Abstract
We explored cortical fields on the upper bank of the Sylvian fissure using functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG) to measure responses to two stimulus conditions: a tactile stimulus applied to the right hand and a tactile stimulus with an additional movement component. fMRI data revealed bilateral activation in S2/PV in response to tactile stimulation alone and source localization of MEG data identified a peak latency of 122 ms in a similar location. During the tactile and movement condition, fMRI revealed bilateral activation of S2/PV and an anterior field, while MEG data contained one source at a location identical to the tactile-only condition with a latency of 96 ms and a second rostral source with a longer latency (136 ms). Furthermore, Region-of-interest analysis of fMRI data identified increased bilateral activation in S2/PV and the rostral area in the tactile and movement condition compared with the tactile only condition. An area of cortex immediately rostral to S2/PV in monkeys has been called the parietal rostroventral area (PR). Based on location, latency, and conditions under which this field was active, we have termed the rostral area of human cortex PR as well. These findings indicate that humans, like non-human primates, have a cortical field rostral to PV that processes proprioceptive inputs, both S2/PV and PR play a role in somatomotor integration necessary for manual exploration and object discrimination, and there is a temporal hierarchy of processing with S2/PV active prior to PR.
Introduction
Primates are especially skilled at manual exploration and use their hands for object manipulation and tactile identification. As in other sensory systems, tactile sensory reception is an active process that requires a precise and efficient interface between receptor arrays and the object to be identified. Thus to discriminate objects, sensory receptors located in abundance on the hands, particularly the fingertips, are moved over the object to be explored, and the object itself is being moved within and between the hands. Discriminating features of the object such as size and shape can be extracted from the shape of the hand and the articulation of the digits with respect to each other, both of which are being re-configured during haptic shape exploration. Thus tactile discrimination and identification require precise manual manipulation and a rapid and efficient interface between both mechanosensory and proprioceptive inputs and the motor system.
Recent noninvasive imaging studies provide a good deal of information regarding areas of the cortex involved in somesthesis (Blankenburg et al. 2003; Fox et al. 1987; Moore et al. 2000) or motor control (Kwong et al. 1992; Sanes et al. 1995; Toma et al. 1999) in anterior parietal and motor cortex, respectively. However, much less is known about sensorimotor integration outside of primary cortical areas. One candidate location for sensorimotor integration is the second somatosensory area (S2) and surrounding cortex, located on the upper bank and parietal operculum of the lateral (Sylvian) sulcus. S2 receives inputs from portions of the thalamus that process inputs from deep receptors of the skin, muscles and joints (such as VPi and VPs) (see Disbrow et al. 2002; Friedman and Murray 1986) and surrounding fields in turn are densely connected with a number of structures in parietal and frontal cortex, including posterior parietal and premotor areas (Disbrow et al. 2003; Krubitzer and Kaas 1990; Lewis and Van Essen 2000).
Recently it has been shown that the S2 region in humans, as described by Penfield (Penfield and Jasper 1954), is actually comprised of a number of cortical fields on the upper bank of the Sylvian sulcus. One of these, the parietal ventral (PV) area, was first described in squirrels (Krubitzer et al. 1986) and is now known to exist in many mammals, including both New (Coq et al. 2004; Krubitzer and Kaas 1990; Qi et al. 2002) and Old World monkeys (Krubitzer et al. 1995) as well as humans (Disbrow et al. 2000; Eickhoff et al. 2006). Area PV, rostral to S2, contains a mirror-symmetric representation of the body surface joined at the hands, face, and feet. Neurons in both S2 and PV have large, overlapping receptive fields, often responding to deep, cutaneous and bilateral stimulation of the skin and joints (Disbrow et al. 2000, 2001; Krubitzer et al. 1995; Robinson and Burton 1980a,c). Recent work by Fitzgerald and colleagues (2006a,b) indicates that neurons in this region respond to an oriented stimulus and that receptive fields cover multiple digits. These investigators propose that these fields combine cutaneous and proprioceptive inputs to determine the size and shape of objects.
Although S2 and PV are clearly involved in tactile processing, converging lines of evidence from multiple species suggest that these fields are also involved in sensorimotor integration. Like S2, PV is densely connected with posterior parietal and premotor cortex as well as portions of the thalamus, such as VPi (Disbrow et al. 2003; Qi et al. 2002), which processes inputs from deep receptors of the hand and forelimb (Kaas et al. 1984). Ablations of S2/PV and surrounding fields in the macaque produce performance deficits for tasks that require sensorimotor integration, such as texture discrimination and shape judgements (Murray and Mishkin 1984). In the human, several studies have demonstrated that activity in the S2 region is modulated by movement of the distal extremities (Inoue et al. 2002; Wasaka et al. 2005; Xiang et al. 1997). This activity could represent feedback information from deep receptors during different body postures. Activity in the human S2 region is also modulated by isometric muscle contraction when the muscle contracted is near the topographic representation of a body structure being concurrently stimulated (Lin et al. 2000).
In addition to areas S2 and PV, two other fields in the lateral sulcus of primates have been identified that may process movement related inputs. Caudal to S2 is area 7b, which has been well described in non-human primates (Dong et al. 1994; Hyvarinen 1981; Leinonen et al. 1979; Neal et al. 1987; Robinson and Burton 1980b,c; Vogt and Vogt 1919). Rostral to PV, an area termed the parietal rostroventral area (PR) has been described in prosimian Galagos (Wu and Kaas 2003), owl monkeys (Stepniewska et al. 2006), marmoset monkeys (Krubitzer and Kaas 1990; Qi et al. 2002), titi monkeys (Coq et al. 2004), and macaque monkeys (Disbrow et al. 2002, 2003). Both PV and S2 have dense connections with PR and 7b, respectively (Coq et al. 2004; Disbrow et al. 2003). Neurons in 7b respond to visual (Dong et al. 1994; Leinonen et al. 1979; Robinson and Burton 1980c) and noxious (Dong et al. 1994; Robinson and Burton 1980c) stimulation, and Robinson and Burton (1980c) reported that passive somatosensory stimulation produced activity in about 50% of neurons in 7b. More recently Fitzgerald and colleagues (2004) described rostral and caudal zones in the S2 region in which single neurons responded well during active grasping of objects.
Less is known about the second area PR, which is located rostral to PV. In macaque monkeys, neurons in regions of cortex bordering S2/PV rostrally and caudally are less sensitive to stimulus orientation than those in S2/PV and are largely unresponsive to simple somatosensory stimuli (Fitzgerald et al. 2006b). In humans, PR and 7b were active during passive tactile stimulation of the hand, but responses were not consistent across subjects (63% of subjects for PR, 31% of subjects in 7B) (see Disbrow et al. 2000). Further, both PR and 7b have connections with premotor, posterior parietal and primary motor cortex in non-human primates (Disbrow et al. 2003; Padberg et al. 2005; Qi et al. 2002). The complex response properties and distributed connection patterns of S2, PV, PR, and 7b make them potential sites for sensorimotor integration, but this hypothesis has never been directly tested.
The purpose of the present study was to determine the sites in human cortex at which tactile, proprioceptive, and potentially motor inputs converge, and the temporal sequence of processing of the different areas associated with this sensorimotor integration using both functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG). Thus we determined whether movement tasks generated activity in S2/PV and neighboring cortical fields such as PR and 7b. Our second issue of interest focuses on the temporal sequence of processing across cortical areas. Although feedforward connections from the lateral sulcus provide indirect evidence that tactile information is sent to primary and premotor cortex to guide movement (see Qi et al. 2002 for review), there is little physiological evidence to support such a claim. The exquisite temporal resolution of MEG recorded during these sensorimotor tasks will provide a reliable measure of activity in these fields of the Sylvian sulcus over time. Combining data from these two techniques will allow us to determine where in Sylvian cortex the convergence of somatosensory and motor inputs occurs, and when this convergence takes place.
