Significance
Blood perfusion is a fundamental property of brain physiology and is known to be higher in adult females than in males. However, it is unknown when such a sex difference emerges during the lifespan, or what biological processes may cause it. In the largest study of brain perfusion yet reported, we establish for the first time to our knowledge that patterns of development of cerebral perfusion during adolescence are markedly different in males and females, and such differences are attributable in part to the effects of puberty. These results may have important implications for neuropsychiatric disorders with adolescent onset and strong gender disparities, such as mood disorders, anxiety disorders, and schizophrenia.
Abstract
Puberty is the defining biological process of adolescent development, yet its effects on fundamental properties of brain physiology such as cerebral blood flow (CBF) have never been investigated. Capitalizing on a sample of 922 youths ages 8–22 y imaged using arterial spin labeled MRI as part of the Philadelphia Neurodevelopmental Cohort, we studied normative developmental differences in cerebral perfusion in males and females, as well as specific associations between puberty and CBF. Males and females had conspicuously divergent nonlinear trajectories in CBF evolution with development as modeled by penalized splines. Seventeen brain regions, including hubs of the executive and default mode networks, showed a robust nonlinear age-by-sex interaction that surpassed Bonferroni correction. Notably, within these regions the decline in CBF was similar between males and females in early puberty and only diverged in midpuberty, with CBF actually increasing in females. Taken together, these results delineate sex-specific growth curves for CBF during youth and for the first time to our knowledge link such differential patterns of development to the effects of puberty.
Blood perfusion is one of the fundamental physiologic properties of any organ and is of particular relevance for the human brain, which receives 15% of cardiac output despite only representing 2% of body mass (1). Prior work has shown that cerebral blood flow (CBF) declines markedly throughout childhood and adolescence (2–4). Along with gray matter loss and white matter expansion (5), CBF thus represents one of the most important properties of brain physiology that changes during youth and may be critical for establishing normative growth charts of brain development. CBF is coupled to regional metabolism (6, 7), changes under cognitive demands (8), responds specifically to psychoactive drugs (9), and is abnormal in a variety of psychiatric conditions including schizophrenia (10) and addiction (11). Thus, characterization of normative trajectories of CBF during adolescent development is highly relevant for understanding both normal brain function and its aberrations in psychopathology.
Growth curves of height, weight, and head circumference used in typical pediatric practice are separated by sex, because the timing and tempo of growth are different among males and females. One reason for this is the influence of puberty, which is the defining biological process of adolescence. Puberty results in divergent, sex-specific maturation that is driven by the influence of steroid and other metabolic hormones including estrogen and testosterone. Prior work has demonstrated sex differences in patterns of structural brain development (5, 12), and a growing body of literature has begun to establish the influence of puberty on this process (13, 14).
In contrast to research on structural brain development, work on cerebral perfusion during development has thus far been relatively sparse. Early research by Kennedy and Sokoloff (15), using a modified Kety–Schmidt nitrous oxide method (16), established that whole-brain CBF was 106 mL⋅100 g−1⋅min−1 in children, compared with 60 mL⋅100 g−1⋅min−1 in adults. Later, CBF was measured on a regional basis using techniques such as 133Xe clearance or 15O PET. However, sample sizes of these studies were limited by the need for ionizing radiation exposure, which is particularly problematic in pediatric populations. Nonetheless, these studies reliably demonstrated that CBF is elevated during childhood then declines throughout adolescence (3, 17, 18). In adulthood, females have higher CBF than males (8, 19). However, prior nuclear imaging studies in youth were too small (typically n = 20–40) to characterize sex differences during development.
Arterial spin labeling (ASL) using MRI permits noninvasive quantification of cerebral perfusion without the use of ionizing radiation (20, 21) but gives comparable gray matter CBF measurements when validated versus PET (22, 23). This feature provides a critical advantage for applications in pediatric populations (4), allowing for a substantial increase in sample size. Using ASL, Taki et al. (24, 25) replicated prior findings of declining perfusion in adolescence and also reported that females had higher perfusion in the posterior cingulate cortex (pCC), owing to a steeper rate of CBF decline in males. However, it is not known whether such effects are limited only to the pCC or whether developmental trajectories of perfusion differ between males and females in other regions, potentially in a complex nonlinear fashion. Divergent trajectories in multiple regions are to be expected, because adult females have higher CBF than males across brain regions beyond the pCC (8, 19, 26, 27). However, it is not yet known when such differences emerge in development.
