Abstract
Objective
To test the hypothesis that hepatitis C virus (HCV)-specific interferon (IFN)γ immune responses are correlated with HCV virological response following treatment in subjects with HIV-1 and HCV co-infection.
Design
Immune responses were studied in a treatment trial comparing standard interferon alfa (IFN) to pegylated interferon alfa (PEG-IFN), each with ribavirin (R).
Methods
Using HCV antigens core, NS3 and NS5, and Candida, enzyme-linked immunosorbent spots on peripheral blood mononuclear cells measured IFNγ and interleukin (IL)-10 production. Immunologic, virologic and clinical variables were modeled using recursive partitioning (CART) to identify factors associated with HCV virological response at week 24 (VR) and week 72 (SVR) in 108 patients.
Results
There were no significant differences in baseline IFNγ immune responses and higher IL-10 to NS3 in subjects with VR versus non-responders. Subjects who had significant decreases in IL-10 responses at week 24 compared to baseline for NS3, NS5, or summed HCV responses were more likely to be VR. Using baseline immunological responses and clinical data in CART models, patients who were randomized to PEG-IFN/Rand had high IL-10 responses to summed HCV proteins were more likely to be VR (73%), whereas those on IFN/R who had low IFNγ responses to Candida were less likely to be VR (5%). The main correlate of SVR for genotype-1 subjects was maintenance of HCV-specific IFNγ responses from baseline to week 72.
Conclusions
In this cohort of subjects with HIV and HCV, a decrease in HCV-specific IL-10 responses and maintenance of IFNγ responses during treatment with IFN were associated with week 24 or 72 virological response.
Keywords: hepatitis C virus, interferon α, cellular immunity, interferon gamma, interleukin 10
Introduction
An estimated 15–30% of persons with HIV infection in the United States are co-infected with hepatitis C virus (HCV) [1]. As people live longer in the era of highly active antiretroviral therapy, there is an increasing percentage of hospitalizations and death being attributed to HCV-related liver disease among persons with HIV [2–4]. Increased interest in treating HCV in the setting of HIV is tempered by early studies that have demonstrate overall lower rates of sustained virological response (SVR), especially in genotype one infection, compared to persons with HCV alone [5–7].
In HCV mono-infection, treatment with interferon has been associated with sustained CD4+ T-cell immune responses in persons with successful clearance of HCV [8,9]. Broadly directed, HCV-specific type 1-like cytokine responses have also been associated with an increased likelihood of SVR in some [8,10–12] but not all studies [13]. One study found that a non-persistent HCV-specific interferon (IFN)γ response concomitant with an increase in type 2-like cytokines interleukin (IL)-10 and IL-4 during interferon alfa-2a with ribavirin was associated with decreased likelihood of SVR as well [8]. One possible explanation for the lower likelihood of SVR in persons with HIV/HCV is depressed cellular immune responses. In HIV co-infection, HCV-specific responses are rarely detected in peripheral blood mononuclear cells (PBMC), except in HIV long-term non-progressors [14–18]. HCV-specific responses are detected more frequently in the intrahepatic compartment in persons co-infected with HIV, but intrahepatic responses cannot be followed during a treatment trial [19].
In the present study, we evaluated CD4+ T-cell responses in 108 subjects enrolled in a randomized trial comparing interferon alfa and pegylated interferon alfa, each with ribavirin, to determine whether pre-treatment antigen-specific immune responses were predictive of viral clearance. We also wanted to determine whether changes in patterns of type 1 and type 2-like cytokine responses during interferon-containing regimens were associated with sustained virological response.
Methods
Human patients
One hundred and thirty three eligible patients were enrolled in the Adult AIDS Clinical Trial Group protocol A5071, a randomized, open-labeled multicenter trial comparing pegylated interferon alfa-2a with ribavirin (PEG-IFN/R) or standard interferon alfa-2a three times a week with ribavirin (IFN/R) [6]. HIV-infected subjects 18 years or older were eligible for trial participation if they had chronic HCV infection confirmed by HCV RNA detection and had not been previously treated with IFN-alfa-containing regimens. A liver biopsy demonstrating abnormal histology consistent with chronic hepatitis C was required within 48 weeks of study entry. Patients were eligible with normal or elevated serum alanine aminotransferase (ALT) levels and were required to have negative tests for hepatitis B surface antigen. Liver biopsies were blindly scored by a central hepatopathologist using the modified Histology Activity Index (HAI).