Methods
fMRI
Subjects
Thirteen healthy subjects (5 men, 8 women, all right handed, aged 25–50 yr) participated in these experiments. Written consent from each participant was provided prior to the experiment, and procedures were approved in advance by the Committee of Human Research at the University of California, San Francisco. Subjects were screened for neurological conditions as well as contraindications for MRI.
Stimuli
There were two stimulus conditions. In the first condition, tactile stimulation was applied to the right hand by moving a smallpored sponge (12.7 × 7.6 × 1.25 cm) back and forth across the surface of the digits and palm at a rate of ∼ 1 Hz. During this task, the subjects were instructed to remain still and hold both their left and right hands flat, palm side up while the investigator applied the tactile stimulus across their right hand. The second condition had a movement component. In this condition, the subject held the sponge in their right hand and squeezed it at a rate of ∼ 1 Hz. For this task, the hand was initially held flat, the sponge was placed in the hand and a power grip was utilized such that digits 2–4 curled toward the palm and digit 1 curled over digits 2 and 3. The subjects were simply instructed to lightly squeeze the sponge, and this instruction yielded the stereotypic movement described in the preceding text for all subjects. For both conditions, the subjects' right forearm and hand were stabilized with a series of cushions so that they could effortlessly hold their hand flat on the surface beside them with the palm facing upward. For the tactile condition, the stimulus was presented at a steady rate by the same experimenter for all subjects. For the tactile + movement condition, all subjects were trained prior to data collection to match their rate of squeezing to approximately the same rate that the tactile stimulus was applied by the experimenter during the tactile condition.
Data acquisition
fMRI was performed using a standard clinical GE 1.5 Tesla scanner and a whole head coil (GE Medical Systems, Milwaukee, WI). Anatomical high-resolution three-dimensional steady precession gradient-recalled (3DSPGR) series (acquisition: axial, interleaved, 256 × 256 matrix, FOV 40 times; 40; 124 slices, 1-mm slice thickness, repetition time = 35 ms, echo time = 6 ms, flip angle = 30°) were acquired, and an echo planar gradient echo imaging sequence (repetition time = 2 s, echo time = 60 ms, flip angle = 60°) was used. A 256 × 128 matrix was used with a field of view of 40 × 20 cm, a slice thickness of 5 mm (0.5-mm gap) and thus a voxel (3-dimensional pixel) size of 1.56 × 1.56 × 5 mm. A single fMRI scan (1 stimulus condition alternated with rest every 20 s) lasted 2 min, 20 s, during which a total of 70 repetitions of the brain image (7 slices) were collected. The brain was scanned from just dorsal to the lateral ventricles to the middle temporal sulcus. During scanning, each subject's head was held in position with a plastic pillow (Olympic Vac-Pac, Olympic Medical, Seattle WA) filled with Styrofoam packing beads. The air was removed from the pillow so that it became rigid and conformed to the contours of the head. Subjects were instructed to remain still, keeping their eyes closed during each scan.
Data analysis and display were done using SPM2 (www.fil.ion.ucl.ac.uk/spm). Statistical maps were generated using a “boxcar” response model smoothed with a hemodynamic response function. The functional data were motion corrected and spatially smoothed with an 8-mm Gaussian window. Data were spatially normalized to a modified EPI template covering the volume of the brain partially acquired during fMRI scans. To generalize our finding to the population (second-level analysis), contrast images of activity between stimulus presentation/movement task and rest for each subject were entered into a one-tailed t-test in SPM2. Statistical maps were thresholded at P < 0.05 and corrected for multiple comparisons (false discovery rate). Coordinates in MNI space were converted to Talairach coordinates using nonlinear transforms (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). Regions of interest (ROIs) were drawn in three areas of the lateral sulcus (PR, S2/PV, 7b), and data were extracted from each subject's spatially normalized data using the MARSBAR toolbox (marsbar.sourceforge.net). Each ROI was defined as a 1-cm sphere centered on the coordinates for S2/PV obtained through the group analysis of the tactile dataset as well as a region 2 cm rostral (PR) and 2 cm caudal (7b) to the S2/PV global maxima. Data were extracted from the three ROIs from each hemisphere for each subject and normalized to percent signal change from baseline (mean signal intensity during rest) prior to statistical analysis. Activations in anterior parietal cortex were evaluated relative to sulcal patterns and the Talairach Daemon database (Lancaster et al. 2000).
MEG
Subjects
Twelve healthy subjects (8 men, 4 women, all right handed, aged 25–50 yr; not the same subjects used for the fMRI study) participated in these experiments. Written consent from each participant was provided prior to the experiment, and procedures were approved in advance by the Committee of Human Research at the University of California, San Francisco. Subjects were screened for neurological conditions as well as contraindications for MEG.
Stimuli
Again, there were two stimulus conditions. Tactile stimuli consisted of pneumatically driven mechanical taps (25 psi) applied through a balloon diaphragm (1-cm diam) placed over the distal fingertip of the subject's right index finger (Fig. 1). The stimulus duration was 30 ms. A pseudo-randomized interstimulus interval (3,500–4,500 ms) was included between taps. In the first condition, 250 taps were applied to the right hand while subjects remained passive. In the second condition, the same tactile stimulus (250 taps, ISI = 3,500–4,500 ms) was used, and a movement component was added. Order of presentation (tactile or tactile + movement) was randomized across subjects, and each condition consisted of a single run lasting an average of 17 min.
Fig. 1.
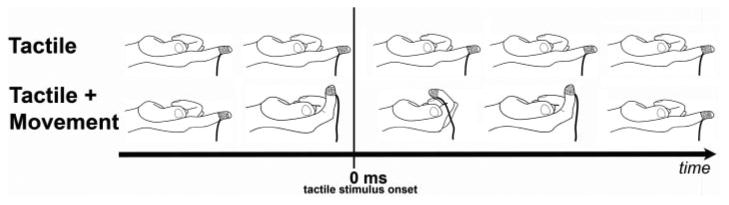
Illustration of pneumatic stimulator used in the magnetoencephalographic (MEG) experiment (
 ) and attached air line (—). In both tactile and tactile + movement conditions, stimuli were delivered at 25 psi for 30 ms to the 2nd digit of the right hand (RD2). During the tactile + movement condition, subjects curled their finger (flexion) prior to the administration of the tactile stimulus. To maintain constant position of the stimulator on the digit pad, surgical tape was used to secure the device to RD2.
) and attached air line (—). In both tactile and tactile + movement conditions, stimuli were delivered at 25 psi for 30 ms to the 2nd digit of the right hand (RD2). During the tactile + movement condition, subjects curled their finger (flexion) prior to the administration of the tactile stimulus. To maintain constant position of the stimulator on the digit pad, surgical tape was used to secure the device to RD2.
In both conditions, the subjects were instructed to remain still and to hold their right hand palm side up with D2 extended. D3-5 remained curled toward, but were not touching, the palm. Subjects held their left hand still in a comfortable resting position. In the first condition, only a tactile stimulus was applied, and data averaging was time locked to stimulus onset. In the second condition, subjects flexed D2 while the other digits remained still. During flexion, the tactile (tap) stimulus was administered, and then subjects extended their finger to the starting position to repeat the movement. Because the balloon diaphragms were attached to the fingers with small plastic clips, it was possible to tap the fingers while the subject moved them. Subjects were trained prior to data collection to ensure that movement of the finger was executed at ∼0.5 Hz, that it was smooth and continuous throughout the recording session, and that the movement across subjects was stereotypic. Each subject coordinated movement of the finger in such a way that the stimulus delivery never coincided with onset of finger movement, that is, the finger tap did not cue the movement (Fig. 1). As in the tactile only condition, data acquisition was time-locked to tactile stimulus onset. Due to technical constraints, the stimulator used in the MEG experiment could not be used for fMRI data collection. Although the types of tactile stimuli (mechanical taps vs. sponge) differ between the fMRI and MEG data sets, both techniques consist of cutaneous tactile stimulation alone, and tactile stimulation with an added movement component. No direct comparisons were made between MEG and fMRI data.