Importantly, no study has investigated whether emerging sex differences in cerebral perfusion seen in adolescence are due to the differential impact of puberty. Studies from both animals and humans provide good reason to suspect that puberty may play a key role in CBF sex differences: Estrogens increase CBF in both animals and humans and also may promote neurogenesis and axonal sprouting (28–31). However, because the progression of age and puberty are correlated, large samples are required to systematically parse the relative influence of each. Here, we investigated developmental patterns of cerebral perfusion in males and females using ASL data from the Philadelphia Neurodevelopmental Cohort (PNC) (32), which constitutes the largest sample of cerebral perfusion yet reported. We hypothesized that differences in cerebral perfusion between males and females would relate to the impact of puberty. As described below, we found pronounced evidence for differential patterns of developmental perfusion in males and females, driven in part by the effects of puberty.
Results
We studied 922 youths aged 8–22 y who were imaged as part of the PNC (Table 1) applying a protocol as detailed elsewhere (32). CBF was measured using pseudocontinuous arterial spin labeling with a spin-echo echo-planar imaging acquisition (33, 34), which provided both greater sensitivity and approximately four times higher spatial resolution than prior developmental studies of CBF. Additionally, CBF quantification incorporated subject-specific modeling of the T1 relaxation time (35), which is known to vary by age and sex and therefore represents a previously unaccounted-for confound in studies of brain perfusion in development. Nonlinear trajectories of CBF differences were flexibly modeled using penalized splines within a general additive model (GAM) (36, 37).
Table 1.
Sample characteristics (n = 922)
| Group | N | Mean age, y (SD) | No. Caucasian | Motion, mm mean relative displacement (SD) |
| Females | 518 | 14.8 (3.50) | 211 | 0.084 |
| Males | 404 | 14.3 (3.58) | 207 | 0.086 |
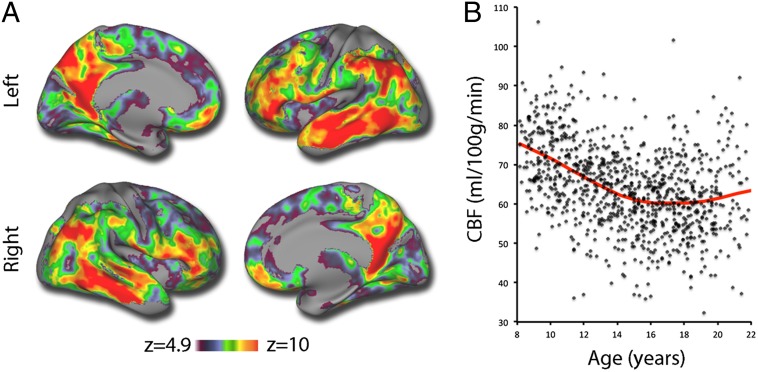
We began by examining age-related differences in CBF. The large sample size allowed us to use the conservative Bonferroni correction for type I error control in all voxelwise analyses. Our results replicate prior reports that CBF declines with age during development. The most significant age-related declines in CBF were seen in areas of heteromodal association cortex (Fig. 1A) that are known hubs of the default mode network (DMN) (38, 39), including the pCC, ventromedial prefrontal cortex, inferior parietal lobule, and lateral temporal cortex. Substantial age-related declines in CBF were also seen within certain hubs of the executive system (i.e., frontal pole and dorsolateral prefrontal cortex). Previously unreported nonlinearities were present throughout: When collapsed across males and females, average gray matter CBF declined most rapidly in late childhood and early adolescence, with a flatter trajectory in midadolescence (ages 15–17 y), followed by a slight increase in CBF during early adulthood (Fig. 1B).
Fig. 1.
Main effect of age on cerebral perfusion. (A) Cerebral perfusion declines throughout the cortex, but most prominently in heteromodal association cortex, including hubs of the default mode network and executive system. Image thresholded at z > 4.9 (Bonferroni P < 0.05), k > 100. (B) Mean gray matter CBF declines in a nonlinear fashion. Data points represent mean gray matter CBF of each subject (n = 922), fit with a penalized spline within a general additive model.
