Abstract
Background
ACTG A5202 randomized treatment-naive individuals to tenofovir-emtricitabine (TDF/FTC) or abacavir-lamivudine (ABC/3TC) combined with efavirenz (EFV) or atazanavir/ritonavir (ATV/r). Individuals in the high screening viral load (VL) stratum (≥100,000 copies/mL) had increased rates of virologic failure with ABC/3TC.
Objective
Compare regimen-specific early virologic response.
Methods
Using Wilcoxon rank-sum tests, we compared regimen-specific VL changes from entry to week 4 in A5202 subjects (n=1813) and from entry to week 1, 2 and 4 in a 179-patient substudy. We evaluated associations between week 4 VL change and time to virologic failure with Cox proportional-hazards models.
Results
TDF/FTC- and ABC/3TC produced similar Week 4 viral load declines in the entire study population and in the high VL stratum. EFV produced greater VL declines from baseline at week 4 than ATV/r (median −2.1 vs. −1.9 log10 copies/mL; p<0.001). In the substudy of subjects with week 1, 2 and 4 VL data, there was no difference in viral load decline in those randomized to TDF/FTC versus ABC/3TC, but EFV resulted in greater VL decline from entry at each of these timepoints than ATV/r. Smaller Week 4 viral load decline was associated with increased risk of virologic failure.
Conclusions
Within all treatment arms, a less robust week 4 virologic response was associated with higher risk for subsequent virologic failure. However, between-regimen differences in week 4 VL declines did not parallel the previously reported differences in longer term virologic efficacy in A5202, suggesting that between-regimen differences in responses were not due to intrinsic differences in antiviral activity.
Keywords: Anti-HIV Agents, HIV Infections/drug therapy/*virology, Treatment Outcome
INTRODUCTION
In the randomized clinical study AIDS Clinical Trials Group (ACTG) A5202, we previously reported that in treatment-naive HIV-1-infected individuals time to virologic failure was similar in those assigned to atazanavir/ritonavir (ATV/r) or efavirenz (EFV).1 However, in the high screening viral load stratum (≥100,000 copies/mL), there was a significantly shorter time to virologic failure in those assigned to abacavir (ABC)/lamivudine (3TC) versus tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) (hazard ratio=2.33; p<0.001), regardless of which third drug was used.2 In contrast, this was not seen in those in the low screening viral load stratum.3
Studies have shown similar findings 4, others have not 5, and there remains debate regarding the optimal nucleoside/tide reverse transcriptase inhibitor (NRTI) combination for treatment-naive HIV-1 infected individuals. The Department of Human Health Services guidelines consider TDF/FTC as the preferred NRTI combination,6 while the International Antiviral Society–USA Panel guidelines also include ABC/3TC as recommend components of first-line therapy for individuals with a viral load of <100,000 copies/mL.7
The chronic phase of untreated HIV infection is characterized by viral replication resulting in a dynamic equilibrium between viral production and clearance, with approximately one-half of the circulating virus replaced daily with newly produced virus.8 Combination antiretroviral therapy (ART) prevents the infection of lymphocytes and monocytes and perturbs this equilibrium. Most viral-dynamics models assume that ART results in complete inhibition of viral replication. If treatment has no effect on the death rate of infected cells or on the rate of plasma virus clearance, decay rates reflect the potency of antiretrovirals in combination. Thus, a comparison of early virologic response between different antiretroviral regimens may provide insights into a regimen's inherent antiviral activity, although antiretrovirals targeting different steps in the viral replication cycle may result in different decay rates independent of their antiviral activity.9 Many, but not all studies, have shown that greater early virologic response (e.g., at 1, 2, and 4 weeks) of a regimen is associated with superior longer term virologic efficacy.10–13 Here, in an attempt to explain the observed primary efficacy difference between TDF/FTC and ABC/3TC in the high viral load stratum, we evaluate regimen-specific early virologic response in ACTG A5202 and in its early viral load substudy.