Data Acquisition
Neuromagnetic fields were recorded in a shielded room using a 37-channel biomagnetometer system (Magnes, BTi, San Diego, CA). Epochs of 500-ms duration (plus 100-ms pre-stimulus) were acquired with a 1.0-Hz high-pass cutoff and a sampling rate of 1 kHz. In all subjects, data were acquired from the left hemisphere during stimulation of the right index finger. The sensor array was positioned over somatosensory cortex and optimized for recording from the S2 region of the lateral sulcus. In both conditions, epoch data that were time-locked to somatosensory stimulus onset were averaged and band-pass filtered (8–40 Hz) before additional analysis.
For the calculation of theoretical source generators, we used brain electric source analysis (BESA) software (BESA 2000, NeuroScan, McLean, VA) in a single spherical shell model. Sources were initially “seeded” near the expected location of primary somatosensory cortex. Sources were fit one at a time over the course of the waveform with no restrictions on source location or orientation. An iterative leastsquares fit was performed with the addition of each dipole into the solution. Additional dipoles, up to five sources, were added until the residual variance (RV), or variance not explained by the model, fell to <10%, at which point no additional sources were added to avoid overfitting noise (Scherg 1992; Scherg and Berg 1991). After dipole locations were fixed, solution amplitude was calculated.
To evaluate the appropriateness of the addition of the final (4th) dipole to the somato-motor condition, the four-dipole solution derived from each subject's tactile + movement run was applied to the data from the tactile only condition. Source waveforms for the fourth dipole in the tactile only data were then subtracted from waveforms from the same dipole location in the tactile + movement data to examine amplitude differences. Remaining late-field sources localizing to cortex in the lateral sulcus as a result of this subtraction would indicate that sensorimotor integration extends beyond processing in just S1 and S2/PV.
The computed dipoles were co-registered to individual subjects' MR images (3DSPGR sequence, TR/TE/Flip angle = 35 ms/6 ms/60°, 1-mm spatial resolution) to determine their location in an anatomic context. To co-register source locations with subjects' MR images, an anatomic reference frame was established using a digital sensor position indicator. Receivers were used to triangulate the signal from the indicator placed at fiducial reference points on each subject's head surface. These points were used to define the MEG reference frame in which the source localization was described. Radiological identification of these fiducials on high-resolution MRI allowed for the transformation of MEG space into the anatomic (MRI) coordinate system and the anatomical registration of the MEG sources. The resolution of this technique is < 1 cm, depending on noise in the data and accuracy of fiducial identification.
Results
Anterior parietal fields
Functional MRI revealed consistent activation in and around the central sulcus in response to tactile stimulation of the hand alone (Fig. 2A). Based on sulcal landmarks and Talaraich coordinates, this area likely includes anterior parietal fields 3b and 1 and potentially 3a and 2 as well although stimulation was predominantly cutaneous. This activation was largely contralateral, although a small region of activation was also seen ipsilateral to stimulation (Fig. 2A). Table 1 contains mean Talairach coordinates for the peak signal intensity for the ROIs. In the MEG data, we identified two peaks in the waveforms (Fig. 3) in which sources localized over the central sulcus of the contralateral hemisphere in response to tactile stimulation (Fig. 2B, Table 2), with mean latencies of 56.9 ± 30.64 (SD) ms and 96.8 ± 33.64 ms. There were no significant differences in the localization coordinates for these two anterior parietal sources, which were located in a region similar to the fMRI activation, in areas 3a, 3b, 1, and 2. The mean residual variance for this two-dipole solution was <10% in 11 of the 12 subjects (RV <10%). In only a single subject was a single source identified in anterior parietal fields.
Fig. 2.
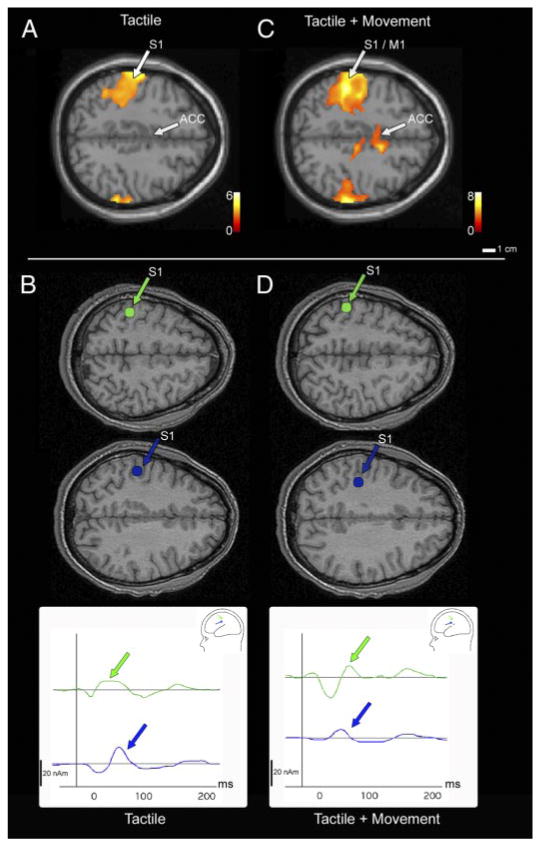
Axial views of activity along the central sulcus (CS) for both a functional magentic resonance imaging (fMRI) second-level (random effects) group analysis and source localizations of MEG responses from a single subject during the tactile (A and B) and tactile + movement (C and D) conditions. For fMRI, activity was present along the CS (likely corresponding to areas 3b and 1) in response to tactile stimulation alone (A). During the tactile + movement condition, activity along the CS was more extensive and likely encompassed primary motor cortex as well as areas 3b and 1; anterior cingulate cortex (ACC) was active as well (C). Group analysis fMRI data are superimposed on the colin.27 atlas. In MEG, earlier latencies localized along the CS (in blue and green) in both the tactile (B) and tactile + movement (D) conditions. Dipole localizations are superimposed on an axial view from the subject's MRI. In all views, the left hemisphere is above, anterior to the right and lateral to the left and right of midline.
Table 1. Talairach coordinates for group fMRI data.
| ML (x) | AP (y) | S (z) | TIValue | |
|---|---|---|---|---|
| Tactile | ||||
| S1 contra | −51 | −27 | 46 | 4.27 |
| S1 ipsi | 63 | −23 | 42 | 4.43 |
| S2/PV contra | −50 | −36 | 20 | 5.56 |
| S2/PV ipsi | 57 | −32 | 18 | 4.37 |
| Tactile + movement | ||||
| S1 contra | −46 | −29 | 42 | 8.02 |
| S1 ipsi | 61 | −29 | 42 | 7.54 |
| M1 contra | −57 | −29 | 38 | 6.26 |
| M1 ipsi | 59 | −16 | 28 | 8.45 |
| S2/PV contra | −53 | −23 | 16 | 5.48 |
| S2/PV ipsi | 59 | −26 | 18 | 6.20 |
| PR contra | −55 | 5 | 13 | 6.82 |
| PR ipsi | 55 | 7 | 16 | 6.62 |
Fig. 3.