The primary endpoint was HCV virological response (HCV RNA < 60 IU/ml) at 24 weeks of treatment (VR). Patients without a VR or histological improvement discontinued the study and the remaining patients were followed for virological response at week 72 (sustained virological response; SVR). This report analysed the subset of patients who had adequate PBMC for analysis at study entry (108 patients). The protocol was reviewed and approved by the Ethics Committee of the Beth Israel Deaconess Medical Center and all participating sites. All subjects participating in the study presented a written informed consent before any study-related procedures. The protocol and all study procedures were conducted in conformity with the ethical guidelines of the Declaration of Helsinki.
Recombinant HCV proteins
The recombinant HCV proteins used were derived from HCV genotype 1b and included core protein [amino acid (aa) 1–115], and non-structural (NS) proteins NS3 (aa 1007–1534), and NS5 (aa 2622–2868) at 1 μg/ml (Mikrogen, GmbH, Munich, Germany).
Enzyme-linked immunosorbent spot assay
PBMCs were isolated by Ficoll-Hypaque (Amersham Biosciences, Piscataway New Jersey, USA) density-gradient centrifugation and cryopreserved. Enzyme-linked immunosorbent spot (ELISpot) assays were performed as described previously [15]. Briefly, plates were coated with primary monoclonal antibody (mAb) [anti- IFNγ, anti-IL-10, (Endogen, Woburn, Massachusetts, USA), or anti-tumor necrosis factor (TNF)α, (BD Pharmingen, San Diego, California, USA)] at a concentration of 10 μg/ml, 2.5 × 105 PBMC and were cultured for 40 h in the presence of the recombinant HCV protein core, and NS proteins NS3 and NS5 at 1 μg/ml. Positive control wells consisted of phytohemaglutinin (PHA; 5 μg/ml; Sigma, St. Louis, Missouri, USA) and Candida cellular antigen (20 μg/ml; Greer Labs, Lenoir, North Carolina, USA). Negative control wells were media alone and buffer. After 48 h the PBMC were removed and 50 μl of biotin-conjugated secondary mAb [anti- IFNγ or anti-IL-10 (Endogen), or anti-TNFα (BD Pharmingen)] were added at 0.2 μg/ml (IFNγ and IL-10) or 0.5 μg/ml (TNFα). Plates were developed and the numbers of spots per well were scored using a dissection microscope. The averaged numbers of spot-forming cells (SFC) in control wells were subtracted from antigen-stimulated wells to correct for spontaneous cytokine production. Persons performing ELISpot assays were blinded to all subject information except the identification number and specimen collection date.
Statistical methods
Data were analyzed in SAS 6.12 (SAS Institute, Cary North Carolina, USA) using non-parametric tests including the Kruskal–Wallis test. All tests were two-tailed and evaluated at a significance level of 0.05. No adjustment for multiple comparisons was made in these exploratory analyses. Recursive partitioning using classification and regression trees (CART) in Splus V3.4 (MathSoft, Inc.; Insightful Corporation; Seattle, Washington, USA) was performed to identify subgroups highly associated with virological response [20]. Clinical variables included in each model were treatment arm, gender, race, entry age, intravenous drug use, CD4+ T-cell count, antiretroviral regimen [protesase inhibitor (PI) versus non-PI], HCV genotype and HIV and HCV viral loads. Immune response data were included as continuous variables and used summed IFNγ or IL-10 responses to HCV proteins core, NS3 and NS5 (HCV-Sum) and responses to Candida. Baseline responses were included in the CART models represented by Fig. 1; both baseline and week 24 responses as well as the difference between baseline and week 24 responses were included in CART models represented by Fig. 2; and baseline, week 24, week 72, and differences between baseline and week 24 and baseline and week 72 responses were included in CART models represented by Fig. 3. CART selected ‘cut points’ associated with virological outcome. These have been characterized as ‘high’ and ‘low’, and the actual value may be specific to this dataset. Numerous models were generated, and parsimonious models with high sensitivity and specificity for virological response are presented. Extensive cross-validation and ‘pruning’ was not performed in order to retain small subgroups with unique characteristics for visual inspection.
Fig. 1. Classification and regression tree (CART) for predicting the outcome of week 24 virological response (VR).