METHODS
ACTG A5202 Study Design
AIDS Clinical Trials Group (ACTG) A5202 was an equivalence study that randomized 1857 HIV-1-infected treatment-naïve subjects to blinded TDF/FTC or ABC/3TC combined with open-label ATV/r or EFV, stratified by screening viral load (≥100,000 or <100,000 copies/mL). Entry criteria for ACTG A5202 included age of ≥16 years, ≤7 days of prior ART, and no evidence of major resistance mutations.1 Resistance testing was only required for recently infected patients and was optional for those with chronic infection. Planned study duration was 96 weeks after the last subject enrolled. The study enrolled between 2005 and 2007 and completed follow-up in 2009 with a median length of follow-up of 138 weeks. The primary efficacy endpoint was time from randomization to virologic failure. Virologic failure was defined as a confirmed viral load of ≥1000 copies/mL between 16 and 24 weeks after randomization or ≥200 copies/mL at or after 24 weeks. Screening viral load assessments prior to study entry were performed locally at each study site. From study entry onward, viral load assessments were performed centrally with the Roche Amplicor Monitor Assay Version 1.5 with a lower limit of quantification (LLQ) of 50 copies/mL.
Viral Load Change from Entry to Week 4 Analysis
All ACTG A5202 subjects with an available week 4 viral load measurement were included in this analysis. We compared the viral load change from entry to week 4 among the four different regimens (and summarized across third drug and by NRTI assignment by combining ATV/r and EFV and then ABC/3TC and TDF/FTC arms, respectively) with the Wilcoxon rank sum test. Viral load values below the Amplicor assay’s lower limit of quantification (LLQ) of 50 copies/ml (occurring in 8% of patients at week 4) were set to 50 copies/mL.
Early Viral Load Substudy
An early viral load substudy was performed at five of the 59 enrolling ACTG A5202 sites. Subjects returned for phlebotomy at entry and weeks 1, 2, and 4 after randomization, and plasma samples from these visits were tested with the Roche COBAS Ampliprep/COBAS TaqMan HIV Test Version 2 with a LLQ of 20 copies/mL. To conserve sample aliquots for additional analyses, samples were diluted 1:1 (0.5mL per sample). The LLQ was therefore set to 40 copies/mL, and copy numbers reported account for this dilution. We compared the viral load changes from entry to values obtained at Weeks 1, 2, and 4 between each of the four regimens using Wilcoxon rank sum tests. Viral load values below Taqman assay’s LLQ were set to 40 copies/mL (occurring in 0%, 0%, and 6% of patients at 1, 2, and 4 weeks, respectively).
Time to Virologic Failure Analysis
In the overall ACTG A5202 study population, the relationship between change in viral load from entry to week 4 and time to virologic failure was evaluated with univariate and multivariable Cox proportional hazards models. Multivariable models were adjusted for age, sex, race/ethnicity, history of intravenous drug use, whether a pre-enrollment genotype was performed, history of AIDS, hepatitis B or C infection, baseline HIV-1 viral load, and baseline CD4+ T-cell count. Given the efficacy difference in TDF/FTC versus ABC/3TC arms in the high viral load stratum, we analyzed the associations separately in the TDF/FTC and ABC/3TC arms.
Ethics
Approval was obtained from each participating sites' institutional review board for the main study and the substudy. All subjects provided written informed consent prior to study entry.
RESULTS
The 1813 enrolled subjects included in this analysis who returned for a Week 4 viral load assessment were similar to the overall study population of ACTG A5202 (Table 1). The median age of the included subjects at baseline was 38 (IQR 31, 45) and 83% were male (Table 1). The median baseline CD4 T-cell count and viral load (by Amplicor) was 229 cells/µL (IQR 91, 333) and 4.7 log10 copies/mL (IQR 4.3, 5.0), respectively. Forty-three percent of subjects were in the high screening viral load stratum (screening viral load of ≥100,000 copies/mL).
