Abstract
The combination of mapping functional cortical neurons by intraoperative cortical stimulation and axonal architecture by diffusion tensor MRI fiber tracking can be used to delineate the pathways between functional regions. In this study the authors investigated the feasibility of combining these techniques to yield connectivity associated with motor speech and naming. Diffusion tensor MRI fiber tracking provides maps of axonal bundles and was combined with intraoperative mapping of eloquent cortex for a patient undergoing brain tumor surgery. Tracks from eight stimulated sites in the inferior frontal cortex including mouth motor, speech arrest, and anomia were generated from the diffusion tensor MRI data. The regions connected by the fiber tracking were compared to foci from previous functional imaging reports on language tasks. Connections were found between speech arrest, mouth motor, and anomia sites and the SMA proper and cerebral peduncle. The speech arrest and a mouth motor site were also seen to connect to the putamen via the external capsule. This is the first demonstration of delineation of subcortical pathways using diffusion tensor MRI fiber tracking with intraoperative cortical stimulation. The combined techniques may provide improved preservation of eloquent regions during neurological surgery, and may provide access to direct connectivity information between functional regions of the brain.
Keywords: Language, Tensor imaging, Fiber tracking
Introduction
Diffusion tensor magnetic resonance imaging (DT-MRI) fiber tracking provides us with the first technique that can noninvasively identify specific white matter tracts in the brain in vivo, and can delineate the subcortical path of these tracts (Basser et al., 2000; Conturo et al., 1999; Jones et al., 1999; Mori et al., 1999). The DT-MRI fiber tracking technique can provide new information about white matter architecture and structural connectivity in normal and pathological conditions. While tracks can be generated, the relation of these tracks to specific functions is unknown. Functional mapping techniques complement the DT-MRI fiber tracking technique by providing seed regions of known functionality from which to initiate tracks.
In each voxel the dominant direction of the white matter tracts can be represented by the direction associated with the fastest diffusion or the maximum eigenvector (Basser et al., 2000). In the case where there are many fibers crossing each other within a voxel, the fiber direction will not be well defined. Therefore fiber tracking algorithms estimate the voxels that contribute to a contiguous tract by linking neighboring pixels that are colinear within a defined angular deviation (Basser et al., 2000; Conturo et al., 1999; Jones et al., 1999; Mori et al., 1999). Intraoperative stimulation (Berger, 1995; Berger and Ojemann, 1992, 1994; Ojemann et al., 1989; Penfield and Rasmussen, 1950; Skirboll et al., 1996) is the gold standard for identifying functional cortical areas but is somewhat limited in its ability to localize subcortical extensions of these neurons. Therefore the subcortical extensions of these functional areas are largely unknown and are often distorted from their normal arrangement by a mass lesion. DT-MRI fiber tracking can localize subcortical tracts. Although much is known about the functional arrangement of the brain in terms of specific cortical locations, within these areas there is much variation between individuals as to the exact location of the neurons associated with specific functions.
The overall aim of this work is to be able to associate functional information with the structural data from DT-MRI fiber tracking. In this manuscript are the results of DT-MRI fiber tracking in a patient undergoing intraoperative mapping during surgery to remove a brain tumor. The data from this patient illustrate the possibility of determination of previously unknown brain connections associated with speech and naming using these combined techniques. New models of motor speech have been formulated based on data from functional studies that suggest a widespread involvement of cortical and subcortical areas for speech. The results herein provide the first in vivo delineation of speech and naming pathways and are consistent with new models of motor speech that indicate the involvement of supplementary motor area, putamen, and corticospinal tracts.
Materials and methods
The patient is a 40-year-old, right-handed male who presented with a grand malseizure that included postictal speech arrest. The magnetic resonance imaging study demonstrated a large noncontrast enhancing intraaxial mass in the left frontotemporal region, consistent with a low-grade glial neoplasm. The patient's seizures were controlled with antiepileptic drugs, and he was referred to the Neurosurgery Service at our institution for a definitive surgical resection. This clinical research protocol was approved by our institutional committee on human research.