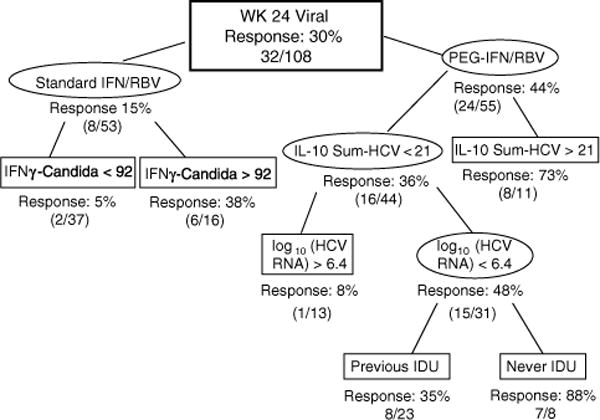
Thirty percent of the overall cohort had week 24 VR. CART identified useful baseline predictors for subgroups with higher or lower frequencies of week 24 VR. All percentages indicate classified subjects with week 24 VR compared to the total subjects in that classified group. IL-10 Sum-HCV = sum of interleukin (IL)-10 responses to hepatitis C virus (HCV) proteins core, NS3 and NS5. Numbers associated with immune response data represent spot-forming cells (SFC)/106 peripheral blood mononuclear cells. IDU, injection drug users; IFN, interferon alfa-2a; PEG-IFN, pegylated interferon alfa-2a; RBV, ribavirin.
Fig. 2. Classification and regression tree (CART) for predicting the outcome of week 24 virological response (VR) in 93 subjects who had immune response data for baseline and week 24 time points.
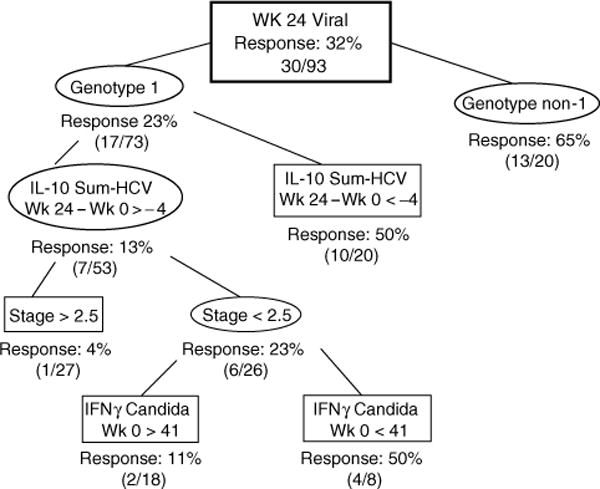
CART identified useful predictors for subgroups with higher or lower frequencies of week 24 VR. IL-10 Sum-HCV = sum of interleukin (IL)-10 responses to hepatitis C virus (HCV) proteins core, NS3 and NS5. Numbers associated with immune response data represent spot-forming cells (SFC)/106 peripheral blood mononuclear cells. IFN, interferon alfa-2a.
Fig. 3. Classification and regression tree (CART) for predicting the outcome of sustained virological response (SVR) in 54 subjects who had immune response data for baseline, week 24 and week 72 time points.
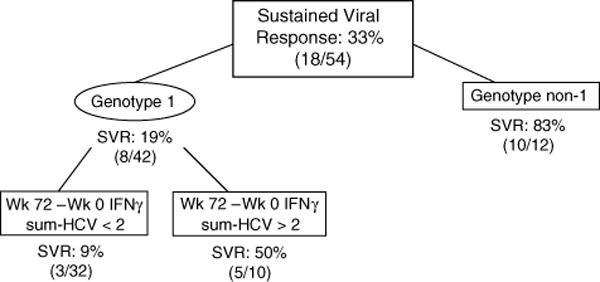
IFNγ Sum-HCV = sum of interferon (IFN)γ responses to hepatitis C virus (HCV) proteins core, NS3 and NS5. Numbers associated with immune response data represent spot-forming cells (SFC)/106 peripheral blood mononuclear cells.