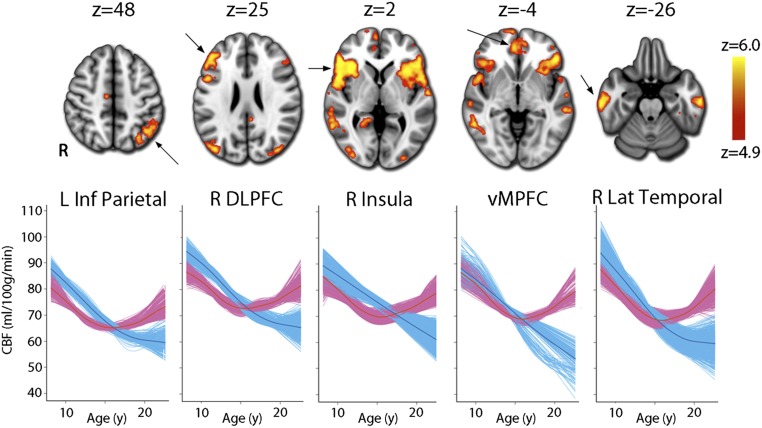
However, this average pattern masked a marked sex difference in developmental trajectories of CBF. A nonlinear age-by-sex interaction was significant in multiple brain regions (Fig. 2, Table 2, and Fig. S1). Divergent patterns of development were again most prominent in hubs of the DMN and executive system but additionally included other brain regions known to be critical for affective processing such as the anterior insula and orbitofrontal cortex (OFC). Across these regions, before age 13 females had lower CBF but males had a more rapid rate of age-related CBF decline, resulting in male CBF becoming lower than female CBF in midadolescence. In late adolescence, female CBF actually began to increase with age, whereas CBF continued to decline in males, accentuating the divergence between the sexes. To exclude the possibility that these results were driven by the effect of the gray matter density model covariate, we reran this analysis with this covariate excluded from the model. Results were highly similar and still exceeded the same statistical threshold, with a spatial correlation between the images of r = 0.78. Because sex differences were so highly dependent on age, a main effect of sex was only present in bilateral auditory cortex, where males had higher CBF than females [right: k = 342, peak Montreal Neurologic Institute (MNI) coordinates: x = 48, y = 4, z = 0; left: k = 336, x = −44, y = −10, z = −4].
Fig. 2.
A voxelwise GAM revealed that the developmental pattern of CBF change differed significantly between males (blue) and females (pink) in multiple regions within heteromodal association cortex (see Table 2 for a complete list). Whereas CBF values declined in males until late adolescence, CBF in females declined until midadolescence but increased thereafter. Images thresholded at z > 4.9 (Bonferroni corrected P < 0.05), k > 100; age plots in bottom row depict GAM fit for each voxel in a specified cluster, stratified by sex and adjusted for model covariates. See Fig. S1 for display of complete results.
Table 2.
Regions where males and females had a significantly different developmental trajectory of CBF (i.e., a nonlinear age-by-sex interaction)
| Region | k | Maximum z | Peak MNI coordinates | ||
| Left insula | 1,749 | 7.59 | −50 | 32 | 0 |
| Right insula | 1,225 | 7.08 | 52 | 28 | 4 |
| Left lateral occipital cortex | 647 | 7.3 | −44 | −82 | 12 |
| Left lateral temporal cortex | 618 | 6.28 | −54 | −48 | −2 |
| Right lateral occipital cortex | 551 | 6.93 | 34 | −90 | 6 |
| Left dorsolateral prefrontal cortex | 514 | 6.54 | −44 | 34 | 22 |
| Right lateral temporal cortex | 451 | 6.74 | 64 | −26 | −16 |
| Left inferior parietal cortex | 262 | 5.92 | −36 | −74 | 46 |
| Right dorsolateral prefrontal cortex | 202 | 6.29 | 48 | 32 | 20 |
| Left thalamus | 159 | 6.84 | −8 | −18 | 16 |
| Ventromedial prefrontal cortex | 145 | 5.79 | −2 | 42 | −8 |
| Left hippocampus | 132 | 6.97 | −30 | −28 | −14 |
| Precuneus | 128 | 5.98 | 18 | −52 | 6 |
| Posterior cingulate cortex | 107 | 5.65 | 4 | −42 | 28 |
| Right thalamus | 106 | 5.9 | 10 | −22 | 16 |
Clusters considered significant if z > 4.9, k > 100 in a sample of 922 subjects.