Intraoperative mapping
The operation was performed under awake surgical conditions, which included a propofol/remifentanyl intravenous mixture, administered during the opening procedure. All medications were discontinued before opening the dura and instituting language mapping. A bipolar electrode attached to a constant current generator eliciting biphasic square wave pulses (60 Hz, 1.25 ms per phase), was applied to the cortex to elicit motor function. At 3 mA of current, stimulation evoked movement of the mouth and orofacial region. The same current was applied to surrounding cortex in front of the face motor area, and this resulted in stimulation-induced speech arrest, which was labeled separately. Electrodes were then placed on the cortical surface and stimulation with currents between 2 and 6 mA were used to determine the appropriate stimulation current to use, i.e., 5 mA, without causing after-discharge potentials during constant electrocortigraphy. For the naming task, the patient was presented with common objects every 4–5 s, during which the cortex was stimulated to functionally inactivate that defined region before having the patient see the common object to be named. Stimulation mapping of the cortex revealed several sites that consistently and repetitively interrupted naming without speech arrest. These sites were labeled separately and considered as anomia sites. To be a positive site for naming, each site had to be tested three times, and anomia found in at least 66% of stimulation sequences per cortical site.
DT-MRI fiber tracking
This patient received presurgical imaging with fiducials for stereotactic neurosurgical guidance system (STEALTH, Medtronic Corp). All imaging was performed on a 1.5 T General Electric Signa scanner with a head coil. The conventional imaging protocol included whole brain 3D volumes that were T2-weighted (1 × 1 × 1.5 mm; TR/TE = 3000/104 ms) and postcontrast T1-weighted images (1 × 1 × 1.5 mm; TR/TE = 34/6 ms). The diffusion tensor imaging data was acquired with an echo-planar spin-echo sequence with axial slices covering the brain from the pons to the vertex (1.5 × 1.5 × 2 mm; TR/TE = 20 s/109 ms; six averages; b value = 1000 s/mm2). The raw images from the DT-MRI sequence were aligned to correct for movement.
Maps of apparent diffusion coefficient, anisotropy, eigenvalues, and eigenvectors were calculated from the diffusion data using software developed in our laboratory. The anisotropy and eigenvectors were used to create diffusion tensor tracks using a program developed by our group and based on the FACT algorithm (Mori et al., 1999). This algorithm follows the principal eigenvector within each voxel changing directions at the voxel boundary. This algorithm allows bifurcation of tracks from within a single voxel since the tracks are represented in continuous space but cannot model fiber crossings. The tracks were terminated if the relative anisotropy value dropped below 0.03 or if the angle between consecutive voxels exceeded 70°. The coordinates of the functional regions identified by intraoperative mapping were used to define starting points for the tracks generated by the DT-MRI algorithm. The starting regions were defined over 4.5 × 4.5 mm in-plane (3 × 3 voxels) and 4–6 mm through-plane (2–3 voxels) since this reflects the effective area receiving bipolar stimulation. Subvoxel seeding of starting regions was used with a three dimensional 6× 6 × 6 grid resulting in 216 starting points per voxel employed. The seed region includes both the grey matter and the adjoining white matter. The tracks produced by the DT-MRI algorithm were overlaid on the anatomic MRI data sets and plotted in a 3D rendering for visualization using the 3D Slicer program (http://www.slicer.org). The 3D renderings of the brain with stimulation and track termination points, and macroanatomic labels, Brodmann areas (BA) were accomplished using the mri3dX program (http://www.aston.ac.uk/lhs/staff/singhkd/mri3dX).
The intraoperative stimulation sites obtained were overlaid on the T1 or T2 imaging data in three orthogonal planes. Using programs developed in our laboratory, coordinates corresponding to the stimulation regions were obtained. These coordinates were then found on the diffusion data to define starting regions of interest. Final adjustment of these regions were obtained by visual inspection in three orthogonal planes to correct for small distortions inherent to the fast imaging technique used to obtain the diffusion tensor imaging data.
Comparison to functional imaging studies
The T1-weighted and T2-weighted EPI volumes were used to align the patients data to the template of the Montreal Neurologic Institute (MNI) using FSL routines (Jenkinson and Smith, 2001) and coordinates from the MNI template were translated to Talairach coordinates (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace. html). The Talairach Daemon (Lancaster et al., 1997, 2000) was used to label the regions from the Talairach coordinates. While the tumor is expected to have some effect on the validity of this procedure, the atlas was used away from the tumor to determine the supplementary and premotor regions, and coordinates relative to the anterior commissure were used to compensate for tumor-related shifts. Visual inspection verified the validity of this approach for medial areas exhibiting similar mass effects as the anterior commissure. The regions connected by the tracks were then compared to the stereotactic coordinates for language function from published functional imaging studies.
