2010). Although the inflammatory response to a single yoga session was not different from a movement or passive video control condition, experienced yoga practitioners did show evidence of lower inflammatory activity than novices, suggesting an effect of more sustained yoga practice on inflammation. Finally, there is limited evidence that other mind-body interventions that incorporate some component of yoga may have anti-inflammatory effects. In particular, mindfulness-based stress reduction (which includes gentle yoga postures), as well as yogic meditation, have recently been shown to reduce inflammatory signaling through the pro-inflammatory NF-κB control pathway (Creswell et al., 2012; Black et al., 2013). However, the few studies to assess gene expression profiles in experienced yoga practitioners did not report effects on inflammation-related genes (Sharma et al., 2008; Qu et al., 2013).
The current study examined the effects of an Iyengar yoga intervention targeted at fatigue on genomic and circulating markers of inflammation in fatigued breast cancer survivors. The primary outcome of this randomized controlled trial was fatigue, and results showed improvements in fatigue and energy among women assigned to the yoga group relative to women assigned to the health education control condition (Bower et al., 2012). In addition to behavioral outcomes, the trial was designed to examine effects on inflammatory processes, which may underlie symptoms of cancer-related fatigue (Bower et al., 2002; Collado-Hidalgo et al., 2006; Orre et al., 2011; Bower et al., 2011b; Alfano et al., 2012). Based on preliminary work suggesting beneficial effects of yoga on inflammation (Pullen et al., 2008; Pullen et al., 2010), we hypothesized that women randomly assigned to the targeted Iyengar yoga intervention would show decreases in markers of inflammatory activity relative to health education controls. We were particularly interested in the NF-κB signaling pathway, given its central role as a regulator of pro-inflammatory gene expression (Aggarwal et al., 2009) and evidence of elevated NF-κB activity in fatigued breast cancer survivors (Bower et al., 2011a). Specifically, we hypothesized that Iyengar yoga would lead to reductions in NF-κB signaling, as has been seen following other mind-body interventions (Creswell et al., 2012; Black et al., 2013). We also hypothesized that this intervention would lead to reductions in circulating markers of inflammation. Further, given the role of the HPA axis and the sympathetic nervous system as key regulators of inflammatory processes (Irwin and Cole, 2011), we evaluated the impact of the yoga intervention on transcription control pathways linked to these systems and on diurnal cortisol production.
METHODS
Yoga Randomized Controlled Trial
Data came from a study of 31 stage 0-II breast cancer survivors who had completed local and/or adjuvant therapy (with the exception of endocrine therapy) at least 6 months previously, had no evidence of active disease, had no other medical conditions or medications that would confound immune evaluation, and were experiencing persistent cancer-related fatigue. The presence of cancer-related fatigue was indicated by scores of 50 or below on the SF-36 energy/fatigue scale, a reliable and valid measure of energy/fatigue in the past month (24), and self-report that fatigue was a consequence of cancer or cancer therapy. Women were randomized to either a 12-week Iyengar yoga intervention (n = 16) or to a health education control condition (n = 15), as previously described (Bower et al., 2012). The yoga intervention, proposed by the Iyengar system to reduce fatigue, emphasized postures and breathing techniques that focus on passive inversions (i.e., supported upside-down postures in which the head is lower than the heart) and passive backbends (i.e., supported spinal extensions) (for description of all study postures, see Bower et al. (2012)). To evaluate the secondary outcomes of inflammation and related HPA activity, participants provided blood and saliva samples at baseline, post-intervention, and 3 months post-intervention. Blood draws were conducted before noon to control for diurnal influences on immune parameters. The research was approved by the UCLA Institutional Review Board and informed consent was obtained from all participants. The ClinicalTrials.gov identifier for this study is NCT00727662.
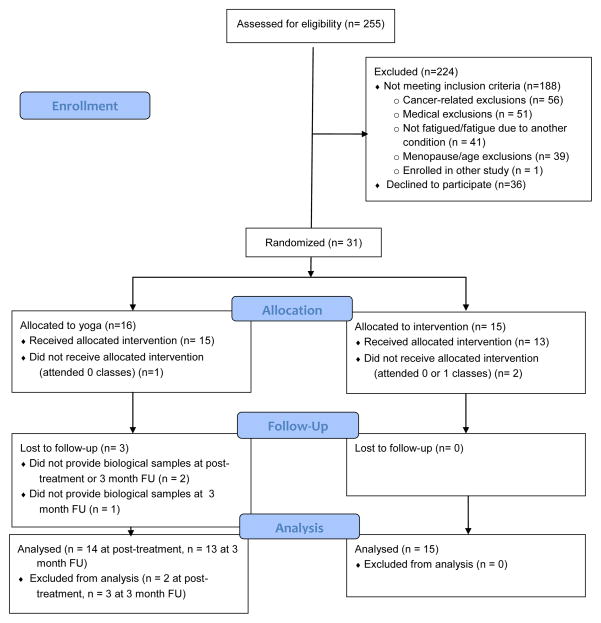
As indicated in Figure 1, three participants randomized to yoga were lost to follow-up, two at the post-intervention assessment and one at the 3 month follow-up. Samples were only analyzed for participants who provided at least one post-treatment assessment. Thus, analyses for inflammatory markers are based on 29 participants at post-intervention and 28 participants at the 3-month follow-up. One additional participant did not provide any saliva samples; thus, analyses for salivary cortisol (diurnal slope) were based on 28 participants at post-intervention and 27 at the 3-month follow-up.