Table 1.
Baseline Characteristics of Included Subjects in ACTG A5202 and the Early Viral Load Substudy
| Characteristic | A5202 with Week 4 RNA Data (n=1813) |
All A5202 (n=1857) |
Early Viral Load Substudy (n=179) |
|---|---|---|---|
| Median Age - Years (IQR) | 38 (31, 45) | 38 (31, 45) | 38 (31, 45) |
| Gender | |||
| Male | 1500 (83%) | 1535 (83%) | 159 (89%) |
| Female | 313 (17%) | 322 (17%) | 20 (11%) |
| Race/Ethnicity | |||
| White Non-Hispanic | 734 (41%) | 746 (40%) | 93 (52%) |
| Black Non-Hispanic | 599 (33%) | 615 (33%) | 39 (22%) |
| Hispanic | 417 (23%) | 429 (23%) | 39 (22%) |
| Other | 58 (4%) | 62 (4%) | 7 (5%) |
| Median CD4+ Count -cells/µL (IQR) | 229 (91, 333) | 230 (90, 334) | 185 (56, 287) |
| Screening Viral Load | |||
| ≥100,000 copies/mL | 783 (43%) | 797 (43%) | 80 (45%) |
| <100,000 copies/mL | 1030 (57%) | 1060 (57%) | 99 (55%) |
| Entry Viral Load (Amplicor) – copies/mL (IQR) | 4.7 (4.3, 5.0) | 4.7 (4.3, 5.0) | 4.7 (4.4, 5.3) |
| Entry Viral Load (Taqman) - copies/mL (IQR) | - | - | 5.2 (4.8, 5.6) |
| Genotype at Entry | |||
| Yes | 814 (45%) | 830 (45%) | 38 (21%) |
| No | 999 (55%) | 1027 (55%) | 141 (79%) |
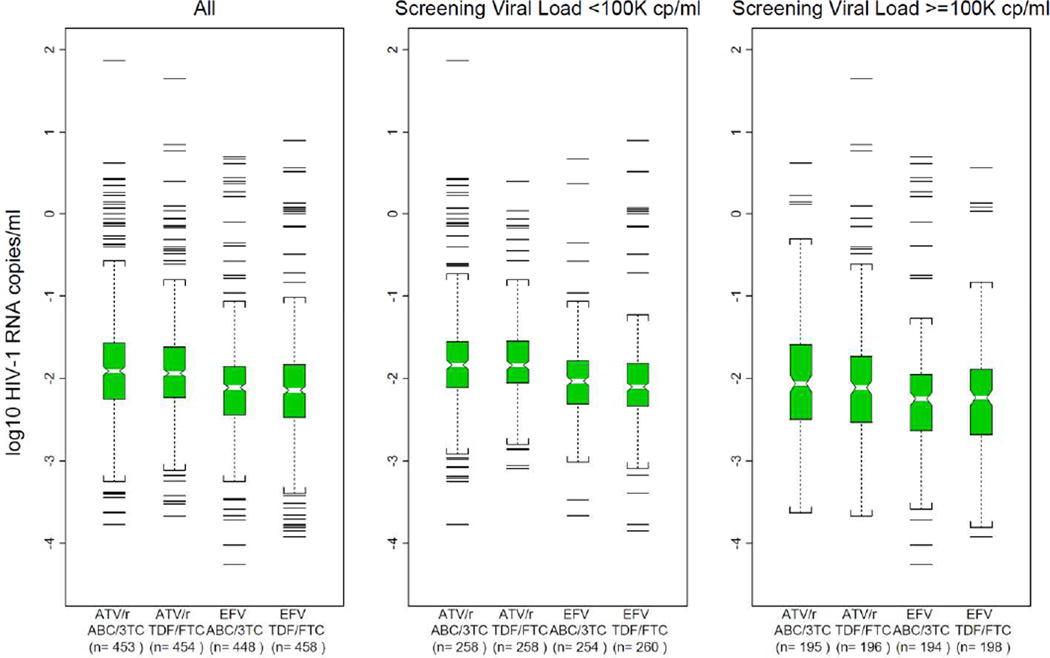
Combining the third drugs, there was no significant difference in the distribution of viral load change from entry to week 4 in those subjects randomized to TDF/FTC vs. those randomized to ABC/3TC (Figure 1; median change −2.0 vs. −2.0 log10 copies/mL; p=0.54) without any evidence of interaction by third drug (p=0.95). Restricting the evaluation to subjects in the high screening viral load stratum, there was also no significant difference in change in viral load from entry to week 4 in subjects randomized to TDF/FTC versus those to ABC/3TC (Figure 1; median change −2.2 vs. −2.2 log copies/mL; p=0.69).