Results
The intraoperative mapping on this patient identified eight regions corresponding to stimulation of jaw/mouth, mouth (two regions), speech arrest, and four regions (one in pars opercularis and three in precentral gyrus) resulting in anomia (inhibited object naming with intact speech). The MNI coordinates and estimated macroanatmical labels are shown (Table 1). DT-MRI fiber tracking resulted in tracks from all eight regions with connectivity to two to four regions for each site. The regions used to seed the tracking algorithm for the inferior precentral gyrus anomia site (26Anomia) and for the speech arrest stimulation sites are shown (Fig. 1A) with a clear demarcation by a sulcus in between these sites. Tracking from each side of the central region identified with the stimulation does not produce tracks that connect beyond the immediate subcortical white matter. The seed regions on a diffusion anisotropy map (Fig. 1B) shows low anisotropy between the speech and anomia regions consistent with the location of a sulcus and the dramatic reduction in tracks generated from this intermediate region gives support to the specificity of the registration between intraoperative mapping and the diffusion fiber tracking seed regions. Note that the anomia site is anterior to the speech arrest site and at the same axial level. Also shown is the color-coded map of the principal eigenvector direction weighted by the relative anisotropy (Fig. 1C) at the level of the arcuate fasciculus, indicating the effects of the lesion on the direction of the principal eigenvector.
Table 1. Macroanatomic labels and MNI coordinates for stimulation regions and corresponding SMA and frontal gyri destinations of fiber tracking in MNI coordinates.
| Stimulation region | Seed region MNI coordinates | Track destination MNI coordinates | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Label | X | Y | Z | Label | X | Y | Z | |
| 21Anomia | PreCG 44 | −57 | 17 | 10 | NA | NA | NA | NA |
| 26Anomia | PreCG 6 | −61 | 2 | 20 | MidFG 6 | −20 | −6 | 66 |
| Speech | PreCG 6 | −64 | −2 | 24 | MedFG 6 | −3 | −3 | 66 |
| 4Mouth | PreCG 6 | −62 | −10 | 34 | SFG 6 | −9 | −9 | 70 |
| 2Jaw/mouth | PostCG 3 | −56 | −17 | 48 | SFG 6 | −14 | −16 | 72 |
| 41Anomia | PreCG 6 | −38 | −14 | 66 | SFG 6 | −10 | −11 | 68 |
| 42Anomia | PreCG 6 | −46 | −8 | 60 | PreCG 6 | −40 | −10 | 64 |
| 3Mouth | PreCG 4 | −62 | −9 | 26 | PreCG 4 | −17 | −35 | 68 |
All terminations are in the SMA proper or BA6 with the exception of tracks from the (3Mouth) mouth motor stimulation in the primary motor area. PreCG: precentral gyrus; MedFG: medial frontal gyrus; SFG: superior frontal gyrus; MidFG: middle frontal gyrus.
Fig. 1.
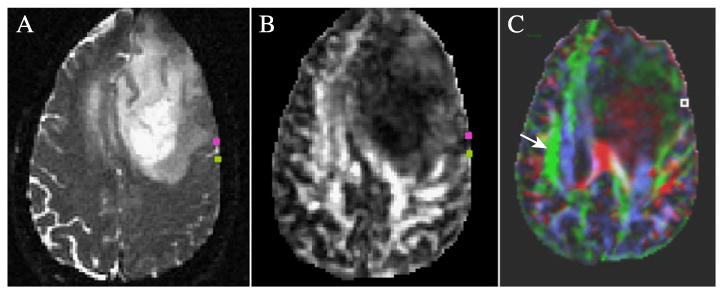
Fiber tracking starting regions for speech arrest (green) and anomia (pink) sites overlaid on (A) T2 image and (B) relative anisotropy map. (C) Illustration of the reduced anisotropy in the arcuate fasciculus and other white matter regions due to the left frontal lesion. The colors indicate the direction of the principal eigenvector (green: anterior–posterior; red: right–left; and blue: inferior–superior) and the intensity denotes the relative anisotropy. The contralateral arcuate fasciculus is indicated by the arrow. The square shows the anomia site in Broca's area that may have connections to Wernicke's area via the arcuate fasciculus but for which no pathways could be tracked.