Figure 1.
CONSORT flow diagram
Gene Expression Profiling and Bioinformatic Analysis
Blood samples for RNA collection were collected by venipuncture into PAXgene Blood RNA tubes. RNA was extracted (Qiagen PAXgene Blood RNA Kit; Qiagen Valencia CA), and subject to genome-wide transcriptional profiling using Illumina HT-12 v4 BeadArrays in the UCLA Neuroscience Genomics Core following the manufacturer’s standard protocol (Illumina Inc., San Diego CA). Quantile-normalized gene expression values were log2-tranformed before analysis. Differentially expressed genes were identified based on ≥ 15% difference in magnitude of pre- to post-intervention change in average gene expression in the treatment group vs. control (i.e., Group × Time interaction) in a mixed effect linear model analysis controlling for age, body mass index (BMI), breast cancer stage, cancer treatment (chemotherapy or endocrine therapy), and time since treatment completion.
TELiS promoter-based bioinformatics analyses (Cole et al., 2005) tested the hypothesis that leukocytes from fatigued breast cancer survivors randomized to yoga would show alterations in global gene expression profiles consistent with 1) decreased activity of the pro-inflammatory transcription factor NF-κB (assessed by prevalence of the TRANSFAC V$NFKB_Q6 nucleotide motif in differentially expressing promoters), 2) increased activity of the anti-inflammatory glucocorticoid receptor (GR) (V$GR_Q6), and 3) decreased activity of CREB transcription factors involved in β-adrenergic signaling by the sympathetic nervous system (SNS) (V$CREB_01). The ratio of response element frequencies in the promoters of up- vs. down-regulated genes was taken as a measure of differential activity of transcription control pathways, and (log) ratios were averaged over 9 different parametric combinations of promoter length (−300, −600, and −1000 to +200 bp upstream of RefSeq-designated transcription start site) and motif detection stringency (TRANSFAC mat_sim values of .80, .90, and .95) to ensure robust results (Cole et al., 2005). To identify the primary cellular sources of differentially expressed genes, we carried out Transcript Origin Analysis as previously described (Cole et al., 2011).
Circulating Inflammatory Markers
Blood samples for circulating inflammatory markers were collected by venipuncture into EDTA tubes, placed on ice, centrifuged for acquisition of plasma, and stored at −80°C for subsequent batch testing. Levels of these markers were quantified at the pre- and post-intervention assessments only due to funding constraints. We focused on downstream markers of pro-inflammatory cytokine activity because they are produced at higher levels and can often be quantified more reliably than the cytokines that induce their production, and may provide a more accurate and stable reflection of cytokine activity (Ferrucci et al., 2004). These included the soluble TNF receptor type II (sTNF-RII), a marker of TNF-α activity (Diez-Ruiz et al., 1995; Faustman and Davis, 2010); the IL-1 receptor antagonist (IL-1ra), a marker of IL-1β activity (Arend et al., 1998); and C reactive protein (CRP), a marker of IL-6 activity (Dayer et al., 2007). All of these markers have been associated with cancer-related fatigue in previous research (Bower et al., 2002; Collado-Hidalgo et al., 2006; Orre et al., 2009; Alexander et al., 2009; Bower et al., 2009). In addition, we assessed IL-6 based on earlier yoga trials showing effects on this cytokine (Pullen et al., 2008; Pullen et al., 2010).
Plasma levels of IL-1ra and sTNF-RII were determined by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocols, with a lower limit of detection of 31 and 234 pg/ml, respectively. IL-6 levels were determined using a high sensitivity ELISA (R&D Systems), with a lower limit of detection of 0.2 pg/ml. CRP levels were determined by a high sensitivity ELISA (Immundiagnostik, ALPCO Immunoassays, Salem, NH) according to the manufacturer’s protocol, but with an extended standard curve to a lower limit of detection of 0.2 mg/L. All samples were run in duplicate. The intra- and inter-assay precision of all tests was <5%, with the exception of IL-6, which had an inter-assay precision of 12%. Inflammatory markers were log transformed before analysis to normalize distributions.