Figure 1.
Box Plot of 4 Week Viral Load Change from Entry by Regimen and Screening Viral Load Strata
Combining the NRTIs, subjects randomized to EFV-containing regimens had a significantly greater decline in viral load from entry to 4 weeks than those randomized to ATV-containing regimens (Figure 1; median change −2.1 vs. −1.9 log10 copies/mL; p<0.001) without evidence of an interaction by assigned NRTIs (p=0.78).
In the early viral load substudy, 179 subjects enrolled. Table 1 displays the baseline characteristics of these subjects. The subjects differed from those ACTG A5202 subjects not enrolled in the substudy in that they were more frequently male and White non-Hispanic, had a lower median baseline CD4+ T-cell count, and less frequently had a pre-enrollment genotype performed. Baseline characteristics were balanced between subjects randomized to each of the four study regimens within the substudy (data not shown).
Patterns of viral load changes in the early viral load substudy mirrored the patterns observed in the overall study. Viral load change from entry to weeks 1, 2, and 4 were similar between those subjects randomized to TDF/FTC versus those randomized to ABC/3TC, whether paired with EFV or ATV/r (Table 2). In the high viral load stratum, there also was no significant difference in viral load change between subjects randomized to TDF/FTC compared to those randomized to ABC/3TC when paired with EFV, although with ATV/r there appeared to be a trend toward a smaller decline in viral load from entry to weeks 1, 2 and 4 in subjects randomized to ABC/3TC compared to those randomized to TDF/FTC (Table 3).
Table 2.
Median Log10 Viral Load Change from Entry by NRTI Arm in the Early Viral Load Substudy (IQR)
| Visit Week |
EFV | ATV/r | ||||
|---|---|---|---|---|---|---|
| TDF/FTC (n=48) |
ABC/3TC (n=44) |
p-value† | TDF/FTC (n=46) | ABC/3TC (n=41) |
p-value† | |
| 1 | −1.6 (−1.7, −1.2) | −1.5 (−1.8, −1.3) | 0.92 | −1.2 (−1.5, −1.0) | −1.2 (−1.4, −0.9) | 0.39 |
| 2 | −2.1 (−2.2, −1.8) | −2.0 (−2.3, −1.8) | 0.98 | −1.9 (−2.1, −1.6) | −1.8 (−2.0, −1.7) | 0.80 |
| 4 | −2.4 (−2.6, −2.1) | −2.4 (−2.6, −2.1) | 0.71 | −2.3 (−2.4, −1.9) | −2.2 (−2.4, −1.9) | 0.78 |
EFV=efavirenz; TDF=tenofovir disoproxil fumarate; FTC=emtricitabine; ABC=abacavir; 3TC=lamivudine; ATV/r=atazanavir/ritonavir;
Calculated with Wilcoxon rank sum test.
Table 3.