All stimulated sites were estimated to be in the precentral gyrus (BA6) with the exception of an anomia site (21Anomia) in Broca's area, a mouth motor site (3Mouth) in the primary motor area (BA4), and the jaw/mouth site in the postcentral gyrus (BA3). The stimulation sites are shown on a 3D rendered T2-weighted volume (Fig. 2). All tracks from the stimulation sites are shown (Fig. 3) and it is clear that there is a smooth transition from one track to the other so that the overall white matter topography changes continuously. Therefore tracks coming from neighboring cortical sites tend to have similar and parallel trajectories. While the trajectories found in this study do tend to be similar and parallel, there are important differences that emerge. For example the speech arrest site is between an anomia site and a mouth motor site. Though occupying distinct voxels for the most part, the tracks from the speech arrest site are similar in connectivity to those of the neighboring mouth motor site but have both some similarity and important differences when compared to tracks from the neighboring anomia site. It is also clear that the regions stimulated and used for tracking represent multiple bundles of coherent axonal pathways with typically about 2–5 mm separating regions of unique trackable pathways.
Fig. 2.
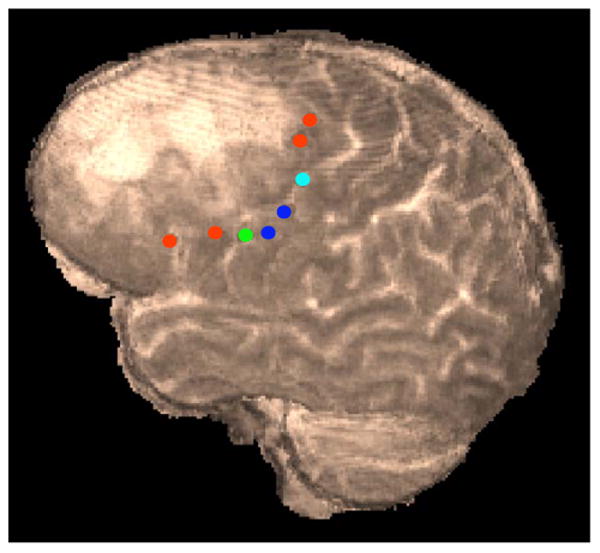
Rendering of anomia (red), mouth motor (blue), jaw/mouth (light blue), and speech arrest (green) stimulation sites on preoperative 3D fastspin-echo volume.
Fig. 3.

Coronal (left) and sagittal (right) views of tracks from anomia (A), mouth motor (M), and speech arrest (S) regions illustrating the internal architecture of white matter tracks. The green area in the sagittal view represents the tumor. Inferior axial and posterior coronal image slices are also shown on the left and an axial plane shown on the right figure.
Subcortical connections
In general, the tracks from the mouth motor (4Mouth), speech arrest, and anomia (26Anomia) sites (Fig. 3) traversed parallel to each other medially from the cortex and then bifurcated before turning sharply to join the pyramidal tract with inferior and superior branches. All three sites resulted in tracks connecting to the SMA and inferiorly to the cerebral peduncle via the posterior limb of the internal capsule (Fig. 4). Additionally as the inferior branch of the speech arrest and mouth motor tracks (Fig. 3) approached the basal ganglia, it bifurcates with a lateral branch into the external capsule that then turns medially traversing the putamen (Fig. 4). The anomia site in Broca's area (BA44) resulted in tracks through the lesion connected superiorly to an ipsilateral cortical region in the inferior frontal gyrus (BA45). Tracks from this anomia site also traveled inferiorly through the lateral globus pallidus, thalamus, and brain stem.
Fig. 4.
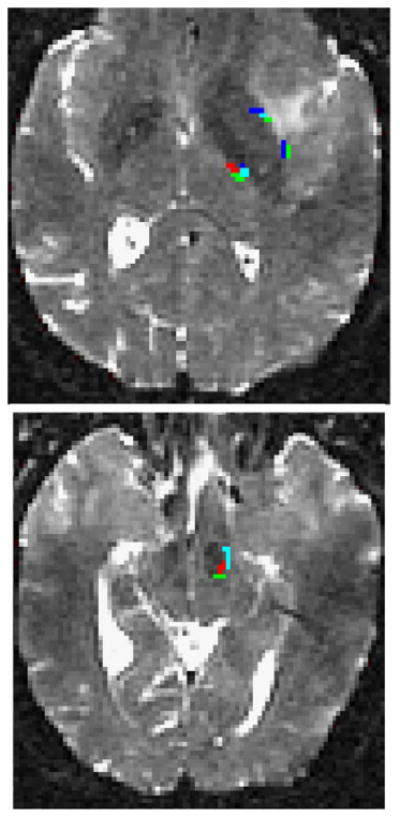
Overlay of tracks from the speech arrest (green), 4Mouth motor (blue), and 26Anomia (red) sites in (upper panel) the internal and external capsules and putamen, and (lower panel) the midbrain. The light blue color arises from voxels containing both blue and green tracks.