Results
Patient characteristics
The parent study had 133 eligible subjects and had VR rates of 44% in the PEG-IFN/R group compared to 15% in the IFN/R group, and SVR rates of 27% in the PEG-IFN/R group compared to 12% in the IFN/R group [6]. Table 1 shows subject characteristics for the 108 subjects in whom cellular immune responses were studied at baseline, divided by whether they had clearance of HCV virus at week 24 (VR) or not (NR). In this subgroup, VR were significantly more likely to have received PEG-IFN/R, be white non-Hispanic, to have never used injection drugs, and to be genotype non-1. In the right columns are characteristics of those subjects in whom immune responses were studied at baseline and week 24 (n = 93), divided by whether or not they achieved week 72 virological response (SVR). Factors associated with SVR were similar to those associated with VR, with the exception that race was no longer significant.
Table 1.
Subject characteristics by week 24 or week 72 virological response.
| Characteristic | Week 24 virological response
|
Week 72 virological responsea
|
||||
|---|---|---|---|---|---|---|
| No response n = 76 |
Response n = 32 |
P value | No response n = 73 |
Response n = 20 |
P value | |
| Treatment | ||||||
| IFN | 45 (85%) | 8 (15%) | 0.002 | 43 (88%) | 6 (12%) | 0.025 |
| PEG-IFN | 31 (56%) | 24 (44%) | 30 (68%) | 14 (32%) | ||
| Gender | ||||||
| Male | 60 (67%) | 30 (33%) | 0.088 | 60 (77%) | 18 (23%) | 0.511 |
| Female | 16 (89%) | 2 (11%) | 13 (87%) | 2 (13%) | ||
| Race | ||||||
| White, non-Hispanic | 32 (59%) | 22 (41%) | 0.050 | 32 (68%) | 15 (32%) | 0.060 |
| Black, non-Hispanic | 29 (81%) | 7 (19%) | 27 (90%) | 3 (10%) | ||
| Other | 15 (83%) | 3 (17%) | 14 (88%) | 2 (13%) | ||
| Age (years) | ||||||
| 13–39 | 12 (55%) | 10 (45%) | 0.207 | 11 (58%) | 8 (42%) | 0.055 |
| 40–49 | 45 (74%) | 16 (26%) | 45 (85%) | 8 (15%) | ||
| > 50 | 19 (76%) | 6 (24%) | 17 (81%) | 4 (19%) | ||
| Injection drug use history | ||||||
| Never | 22 (56%) | 17 (44%) | 0.027 | 26 (67%) | 13 (33%) | 0.023 |
| Previous use | 54 (78%) | 15 (22%) | 47 (87%) | 7 (13%) | ||
| CD4 cell count (cells/μl) | ||||||
| 100–299 | 18 (82%) | 4 (18%) | 0.274 | 18 (90%) | 2 (10%) | 0.353 |
| 300–500 | 23 (62%) | 14 (38%) | 23 (72%) | 9 (28%) | ||
| > 500 | 35 (71%) | 14 (29%) | 32 (78%) | 9 (22%) | ||
| Antiretroviral regimen | ||||||
| None; missing | 8 (62%) | 5 (38%) | 0.088 | 9 (75%) | 3 (25%) | 0.158 |
| antiretroviral + protease inhibitor | 46 (79%) | 12 (21%) | 43 (86%) | 7 (14%) | ||
| antiretroviral, no protease inhibitor | 22 (59%) | 15 (41%) | 21 (68%) | 10 (32%) | ||
| HIV RNA load | ||||||
| Undetectable | 45 (70%) | 19 (30%) | 1.000 | 45 (83%) | 9 (17%) | 0.208 |
| Detectable | 31 (70%) | 13 (30%) | 28 (72%) | 11 (28%) | ||
| HCV genotype | ||||||
| Genotype 1 | 66 (78%) | 19 (22) | 0.004 | 64 (88%) | 9 (12%) | 0.0002 |
| Genotype non-1 | 10 (43%) | 13 (57%) | 9 (45%) | 11 (55%) | ||
| Log10 (HCV RNA) IU/ml (median value at baseline) | 6.29 | 6.23 | 0.185 | 6.28 | 6.22 | 0.387 |
| Liver histology HAI A–D (inflammation) at baseline | ||||||
| Score 0–5 | 42 (71%) | 17 (29%) | 1.000 | 42 (84%) | 8 (16%) | 0.303 |
| Score > 5 | 34 (71%) | 14 (29%) | 31 (74%) | 11 (26%) | ||
| Missing | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) | ||
| Liver histology HAI E (fibrosis) at baseline | ||||||
| Score 0–2 | 41 (66%) | 21 (34%) | 0.204 | 40 (77%) | 12 (23%) | 0.608 |
| Score 3–6 | 35 (78%) | 10 (22%) | 33 (83%) | 7 (18%) | ||
| Missing | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) | ||
Subjects with week 72 data had baseline and week 24 immunological data available. IFN, interferon alfa-2a; PEG-IFN, pegylated interferon alfa-2a; HCV, hepatitis C virus; HCV, hepatitis C virus; HAI, Histology Acitivity Index.