Salivary Cortisol
Participants collected saliva samples at home using cotton swabs, or “Salivettes” (Sarstedt, Inc.) for determination of diurnal changes in salivary cortisol. Subjects were instructed to collect samples upon awakening, 30 minutes later, 8 hours later, and at bedtime for two consecutive days. Participants were instructed not to eat, drink, or brush their teeth for at least 15 minutes before sampling. The importance of collecting samples at designated times was emphasized at the initial appointment, and questionnaires were administered on each collection day to verify collection times and compliance with other instructions. Previous studies have demonstrated the reliability of self-reported saliva collection times among cancer patients and older populations (Kraemer et al., 2006; Dedert et al., 2012).
Participants stored samples in the refrigerator until both collection days were completed and then returned the samples to our laboratory either in person or via express mail delivery service. All samples were received within one week of collection; internal studies conducted by our laboratory have shown that saliva samples can be stored at room temperature for at least 7 days with no changes in salivary cortisol values, consistent with findings from other laboratories (Kirschbaum and Hellhammer, 1989). After receipt, saliva samples were frozen and stored at −80°C until analysis. After thawing, salivettes were centrifuged at 3,000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary concentrations were measured using commercially available chemiluminescence-immunoassay with high sensitivity (IBL International, Hamburg, Germany). The intra and interassay coefficients for cortisol were below 8%. All samples from a participant were analyzed in the same assay to minimize variability.
Cortisol values were log transformed and averaged across the two collection days after confirming via multilevel analyses that there was minimal between-day variability. The two measures of interest were diurnal cortisol slope and total daily cortisol output, as indexed by area under the curve. Diurnal cortisol slope was calculated by regressing hours since awakening on logged cortisol values after removing the 30 minutes post-awakening cortisol value. Area under the curve (AUC) was calculated with respect to ground using the trapezoidal formula with all four cortisol values (Pruessner et al., 2003).
Statistical Analyses
Mixed effect linear models were used to analyze all outcomes, with treatment (yoga, health education) and time as the independent factors. Note that there were three time points (baseline, post-intervention, and 3-month follow-up) for gene expression and cortisol measures, and two time points (baseline, post-intervention) for the circulating inflammatory markers due to funding constraints. Analyses controlled for age, BMI, breast cancer stage (0/I/II), use of chemotherapy (yes/no), use of endocrine therapy (yes/no), and time since treatment completion. For substantive interpretation of intervention-induced changes in cortisol level and gene expression within the overall 2 (Group) × 3 (Time) factorial design, we conducted follow-up planned contrast analyses of group differences in the repeated measures Time effect as previously described (Rosenthal & Rosnow, 1985). The 1-df contrast score (coefficients: baseline −1, post-intervention +.5, 3-month follow-up +.5), represents the magnitude of change from baseline to the average of post-intervention and 3-month follow-up values. Gene transcripts showing >15% difference between groups in the value of that contrast score were subject to bioinformatic interpretation (e.g., TELiS promoter analyses). To verify that similar changes in transcriptome regulation were observed at both immediate post-intervention and 3-month follow-up timepoints, we also conducted separate reparameterized analyses of group differences in the magnitude of change in gene expression from baseline to post-intervention, and from baseline to 3-month follow-up (again thresholding differential gene expression at 1.15-fold difference between groups and conducting TELiS promoter bioinformatics on the resulting gene lists). Because TELiS promoter analyses require mapping transcripts to a named human gene for analysis of promomoter DNA sequences, differentially expressed transcripts that did not map to a named human gene were excluded from analysis.
RESULTS
Participant characteristics and intervention effects on behavioral outcomes including the primary outcome of fatigue have previously been reported (Bower et al., 2012). Briefly, participants in the current study were on average 54 years old (SD = 5.4) and had been diagnosed with breast cancer on average 3.6 years previously (SD = 3.7). The majority had been diagnosed with either Stage 1 (35%) or Stage II (38%) cancer and had received chemotherapy (52%) and endocrine therapy (69%). Groups were balanced on demographic and disease-related characteristics at baseline with no significant group differences on these factors (all p > .05). Intervention adherence was good and comparable across groups, with women randomized to yoga attending an average of 78% of classes and women randomized to health education attending an average of 77% of classes. In terms of intervention effects on primary outcomes, there was a significant decrease in fatigue severity from baseline to post-intervention and over a 3 month follow-up in the yoga group relative to controls (Bower et al., 2012).
Genome-wide transcriptional profiling was carried out on blood samples collected at study baseline, post-intervention, and 3-month follow-up. Analyses comparing the magnitude of pre- to post-intervention change in gene expression across conditions identified 435 gene transcripts as showing ≥ 15% differential change over time (282 transcripts relatively up-regulated from baseline to the average of post-intervention and 3-month follow-up in the yoga intervention participants relative to controls, and 153 transcripts relatively down-regulated; listed in Supplementary Data Table 1). Particularly prominent among intervention-down-regulated genes were transcripts involved in Type I interferon responses (e.g., GBP1, GBP2, GBP4, GBP5, IFI44, IFI44L, IFIH1, IFIT1, IFIT2, IFIT3, IFIT5, IFITM3, OAS1, OAS2, and STAT1).