Connections to the SMA
The MNI coordinates and estimated macroanatomical labels (Table 1) and overlays of the termination of tracks in the SMA and surrounding areas are shown (Fig. 5). All stimulated sites produced tracks that terminated in the SMA proper (Vorobiev et al., 1998) with the exception of tracks from the 3Mouth site, which begin and end in the primary motor cortex area (BA4), and the anomia site in Broca's area for which no superior going tracks were delineated. In the SMA proper, the tracks from the speech arrest site terminate in medial frontal gyrus (BA6), 4Mouth and 2Jaw/mouth motor tracks connect more posteriorly, while the 26Anomia tracks are more lateral in the middle frontal gyrus (BA6). The 42Anomia site connects to the region near the 41Anomia site, and then tracks into the same region of the SMA occupied by tracks from the speech arrest site described above (Fig. 3).
Fig. 5.
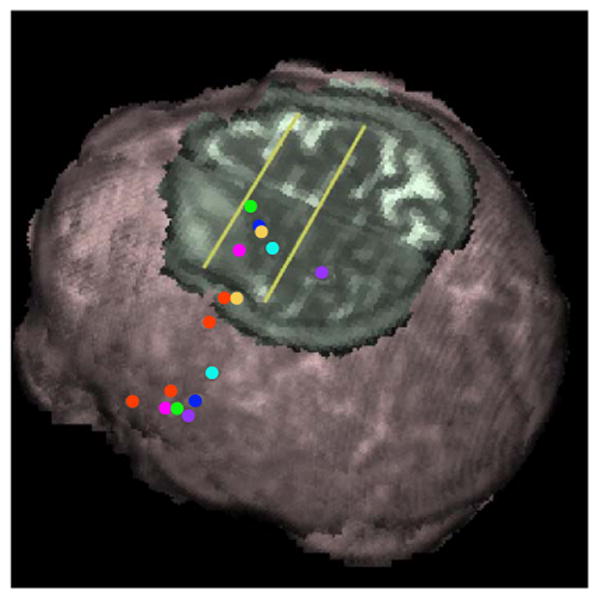
Projections of destinations of fiber tracks in the SMA and frontal gyri from stimulation sites onto an axial view of the T2 image normalized to the MNI template and at z position 68 mm. The yellow lines indicate anterior– posterior positions of the VCA (y = 0 mm) and VCP planes (y = − 22 mm), which nominally defines the SMA proper. Points on the brain surface are stimulation points and points on the axial cutout are fiber track destinations with the exception of the yellow anomia site for which the most lateral dot indicates the stimulation site. 3Mouth: purple; 2Jaw/Mouth: light blue; 41Anomia: yellow; 4Mouth: blue; 10Speech: green; 26Anomia: pink; 21Anomia: red (no superior connections); 42Anomia: red.
Discussion
This study represents the first human in vivo subcortical delineation of pathways involved in speech and naming using DT-MRI fiber tracking initiated from intraoperative cortical stimulation sites. The results indicate the involvement of the supplementary motor cortex, putamen, and cerebral peduncle in motor speech and illustrate the similar but distinct white matter connectivity associated with speech arrest, anomia, and mouth motor function. The identified pathways include cortico-spinal, corticobulbar, and primary/supplementary motor association tracts, and cortico-striatal connections. The particular locations to which these tracks project have correlated very well with knowledge from intraoperative stimulation and recordings and locations from other functional language studies and are within the expected deviation between these studies due to registration, anatomic, and functional variability (Xiong et al., 2000). The feasibility of using structural connectivity data from DT-MRI fiber tracking in conjunction with functional techniques to explore unknown facets of brain architecture and functional connectivity has been shown.