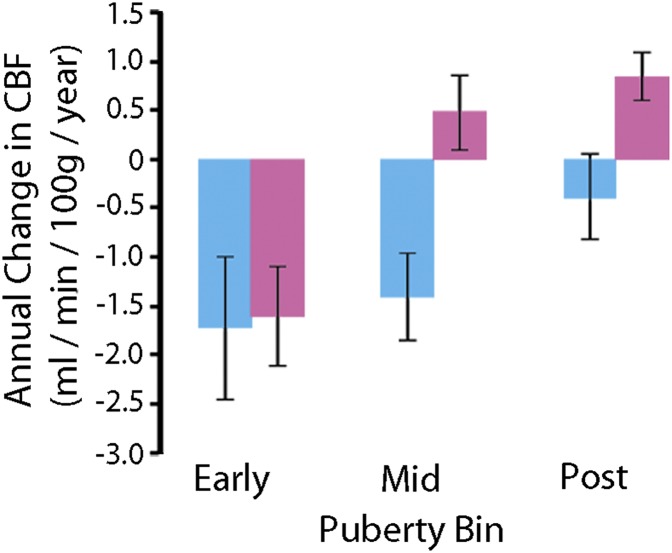
These results reveal fundamentally divergent patterns of age-related changes in CBF for males and females during late adolescence. We next investigated whether sex differences in age-related effects were in part due to the impact of puberty. As part of the PNC, subjects over 10 y old completed a computerized self-report regarding their pubertal development. As expected, in females pubertal status advanced at earlier ages than in males (t[764] = 4.23; P = 2.6 × 10−5; Table 3). We focused on regions that demonstrated a significant age-by-sex interaction effect (Table 2) and investigated whether sex-specific patterns of CBF change with age could be attributed to puberty. Notably, developmental effects varied by sex during puberty (Fig. 3), with males and females having similar declines in CBF in early puberty (t[764] = 0.1, corrected P = 0.89) but differing during the midpubertal (t[764] = 3.2, corrected P = 0.001) and postpubertal (t[764] = 2.4, corrected P = 0.01) periods. Notably, these results were similar regardless of whether Tanner stages were grouped into bins or left ungrouped (see SI Methods and Table S1 for further details). Additionally, general effects of growth did not drive these findings: Results were unchanged when height or body mass index was added as a covariate. Overall, these results suggest that the occurrence of puberty in females is associated with a marked alteration in the developmental trajectory of cerebral perfusion and that perfusion diverges between females and males during the midpubertal period.
Table 3.
Sample by pubertal stage (n = 769)
| Pubertal stage | No. female | No. male | Mean age, females (SD) | Mean age, males (SD) |
| Early | 84 | 107 | 12.6 (2.1) | 12.2 (1.3) |
| Mid | 109 | 109 | 15.1 (2.5) | 15.6 (2.1) |
| Post | 255 | 115 | 17.0 (2.5) | 18.0 (2.1) |
Fig. 3.
Impact of puberty on sex-specific patterns of CBF change. Age-related differences in CBF diverge in males (blue) and females (pink) with advancing pubertal development. Error bars represent SEs.
Discussion
This study demonstrates that normative developmental patterns of cerebral perfusion differ markedly between males and females, in part owing to the divergent impact of puberty. CBF values are similar and decline at the same rate in both males and females during early puberty but diverge markedly by the midpubertal period. These results expand our knowledge regarding the normative development of cerebral perfusion and may have substantial relevance for understanding both normative cognitive development and abnormalities related to neuropsychiatric disorders.
Prior studies in adults have reported higher levels of brain perfusion in females (8, 19, 26, 27). However, it was not known at what point in the lifespan this difference appeared. The current results demonstrate that this disparity begins in adolescence and is specifically associated with puberty. Taki et al. (24, 25) recently reported a sex difference in the posterior cingulate. Using a region of interest analysis, they found that the slope of CBF age-related decline in the posterior cingulate was steeper for males than for females (24, 25). We found that such age-by-sex interactions are present not just in the posterior cingulate but are widespread across heteromodal association cortex, including critical hubs of the default mode network, executive system, and limbic system.
Subsequent analyses focused on these regions and demonstrated puberty modulated the age-related changes in CBF in a sex-specific manner. CBF declined at a similar rate in males and females in early puberty but was markedly different thereafter; it continued to decline in males but in fact increased with age in females. This finding suggests that much of the observed nonlinear age-by-sex interaction may be specifically due to the effects of puberty, which seems to alter the trajectory of developmental changes in females, prompting a dramatic reversal from falling CBF values to an actual increase in CBF postpuberty. This effect was present regardless of whether weight and body mass index were included in the model as covariates. We speculate that this may be due to the influence of rising levels of estrogens during female puberty.
Data from studies in humans and animal models accord with this possibility. Specifically, estrogens increase CBF in both animals and humans undergoing controlled ovarian stimulation (40–42). The loss of estrogen associated with menopause has been linked to reduced reactivity of cerebrovasculature (43). However, we do not believe our findings are primarily attributable to estrogen-related changes in vascular reactivity, because such effects would be unlikely to have the regional specificity observed here (e.g., OFC). Furthermore, in animal models estrogens have a potent impact on neurons at the cellular level, preserving dendritic spines and axonal sprouting in hippocampus subfield CA1 (28, 29) and affecting neurogenesis in the medial temporal lobe (30, 31). Further research is necessary to link such findings to the large-scale regional changes in perfusion observed in the present study. Although preclinical data regarding androgens is less extensive, testosterone has conversely been linked to diminished vascular dilation (44). However, such effects are unlikely to explain the present data, because age-related CBF declines were most prominent in the prepubertal period, before maximal androgen exposure.