Median Log10 Viral Load Change from Entry among Individuals in High Viral Load Stratum (≥100,000 copies/mL) in the Early Viral Load Substudy (IQR)
| Visit Week |
EFV | ATV/r | ||||
|---|---|---|---|---|---|---|
| TDF/FTC (n=26) |
ABC/3TC (n=18) |
p-value† | TDF/FTC (n=18) | ABC/3TC (n=18) |
p-value† | |
| 1 | −1.5 (−1.7, −1.2) | −1.5 (−1.8, −1.3) | 0.64 | −1.4 (−1.6, −1.1) | −1.1 (−1.5, −0.9) | 0.18 |
| 2 | −2.1 (−2.3, −1.8) | −2.1 (−2.4, −1.8) | 0.58 | −2.0 (−2.2, −1.8) | −1.8 (−1.95, −1.6) | 0.11 |
| 4 | −2.6 (−2.9, −2.3) | −2.5 (−2.8, −2.2) | 0.63 | −2.4 (−2.6, −2.2) | −2.1 (−2.4, −1.9) | 0.08 |
EFV=efavirenz; TDF=tenofovir disoproxil fumarate; FTC=emtricitabine; ABC=abacavir; 3TC=lamivudine; ATV/r=atazanavir/ritonavir;
Calculated using Wilcoxon rank sum test.
In the early viral load substudy, subjects randomized to EFV-containing regimens had greater decreases from entry in viral load than those assigned to ATV/r-containing regimens. This difference was apparent by week 1 (median change −1.5 log10 vs. −1.2 log10; p<0.001) and was maintained at both week 2 (median change −2.0 log10 vs. −1.8 log10; p<0.001) and week 4 (median change −2.4 log10 vs. −2.2 log10; p=0.003).
In the overall ACTG A5202 study, combining across the third drugs, a smaller viral load decline from entry to week 4 was associated with increased risk of study-defined virologic failure in individuals on both TDF/FTC- and ABC/3TC-containing regimens with a hazard ratio (HR) for virologic failure per 1 log10 copies/mL less decline in viral load of 1.8 (95% CI:1.4, 2.3) and of 1.3 (95% CI:1.1, 1.7), respectively. There was no evidence that this association differed by third drug assignment (p-value for interaction of 0.66 for TDF/FTC-containing arms and of 0.53 for ABC/3TC-containing arms). After adjusting for baseline covariates, the relationship between viral load change from entry to week 4 and virologic failure was maintained in both TDF/FTC- and ABC/3TC-containing regimen arms with adjusted HR of virologic failure per 1 log10 copies/mL less decline in viral load of 1.8 (95% CI: 1.4, 2.3) and of 1.9 (95% CI: 1.5, 2.4), respectively.
DISCUSSION
In this protocol-specified secondary analysis of ACTG A5202 and its viral load substudy, we found no significant difference in early viral load change between the NRTI arms, including in the high viral load stratum. In contrast, assignment to EFV resulted in a small but significantly greater early viral load decline than ATV/r. We found a smaller week 4 virologic response was associated with an increased risk for subsequent virologic failure.