The primary analyses examined intervention effects on transcription control pathways related to inflammation. In TELiS promoter-based bioinformatic analyses of genes showing differential change in expression over time in yoga vs. control subjects, results indicated reduced activity of NF-κB (mean prevalence ratio = 0.65 ± 0.10 standard error, p = .0211), increased activity of the glucocorticoid receptor (mean ratio = 1.28 ± 0.07 standard error, p = .0040), and reduced activity of CREB (mean ratio = 0.80 ± 0.07 standard error, p = .0314) (see Figure 2A). Although not initially hypothesized, results also indicated significant down-regulation of interferon-activated transcription factors (V$ISRE: mean ratio = 0.29 ± 0.08 standard eror, p = .0009). Transcript origin analyses identified genes down-regulated in intervention participants as originating primarily from monocytes (p = .0178) and NK cells (p = .0002) (see Figure 2B). No leukocyte subset was identified as predominately contributing intervention up-regulated genes (all p > .16). Similar results emerged in separate analyses of change in gene expression from baseline to the immediate post-intervention assessment (yoga – control group difference in change over time: NF-κB = 0.47 ± .20, p = .0263; GR = 1.45 ± .08, p = .0061; ISRE = 0.42 ± .11, p < .0001; although CREB did not differ significantly at this time-point: CREB = 1.02 ± .21, p = .6629) and from baseline to 3-month follow-up (yoga – control group difference in change over time: NF-κB = 0.57 ± .38, p = .0018; GR = 1.09 ± .02, p = .0123; ISRE = 0.58 ± .18, p = .0105; CREB = 0.70 ± 1.03, p < .0001).
Figure 2.
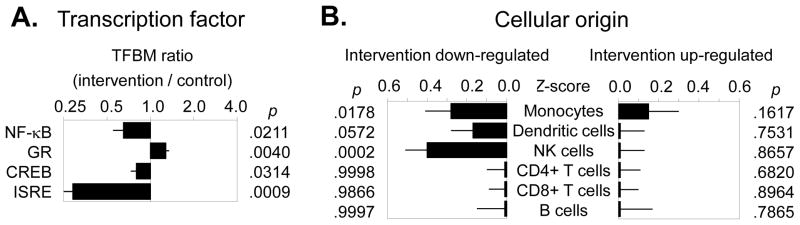
(A) Bioinformatics analysis of transcription factor activity indicated reduced activity of NF-κB, increased activity of the glucocorticoid receptor (GR), and reduced activity of CREB in the yoga group vs. health education controls. In addition, analyses revealed reduced activity of interferon-related transcription factors (ISRE). (B) Transcript origin analyses identified genes down-regulated in intervention participants as originating primarily from monocytes and NK cells.
The observed group × time interaction in transcription control pathway activity was driven predominately by down-regulation of pro-inflammatory signaling in the intervention group. The two groups did not differ at baseline in indicated activity of NF-κB, GR, or ISRE (all p > .05) and CREB activity was indicated to be lower in the yoga group than in control participants at baseline (0.75 ± .07, p = .0125; i.e., prior to the subsequent further reduction following the intervention). Within yoga group participants, analyses indicated significant reductions over time in activity of NF-κB (0.45 ± .07, p = .0003) and CREB (0.69 ± .05, p = .0009), and significant increases over time in activity of the GR (1.26 ± .06, p = .0026) and ISRE factors (1.23 ± .08, p = .0193). Control group participants showed no significant change over time in the activity of any of these transcription control pathways (all p > .05).
Intervention Effects on Circulating Inflammatory Markers
Levels of inflammatory markers at baseline and post-intervention are shown in Table 1. Groups were comparable at baseline on all markers, with no significant group differences (all p > .05). Results of mixed model analyses showed a significant group × time interaction for sTNF-RII (p = .032), controlling for age, BMI, breast cancer stage, cancer treatment, and time since treatment completion. Post-hoc examination of within-group changes in sTNF-RII showed a significant increase in the health education group (p < .05), whereas levels remained relatively stable in the yoga group. A similar pattern was observed for IL-1RA, with significant increases observed in the health education group, although the overall group × time interaction was not significant (p = .16). There was no group × time interaction for either IL-6 or CRP (ps > .40).
Table 1.
Mean Levels of Inflammatory Markers in Yoga and Health Education Groups
| Inflammatory Markers | Baseline Mean (SD) |
Post-intervention Mean (SD) |
p for group × time interaction* |
|---|---|---|---|
| soluble TNF receptor type II (pg/ml) | 0.03 | ||
| Iyengar Yoga | 2261 (297) | 2188 (255) | |
| Health Education | 2123 (390) | 2506 (845) | |
|
| |||
| IL-1 receptor antagonist (pg/ml) | 0.16 | ||
| Iyengar Yoga | 220.9 (95) | 230.9 (96) | |
| Health Education | 279.4 (250) | 349.9 (272) | |
|
| |||
| IL-6 (pg/ml) | 0.87 | ||
| Iyengar Yoga | 1.03 (0.54) | 1.19 (0.83) | |
| Health Education | 1.37 (0.69) | 1.64 (1.22) | |
|
| |||
| CRP (mg/L) | 0.70 | ||
| Iyengar Yoga | 2.85 (6.39) | 2.22 (3.92) | |
| Health Education | 1.52 (2.08) | 2.38 (3.59) | |
Raw values for inflammatory markers are provided here for descriptive purposes; log transformed values were used in all analyses.