The supplementary motor area has been previously shown to result in speech arrest and/or anomia when stimulated (Baumgartner et al., 1996; Berger, 1995; Berger and Ojemann, 1992, 1994; Fried et al., 1991; Ojemann et al., 1989; Penfield and Rasmussen, 1950; Skirboll et al., 1996), resected (Fontaine et al., 2002; Krainik et al., 2003; Laplane et al., 1977; Rostomily et al., 1991; Zentner et al., 1996), or activated in functional imaging speech experiments (Alexander et al., 1986; Blank et al., 2002; Bookheimer et al., 2000; Corson et al., 2001; Etard et al., 2000; Fiez and Petersen, 1998; Fontaine et al., 2002; Krainik et al., 2003; Petersen et al., 1989; Rosen et al., 2000; Wildgruber et al., 2001; Xiong et al., 2000). Hence the existence of white matter connectivity between the supplementary motor area and the anomia and speech arrest sites in the inferior precentral gyrus is expected.
The track from the speech arrest (interrupted counting) site in the SMA was found to correspond to foci from fMRI and PET studies of language tasks; in particular it was 7 mm from the foci found in a study utilizing internally cued counting (Blank et al., 2002) and 5–9 mm from foci identified in other automatic speech tasks (Bookheimer et al., 2000; Corson et al., 2001). In a metaanalysis (Fiez and Petersen, 1998), motoric aspects of speech were related to foci that were 5 mm from the speech arrest site, 7 mm from the mouth motor site, and 6 mm from the SMA destination of the track from the speech arrest site. Furthermore the speech arrest track was less than 1 mm posterior to the coronal plane passing through the anterior commissure (VCA), while the tracks from the mouth motor and jaw/mouth sites in the SMA were 5 mm posterior to the VCA plane. This arrangement is consistent with studies suggesting somatotopy of the SMA proper (Fontaine et al., 2002; Krainik et al., 2003) with the most anterior region directly posterior to the VCA implicated in language and face/mouth motor aspects directly posterior to this language area.
The anomia site in Broca's area (BA44) resulted in tracks through the lesion connected superiorly to an ipsilateral cortical region in the inferior frontal gyrus (BA45). The Talairach coordinates for this site and its association connected cortical site coincides with fMRI activation foci from novel naming tasks (van Turennout et al., 2003). Of notable interest is the absence of connections to Wernicke's area via the arcuate fasciculus. While it is clear that anomia is possible without involvement of Wernicke's and Broca's areas as described by the Wernicke–Geschwind model (Geschwind, 1965), we would have expected to see connectivity to Wernicke's area for the anomia site in Broca's area. This was further investigated by tracking from regions believed to correspond to Wernicke's area. The tracks believed to correspond to the arcuate fasciculus traversed anteriorly into the lesion and came close to, but did not connect to the anomia site in Broca's area presumably due to the effects of the lesion on the tract (Fig. 1). Similarly, the possibility of observing more anterior going tracks was limited by the lesion in the pre-SMA region.
We hypothesize that the tracks between the speech arrest cortical site and the basal ganglia may be part of the cortex-basal ganglia motor loop that has been previously shown to be involved in speech (Friederici et al., 2003; Kotz et al., 2002; Pickett et al., 1998). This loop is important for higher-order motor control, and goes from the motor cortex to the putamen via the external capsule. The anterior and inferior putamen traversed by tracks from the speech arrest site likely represent motor aspects of speech and is only 4 mm from fMRI foci (Kotz et al., 2002) while the superior and medial putamen traversed by the tracks from the anomia site in pars opercularis was 6 mm from the lexically important foci and were also identified with syntactic processes (Friederici et al., 2003).
Our results suggest continuity to the topography of white matter tracts on the scale interrogated by DT-MRI fiber tracking that results in similarity in the white matter proximal to the neighboring cortical regions. However, at some point, these tracts do diverge to connect to other regions but continue to maintain a smooth change in the overall white matter fiber structure. We believe that this organization of large white matter fiber bundles is due to the intrinsic link between cortical location and white matter organization; that is, cortical organization is arranged so that large-scale white matter organization can be continuous and orderly in some sense. Within this regular framework, the different regions stimulated here connect differently depending on the location of cortical stimulation as well as tracks from widely separated sites converging as in the medial frontal part of the supplementary motor area.