Our findings are consistent with a study from Haubrich and colleagues in ACTG A5142, which found a small, but significantly greater early viral load decline in individuals on non-nucleoside reverse transcriptase inhibitor (NNRTI)-based therapy compared to individuals on ritonavir-boosted protease inhibitor (PI/r)-based therapy.13 In this previous study, NNRTI-based therapy resulted in faster Phase 1 viral decay than PI/r-based therapy.13 Our results support this observation as a significantly greater viral load decline from entry between those on EFV compared to ATV/r was already apparent at week 1 - a timepoint that can be used as a surrogate for Phase 1 decay.13 It has been postulated that the slower early decrease in viral load with PI- versus NNRTI-based regimens may be due to PIs acting later in the virus replication cycle than NNRTIs, resulting in slower inhibition of productive virus replication. More importantly and somewhat paradoxically, the diminished early response to PI-based therapy as compared to NNRTI-based therapy did not result in an increased rate of subsequent virologic failure with the PI/r-based regimen in ACTG A5202, as was seen in ACTG A5142.14
Randomized comparisons between TDF/FTC and ABC/3TC show conflicting results, depending on the third drug used.2,4,5,15 In the ASSERT study with EFV as the third drug among 385 treatment-naive subjects, a significantly lower proportion achieved a viral load of <50 copies/mL at 48 weeks in the ABC/3TC arm (59%) compared to in the TDF/FTC arm (71%).4 Alternatively, in the HEAT study with lopinavir/ritonavir as the third drug among 688 treatment-naive subjects, ABC/3TC was shown to be non-inferior to TDF/FTC with 68% and 67% of subjects, respectively, achieving a viral load of <50 copies/mL at 48 weeks, with similar outcomes also seen in individuals with a viral load of ≥100,000 copies/mL.5 In a small study of 109 subjects with ATV/r as the third drug, the time to virologic failure was not significantly different in those randomized to ABC/3TC versus TDF/FTC (HR=2.1; 95% CI: 0.7, 6.1), although nearly 100% of patients had a baseline viral load of <100,000 copies/mL.15 Additionally, subgroup analyses from the randomized THRIVE and SPRING-2 studies did not show a difference in efficacy between subjects given ABC/3TC vs. TDF/FTC, although the NRTIs were investigator-selected in these studies and not allocated through randomization.16,17
The early decline in plasma HIV-1 RNA from baseline does not appear to explain the difference in primary efficacy outcomes observed in ACTG A5202 between the NRTIs. In fact, we found no significant differences in the early virologic responses to TDF/FTC and ABC/3TC, including in the high viral load stratum. This suggests that the increased rates of virologic failure with ABC/3TC in the high viral load stratum may not have been primarily due to differences in antiviral activity between ABC/3TC and TDF/FTC, but by factors such as long-term adherence, pharmacokinetics/pharmacodynamics, and toxicity. Our study results call into question the value of using early virologic response as a surrogate for expected longer term virologic outcome when comparing the efficacy of different regimens. Indeed, in ACTG A5202, early virologic responses were the converse of the overall study outcome: TDF/FTC and ABC/3TC had comparable early VL responses, yet ABC/3TC had a higher rate of virologic failure; by contrast, EFV had a greater VL response than ATV/r, yet the two strategies had similar rates of virologic failure in this equivalence study. Similarly, clinical trials that compare integrase inhibitor- to non-integrase inhibitor-based regimens invariably show a faster viral load decline in the integrase treatments, yet the ultimate study outcomes may be comparable.18–20
In monotherapy studies, TDF and ABC produced 1.5 and 1.1 log10 copies/mL reductions in viral load, respectively.21,22 In vitro, FTC has been shown to be more potent than 3TC.23 However, the most clinically relevant difference between the medications may be the longer intracellular half-life of TDF/FTC versus ABC/3TC.6 Pharmacokinetic differences in regimens have manifested as higher rates of virologic failure in only the subset of subjects with high viral loads in a number of recent clinical trials,16,24,25 suggesting that the shorter half-life of ABC/3TC could be a plausible mechanism for the overall findings of ACTG A5202.