P values represent effects for group × time interactions controlling for age, BMI, breast cancer stage, cancer treatment, and time since treatment completion.
Intervention Effects on Salivary Cortisol Measures
Salivary cortisol measures at baseline, post-intervention, and 3 month follow-up are shown in Table 1. Mixed model analyses were conducted to examine intervention effects on diurnal cortisol slope and area under the curve (AUC). There were no significant differences between groups at baseline on these measures (ps > .05). In analyses adjusting for age, body mass index, breast cancer stage, cancer treatment, and time since treatment completion, there was no significant group × time interaction for either outcome. Planned contrasts also found no significant differences in the magnitude of change in cortisol slope or AUC from baseline to the average of both follow-up timepoints (ps > .05).
DISCUSSION
Results from this randomized, controlled trial provide initial evidence that an Iyengar-based yoga intervention designed to ameliorate fatigue not only improves that symptom but also leads to alterations in molecular signaling pathways associated with inflammation in fatigued breast cancer survivors. Specifically, promoter-based bioinformatics analysis indicated decreased pro-inflammatory NF-κB-related gene expression among women randomized to 12 weeks of Iyengar yoga relative to health education controls. Previous observational studies have documented elevated inflammatory activity in breast cancer survivors with persistent fatigue relative to non-fatigued controls, including increased NF-κB-signaling (Bower et al., 2011a). Results from the current trial suggest that a yoga intervention targeting fatigue may help dampen inflammatory signaling in this patient population.
The intervention also had an impact on circulating inflammatory markers, particularly the soluble TNF receptor type II. Women randomized to yoga showed relatively stable plasma levels of sTNF-RII over the 12-week intervention, whereas women in the health education group showed increases in sTNF-RII over this period. The sTNF-RII is shed from a cell surface following engagement by the proinflammatory cytokine TNF-α, and therefore serves as a plasma marker of TNF activity (Diez-Ruiz et al., 1995). We have previously shown elevated concentrations of sTNF-RII in fatigued breast cancer survivors (Bower et al., 2002; Bower et al., 2011b), suggesting that this marker may have particular relevance for cancer-related fatigue. A similar pattern was observed for IL-1RA, which remained stable in the yoga group and increased in the health education group, although this effect was not significant. No intervention effects on IL-6 or CRP were observed.
Only a handful of previous studies have examined yoga effects on markers of inflammation. There is preliminary evidence that mind-body interventions that incorporate some component of yoga (i.e., mindfulness-based stress reduction, yogic meditation) reduce inflammatory signaling through the NF-κB control pathway (Creswell et al., 2012; Black et al., 2013), consistent with our results. In addition, there is preliminary evidence that yoga can influence circulating inflammatory markers. Two randomized trials conducted with heart failure patients found decreases in CRP and IL-6 after an 8-week yoga intervention (Pullen et al., 2008; Pullen et al., 2010); of note, initial levels of these markers were considerably higher in the heart failure patients relative to the breast cancer survivors in our trial, which may explain why changes in CRP and IL-6 were not observed in the current study. In a non-randomized yoga trial conducted among patients with more comparable levels of inflammatory markers, there was a significant decrease in plasma levels of TNF-α but no change in plasma IL-6 among female participants (Yadav et al., 2012). This pattern is generally similar to our results, with intervention effects observed for sTNF-RII but not for IL-6, although in our trial this effect was driven by an increase in sTNF-RII levels among control group participants. Previous studies comparing movement-based therapies to health education have also shown increases in inflammatory markers in the control group. For example, Nicklas and colleagues observed increases in circulating levels of CRP and IL-6 among older individuals randomized to a health education control condition at the 6 month assessment relative to those randomized to exercise, whose levels remained relatively stable at this assessment point (Nicklas et al., 2008). In observational studies of older adults, chronic stress is associated with immune dysregulation, including an increase in circulating inflammatory markers over time (Kiecolt-Glaser et al., 2003). It is possible that we are observing a similar trend in TNF-α activity among control group participants in the current study, which is buffered among yoga group participants. Longer-term follow-up is needed to determine whether circulating markers eventually decline in the yoga group, following the decrease in inflammatory signaling noted here in transcriptome-based bioinformatics.