When seed or start points are defined, generally many voxels will be linked including those that may not be part of the same tissue tract. This problem arises from the intrinsic differences in size between the imaging voxels and the individual axons. Directional information of small fiber bundles may not be measurable when these fiber tracts share voxels with larger bundles whose maximum diffusion direction dominate that voxel. This gives a set limitation to the size of white matter fiber bundles that can be reliably tracked with DT-MRI techniques. The algorithm that we used for the analysis of the data in this report cannot give reliable results when tracks cross each other but do show results of tracks wrapping around each other. Developing algorithms that can reliably reproduce crossing fiber tracts is an active and vital area of research currently with the emphasis on using higher angular resolution diffusion acquisitions and interpolative techniques. Nonetheless, on the scale of the fiber bundles investigated here, most areas of the brain do not exhibit crossing fibers and therefore many tracts can be accurately delineated. While “gold standards” for comparison of DT-MRI tracks have been difficult to establish, the results from several groups have been very compelling including studies in rat brain (Xue et al., 1999), developing mice brain (Mori et al., 2001; Pierpaoli et al., 2001). The validation of tracks produced by the DT-MRI fiber tracking technique remains a difficult issue and to date there are no published studies like the one presented here that aid in the understanding and validation of the technique in humans. While this study demonstrates the potential for delineation of novel pathways, further development in data acquisition, tracking methods, and registration with intraoperative mapping is needed. Accuracy and completeness of the identified pathways are influenced by all these considerations. Validation will be best ascertained in the future by having both cortical and subcortical stimulation points on the same tracks, clear correlation with patients' functional status, and reproducibility in multiple individuals.
The importance of intraoperative cortical mapping has been established (Berger, 1995; Berger and Ojemann, 1992, 1994; Ojemann et al., 1989), but it is clear that postoperative preservation of function also depends on identification of subcortical white matter tracts from eloquent cortex within the region of resection (Skirboll et al., 1996). Other functional cortical mapping techniques like functional magnetic resonance imaging (Roux et al., 2001) and magnetic source imaging (Schiffbauer et al., 2001) have recently been investigated as noninvasive presurgical means of identifying eloquent cortical regions. The results have been promising, but to date, the best conclusion is that these methods can guide the surgeon in performing intraoperative stimulation but cannot replace cortical mapping. The primary concern with these alternate methods is the lack of precise localization of functional neurons. Hence intraoperative mapping remains the gold standard for identification of functional cortex.
We have demonstrated the delineation of axonal connections between language and motor regions using DT-MRI fiber tracking combined with intraoperative mapping, in particular the association connections to the SMA and the basal ganglia from speech arrest and mouth motor sites and SMA connectivity and pyramidal tracks associated with anomia sites. This ongoing study may establish DT-MRI fiber tracking as a useful clinical technique to identify white matter tracts before surgical procedures. This will result in the preservation of vital functional connections when performing surgery for brain tumors and other disorders. The feasibility of combining intraoperative mapping and diffusion tensor imaging fiber tracking has been shown here and promises to be an important technique to aid in surgical resections and reduce operative morbidity, and to gain new scientific insights into the functional connectivity of the human brain.
Acknowledgments
The support for Roland G. Henry by NIH/NCI K01 CA76998 is acknowledged. Thanks to Maria Luisa Gorno-Tempini and Howard Rosen for discussions on fMRI with language paradigms and to William P. Dillon for helpful critique and discussions.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Basser P, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tracking using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Baumgartner C, et al. Supplementary motor area seizures: propagation pathways as studied with invasive recordings. Neurology. 1996;46:508–514. doi: 10.1212/wnl.46.2.508. [DOI] [PubMed] [Google Scholar]
- Berger MS. Functional mapping guided resection of low-grade gliomas. In: Loftus C, editor. Clinical Neurosurgery. Vol. 42. Williams and Wilkins; Baltimore: 1995. pp. 437–452. [PubMed] [Google Scholar]
- Berger MS, Ojemann GA. Intraoperative brain mapping techniques in neuro-oncology. Stereotact Funct Neurosurg. 1992;58:153–161. doi: 10.1159/000098989. [DOI] [PubMed] [Google Scholar]