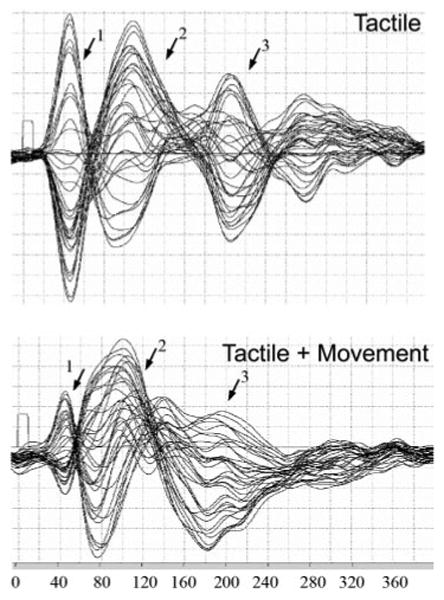
Graph of MEG data shows magnetic field strength over time from all 37 sensors drawn with a common baseline. Top: activation in response to tactile stimulation of the 2nd digit of the right hand (RD2). ↓, S1 (1, 2) and late-field (3) S2/PV peaks. Bottom: activation in response to finger flexion concurrent with RD2 tactile stimulation. ↓, primary somatosensory (1, 2) peaks as well as a late-field signal (3) that resulted in source localization in both S2/PV and PR.
Table 2. Locations for MEG source estimates.
| ML (x) | AP (y) | SI (z) | Latency | nA | |
|---|---|---|---|---|---|
| Tactile | |||||
| S1 (Early) | −37.3 ± 4.9 | 10.5 ± 9.3 | 95.8 ± 8.9 | 56.9 ± 30.6 | 9.8 ± 4.3 |
| S1 (Late) | −30.7 ± 21.4 | 8.5 ± 10.0 | 95.6 ± 13.3 | 97.8 ± 33.6 | 16.0 ± 9.2 |
| S2/PV | −42.7 ± 4.6 | 11.2 ± 7.3 | 67.5 ± 6.0 | 121.9 ± 42.0 | 19.8 ± 8.2 |
| Tactile + movement | |||||
| S1 (Early) | −31.8 ± 8.7 | 12.5 ± 8.1 | 96.2 ± 10.5 | 65.3 ± 12.9 | 10.4 ± 7.7 |
| S1 (Late) | −36.2 ± 7.7 | 7.1 ± 10.7 | 91.1 ± 13.5 | 89.2 ± 24.1 | 10.8 ± 6.5 |
| S2/PV | −38.1 ± 8.9 | 10.5 ± 8.1 | 72.7 ± 8.2 | 96.4 ± 26.9 | 17.6 ± 10.3 |
| PR | −37.3 ± 10.1 | 19.6 ± 10.2 | 64.4 ± 11.6 | 136.3 ± 43.4 | 14.8 ± 6.9 |
Values are means ± SD. MEG, magnetoencephalography.
The tactile and movement stimulus resulted in an increased area of fMRI activation in and around the central sulcus (Fig. 2C). A second-level group analysis revealed two overlapping ROIs corresponding to primary motor cortex (M1) and anterior parietal somatosensory cortex bilaterally (Table 1). As with anterior parietal fields, M1 was defined by its location relative to the central sulcus and its Talairach coordinates. Further, significant activation was observed in anterior cingulate cortex (ACC; Fig. 2C). Similarly, in the MEG experiments, two sources were identified during tactile and movement stimulation localizing to anterior parietal cortex with mean response latencies of 65.3 ± 12.9 and 89.2 ± 24.1 ms. The mean locations and latencies of these sources were not significantly different from the locations of the two anterior parietal sources during the tactile only condition and thus were in the presumptive areas 3a, 3b, 1, and 2 (Fig. 2D). Given that movement onset preceded tactile stimulation and that a movement-evoked response was not time-locked to tactile stimulation, it is not surprising that we were unable to either localize the activity in motor cortex identified in the fMRI study or demonstrate “gating effects” described elsewhere (Forss and Jousmaki 1998; Nakata et al. 2003) that have been shown to strongly attenuate activity in S1 prior to movement onset (Wasaka et al. 2005). There was no significant difference in amplitude of the early S1 peak between tactile + movement and tactile only conditions (9.8 ± 4.29 vs. 10.4 ± 7.7 nA, respectively). The amplitude of the late S1 peak (Fig. 3), however, was reduced somewhat in the tactile + movement condition as compared with the tactile only condition (10.75 ± 6.51 vs. 15.96 ± 9.22 nA, respectively) although the difference was not significant and the variance was large.
Sylvian fissure
In the fMRI experiment, a second-level random effects group analysis identified somatosensory cortex along the upper bank of the Sylvian fissure of both hemispheres active in response to tactile stimulation alone (Fig. 4A, Table 1). One large area of activation was identified in all subjects, although the location of this activation varied slightly between subjects (Fig. 5). The Talairach coordinates for this active region correspond to the hand representations in S2 and PV as has been previously characterized in detail in humans (Disbrow et al. 2000, 2001; Ruben et al. 2001). Although ipsilateral activation was observed in 10 of the 13 subjects, in 4 of these subjects, the signal magnitude (percent signal change) of the ipsilateral activation was slightly smaller than the contralateral activation. Three of 13 subjects also had an additional area of activation rostral to S2/PV in response to tactile stimulation only—an example is shown in Fig. 5. In the MEG experiment, a single magnetic source was identified on the upper bank of the Sylvian fissure (Fig. 4B, Table 2) in the tactile-only condition of 11 subjects with a mean latency of 121 ± 42.0 ms. In only 1 of the 12 subjects was this three-dipole model insufficient, requiring a fourth source to be fit to the data that localized rostral to the S2/PV source. The three-dipole solution (anterior parietal fields and S2/PV) accounted for an average of 92.8% of the variance. The mean location of this single source was significantly anterior (8.7 mm, P < 0.05) and inferior (28.2 mm, P < 0.001) to the anterior parietal sources and corresponded to the location of the fMRI activation in S2/PV.
Fig. 4.
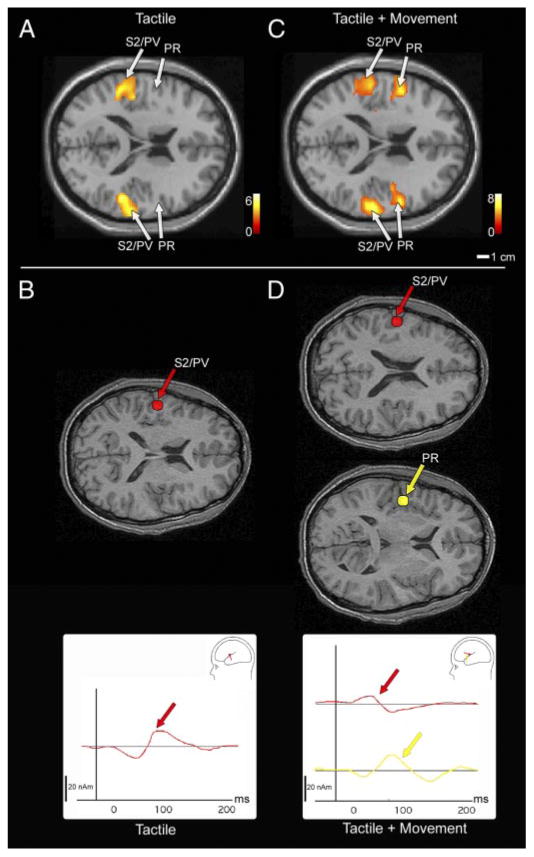
Axial views of activity along the Sylvian fissure (SF) for both a fMRI second-level (random effects) group analysis and source localizations of MEG responses from a single subject during the tactile (A and B) and tactile + movement (C and D) conditions. For fMRI, bilateral activity on the upper bank of the SF corresponding to S2/PV was consistent in all subjects in the tactile only condition (A). In the tactile + movement condition, both S2/PV and an anterior portion of cortex in area PR were active in both hemispheres in the fMRI group analysis (C). Group analysis fMRI data are superimposed on the colin.27 atlas. In MEG, during the tactile-only condition, a single late-field source localized to S2/PV (B; in red), whereas in the tactile + movement condition both the S2/PV source as well as a 4th source (D; in yellow) localizing rostral to S2/PV in area PR was identified. The PR source was absent during the tactile only condition. Dipole localizations are superimposed on an axial view from the subject's MRI. In all views, left hemisphere is above, anterior to the right and lateral to the left and right of midline.