What are the mechanisms through which yoga might influence inflammatory processes? The current results suggest two potential pathways. First, we found that yoga significantly increased the expression of genes bearing glucocorticoid receptor (GR) response elements, indicating increased glucocorticoid signal transduction. Previous research has documented decreased GR-mediated gene expression among breast cancer survivors with persistent fatigue, which may contribute to chronic inflammation (Bower et al., 2011a). If this intervention causes glucocorticoid receptors to become more sensitive to the anti-inflammatory effects of cortisol, allowing more of this hormonal signal to be “heard” by the cell, this may lead to decreases in inflammatory signaling such as those observed in the current trial. Of note, we did not find significant changes in cortisol production following the yoga intervention, as indexed by diurnal cortisol slope and total daily cortisol production (AUC). In addition, effects of yoga on GR signaling remained significant in analyses controlling for salivary cortisol measures. The few previous yoga trials that have collected saliva samples over the course of the day for assessment of salivary cortisol have yielded inconsistent results, with intervention effects seen at specific times of day but not on summary measures of cortisol rhythm (slope) or output (AUC) (Vadiraja et al., 2009; Banasik et al., 2011). Overall, our results suggest that yoga may influence the sensitivity of GRs, rather than daily cortisol production, although further research is needed to determine the impact of yoga on specific aspects of the HPA axis. It may also be helpful to assess blood cortisol levels, which may provide a more robust measure of yoga effects (Yadav et al., 2012).
A second potential pathway for yoga’s anti-inflammatory effects may be alterations in sympathetic nervous system signaling. Bioinformatic analyses indicated reduced activity of CREB family transcription factors in yoga arm participants, which may indicate reduced sympathetic nervous system signaling through β-adrenergic receptors (Sanders and Straub, 2002). β-adrenergic signaling can activate NF-κB and upregulate transcription of pro-inflammatory cytokine genes (Bierhaus et al., 2003; Irwin and Cole, 2011); thus, decreases in CREB factors may lead to reductions in inflammatory processes. Two previous studies have documented alterations in autonomic nervous system function among experienced yoga practitioners following one or more sessions of Iyengar-based yoga, including reduced sympathetic and increased parasympathetic activity (Khattab et al., 2007; Kiecolt-Glaser et al., 2010). Other practices involving meditative movement, such as Tai Chi, also lead to decreases in sympathetic activity among experienced practitioners (Motivala et al., 2006). The impact of yoga on autonomic nervous system activity and corresponding changes in inflammation is a promising direction for future research.
In addition to effects on inflammatory and neuroendocrine-related signaling pathways, results indicated down-regulation of genes involved in Type I interferon (IFN) responses and reductions in IFN-related transcription factors. Treatment with IFN-α is known to cause symptoms of fatigue in patients with melanoma and hepatitis C (Capuron and Miller, 2004), though links with cancer-related fatigue outside the context of IFN treatment have not previously been documented. Fatigue is associated with elevated antibody titers to cytomegalovirus (CMV) in breast cancer patients (Fagundes et al., 2012), suggesting an association between latent viral reactivation (and possible increases in IFN signaling pathways) and cancer-related fatigue. Regardless of etiology, the present data suggest an additional biological signaling dynamic beyond classical pro-inflammatory cytokines that may contribute to cancer-related fatigue and potentially mediate the salutary effects of yoga.
This study had several limitations, most notably the small, highly selected sample. For this initial trial, we restricted participation to breast cancer survivors with persistent cancer-related fatigue who were otherwise healthy and willing to participate in a time-consuming intervention program. Given the limited sample size in this study, the present gene expression analyses are not powered to discover new statistically significant associations between experimental condition and the expression of any given transcript. The sets of differentially expressed genes reported here serve only as inputs into higher-order gene set-based bioinformatics analyses testing a limited number of a priori hypotheses regarding shared transcription factor promoter motifs (i.e., inflammation-related NF-κB, GR, and CREB factors) and shared cellular origin (i.e., pro-inflammatory monocytes) as documented in previous gene expression reference studies. None of the specific transcript-experimental condition point estimates in Supplemental Table 1 should be interpreted as statistically reliable. As a related limitation, the number of biological outcomes examined was high relative to the number of participants; though all outcomes were selected based on a priori hypotheses about biological underpinnings of fatigue, results should be regarded are preliminary. It will be important to replicate these findings in a larger trial, and to determine if effects are generalizable to a broader group of breast cancer survivors.
In addition, although the inclusion of an active control group is an important strength of the study, the yoga and health education conditions were not matched for class frequency or duration and it is possible that benefits seen in the yoga group may be attributable in part to the higher number of intervention hours received. Future studies should match class frequency and duration across conditions. It will also be informative to compare yoga to more physically-oriented control conditions (e.g., stretching, walking) and to relaxation to probe the active components of this intervention. Finally, future research should include a longer-term follow-up to determine the persistence of effects on behavioral and biological outcomes.