- Berger MS, Ojemann GA. Techniques of functional localization during removal of tumor involving the cerebral hemispheres. In: Loftus C, Traynelis V, editors. Intraoperative Monitoring Techniques in Neurosurgery. McGraw-Hill; New York: 1994. pp. 113–127. [Google Scholar]
- Blank SC, et al. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton TA, Gaillard W, Theodore WH. Activation of language cortex with automatic speech tasks. Neurology. 2000;55:1151–1157. doi: 10.1212/wnl.55.8.1151. [DOI] [PubMed] [Google Scholar]
- Conturo TE, et al. Tracking neronal fiber pathways in the living human brain. Proc Natl Acad Sci. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson B, et al. Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. J Cogn Neurosci. 2001;13(2):272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- Etard O, et al. Picture naming without Broca's and Wernicke's area. NeuroReport. 2000;11:617–622. doi: 10.1097/00001756-200002280-00036. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc Natl Acad Sci. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine D, Capelle L, Duffau H. Somatotopy of the supplementary motor area: evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery. 2002;50:297–305. doi: 10.1097/00006123-200202000-00011. [DOI] [PubMed] [Google Scholar]
- Fried I, et al. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnection syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones DK, Simmons A, Williams SCR, Horsfield MA. Noninvasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical-semantic network by auditory semantic priming: an event-related functional MRI study. NeuroImage. 2002;17:1761–1772. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- Krainik A, et al. Postoperative speech disorder after medial frontal surgery, Role of the supplementary motor area. Neurology. 2003;60:587–594. doi: 10.1212/01.wnl.0000048206.07837.59. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerln JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. NeuroImage. 1997;5(4):S633. [Google Scholar]
- Lancaster JL, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci. 1977;34:301–314. doi: 10.1016/0022-510x(77)90148-4. [DOI] [PubMed] [Google Scholar]
- Mori S, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S, et al. Diffusion tensor imaging of the developing mouse brain. Magn Reson Med. 2001;46:18–23. doi: 10.1002/mrm.1155. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man. McMillan; New York: 1950. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintum M, Raichle ME. Positron emission tomographic studies of cortical anatomy of singleword processing. Nature. 1989;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Pickett ER, Kuniholm E, Protopapas A, Friedman J, Lieberman P. Selective speech motor, syntax and cognitive deficits associated with bilateral damage to the putamen and the head of the caudate nucleus: a case study. Neuropsychologia. 1998;36(2):173–188. doi: 10.1016/s0028-3932(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Ojemann JG, Ollinger JM, Petersen SE. Comparison of brain activation during word retrieval done silently and aloud using fMRI. Brain Cogn. 2000;42:201–217. doi: 10.1006/brcg.1999.1100. [DOI] [PubMed] [Google Scholar]
- Rostomily RC, Berger MS, Ojemann GA, Lettich E. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 1991;75:62–68. doi: 10.3171/jns.1991.75.1.0062. [DOI] [PubMed] [Google Scholar]
- Roux FE, et al. Methodological and technical issues for integrating functional magnetic resonance imaging data in a neuronavigational system. Neurosurgery. 2001;49:1145–1156. doi: 10.1097/00006123-200111000-00025. [DOI] [PubMed] [Google Scholar]
- Schiffbauer H, Ferrari P, Rowley HA, Berger MS, Roberts TPL. Functional activity within brain tumors: a magnetic source imaging study. Neurosurgery. 2001;49:1333–1342. doi: 10.1097/00006123-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn R. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678–685. [PubMed] [Google Scholar]
- van Turennout M, Bielamowicz L, Martin A. Modulation of neural activity during object naming: effects of time and practice. Cereb Cortex. 2003;13:381–391. doi: 10.1093/cercor/13.4.381. [DOI] [PubMed] [Google Scholar]
- Vorobiev V, Govoni P, Rizzolatti G, Matelli M, Luppino G. Parcellation of human mesial area 6: cytoarchitectonic evidence for three separate areas. Eur J Neurosci. 1998;10:2199–2203. doi: 10.1046/j.1460-9568.1998.00236.x. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Grodd W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: effects of syllable repetition rate evaluated by fMRI. NeuroImage. 2001;13:101–109. doi: 10.1006/nimg.2000.0672. [DOI] [PubMed] [Google Scholar]
- Xiong J, et al. Intersubject variability in cortical activations during a complex language task. NeuroImage. 2000;12:326–339. doi: 10.1006/nimg.2000.0621. [DOI] [PubMed] [Google Scholar]
- Xue R, van Zijl P, Crain B, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med. 1999;42:1123. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Zentner J, Hufnagel A, Pechstein U, Wolf HK, Schramm J. Functional results after resective procedures involving the supplementary motor area. J Neurosurg. 1996;85:542–549. doi: 10.3171/jns.1996.85.4.0542. [DOI] [PubMed] [Google Scholar]