Cellular immune responses
We next determined whether baseline immune responses were associated with week 24 VR. Overall, HCV-specific immune responses were of low frequency with median values of zero SFC/106 PBMC for both IFNγ and IL-10 for all HCV antigens, as consistent with prior reports of infrequent responses in persons with chronic HCV infection (data not shown) [9,14,15,17]. There were no significant differences in baseline IFNγ immune responses between VR and NR (Table 2). Subjects with VR had significantly higher IL-10 to NS3 (P = 0.03) and a trend to higher summed IL-10 responses to HCV proteins (P = 0.09) at baseline. We then examined whether changes in immune responses at week 24 compared to baseline were related to virological responses. We found that week 24 VR were significantly more likely to have a decrease in IL-10 secretion to NS3 (P = 0.01), a trend to less IL-10 to NS5 (P = 0.06) and less IL-10 to summed HCV responses (P = 0.05) (Table 2). There were no significant differences in immune responses between week 72 SVR and those failing to sustain response (data not shown).
Table 2.
Differences in immune responses in subjects with week 24 virological response (VR) versus no response (NR).
| Antigen | Baseline immune responses
|
Change in immune response, week 24 – baselinec
|
||||
|---|---|---|---|---|---|---|
| Week 24 NR median (5%, 95% range) |
Week 24 VR median (5%, 95% range) |
P valuea | Week 24 NR median, (5%, 95% range) |
Week 24 VR median (5%, 95% range) |
P value | |
| INFγ-core | 0 (0, 8) | 0 (0, 3) | 0.19 | 0 (−7, 3) | 0 (−1, 7) | 0.29 |
| IFNγ-NS3 | 0 (0, 9) | 0 (0, 23) | 0.66 | 0 (−7, 5) | 0 (−23, 5) | 0.63 |
| IFNγ-NS5 | 0 (0, 11) | 0 (0, 13) | 0.45 | 0 (−4, 3) | 0 (−13, 1) | 0.22 |
| IFNγ-Sumb | 1 (0, 20) | 1 (0, 24) | 0.77 | 0 (−13, 4) | 0 (−24, 5) | 0.75 |
| IFNγ-Candida | 35 (0, 415) | 15 (0, 178) | 0.60 | −3 (−163, 103) | −9 (−119, 93) | 0.43 |
| IL10-core | 0 (0, 7) | 0 (0, 33) | 0.30 | 0 (−7, 1) | 0 (−4, 3) | 0.84 |
| IL10-NS3 | 0 (0, 23) | 0 (0, 111) | 0.03 | 0 (−27, 59) | −1 (−111,3) | 0.01 |
| IL10-NS5 | 0 (0, 17) | 0 (0, 49) | 0.28 | 0 (−17, 7) | 0 (−49, 0) | 0.06 |
| IL10-Sumb | 0 (0, 63) | 1 (0, 116) | 0.09 | 0 (−55, 69) | −3 (−116, 3) | 0.05 |
| IL10-Candida | 1 (0, 243) | 0 (0, 456) | 0.95 | 0 (−243, 65) | 0 (−273, 11) | 0.84 |
P values calculated using the non-parametric Kruskal–Wallis test.
Sum of responses [spot forming cells (SFC)] to hepatitis C virus (HCV) proteins core, NS3 and NS5.
Numbers of SFC at baseline subtracted from SFC at week 24 (negative number indicates decreased response).