Fig. 5.
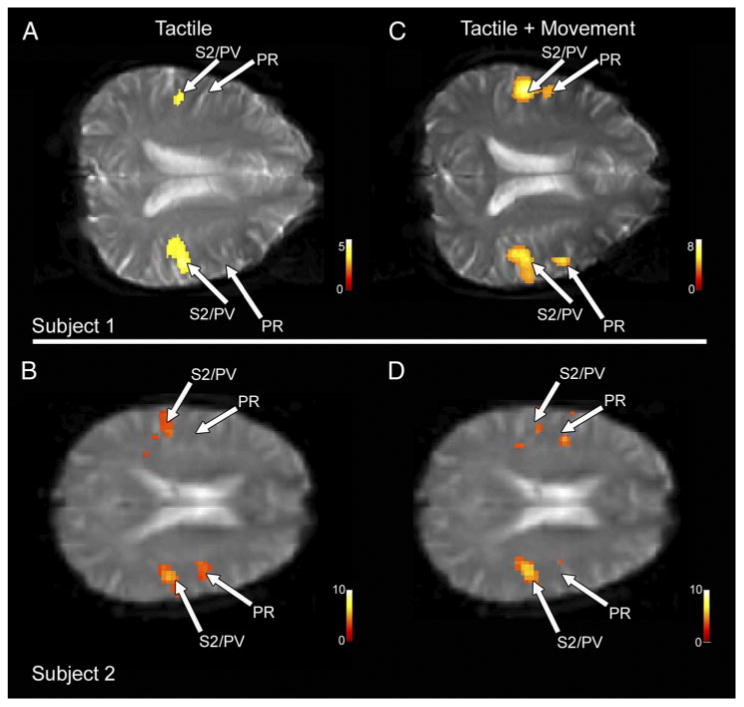
fMRI data from 2 subjects from the tactile (A and B) and tactile + movement (C and D) conditions. While tactile stimulation consistently drove activity in S2/PV alone (A and B), in 3 of the 13 subjects, passive tactile stimulation resulted in activation in PR as well (B). Bilateral PR (anterior to S2/PV) was active in the majority of subjects during the tactile + movement condition (C and D), although bilateral activation was not robust in 4 of the 13 subjects (D). Views are axial, with left hemisphere above, anterior to the right of the figure and lateral left and right of midline.
During the tactile and movement condition, a second-level group analysis of fMRI data indicated significant activation in S2/PV in both hemispheres. S2/PV was active contralaterally in all (13/13) subjects. A ROI analysis of the group data identified a significant difference in percent signal change between the tactile and tactile + movement task in the contralateral hemisphere (P < 0.05). In the ipsilateral hemisphere, 11 of 13 subjects showed activation in S2/PV during tactile + movement stimulation, and this activity was significantly greater than when a tactile stimulus was presented alone (P < 0.01).
For the movement condition, we identified a region of cortex rostral to S2/PV in both hemispheres that was significantly active in a group analysis (Fig. 4C). The Talairach coordinates for this more anterior region of cortex were ∼2 cm rostral to S2/PV. Based on the location and the stimulus conditions to which it was active, we have termed this region of human cortex PR. PR was active in 11 of 13 subjects in the contralateral hemisphere and 9 of 13 subjects in the ipsilateral hemisphere (see examples in Fig. 5). Although 2 of 13 subjects had an additional area of activation posterior to S2/PV, cortex in this putative area 7b was not consistently active in the group analysis (Fig. 4C). Data extracted from 7b of the left and right hemispheres in an ROI analysis demonstrated that there was no significant difference in 7b activity between the tactile and tactile + movement conditions (contralateral hemisphere, P = 0.13; ipsilateral hemisphere, P = 0.7).
Similar responses in the Sylvian fissure during the tactile and movement condition were identified using MEG. Both one- and two-source models seeded in the Sylvian fissure were used to analyze the data based on the fMRI results (e.g., Ahlfors et al. 1999). With a single source in the S2/PV region, the mean residual variance was 12.8%, with a residual variance >10% in 9 of the 12 subjects. To reduce the residual variance below our threshold of 10%, a second source in the S2 region was added and reduced the mean residual variance to 5.4%. The first source had a mean latency of 96.4 ± 26.9 ms and localized to the S2/PV area identified in the tactile only condition (Table 2). There was no significant difference for this S2/PV source in either source location or mean latency between the tactile and tactile + movement conditions. The second source in the Sylvian fissure was located rostral to the S2/PV source and corresponded to the location of activation in the fMRI dataset that we identified as PR (Fig. 4D). PR was located a significant distance anterior (9.1 ± 6.5 mm) to the S2/PV source (P < 0.01, Table 2, Fig. 4) and tended to be inferior [8.3 ± 14.3 mm, P = 0.08], although this difference was not significant. Also, the PR source had a significantly longer latency (136.3 ± 43.4 ms, P < 0.05) than the latency of the peak in S2/PV.
Two additional analyses confirmed that activity in PR was present only during the tactile + movement condition. fMRI data were extracted from ROIs centered on PR in the left and right hemispheres. Percentage signal change was significantly larger in both the contralateral (P < 0.01) and ipsilateral PR (P < 0.05) during tactile and movement versus tactile alone stimulation. In the MEG experiment, the four source solution defined in the tactile + movement condition for each subject was applied to their tactile only data, and the residual data from the fourth dipole was then subtracted from a waveform at the same source location in the tactile + movement data. Ten of 12 subjects showed a residual peak in the PR latency range, indicating a significant contribution of the fourth source in the model of the tactile-movement data (Fig. 6). Thus both fMRI and MEG data show a second source in the Sylvian fissure selectively active during the tactile + movement condition.
Fig. 6.
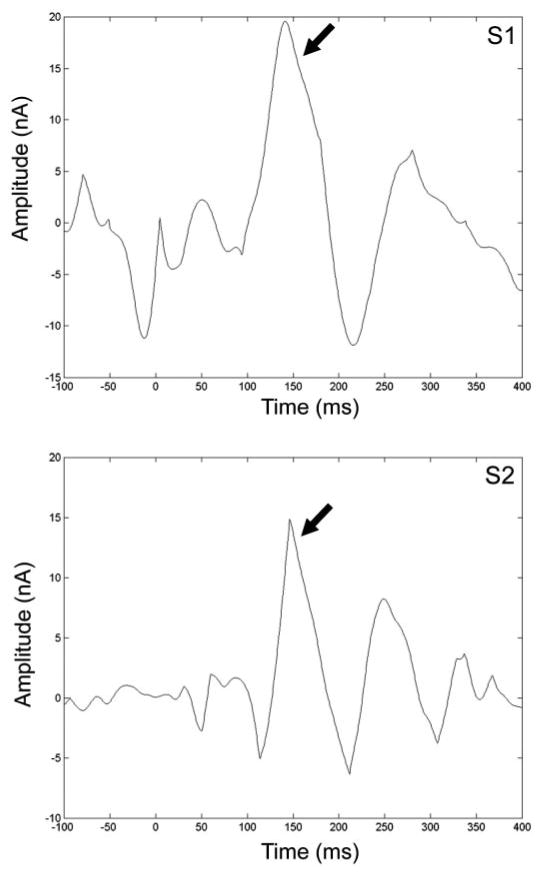
Subtraction waveforms ([tactile + movement] − [tactile]) from 2 subjects showing changes in field strength over time at the location of the 4th dipole defined in each subject's tactile + movement solution. In both subjects, a late-field peak (↓) was identified in the tactile + movement data that could not be accounted for by data present in the tactile only condition at the same source location.