Some limitations of this study should be noted. Subjects were not directly observed taking medication and baseline genotypes were not performed for all individuals in the study, possibly obscuring any potential differences in regimen potencies; however, these limitations would presumably apply similarly across all randomized treatment arms. The association analysis is based on data collected post-randomization and restricted to those who were able to return for their Week 4 viral load sample. The virologic failure definition for A5202 included the relatively atypical criteria of a viral load of ≥1000 copies/mL between 16 and 24 weeks after randomization, as well as the more typical threshold of ≥200 copies/mL at or after 24 weeks. We did not separately assess the relationship between week 4 virologic response and early or late virologic failure. Additionally, for the early viral load substudy, the sample size was relatively small and we did not sample sufficient timepoints to calculate Phase 1 and Phase 2 decay rates. However, previous studies have shown that single early viral load measurements can be used as surrogates for the more complex sampling and modeling involved in estimating the phases of decay.13
CONCLUSION
In summary, we have shown that smaller four-week decline in viral load was associated with a higher rate of subsequent virologic failure. EFV resulted in a slightly greater decline in early viral loads than ATV/r, but this did not parallel a difference in virologic failure in individuals on these medications. By contrast, there was no significant difference observed in early response between the TDF/FTC and ABC/3TC arms, including in the high viral load stratum. This would suggest that differences in intrinsic antiviral potency alone do not explain the increased rates of virologic failure in those assigned ABC/3TC versus TDF/FTC in the high viral load stratum of ACTG A5202, raising the possibility that other factors, such as pharmacokinetics, barrier to resistance, and tolerability may have contributed.
ACKNOWLEDGEMENTS
Parts of this work were previously presented at the 18th Conference on Retroviruses and Opportunistic Infections in February of 2011 in Boston, MA (Abstract #535).
We would like to thank the study participants of ACTG A5202.
Supported by grants (AI38858, to the AIDS Clinical TrialsGroup [ACTG] Central Group; AI68636, to the ACTG Network; and AI68634 and AI38855, to the ACTG Statistical and Data Analysis Center) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, with additional support from the General Clinical Research Center units funded by the National Center for Research Resources and GlaxoSmithKline, Inc., Gilead Sciences, Inc., Bristol-Myers Squibb Company, and Abbott Labs.
Dr. Grant has received grant support from Bristol-Myers Squibb. Dr. Tierney is a paid member of a Data Monitoring Committee for a Tibotec-sponsored hepatitis C drug study. Dr. Daar has received research grants from Abbott, Gilead, Merck, and Viiv and has served as a consultant for Bristol-Myers Squibb, Gilead, Janssen, and Viiv. Dr. Sax has received grant support from GlaxoSmithKline, Pfizer, and Merck Laboratories, consulting fees from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Laboratories, and Tibotec, and serves as a member of the Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Dr. Collier receives research support from Merck, received past research support from Schering-Plough, previously owned stock in Abbott, Bristol-Myers-Squibb, Johnson & Johnson, and Pfizer, and was a past Data and Safety Monitoring Board member of a Merck & Co.-sponsored study. Dr. Fischl has received grant support from Abbott Laboratories, Bionor Immuno AS, Bristol-Myers Squibb, Cytheris, GlaxoSmithKline, and Tibotec and grant support and lecture and consulting fees from Merck Laboratories. Dr. Zolopa has received research grant support from Gilead Sciences and GlaxoSmithKline, consulting fees as an advisory board member for Bristol-Myers Squibb, Gilead Sciences, and Janssen Therapeutics. Dr. Katzenstein has received grant support from Pfizer, Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences.