Multivariable modeling of clinical and immune parameters with VR and SVR
Next, we used CART to look for associations between immune responses and other clinical variables since factors such as age, gender, race, or HIV status could affect both immune responses and ability to clear HCV viremia with interferon alfa treatment. CART selects the ‘best predictor’ of an outcome from a dataset, then, for a given branch, selects the next best predictor. Figure 1 shows the model for predicting week 24 VR using baseline IFNγ and IL-10 summed HCV responses, Candida responses, and clinical variables described in Methods. The overall VR was 30%. Subjects who received PEG-IFN/R and had high baseline summed IL-10 HCV responses had a 73% likelihood of VR. Subjects who received PEG-IFN/R and had lower baseline summed IL-10 HCV responses and high HCV viral load at baseline had an 8% likelihood of VR. In the standard IFN/R arm, subjects with high IFNγ secretion to Candida had a 2.5-fold increase in VR (38%) compared with the parent group (15%), and substantially higher than the subjects with low IFNγ secretion to Candida (5%). The overall sensitivity of this model was 0.91 with a specificity of 0.62.
We then incorporated changes in immune responses from baseline to week 24 in addition to baseline variables to determine factors associated with week 24 VR. As shown in Fig. 2, the best predictor of week 24 VR was HCV genotype, where subjects with genotype non-1 had a 65% VR. For subjects with genotype 1, a decrease in IL-10 responses to summed HCV proteins of at least 4 SFC/106 PBMC from baseline to week 24 was associated with a 50% VR. Conversely, those who maintained HCV-specific IL-10 responses during treatment and had higher fibrosis scores only had a 4% VR. The overall sensitivity of this model was 0.90 with a specificity of 0.67.
Correlates of week 72 SVR in subjects who had immune responses studied at baseline, week 24 and week 72 (n = 54) were studied (Fig. 3). The available number of subjects was lower as subjects with no response at week 24 did not have additional PBMC collected. 33% of these 54 subjects had an SVR (these are subjects who had had a week 24 virological or histological response and therefore remained in the study past week 24). Subjects who were genotype non-1 had an 83% SVR. For subjects who were genotype 1, maintenance of HCV-specific IFNγ responses from baseline to week 72 (those subjects that gained at least 2 SFC/106 PBMC) was associated with an SVR of 50% whereas those that did not maintain IFNγ responses to HCV proteins had an SVR of 9%. The overall sensitivity of this model was 0.83 with a specificity of 0.80.
Discussion
Studies of immunological predictors of virological response after interferon-based treatment in HCV mono-infection have often focused solely on type-1 IFNγ responses [11–13]. However, at least two studies of persons with HCV mono-infection undergoing interferon alfa treatment have shown that lack of, or loss of IL-10 responses along with increases in IFN responses were important predictors for clearance of HCV [8,9]. There are scant data on immunological predictors of interferon-based treatment outcome in HIV/HCV co-infection. Legrand et al. analyzed HCV-specific proliferative responses with respect to outcome of treatment with IFN/R, and found that subjects with persistent proliferative responses during treatment were more likely to have an SVR, but this association was confined to those with genotype 3 infection [21]. In contrast, Alatrakchi et al. did not find enhancement of HCV-specific IFNγ during treatment with IFN/R in persons with HIV/HCV co-infection. In fact, when IFNγ responses were detected at baseline, frequencies decreased in all cases at 6 months on IFN/R [22]. Of note, in both of these studies, there were concomitant decreased responses to other antigens, including HIV P24 antigen, cytomegalovirus, and tuberculin during treatment with IFN/R.
In this cohort of patients with HIV and HCV co-infection undergoing treatment with interferon alfa plus ribavirin, the overall SVR was 19%, which is substantially lower than what is typically found in the treatment of HCV mono-infection [23,24]. Clinical predictors of SVR in this trial have already been published, and include randomization to PEG-IRN/R, genotype non-1, no previous injection drug use, and HIV viral load greater than 50 copies/ml [6]. We wanted to determine if type 1 and type 2-like immune responses could augment clinical parameters in predicting the primary outcome of this trial, namely week 24 virological responses.
Not surprisingly, the strongest predictor of week 24 virological response was randomization to the PEG-IFN/R arm. In that arm, the next best predictor of VR was robust HCV-specific IL-10 responses at baseline (Fig. 1), and in those subjects with lower IL-10 responses, a combination of lower HCV viral load and no injection drug use history predicted outcome. In contrast, when dynamic immune responses between week 24 and baseline were added to the model (Fig. 2), loss of HCV-specific IL-10 responses during treatment emerged as an important correlate of VR.