Discussion
In the present study, tactile stimulation across the surface of the right hand produced activity in anterior parietal fields 3a, 3b, 1, and 2 and bilateral activity in S2/PV in all subjects as measured using fMRI and MEG. However, when a movement task was conducted in the presence of tactile stimulation, an additional region on the upper bank of the Sylvian fissure, PR, was also consistently active. fMRI results demonstrated that this additional active area was 1 cm rostral to S2/PV (Table 1), and the MEG data indicated that it was active later than sources in S2/PV (Table 2). A ROI analysis of fMRI data revealed an increase in the percent signal change during the tactile and movement condition versus tactile condition alone in both PR and S2/PV bilaterally, whereas in area 7b, neither the tactile nor the tactile + movement condition resulted in consistent activity. In conjunction with previous studies in human and non-human primates, these findings suggest that humans, like non-human primates, have a cortical field rostral to PV that processes proprioceptive inputs, both S2/PV and PR play a role in proprioception necessary for discrimination of object size and shape, and there is a temporal hierarchy of processing with S2/PV being activated prior to PR.
Factors that contribute to the activation in S2/PV and PR
A clear result from our study was that the S2/PV region was consistently active under the tactile stimulation condition alone, whereas both S2/PV and PR were consistently active under the tactile + movement condition. This result demonstrates that S2/PV is involved in processing tactile information, whereas PR is involved in proprioception and that both are associated with the motor system, possibly by integrating cutaneous, proprioceptive, and motor inputs necessary for fine motor control. There are several factors that may contribute to the activation of these fields during movement. For example, in S2 and PV, the most obvious is that these fields are processing proprioceptive inputs from the muscles and joints in addition to cutaneous inputs. Receptors from each would be active during the tactile + movement task. The notion that these fields are processing proprioceptive input is supported by neuroanatomical studies in macaque monkeys that demonstrate that S2 and PV receive inputs from nuclei of the thalamus such as VPi and VPs that ultimately receive inputs from proprioceptors (Disbrow et al. 2002; Qi et al. 2002) as well as thalamic nuclei and cortical fields that process cutaneous inputs such as VP (Friedman and Murray 1986; Jones and Powell 1970) and areas 3b and 1 (Cusick et al. 1989; Disbrow et al. 2003). Thus both fields have access to cutaneous and proprioceptive inputs, and information regarding the relative position of the digits, joint articulation, and muscle contraction are necessary for object manipulation and ultimately determination of object size and shape. Consistent with the role of S2 and PV in these abilities, recent electrophysiological recording studies in macaque monkeys demonstrate that neurons in the S2/PV region respond to an oriented stimulus and have large receptive fields that cover multiple digits (Fitzgerald et al. 2006a,b). These investigators propose that these fields combine cutaneous and proprioceptive inputs to determine size and shape of objects.
The notion that S2/PV is not only part of the somatosensory system but equally involved in motor processing and integration of inputs comes from previous work in humans that demonstrates that the S2 region is active not only during passive and active finger movements (Wasaka et al. 2005; Xiang et al. 1997) but during movement imagery as well (Kakigi et al. 1997). Further, Forss and Jousmaki (1998) reported that although electrical median nerve stimulation drove activity in S2/PV of both hemispheres, this response was enhanced in both hemispheres when combined with isometric muscle contraction of the hand. The S2 region is also active during complex tasks involving discriminations of roughness and length of an object (Ledberg et al. 1995) and is associated with complex object manipulation in monkeys (Murray and Mishkin 1984) and humans (Binkofski et al. 1999) although the amplitude of response in humans is not modulated by the complexity of the movement (Huttunen et al. 1996).
Our results suggest that, like S2 and PV, PR also plays a major role in integrating different subclasses of inputs across the hand to enable on-line manual alterations necessary for active exploration and discrimination of features of an object. This result is supported by recent work in monkeys (Fitzgerald et al. 2004) that demonstrates that neurons located in both the anterior (possibly PR) and posterior extents of the S2 region responded to proprioceptive stimulation with some cells active during grasping. In contrast, cells in the central region, possibly corresponding to S2/PV, responded best to cutaneous stimulation. Data from neuroanatomical studies in monkeys demonstrate that both S2 and PV project to a rostral field on the upper bank of the lateral sulcus, presumably PR (Coq et al. 2004; Disbrow et al. 2003; Friedman et al. 1986; Krubitzer and Kaas 1990), and it is PR that sends information directly to motor cortex (Fig. 7) (Qi et al. 2002). Further, PV in macaque monkeys receives and sends projections to premotor cortex (Disbrow et al. 2003).
Fig. 7.
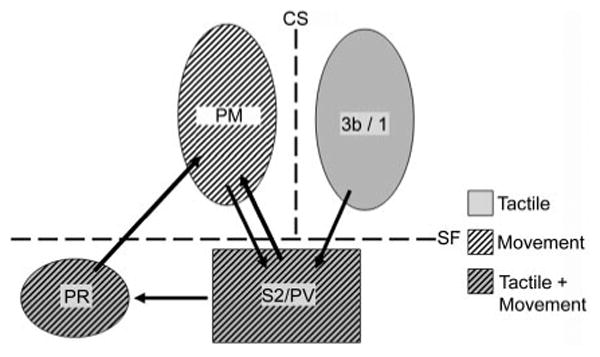
Summary of processing between somatosensory and motor areas during sensorimotor integration in humans. Converging tactile and movement information from cortex along the central sulcus (CS) is integrated in the S2/PV regions on the upper bank of the Sylvian fissure (SF). Sensorimotor information is then relayed to an anterior cortical field, the parietal rostroventral region. Conventions as in previous figures.
Another factor that likely contributes to the activation in S2/PV and PR is the difference in attention demand between the tactile alone condition and the tactile + movement condition with the latter task having a larger demand. Support for this proposition comes from studies in both human and non-human primates. In monkeys, the majority of neurons in the S2/PV region of cortex change both their firing rate and the form of response with attention directed to a tactile discrimination task versus a visual task (Hsiao et al. 1993). Although a different paradigm was utilized, similar attention effects on neuronal firing were observed in the S2 region by Burton and colleagues (Burton et al. 1997). In humans, the magnitude of blood flow, measured with positron emission tomography (PET), increases in the S2 region with tasks requiring either directed or split attention (Burton et al. 1999), and in MEG studies, tasks that require active attention increase the activity in the S2 region (Mima et al. 1989).
Serial processing and sensorimotor integration
The late-field latency of sources localizing to the S2/PV region in the human Sylvian fissure in both the tactile alone and tactile + movement conditions suggest that they are active after processing in anterior parietal fields. In humans, connections between S1 and S2 have never been directly examined, but such connectivity has been inferred previously based on the latency difference between the somatosensory-evoked fields (SEFs) from S1 and the S2 region as measured using EEG and MEG recordings (see Lin and Forss 2002 for a review). Although there is increasing evidence for parallel processing in the somatosensory system (Karhu and Tesche 1999; Zhang et al. 1996, 2001), support for serial processing between primary and secondary somatosensory fields comes from neuroanatomical studies of connectivity, cortical deactivation studies, and MEG studies in macaque monkeys and humans (Bohlhalter et al. 2002; Felleman and Van Essen 1991; Garraghty et al. 1990; Inui et al. 2004; Pons et al. 1992). More recent studies have shown that although both S2 and PV receive afferents from areas 3b and 1, which process cutaneous inputs, and from area 3a, which processes inputs from proprioceptors (Disbrow et al. 2003; Qi et al. 2002), they also project to posterior parietal areas (Disbrow et al. 2003), including cortex representing the hands in the anterior intraparietal sulcus (AIP) (Lewis and Van Essen 2000).