The robust results of this study were enabled by the large sample size, advances in image acquisition and CBF quantification, and the analytic techniques used. The present sample is nearly five times larger than the studies by Taki et al. (24, 25), which previously were the largest studies of the development of brain perfusion, and 10–20 times larger than in other studies of perfusion in development. The sample size of the PNC resulted in a substantial increase in sensitivity; whereas prior studies often used regional analyses, the power of this sample allowed voxelwise analyses with greater sensitivity to detect real effects (e.g., low type II error) while simultaneously minimizing type I error with a conservative Bonferroni correction.
It should be noted, however, that larger samples are similarly sensitive to the influence of confounding variables. Accordingly, we accounted for confounds including subject motion in our model, and for the first time to our knowledge accounted for variations in gray matter density on a voxelwise basis. Furthermore, this is the first large study, to our knowledge, to account for known age- and sex-related differences in the T1 relaxation time. Better localization of effects was allowed by improved spatial resolution, which was four times that of prior developmental CBF studies. Additionally, the application of general additive models provided a powerful and statistically rigorous test for nonlinear differences in the trajectories of male and female subjects.
The present results add to a rapidly expanding body of literature demonstrating the impact of puberty on the developing brain (14, 45–47). Sex differences in brain structure are well documented (48, 49) and are increasingly tied to developmental effects related to puberty (13, 46, 50–53). In particular, several studies have found that puberty in females and rising estrogen is related to increased gray matter in structures such as the hippocampus (51, 52, 54) that are known to have high density of gonadotropin receptors (55). Because CBF is higher in gray matter than in white matter, these findings underscore the necessity of accounting for changes in brain structure when modeling developmental changes in CBF.
Similarly, functional connectivity is known to evolve rapidly with development (56–58) and exhibits prominent sex differences (59, 60). Furthermore, functional connectivity may be affected by sex steroids (45). Because brain perfusion and functional connectivity have been shown to be related in critical network hubs (61), future work should explore the relevance of the observed changes in cerebral perfusion for the evolution of brain network topology in adolescence.
Notably, many of the regions with a significantly different pattern of development between the sexes were among those with the highest CBF (Fig. S2) and also exhibited the greatest age-related decline in CBF (as in Fig. 1). CBF decline in childhood and adolescence has previously been found to be tightly linked to the decline of glucose metabolism (62). Furthermore, regions including the DMN and the executive system have also been identified as important sites of aerobic glycolysis (63), which provides 10–12% of the glucose used in the human brain; regions with a high glycolytic index are also characterized by persistent expression of genes typical of infancy (“transcriptional neoteny”) (62). However, although the correspondence between age-related declines in CBF and neotenous regions is striking, several brain regions that show the strongest sex differences in development such as the anterior insula are distinct from this network. Nonetheless, most prior studies of brain metabolism have not specifically investigated sex differences in development, providing fertile ground for future research.
Several limitations should be noted. First, all data presented here are cross-sectional; further description of developmental trajectories requires longitudinal data (64). Second, mechanistic interpretation is limited by the lack of information regarding circulating hormone levels in participants. Therefore, although preclinical and some clinical studies suggest roles for gonadal hormones in evolution of cerebral perfusion during adolescence, the presence of any such link in the present data must remain speculative. Other neuroendocrine factors such as leptin (65) could potentially drive the observed effects and should be evaluated in future studies. Third, whereas this study used a validated self-report assessment of pubertal development, such measures are less accurate than an examination by a trained physician.
These limitations notwithstanding, the results establish that males and females have markedly different normative trajectories of cerebral perfusion and that such differences may in part be due to the impact of puberty. Further study is needed to understand how the observed effects may relate to known sex differences in cognition, most notably social cognition (66). Many of the regions that show a prominent sex difference in developmental trajectory are higher-order association cortex areas that are also involved in social cognition tasks such as emotion identification and emotional face memory (67–70). We speculate that puberty-related, sex-specific changes in perfusion may in part be related to female superiority on these tasks.