It was difficult to study predictors of SVR because subjects without VR or histological improvement discontinued the clinical study without providing week 72 PBMCs for immunological studies. This may explain why only one predictor of SVR in the parent study, HCV genotype, [6] emerged as a predictor of SVR in this subgroup. However, it was interesting that, after HCV genotype, the next best predictor of SVR was maintenance of IFNγ responses to HCV proteins at week 72 relative to baseline. Of note, IFNγ had mixed roles in this cohort. Higher IFNγ responses to Candida were predictive of week 24 VR in subjects treated with IFN/R (Fig. 1). In a second model (Fig. 2), lower IFNγ responses to Candida were predictive of week 24 VR, but this result was only for those patients who were genotype 1, did not substantially decrease their IL-10 responses to HCV during treatment, and had lower fibrosis scores on entry liver biopsy.
The overall lower VR and SVR rates seen in this study, compared with rates seen in HCV mono-infection trials, is not purely due to the degree of immunosuppression, as CD4 cell count did not emerge as a predictor of outcome in this study, nor in the parent study [6]. The data in this cohort suggest that the balance of type 1 and type 2 immune responses during the course of IFN/R treatment is a critical factor in determining clearance of HCV. We previously showed decreased intrahepatic HCV-specific IL-10 production in persons with HIV/HCV in comparison with those with HCV alone [19]. IL-10 is secreted by numerous cell types including T regulatory (Treg) cells, and suppresses production of proinflammatory cytokines [25]. It may be that suppression of Treg cells during IFN/R treatment is associated with improved outcomes, although the current study was not designed to test this hypothesis.
In conclusion, successful clearance of HCV in this HIV-co-infected cohort was predicted by clinical factors such as treatment arm and HCV genotype, but also by suppression of IL-10 responses on treatment, or maintenance of IFNγ responses after completion of treatment. Improvement in rates of SVR in HIV/HCV-co-infected persons may necessitate suppression of type 2 responses as well as augmentation of type 1 responses. A better understanding of immunological correlates of HCV clearance may help target new therapies, especially for our difficult-to-treat genotype 1 HIV/HCV-co-infected patients.
Acknowledgments
We want to thank the subjects and the following study sites for their participation in this study: Harvard University, New York University/Bellevue, Stanford University, University of Cincinnati, University of Rochester Medical Center, University of Minnesota, Washington University, Indiana University Hospital, Northwestern University, Beth Israel Medical Center, University of North Carolina, University of Texas Southwestern Medical Center, University of Hawaii, University of Colorado Health Sciences Center, University of Pennsylvania, Columbia University, University of California, San Diego, San Francisco General Hospital, and University of Miami. We thank Roche Laboratories, Inc. for financial support of the clinical portion of AACTG 5071.
Sponsorship: Supported by the National Institutes of Health (DA14495-01 to C.S.G.; AI38855 to T.L. and J.W.A.), as well as funding from the AIDS Clinical Trial Group (AI 38858 to M.J.K. and R.C.).
Footnotes
Presented in part at the Eleventh Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, 2004; Abstract No.111.
References
- 1.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 2.Soriano V, Garcia-Samaniego J, Valencia E, Rodriguez-Rosado R, Munoz F, Gonzalez-Lahoz J. Impact of chronic liver disease due to hepatitis viruses as cause of hospital admission and death in HIV-infected drug users. Eur J Epidemiol. 1999;15:1–4. doi: 10.1023/a:1007506617734. [DOI] [PubMed] [Google Scholar]
- 3.Gebo KA, Diener-West M, Moore RD. Hospitalization rates differ by hepatitis C satus in an urban HIV cohort. J Acquir Immune Defic Syndr. 2003;34:165–173. doi: 10.1097/00126334-200310010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 6.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 8.Kamal SM, Fehr J, Roesler B, Peters T, Rasenack JW. Peginterferon alone or with ribavirin enhances HCV-specific CD4 Thelper 1 responses in patients with chronic hepatitis C. Gastroenterology. 2002;123:1070–1083. doi: 10.1053/gast.2002.36045. [DOI] [PubMed] [Google Scholar]