References
- 1.Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Annals of internal medicine. 2011 Apr 5;154(7):445–456. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sax PE, Tierney C, Collier AC, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. The New England journal of medicine. 2009 Dec 3;361(23):2230–2240. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sax PE, Tierney C, Collier AC, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. The Journal of infectious diseases. 2011 Oct 15;204(8):1191–1201. doi: 10.1093/infdis/jir505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults-48-week results from the ASSERT study. Journal of acquired immune deficiency syndromes (1999) 2010 Sep;55(1):49–57. doi: 10.1097/QAI.0b013e3181dd911e. [DOI] [PubMed] [Google Scholar]
- 5.Smith KY, Patel P, Fine D, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS (London, England) 2009 Jul 31;23(12):1547–1556. doi: 10.1097/QAD.0b013e32832cbcc2. [DOI] [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 7.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection-2012 recommendations of the International Antiviral Society-USA panel. JAMA : the journal of the American Medical Association. 2012 Jul 25;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 8.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 9.Murray JM, Emery S, Kelleher AD, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS (London, England) 2007 Nov 12;21(17):2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]
- 10.Kuritzkes DR, Ribaudo HJ, Squires KE, et al. Plasma HIV-1 RNA dynamics in antiretroviral-naive subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. The Journal of infectious diseases. 2007 Apr 15;195(8):1169–1176. doi: 10.1086/512619. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Lathey J, Ruan P, et al. Relationship of plasma HIV-1 RNA dynamics to baseline factors and virological responses to highly active antiretroviral therapy in adolescents (aged 12-22 years) infected through high-risk behavior. The Journal of infectious diseases. 2004 Feb 15;189(4):593–601. doi: 10.1086/381500. [DOI] [PubMed] [Google Scholar]
- 12.van Leth F, Huisamen CB, Badaro R, et al. Plasma HIV-1 RNA decline within the first two weeks of treatment is comparable for nevirapine, efavirenz, or both drugs combined and is not predictive of long-term virologic efficacy: A 2NN substudy. Journal of acquired immune deficiency syndromes (1999) 2005 Mar 1;38(3):296–300. [PubMed] [Google Scholar]
- 13.Haubrich RH, Riddler SA, Ribaudo H, et al. Initial viral decay to assess the relative antiretroviral potency of protease inhibitor-sparing, nonnucleoside reverse transcriptase inhibitor-sparing, and nucleoside reverse transcriptase inhibitor-sparing regimens for first-line therapy of HIV infection. AIDS (London, England) 2011 Nov 28;25(18):2269–2278. doi: 10.1097/QAD.0b013e32834d0c20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. The New England journal of medicine. 2008 May 15;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishijima T, Takano M, Ishisaka M, et al. Abacavir/Lamivudine versus Tenofovir/Emtricitabine with Atazanavir/Ritonavir for Treatment-naive Japanese Patients with HIV-1 Infection: A Randomized Multicenter Trial. Internal medicine (Tokyo, Japan) 2013;52(7):735–744. doi: 10.2169/internalmedicine.52.9155. [DOI] [PubMed] [Google Scholar]
- 16.Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011 Jul 16;378(9787):229–237. doi: 10.1016/S0140-6736(11)60983-5. [DOI] [PubMed] [Google Scholar]
- 17.Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013 Mar 2;381(9868):735–743. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 18.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009 Sep 5;374(9692):796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 19.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012 Jun 30;379(9835):2439–2448. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 20.DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012 Jun 30;379(9835):2429–2438. doi: 10.1016/S0140-6736(12)60918-0. [DOI] [PubMed] [Google Scholar]
- 21.Louie M, Hogan C, Hurley A, et al. Determining the antiviral activity of tenofovir disoproxil fumarate in treatment-naive chronically HIV-1-infected individuals. AIDS (London, England) 2003 May 23;17(8):1151–1156. doi: 10.1097/00002030-200305230-00006. [DOI] [PubMed] [Google Scholar]
- 22.Saag MS, Sonnerborg A, Torres RA, et al. Antiretroviral effect and safety of abacavir alone and in combination with zidovudine in HIV-infected adults. Abacavir Phase 2 Clinical Team. AIDS (London, England) 1998 Nov 12;12(16):F203–F209. doi: 10.1097/00002030-199816000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Richman DD. Antiretroviral activity of emtricitabine, a potent nucleoside reverse transcriptase inhibitor. Antiviral therapy. 2001 Jun;6(2):83–88. [PubMed] [Google Scholar]
- 24.Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011 Jul 16;378(9787):238–246. doi: 10.1016/S0140-6736(11)60936-7. [DOI] [PubMed] [Google Scholar]
- 25.Eron JJ, Jr, Rockstroh JK, Reynes J, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. The Lancet infectious diseases. 2011 Dec;11(12):907–915. doi: 10.1016/S1473-3099(11)70196-7. [DOI] [PubMed] [Google Scholar]