In addition to the direct, feed-forward connections between S1 and S2/PV demonstrated in monkeys, S2/PV also receives inputs from divisions of the thalamus that process inputs from cutaneous and deep receptors (e.g., VP, VPi and VPs) (see Disbrow et al. 2002; Friedman and Murray 1986; Qi et al. 2002) as well as from nuclei associated with the motor system (e.g., VL) (Disbrow et al. 2002; Friedman and Murray 1986; Jones and Powell 1970). These data suggest that S2/PV receives convergent inputs from different sources of kinesthetic, somatic, and motor systems and has associations with subcortical and cortical motor structures. These anatomical pathways could provide the substrate for fast and precise manual movements that require constant on-line feedback from mechanosensory and proprioceptive systems.
In the present study, MEG sources localizing rostral to S2/PV in the tactile-movement condition, in PR, occurred even later than their S2/PV counterparts, by an average of 30 ms. Studies done in non-human primates have indicated that PR has connections with both S2/PV and primary motor cortex (Coq et al. 2004; Disbrow et al. 2003; Qi et al. 2002). Therefore the late-field sources we identified in PR could represent a later stage of processing in tactile-motor integration, combining deep somatic, cutaneous, and kinesthetic information from S2/PV for eventual relay to motor cortex (Fig. 7).
The network of connectivity among S2/PV, PR, and motor cortex has led some investigators to suggest that specialization within primate somatosensory cortex is eventually integrated in PR for motor output (see Krubitzer and Kaas 1990). Our data suggest that in humans, area PR plays an integral role in combining multiple somatic inputs from a variety of sources and that the integration of these inputs occurs after somesthetic processing in anterior parietal fields and S2/PV.
Acknowledgments
The authors thank H. Ingraham for assistance with data collection.
Grants: This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS-35103 to L. A. Krubitzer and R01 NS-44590 to E. A. Disbrow and S. S. Nagarajan.
References
- Ahlfors SP, Simpson GV, Dale AM, Belliveau JW, Liu AK, Korvenoja A, Virtanen J, Huotilainen M, Tootell RBH, Aronen HJ, Ilmoniemi RJ. Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. J Neurophysiol. 1999;82:2545–2555. doi: 10.1152/jn.1999.82.5.2545. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolati G, Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 1999;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruben J, Meyer R, Schwiemann J, Villringer A. Evidence for a rostral-to-caudal somatotopic organization in human primary somatosensory cortex with mirror-reversal in areas 3b and 1. Cereb Cortex. 2003;13:987–993. doi: 10.1093/cercor/13.9.987. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Fretz C, Weder B. Hierarchical versus parallel processing in tactile object recognition: a behavioral-neuroanatomical study of aperceptive tactile agnosia. Brain. 2002;125:2537–2548. doi: 10.1093/brain/awf245. [DOI] [PubMed] [Google Scholar]
- Burton H, Abend NS, MacLeod AMK, Sinclair RJ, Snyder AZ, Raichle ME. Tactile attention tasks enhance activation in somatosensory regions of parietal cortex: a positron emission tomography study. Cereb Cortex. 1999;9:662–674. doi: 10.1093/cercor/9.7.662. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, Hong SY, Pruett JR, Jr, Whang KC. Tactile-spatial and cross-modal attention effects in the second somatosensory and 7b cortical areas of rhesus monkeys. Somatosens Mot Res. 1997;14:237–267. doi: 10.1080/08990229770971. [DOI] [PubMed] [Google Scholar]
- Coq JO, Qi HX, Collins CE, Kaas JH. Anatomical and functional organization of somatosensory areas of the lateral fissure of the New World titi monkey (Callicebus moloch) J Comp Neurol. 2004;476:363–387. doi: 10.1002/cne.20237. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Wall JT, Felleman DJ, Kaas JH. Somatotopic organization of the lateral sulcus of owl monkeys: Area 3b, s-II, and a ventral somatosensory area. J Comp Neurol. 1989;282:3169–190. doi: 10.1002/cne.902820203. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Slutsky D, Krubitzer L. Thalamocortical connections of the parietal ventral area (PV) and the second somatosensory area (S2) in macaque monkeys. Thalamus Relat Syst. 2002;1:289–302. [Google Scholar]
- Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol. 2000;418:1–21. doi: 10.1002/(sici)1096-9861(20000228)418:1<1::aid-cne1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Roberts T, Poeppel D, Krubitzer L. Evidence for interhemispheric processing of inputs from the hands in human S2 and PV. J Neurophysiol. 2001;85:2236–2244. doi: 10.1152/jn.2001.85.5.2236. [DOI] [PubMed] [Google Scholar]
- Dong WK, Chudler EH, Sugiyama K, Roberts VJ, Hayashi T. Somatosensory, multisensory and task-related neurons in cortical area 7b (PF) of unanesthetized monkeys. J Neurophysiol. 1994;72:542–564. doi: 10.1152/jn.1994.72.2.542. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Pramodsingh HT, Hsiao SS. Receptive fields properties of the macaque second somatosensory cortex: Representation of orientation on different finger pads. J Neurosci. 2006a;26:6473–6484. doi: 10.1523/JNEUROSCI.5057-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Pramodsingh HT, Hsiao SS. Receptive fields properties of the macaque second somatosensory cortex: RF size, shape and somatotopic organization. J Neurosci. 2006b;26:6485–6495. doi: 10.1523/JNEUROSCI.5061-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field properties of the macaque second somatosensory cortex: evidence for multiple functional representations. J Neurosci. 2004;24:11193–11204. doi: 10.1523/JNEUROSCI.3481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss N, Jousmaki V. Sensorimotor integration in human primary and secondary somatosensory cortices. Brain Res. 1998;781:259–267. doi: 10.1016/s0006-8993(97)01240-7. [DOI] [PubMed] [Google Scholar]
- Fox PT, Burton H, Raichle ME. Mapping human somatosensory cortex with positron emission tomography. J Neurosurg. 1987;67:34–43. doi: 10.3171/jns.1987.67.1.0034. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA. Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the macaque. J Comp Neurol. 1986;252:348–373. doi: 10.1002/cne.902520305. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O'Neill B, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol. 1986;252:323–347. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Pons TP, Kaas JH. Ablations of area 3b (SI proper) and area 3a of somatosensory cortex in marmosets deactivate the second and parietal ventral somatosensory areas. Somatosens Mot Res. 1990;7:125–135. doi: 10.3109/08990229009144703. [DOI] [PubMed] [Google Scholar]
- Hsiao SS, O'Shaughnessy DM, Johnson KO. Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J Neurophysiol. 1993;70:444–447. doi: 10.1152/jn.1993.70.1.444. [DOI] [PubMed] [Google Scholar]
- Huttunen J, Wikstrom H, Korvenoja A, Seppalainen AM, Aronen H, Ilmoniemi RJ. Significance of the second somatosensory cortex in sensorimotor integration: enhancement of sensory responses during finger movements. Neuroreport. 1996;7:1009–1012. doi: 10.1097/00001756-199604100-00011. [DOI] [PubMed] [Google Scholar]
- Hyvarinen J. Regional distribution of functional in parietal association area 7 of the monkey. Brain Res. 1981;206:287–303. doi: 10.1016/0006-8993(81)90533-3. [DOI] [PubMed] [Google Scholar]
- Inoue K, Yamashita T, Harada T, Nakamura S. Role of human SII cortices in sensorimotor integration. Clin Neurophysiol. 2002;113:1573–1578. doi: 10.1016/s1388-2457(02)00162-1. [DOI] [PubMed] [Google Scholar]