As the present results indicate, measurement of neurobiological markers such as brain perfusion has the potential to uncover specific mechanisms that may underlie developmental traits that are different between males and females. Furthermore, because neuropsychiatric disorders are increasingly conceptualized as neurodevelopmental in origin, accurate growth charts of critical aspects of brain development such as brain perfusion are necessary to identify those individuals that “fall off the curve” and may be at risk. Our results underscore the fact that patterns of growth are different for males and females; analyses that do not consider sex differences may suffer from both reduced sensitivity and specificity. The present results have potential relevance to a wide range of psychiatric disorders that often manifest following puberty and have marked sex disparities, including depression, anxiety disorders, and schizophrenia (71–74). Future research could test the hypothesis that increased perfusion in postpubertal females may be linked to the greater risk in females for mood and anxiety disorders and a lower risk of schizophrenia.
Methods
Participants and Assessment.
As described in detail elsewhere (32), the PNC is a collaboration between the Center for Applied Genomics at Children’s Hospital of Philadelphia (CHOP) and the Brain Behavior Laboratory at the University of Pennsylvania (Penn). All subjects or their parent or guardian provided informed consent and minors provided assent; study procedures were approved by the institutional review boards of both Penn and CHOP. Clinical and imaging inclusion and exclusion criteria yielded a final sample of 922 subjects. Pubertal status was evaluated using an abbreviated version of a self-report measure of pubertal status that was computerized and self-administered (75). Puberty data were available for 779 subjects; subjects below age 10 did not complete this assessment, limiting the number of younger children who were assessed as prepubertal. Puberty was coded as a categorical variable with three levels: Tanner stages 1–3 were collapsed to produce similar bin sizes and considered early puberty (50, 52), whereas Tanner stage 4 was considered midpubertal and Tanner stage 5 was considered postpubertal. For further details, see SI Methods and Table S1.
Image Acquisition, Preprocessing, and CBF Quantification.
Neuroimaging acquisition and preprocessing were as previously described (32); see SI Methods for further details. CBF was quantified from control-label pairs using ASLtbx (76), according to the following equation:
where f is CBF, ΔM is the difference signal between the control and label acquisitions, R1a is the longitudinal relaxation rate of blood, τ is the labeling time, ω is the postlabeling delay time, α is the labeling efficiency, λ is the blood/tissue water partition coefficient, and M0 is approximated by the control image intensity (76). We set α = 0.85, λ = 0.9 g/mL, τ = 1.6 s, and ω = 1.2 s. Because prior work has shown that the T1 relaxation time changes substantially in development and varies by sex, this parameter was set according to the methods outlined in Wu et al. (35). Prior validation studies have shown that this procedure enhances accuracy and reliability in pediatric populations (77); for further details see SI Methods.
Group-Level Analysis of Age and Sex Effects.
Following normalization to template space (SI Methods), trajectories of age-related CBF differences were flexibly modeled using penalized splines within a GAM (36, 37). Such an approach allows for nonlinearities in the relationship between age and CBF without specifying a predefined set of functions (such as polynomials). Importantly, the GAM assesses a penalty with increasing nonlinearity to avoid overfitting the data. Furthermore, this approach allowed us to ascertain whether the shape of the nonlinear age trajectory was significantly different between males and females. Within this model we controlled for covariates of no interest as well as known confounds, including race, in-scanner motion, and gray-matter density (calculated on a voxelwise basis); for further details see SI Methods. Type I error control was achieved using Bonferroni correction; thus, corrected P < 0.05 corresponds to uncorrected P < 4.56 × 10−7, or z > 4.9. For display, results were projected to the cortical surface of the Population-Average, Landmark- and Surface-based atlas with Caret (78); axial slices were displayed using Mango.
Analysis of the Effect of Puberty.
The above analysis revealed that the pattern of age-related change in male and female CBF was markedly different at multiple loci, primarily within heteromodal association cortex (Table 2 and Results). We next evaluated whether these divergent patterns of development were due to the impact of puberty. This analysis included 779 subjects ages 10 and over for whom puberty self-report measures were available. Because the pattern of results in all 17 regions were quite similar, we averaged the CBF across all clusters and used a linear model to examine whether the effect of age on CBF differed by sex and pubertal bin. We hypothesized that age effects would be similar during early puberty but differ with advancing pubertal stage. Covariates considered were as in the voxelwise analyses described above. Height, weight, and body mass index were available for a subset of 557 subjects; results were unchanged when these variables were added as model covariates. We tested for a significant difference between age coefficients at each puberty and sex bin while adjusting for covariates using the least squares means procedure in R; a Tukey’s correction for multiple comparisons was applied.