Our findings add to a small but growing body of literature on the anti-inflammatory effects of mind-body interventions and specifically the molecular processes that regulate expression of inflammation-related genes. Randomized controlled trials of cognitive-behavioral stress management for breast cancer patients (Antoni et al., 2012), mindfulness-based stress reduction for older adults (Creswell et al., 2012), and Kirtan Kriya Meditation (a yogic meditation chanting practice) for family dementia caregivers (Black et al., 2013) have all documented significant reductions in NF-κB-related gene expression. Results from the current trial suggest that an Iyengar-based, restorative yoga program also leads to alterations in inflammation-related transcriptional control pathways, which may have enduring effects on inflammatory processes (Slavich and Cole, 2013). Given the importance of inflammation for behavioral symptoms (Miller et al., 2008) and disease outcomes (Pierce et al., 2009) in cancer patients, interventions that reduce inflammation hold significant promise in this population.
Supplementary Material
Table 2.
Mean levels of Salivary Cortisol Measures in Yoga and Health Education Groups
| Salivary Cortisol | Baseline Mean (SD) |
Post-intervention Mean (SD) |
3 month follow-up Mean (SD) |
p for group × time interaction |
|---|---|---|---|---|
| Diurnal slope | 0.37 | |||
| Iyengar yoga | −.117 (.053) | −.102 (.082) | −.124 (.047) | |
| Health Education | −.104 (.049) | −.129 (.097) | −.093 (.057) | |
|
| ||||
| Area under the curve | 0.64 | |||
| Iyengar yoga | 28.75 (7.21) | 32.08 (7.64) | 29.85 (5.75) | |
| Health education | 28.49 (4.22) | 29.27 (5.85) | 28.18 (5.66) | |
|
| ||||
| Cortisol levels at each assessment (nmol/l) | ||||
| Iyengar yoga | ||||
| Waking | 18.80 (6.97) | 18.25 (9.60) | 22.30 (12.60) | |
| 30-minutes post waking | 25.38 (9.04) | 27.56 (9.54) | 24.33 (10.20) | |
| 8 hours post waking | 6.21 (2.30) | 8.84 (5.17) | 6.90 (3.36) | |
| Bedtime | 3.43 (1.82) | 5.35 (4.35) | 3.45 (1.72) | |
| Health education | ||||
| Waking | 14.28 (6.40) | 16.64 (4.41) | 13.71 (5.81) | |
| 30-minutes post waking | 20.66 (9.71) | 21.61 (7.89) | 20.59 (8.84) | |
| 8 hours post waking | 5.75 (1.71) | 7.20 (3.24) | 5.57 (2.21) | |
| Bedtime | 3.11 (2.07) | 4.22 (3.97) | 2.98 (1.24) | |
P values represent effects for group × time interactions in analyses controlling for age, BMI, breast cancer stage, cancer treatment, and time since treatment completion. Raw cortisol values at each assessment point are provided for descriptive purposes; log-transformed values were used for computation of diurnal slope and AUC.
Acknowledgments
We wish to acknowledge Manouso Manos, Senior Iyengar yoga instructor, who provided guidance and support in designing the yoga intervention for this study. We also thank Dr. Clemens Kirschbaum for conducting the cortisol assays and Dr. Elizabeth Breen for conducting the assays for the circulating inflammatory markers. In addition, we thank Lindsey Knowles for her assistance with constructing the diurnal cortisol variables.
ROLE OF FUNDING SOURCES
This study was supported by NCCAM/NIH U01 AT003682, the Oppenheimer Seed Grant Program in Complementary, Alternative, and Integrative Medicine at UCLA, and the Inflammatory Biology Core and the UCLA Older Americans Independence Center (NIH/NIA Grant P30-AG028748).
Footnotes
CONTRIBUTORS
JB designed and oversaw the conduct of the study, including data collection, analysis, and interpretation. GG collaborated on study design and intervention implementation, oversaw medical aspects of the study, and collaborated on data analysis, and interpretation. AC conducted all cortisol analyses. DG oversaw data collection. BS designed and conducted the yoga classes. PA and MI collaborated on study design, conduct, data analysis, and interpretation. RO conducted data analyses. JA conducted the gene expression assays. SC conducted the gene expression analyses. All authors contributed to and have approved the final manuscript.