- 9.Cramp ME, Carucci P, Rossol S, Chokshi S, Maertens G, Williams R, et al. Hepatitis C virus (HCV) specific immune responses in anti-HCV positive patients without hepatitis C viraemia. Gut. 1999;44:424–429. doi: 10.1136/gut.44.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leroux-Roels G, Esquivel CA, DeLeys R, Stuyver L, Elewaut A, Philippe J, et al. Lymphoproliferative responses to hepatitis C virus core, E1, E2, and NS3 in patients with chronic hepatitis C infection treated with interferonalfa. Hepatology. 1996;23:8–16. doi: 10.1002/hep.510230102. [DOI] [PubMed] [Google Scholar]
- 11.Lohr HF, Gerken G, Roth M, Weyer S, Schlaak JF, Meyer zum Buschenfelde KH. The cellular immune responses induced in the follow-up of interferon-alpha treated patients with chronic hepatitis C may determine the therapy outcome. J Hepatol. 1998;29:524–532. doi: 10.1016/s0168-8278(98)80146-3. [DOI] [PubMed] [Google Scholar]
- 12.Missale G, Cariani E, Lamonaca V, Ravaggi A, Rossini A, Bertoni R, et al. Effects of interferon treatment on the antiviral T-cell response in hepatitis C virus genotype 1b- and genotype 2c-infected patients. Hepatology. 1997;26:792–797. doi: 10.1053/jhep.1997.v26.pm0009303515. [DOI] [PubMed] [Google Scholar]
- 13.Barnes E, Harcourt G, Brown D, Lucas M, Phillips R, Dusheiko G, et al. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743–754. doi: 10.1053/jhep.2002.35344. [DOI] [PubMed] [Google Scholar]
- 14.Alatrakchi N, Di Martino V, Thibault V, Autran B. Strong CD4 Th1 responses to HIV and hepatitis C virus in HIV-infected long-term non-progressors co-infected with hepatitis C virus. AIDS. 2002;16:713–717. doi: 10.1097/00002030-200203290-00006. [DOI] [PubMed] [Google Scholar]
- 15.Graham CS, Wells A, Liu T, Sherman KE, Peters M, Chung RT, et al. Antigen-specific immune responses and liver histology in HIV and hepatitis C coinfection. AIDS. 2005;19:767–773. doi: 10.1097/01.aids.0000168970.80551.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauer GM, Nguyen TN, Day CL, Robbins GK, Flynn T, McGowan K, et al. Human immunodeficiency virus type 1-hepatitis C virus coinfection: intraindividual comparison of cellular immune responses against two persistent viruses. J Virol. 2002;76:2817–2826. doi: 10.1128/JVI.76.6.2817-2826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdez H, Anthony D, Farukhi F, Patki A, Salkowitz J, Heeger P, et al. Immune responses to hepatitis C and non-hepatitis C antigens in hepatitis C virus infected and HIV-1 coinfected patients. AIDS. 2000;14:2239–2246. doi: 10.1097/00002030-200010200-00004. [DOI] [PubMed] [Google Scholar]
- 18.Valdez H, Carlson NL, Post AB, Asaad R, Heeger PS, Lederman MM, et al. HIV long-term non-progressors maintain brisk CD8 T cell responses to other viral antigens. AIDS. 2002;16:1113–1118. doi: 10.1097/00002030-200205240-00004. [DOI] [PubMed] [Google Scholar]
- 19.Graham CS, Curry M, He Q, Afdhal N, Nunes D, Fleming C, et al. Comparison of HCV-specific intrahepatic CD4+ T cells in HIV/HCV versus HCV. Hepatology. 2004;40:125–132. doi: 10.1002/hep.20258. [DOI] [PubMed] [Google Scholar]
- 20.Breiman LFJ, Olshen RA, Stone JC. Classification and regression trees. Belmont, CA: Wadsworth Publishing Company, Inc.; 1984. [Google Scholar]
- 21.Legrand E, Neau D, Galperine T, Trimoulet P, Moreau JF, Pitard V, et al. CD4 T lymphocyte proliferative responses to hepatitis C virus (HCV) antigens in patients coinfected with HCV and human immunodeficiency virus who responded to anti-HCV treatment. J Infect Dis. 2002;186:302–311. doi: 10.1086/341659. [DOI] [PubMed] [Google Scholar]
- 22.Alatrakchi N, Di Martino V, Thibault V, Benhamou Y, Katlama C, Poynard T, et al. Decreased frequencies of virus-specific T helper type 1 cells during interferon alpha plus ribavirin treatment in HIV-hepatitis C virus co-infection. AIDS. 2004;18:121–123. doi: 10.1097/00002030-200401020-00015. [DOI] [PubMed] [Google Scholar]
- 23.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 24.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 25.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]