Supplementary Material
Acknowledgments
We thank the acquisition and recruitment team: Jeff Valdez, Raphael Gerraty, Marisa Riley, Jack Keefe, Nick DeLeo, Elliott Yodh, and Rosetta Chiavacci. We also thank Aaron Alexander-Bloch and Phil Reiss for advice on implementation of the general additive model and Peter Schmidt and Armin Raznahan for discussion. This work was supported by National Institute of Mental Health RC2 Grants MH089983 and MH089924 and T32 Grant MH019112. T.D.S. was supported by Grant K23MH098130 and the Marc Rapport Family Investigator grant through the Brain and Behavior Foundation. D.H.W. was supported by Grant K23MH085096, American Psychiatric Institute for Research and Education, and the Sidney R. Baer, Jr. Foundation through the Brain and Behavior Foundation. J.A.D. and M.A.E. were supported in part by National Institute of Biomedical Imaging and Bioengineering Grant P41 EB015893. R.T.S. was supported in part by National Institute of Neurological Disorders and Stroke Grant NS085211.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in database of Genotypes and Phenotypes (dbGaP), www.ncbi.nlm.nih.gov/gap (accession no. phs000607.v1.p1).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400178111/-/DCSupplemental.
References
- 1.Bouma GJ, Muizelaar JP. Relationship between cardiac output and cerebral blood flow in patients with intact and with impaired autoregulation. J Neurosurg. 1990;73(3):368–374. doi: 10.3171/jns.1990.73.3.0368. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy C, Grave GD, Juhle JW, Sokoloff L. Changes in blood flow in the component structures of the dog brain during postnatal maturation. J Neurochem. 1972;19(10):2423–2433. doi: 10.1111/j.1471-4159.1972.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa A, et al. [Regional cerebral blood flow in children—normal value and regional distribution of cerebral blood flow in childhood] No To Shinkei. 1987;39(2):113–118. Japanese. [PubMed] [Google Scholar]
- 4.Wang J, et al. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18(4):404–413. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- 5.Lenroot RK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA. 1986;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 8.Gur RC, et al. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217(4560):659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 9.Wolf DH, et al. Oral alprazolam acutely increases nucleus accumbens perfusion. Mol Psychiatry. 2013;18(9):960–961. doi: 10.1038/mp.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinkham A, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. 2011;194(1):64–72. doi: 10.1016/j.pscychresns.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27(51):14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giedd JN, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4-18 years. J Comp Neurol. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Blanton RE, et al. Pubertal stage and brain anatomy in girls. Neuroscience. 2012;217:105–112. doi: 10.1016/j.neuroscience.2012.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giedd JN, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254-255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; normal values for cerebral blood flow and cerebral metabolic rate in childhood. J Clin Invest. 1957;36(7):1130–1137. doi: 10.1172/JCI103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: Theory, procedure, and normal values. J Clin Invest. 1948;27(4):476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiron C, et al. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33(5):696–703. [PubMed] [Google Scholar]
- 18.Takahashi T, Shirane R, Sato S, Yoshimoto T. Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR Am J Neuroradiol. 1999;20(5):917–922. [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med. 2012;68(3):912–922. doi: 10.1002/mrm.23286. [DOI] [PubMed] [Google Scholar]
- 20.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre GK, Detre JA. The development and future of perfusion fMRI for dynamic imaging of human brain activity. Neuroimage. 2012;62(2):1279–1285. doi: 10.1016/j.neuroimage.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Xu G, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR Biomed. 2010;23(3):286–293. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye FQ, et al. H(2)(15)O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med. 2000;44(3):450–456. doi: 10.1002/1522-2594(200009)44:3<450::aid-mrm16>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Taki Y, et al. Correlation between gray matter density-adjusted brain perfusion and age using brain MR images of 202 healthy children. Hum Brain Mapp. 2011;32(11):1973–1985. doi: 10.1002/hbm.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taki Y, et al. Gender differences in partial-volume corrected brain perfusion using brain MRI in healthy children. Neuroimage. 2011;58(3):709–715. doi: 10.1016/j.neuroimage.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Gur RC, et al. Lateralized changes in regional cerebral blood flow during performance of verbal and facial recognition tasks: Correlations with performance and “effort”. Brain Cogn. 1997;33(3):388–414. doi: 10.1006/brcg.1997.0921. [DOI] [PubMed] [Google Scholar]
- 27.Ragland JD, et al. Hemispheric activation of anterior and inferior prefrontal cortex during verbal encoding and recognition: A PET study of healthy volunteers. Neuroimage. 2000;11(6 Pt 1):624–633. doi: 10.1006/nimg.2000.0577. [DOI] [PubMed] [Google Scholar]
- 28.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10(4):1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse JK, Scheff SW, DeKosky ST. Gonadal steroids influence axon sprouting in the hippocampal dentate gyrus: A sexually dimorphic response. Exp Neurol. 1986;94(3):649–658. doi: 10.1016/0014-4886(86)90244-x. [DOI] [PubMed] [Google Scholar]