CONFLICT OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Wilder Smith A, Meeske K, McTiernan A, Bernstein L, Baumgartner KB, Ulrich CM, Ballard-Barbash R. Fatigue, Inflammation, and ω-3 and ω-6 Fatty Acid Intake among Breast Cancer Survivors. J Clin Oncol. 2012;30:1280–1287. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo JM, Cole SW. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23:135–142. doi: 10.1111/j.1745-7599.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci US A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Cole SW, Irwin MR, Breen E, St Cyr NM, Nazarian N, Khalsa DS, Lavretsky H. Yogic meditation reverses NF-kappaB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38:348–355. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011a;25:147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011b;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, Greendale G. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118:3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Woolery A, Sternlieb B, Garet D. Yoga for cancer patients and survivors. Cancer Control. 2005;12:165–171. doi: 10.1177/107327480501200304. [DOI] [PubMed] [Google Scholar]
- Bussing A, Michalsen A, Khalsa SB, Telles S, Sherman KJ. Effects of yoga on mental and physical health: a short summary of reviews. Evid Based Complement Alternat Med. 2012a;2012:165410. doi: 10.1155/2012/165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussing A, Ostermann T, Ludtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012b;13:1–9. doi: 10.1016/j.jpain.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci US A. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics. 2005;21:803–810. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26:1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer E, Dayer JM, Roux-Lombard P. Primer: the practical use of biological markers of rheumatic and systemic inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:512–520. doi: 10.1038/ncprheum0572. [DOI] [PubMed] [Google Scholar]
- Dedert E, Lush E, Chagpar A, Dhabhar F, Segerstrom S, Spiegel D, Dayyat E, Daup M, McMasters K, Sephton S. Stress, Coping, and Circadian Disruption Among Women Awaiting Breast Cancer Surgery. Ann Behav Med. 2012;44:10–20. doi: 10.1007/s12160-012-9352-y. [DOI] [PubMed] [Google Scholar]
- Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Alfano CM, Bennett JM, Povoski SP, Lipari AM, Agnese DM, Yee LD, Carson WE, III, Farrar WB, Malarkey WB, Kiecolt-Glaser JK. Fatigue and herpesvirus latency in women newly diagnosed with breast cancer. Brain Behav Immun. 2012;26:394–400. doi: 10.1016/j.bbi.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9:482–493. doi: 10.1038/nrd3030. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Ble A, Bandinelli S, Lauretani F, Suthers K, Guralnik JM. A flame burning within. Aging Clin Exp Res. 2004;16:240–243. doi: 10.1007/BF03327390. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab K, Khattab AA, Ortak J, Richardt G, Bonnemeier H. Iyengar yoga increases cardiac parasympathetic nervous modulation among healthy yoga practitioners. Evid Based Complement Alternat Med. 2007;4:511–517. doi: 10.1093/ecam/nem087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF, Glaser R. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72:113–121. doi: 10.1097/PSY.0b013e3181cb9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci US A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Giese-Davis J, Yutsis M, O’Hara R, Neri E, Gallagher-Thompson D, Taylor CB, Spiegel D. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatr Psychiatry. 2006;14:325–333. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Sollers J, Thayer J, Irwin MR. Tai Chi Chih acutely decreases sympathetic nervous system activity in older adults. J Gerontol A Biol Sci Med Sci. 2006;61:1177–1180. doi: 10.1093/gerona/61.11.1177. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise Training and Plasma C-Reactive Protein and Interleukin-6 in Elderly People. J Amer Geriatrics Society. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fosså SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain, Behav, Immun. 2009;23:868–874. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Orre IJ, Reinertsen KV, Aukrust P+, Dahl AA, Fosså SD, Ueland T, Murison R. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71:136–141. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pullen PR, Nagamia SH, Mehta PK, Thompson WR, Benardot D, Hammoud R, Parrott JM, Sola S, Khan BV. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail. 2008;14:407–413. doi: 10.1016/j.cardfail.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Pullen PR, Thompson WR, Benardot D, Brandon LJ, Mehta PK, Rifai L, Vadnais DS, Parrott JM, Khan BV. Benefits of yoga for African American heart failure patients. Med Sci Sports Exerc. 2010;42:651–657. doi: 10.1249/MSS.0b013e3181bf24c4. [DOI] [PubMed] [Google Scholar]
- Qu S, Olafsrud SM, Meza-Zepeda LA, Saatcioglu F. Rapid gene expression changes in peripheral blood lymphocytes upon practice of a comprehensive yoga program. PLoS ONE. 2013;8:e61910. doi: 10.1371/journal.pone.0061910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub JA. Psychophysiologic effects of Hatha Yoga on musculoskeletal and cardiopulmonary function: a literature review. J Altern Complement Med. 2002;8:797–812. doi: 10.1089/10755530260511810. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rubin DB. Contrast analysis: Focused comparisons in the analysis of variance. Cambridge University Press; Cambridge, UK: 1985. [Google Scholar]
- Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- Sharma H, Datta P, Singh A, Sen S, Bhardwaj NK, Kochupillai V, Singh N. Gene expression profiling in practitioners of Sudarshan Kriya. J Psychosom Res. 2008;64:213–218. doi: 10.1016/j.jpsychores.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The Emerging Field of Human Social Genomics. Clin Psychol Sci. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadiraja HS, Raghavendra RM, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, Gopinath KS, Srinath BS, Vishweshwara MS, Madhavi YS, Ajaikumar BS, Ramesh BS, Nalini R, Kumar V. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8:37–46. doi: 10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- Yadav RK, Magan D, Mehta N, Sharma R, Mahapatra SC. Efficacy of a short-term yoga-based lifestyle intervention in reducing stress and inflammation: preliminary results. J Altern Complement Med. 2012;18:662–667. doi: 10.1089/acm.2011.0265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.