- Inui K, Wang X, Tamura Y, Kaneoke Y, Kakigi R. Serial processing in the human somatosensory system. Cereb Cortex. 2004;14:851–857. doi: 10.1093/cercor/bhh043. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. Connexions of somatic sensory cortex of the rhesus monkey. III. Thalamic connexions. Brain. 1970;93:37–56. doi: 10.1093/brain/93.1.37. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Nelson RJ, Sur M, Dykes RW, Merzenich MM. The somatotopic organization of the ventroposterior thalamus of the squirrel monkey, Saimiri sciureus. J Comp Neurol. 1984;226:111–140. doi: 10.1002/cne.902260109. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Shimojo M, Hoshiyama M, Koyama S, Watanabe S, Naka D, Suzuki H, Nakamura A. Effects of movement and movement imagery on somatosensory evoked magnetic fields following posterior tibial nerve stimulation. Brain Res Cogn Brain Res. 1997;5:241–253. doi: 10.1016/s0926-6410(97)00002-5. [DOI] [PubMed] [Google Scholar]
- Karhu J, Tesche CD. Simulatenous early processing of sensory input in human primary (SI) and secondary (SII) somatosensory cortices. J Neurophysiol. 1999;81:2017–2025. doi: 10.1152/jn.1999.81.5.2017. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci. 1995;15:3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Sesma MA, Kaas JH. Microelectrode maps, myeloarchitecture and cortical connections of three somatotopically organized representations of the body surface in the parietal cortex of squirrels. J Comp Neurol. 1986;250:403–430. doi: 10.1002/cne.902500402. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledberg A, O'Sullivan BT, Kinomura S, Roland PE. Somatosensory activations of the parietal operculum of man. A PET study. Eur J Neurosci. 1995;7:1934–1941. doi: 10.1111/j.1460-9568.1995.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Leinonen L, Hyvarinen J, Nyman G, Linnankoski I. Functional properties of neurons in lateral part of associative area 7 in awake monkeys. Exp Brain Res. 1979;34:299–320. doi: 10.1007/BF00235675. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lin YY, Simoes C, Forss N, Hari R. Differential effects of muscle contraction from various body parts on neuromagnetic somatosensory responses. Neuroimage. 2000;11:334–340. doi: 10.1006/nimg.1999.0536. [DOI] [PubMed] [Google Scholar]
- Lin YY, Forss N. Functional characterization of human second somatosensory cortex by magnetoencephalography. Behav Brain Res. 2002;135:141–145. doi: 10.1016/s0166-4328(02)00143-2. [DOI] [PubMed] [Google Scholar]
- Mima T, Nagamine T, Nakamura K, Shibasaki H. Attention modulates both primary and secondary somatosensory cortical activities in humans: a magnetoencephalographic study. J Neurophysiol. 1998;80:2215–2221. doi: 10.1152/jn.1998.80.4.2215. [DOI] [PubMed] [Google Scholar]
- Moore CI, Stern CE, Corkin S, Fischl B, Gray AC, Rosen BR, Dale AM. Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol. 2000;84:558–569. doi: 10.1152/jn.2000.84.1.558. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res. 1984;11:67–83. doi: 10.1016/0166-4328(84)90009-3. [DOI] [PubMed] [Google Scholar]
- Nakata H, Inui K, Wasaka T, Nishihira Y, Kakigi R. Mechanisms of differences in gating effects on short- and long-latency somatosensory evoked potentials relating to movement. Brain Topogr. 2003;15:211–222. doi: 10.1023/a:1023908707851. [DOI] [PubMed] [Google Scholar]
- Neal JW, Pearson RC, Powell TP. The cortico-cortical connections of area 7b, PF, in the parietal lobe of the monkey. Brain Res. 1987;419:341–346. doi: 10.1016/0006-8993(87)90605-6. [DOI] [PubMed] [Google Scholar]
- Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: do New World monkeys have an area 2? Cereb Cortex. 2005;15:1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- Penfield W, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain. Boston, MA: Little, Brown; 1954. [Google Scholar]
- Pons TP, Garraghty PE, Mishkin M. Serial and parallel processing of tactual information in somatosensory cortex in monkeys. J Neurophysiol. 1992;68:518–527. doi: 10.1152/jn.1992.68.2.518. [DOI] [PubMed] [Google Scholar]
- Qi HX, Lyon DC, Kaas JH. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus) J Comp Neurol. 2002;443:168–182. doi: 10.1002/cne.10113. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatotopographic organization in the second somatosensory area of M. fascicularis. J Comp Neurol. 1980a;192:43–67. doi: 10.1002/cne.901920104. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory, and granular insula of M. fascicularis. J Comp Neurol. 1980b;192:69–92. doi: 10.1002/cne.901920105. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas of M. fascicularis. J Comp Neurol. 1980c;192:93–108. doi: 10.1002/cne.901920106. [DOI] [PubMed] [Google Scholar]
- Ruben J, Schwiemann J, Deuchert M, Meyer R, Krause T, Curio G, Villringer K, Kurth R, Villringer A. Somatotopic organization of human secondary somatosensory cortex. Cereb Cortex. 2001;11:463–473. doi: 10.1093/cercor/11.5.463. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S. Shared neural substrates controlling hand movements in human motor cortex. Science. 1995;268:1775–1777. doi: 10.1126/science.7792606. [DOI] [PubMed] [Google Scholar]
- Scherg M. Functional imaging and localization of electromagnetic brain activity. Brain Topogr. 1992;5:103–111. doi: 10.1007/BF01129037. [DOI] [PubMed] [Google Scholar]
- Scherg M, Berg P. Use of prior knowledge in brain electromagnetic source analysis. Brain Topogr. 1991;4:143–150. doi: 10.1007/BF01132771. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Ipsilateral cortical connections of dorsal and ventral premotor areas in New World owl monkeys. J Comp Neurol. 2006;495:691–708. doi: 10.1002/cne.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma K, Honda M, Hanakawa T, Okada T, Fukuyama H, Ikeda A, Nishizawa S, Konishi J, Shibasaki H. Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: an event-related fMRI study. J Neurosci. 1999;19:3527–3534. doi: 10.1523/JNEUROSCI.19-09-03527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt O, Vogt C. Ergebnisse unserer Hirnforschung. J Psychol Neurol. 1919;25:277–462. [Google Scholar]
- Wasaka T, Nakata H, Akatsuka K, Kida T, Inui K, Kakigi R. Differential modulation in human primary and secondary somatosensory cortices during the preparatory period of self-initiated finger movement. Eur J Neurosci. 2005;22:1239–1247. doi: 10.1111/j.1460-9568.2005.04289.x. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Somatosensory cortex of prosimian Galagos: physiological recording, cytoarchitecture and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 2003;457:263–292. doi: 10.1002/cne.10542. [DOI] [PubMed] [Google Scholar]
- Xiang J, Hoshiyama M, Koyama S, Kaneoke Y, Suzuki H, Watanabe S, Naka D, Kakigi R. Somatosensory evoked magnetic fields following passive finger movement. Brain Res Cogn Brain Res. 1997;6:73–82. doi: 10.1016/s0926-6410(97)00017-7. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Murray GM, Turman AB, Mackie PD, Coleman GT, Rowe MJ. Parallel processing in cerebral cortex of the marmoset monkey: effect of reversible S1 inactivation on tactile responses in SII. J Neurophysiol. 1996;76:3633–3655. doi: 10.1152/jn.1996.76.6.3633. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Zachariah MK, Coleman GT, Rowe MJ. Hierarchical equivalence of somatosensory areas I and II for tactile processing in the cerebral cortex of the marmoset monkey. J Neurophysiol. 2001;85:1823–1835. doi: 10.1152/jn.2001.85.5.1823. [DOI] [PubMed] [Google Scholar]