- 30.Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Brain Res Rev. 2008;57(2):342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satterthwaite TD, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Wang DJJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging. 2011;33(4):940–949. doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60(6):1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W-C, et al. In vivo venous blood T1 measurement using inversion recovery true-FISP in children and adults. Magn Reson Med. 2010;64(4):1140–1147. doi: 10.1002/mrm.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004;99(467):673–686. [Google Scholar]
- 37.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol. 2011;73(1):3–36. [Google Scholar]
- 38.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 40.Belfort MA, et al. Hormonal status affects the reactivity of the cerebral vasculature. Am J Obstet Gynecol. 1995;172(4 Pt 1):1273–1278. doi: 10.1016/0002-9378(95)91492-7. [DOI] [PubMed] [Google Scholar]
- 41.Shamma FN, Fayad P, Brass L, Sarrel P. Middle cerebral artery blood velocity during controlled ovarian hyperstimulation. Fertil Steril. 1992;57(5):1022–1025. [PubMed] [Google Scholar]
- 42.Nevo O, Soustiel JF, Thaler I. Cerebral blood flow is increased during controlled ovarian stimulation. Am J Physiol Heart Circ Physiol. 2007;293(6):H3265–H3269. doi: 10.1152/ajpheart.00633.2007. [DOI] [PubMed] [Google Scholar]
- 43.Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M. Age and sex differences in cerebral hemodynamics: A transcranial Doppler study. Stroke. 1998;29(5):963–967. doi: 10.1161/01.str.29.5.963. [DOI] [PubMed] [Google Scholar]
- 44.Gonzales RJ, Krause DN, Duckles SP. Testosterone suppresses endothelium-dependent dilation of rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2004;286(2):H552–H560. doi: 10.1152/ajpheart.00663.2003. [DOI] [PubMed] [Google Scholar]
- 45.Peper JS, van den Heuvel MP, Mandl RCW, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: A review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: A mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Blakemore S-J, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gur RC, et al. Sex differences in brain gray and white matter in healthy young adults: Correlations with cognitive performance. J Neurosci. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Bellis MD, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11(6):552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 50.Bramen JE, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21(3):636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu S, Pruessner JC, Coupé P, Collins DL. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. Neuroimage. 2013;74:276–287. doi: 10.1016/j.neuroimage.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 52.Neufang S, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen T-V, et al. Brain Development Cooperative Group Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013;23(6):1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satterthwaite TD, et al. Sex differences in the effect of puberty on hippocampal morphology. J Am Acad Child Adolesc Psychiatry. 2014;53(3):341. doi: 10.1016/j.jaac.2013.12.002. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64(1):34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- 56.Dosenbach NUF, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fair DA, et al. Functional brain networks develop from a “local to distributed” organization. PLOS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Satterthwaite TD, et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satterthwaite TD, et al. Linked sex differences in cognition and functional connectivity in youth. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu036. 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuo X-N, et al. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30(45):15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci USA. 2013;110(5):1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19(1):49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaishnavi SN, et al. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157(2):163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- 65.Blum WF, et al. Plasma leptin levels in healthy children and adolescents: Dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82:2904–2910. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- 66.Gur RC, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 68.Satterthwaite TD, et al. Frontolimbic responses to emotional face memory: The neural correlates of first impressions. Hum Brain Mapp. 2009;30(11):3748–3758. doi: 10.1002/hbm.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satterthwaite TD, et al. Opposing amygdala and ventral striatum connectivity during emotion identification. Brain Cogn. 2011;76(3):353–363. doi: 10.1016/j.bandc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45(1):174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Aleman A, Kahn RS, Selten J-P. Sex differences in the risk of schizophrenia: Evidence from meta-analysis. Arch Gen Psychiatry. 2003;60(6):565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- 72.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- 73.Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: Gender and psychopathology. Annu Rev Clin Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- 74.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 76.Wang Z, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26(2):261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain V, et al. Longitudinal reproducibility and accuracy of pseudo-continuous arterial spin-labeled perfusion MR imaging in typically developing children. Radiology. 2012;263(2):527–536. doi: 10.1148/radiol.12111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Essen DC, et al. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8(5):443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.