Significance
Tetramethylenedisulfotetramine (TETS) is a feared chemical threat agent because of its high convulsant toxicity, ease of synthesis, and availability even though it is banned as a rodenticide. Earlier physiological evidence indicating action as a GABA receptor antagonist and inhibitor of [35S]TBPS and [3H]EBOB binding is confirmed here by radiosynthesis of [14C]TETS and defining its binding site in rat brain membranes by accelerator mass spectrometry and toxicant specificity studies on inhibition of [14C]TETS and [3H]EBOB binding. TETS undergoes specific and unique polar interactions inside the 1′2′ ring pore region instead of the 2′,6′, and 9′ site for insecticides. This study helps define GABAAR sites for potential antidotes acting to prevent TETS binding or displace it from its binding site.
Keywords: neurotoxicity, convulsant, molecular modeling
Abstract
Use of the highly toxic and easily prepared rodenticide tetramethylenedisulfotetramine (TETS) was banned after thousands of accidental or intentional human poisonings, but it is of continued concern as a chemical threat agent. TETS is a noncompetitive blocker of the GABA type A receptor (GABAAR), but its molecular interaction has not been directly established for lack of a suitable radioligand to localize the binding site. We synthesized [14C]TETS (14 mCi/mmol, radiochemical purity >99%) by reacting sulfamide with H14CHO and s-trioxane then completion of the sequential cyclization with excess HCHO. The outstanding radiocarbon sensitivity of accelerator mass spectrometry (AMS) allowed the use of [14C]TETS in neuroreceptor binding studies with rat brain membranes in comparison with the standard GABAAR radioligand 4′-ethynyl-4-n-[3H]propylbicycloorthobenzoate ([3H]EBOB) (46 Ci/mmol), illustrating the use of AMS for characterizing the binding sites of high-affinity 14C radioligands. Fourteen noncompetitive antagonists of widely diverse chemotypes assayed at 1 or 10 µM inhibited [14C]TETS and [3H]EBOB binding to a similar extent (r2 = 0.71). Molecular dynamics simulations of these 14 toxicants in the pore region of the α1β2γ2 GABAAR predict unique and significant polar interactions for TETS with α1T1′ and γ2S2′, which are not observed for EBOB or the GABAergic insecticides. Several GABAAR modulators similarly inhibited [14C]TETS and [3H]EBOB binding, including midazolam, flurazepam, avermectin Ba1, baclofen, isoguvacine, and propofol, at 1 or 10 μM, providing an in vitro system for recognizing candidate antidotes.
Severe poisonings in a German furniture factory in the 1940s were traced to wool impregnated with the resinous reaction product of sulfamide (NH2SO2NH2) and formaldehyde (HCHO). The causative agent was identified as tetramethylenedisulfotetramine (TETS, also known as tetramine) which was then developed as a rodenticide (now illegal) and continues to be of concern as a chemical threat agent. The chronology of TETS chemistry and toxicology is given briefly here and more extensively in SI Appendix, section S1.
TETS was first synthesized more than 80 y ago (1–3). Structure–activity studies showed that any structural modification greatly reduces the toxicity (4). The need to understand the distribution and fate of TETS led to 14C radiosynthesis in 1967 (5) by an undisclosed method, but the product was only 80% pure, limiting the interpretation of biological experiments. Analysis is achieved by liquid chromatography/MS (6) or when ultrahigh sensitivity is required and [14C]TETS is available by accelerator mass spectrometry (AMS) (7), as reported here.
TETS is highly toxic to mammals with an i.p. LD50 of 0.11–0.22 mg/kg in mice and rats, leading to its use as a rodenticide until it was banned worldwide in the early 1990s (2, 8, 9). However, it is still available illegally and responsible for accidental or intentional poisonings in China and other countries. The estimated lethal dose of 7–10 mg in adult humans coupled with its ease of synthesis and stability serve as the basis for the chemical threat concern (10–15). Neurotoxicity is sometimes alleviated or antidoted by compounds modulating the target site to reduce disruption by the toxicant. TETS toxicity is reported to be alleviated in rodents or humans by diazepam, barbiturates, allopregnanolone, and sodium 2,3-dimercapto-1- propanesulfonate (NaDMPS), some of which are GABAAR modulators (16–24) (SI Appendix, section S1). TETS is one of several small-cage convulsants, a group that also includes bicyclophosphorus compounds, such as the even more toxic t-butylbicyclophosphate (TBPO) and t-butylbicyclophosphorothionate (TBPS) (25) (Fig. 1).
Fig. 1.
Structures of three radioligands and unlabeled insecticides or convulsants with number designations. Two numbers for TETS (1/2) and fluralaner (10/11) refer to different concentrations considered later.
TETS is a noncompetitive antagonist of the GABA type A receptor (GABAAR) based on multiple physiological and toxicological criteria* (20, 26–29) (SI Appendix, section S1) and GABAAR assays with two trioxabicyclooctane radioligands, [35S]TBPS (30), and 4-n-[3H]propyl-4′-ethynylbicycloorthobenzoate ([3H]EBOB) (31) (Fig. 1). TETS is a competitive inhibitor of [3H]EBOB binding with a potency in rat brain GABAAR consistent with its toxicity in mice (4, 32). However, it does not inhibit human GABAAR recombinant β3 homopentamer assayed with [3H]EBOB (33), which has a structure–activity relationship for inhibitors similar to that for the housefly GABAR (34). These deductions are based on the use of [35S]TBPS and [3H]EBOB to assay the action of TETS.
Direct observation of the TETS binding site requires the use of TETS as the radioligand. Radioligand binding studies for neuroreceptors as toxicant targets normally require high specific activities (>10 Ci/mmol) such as 3H labeling, which is not available to date for TETS. [14C]TETS reported here has a specific activity of 14 mCi/mmol, which is only about 0.02–0.1% the level normally used for radioligand binding assays. The required detection could only be achieved with [14C]TETS by greatly enhanced sensitivity resulting from AMS, which to our knowledge has never been used before in neuroreceptor radioligand binding assays.
This study characterizes the [14C]TETS binding site in rat brain GABAAR. TETS and EBOB are compared as an unknown versus a standard cage convulsant radioligand that may have some common features in their binding sites. Rat brain is used because TETS is primarily a rodenticide. The same experiments are run with [14C]TETS and [3H]EBOB so that only the radioligand and analytical method are varied to best evaluate the utility of the new radioligand and the AMS analysis technique in defining the mechanism of TETS toxicity. The [14C]TETS binding assay allows verification of the mode of action, definition of the pharmacological profile, localization of the binding site, and characterization of potential antidotes or alleviating agents.
Results and Discussion
Synthesis of [13C]TETS and [14C]TETS.
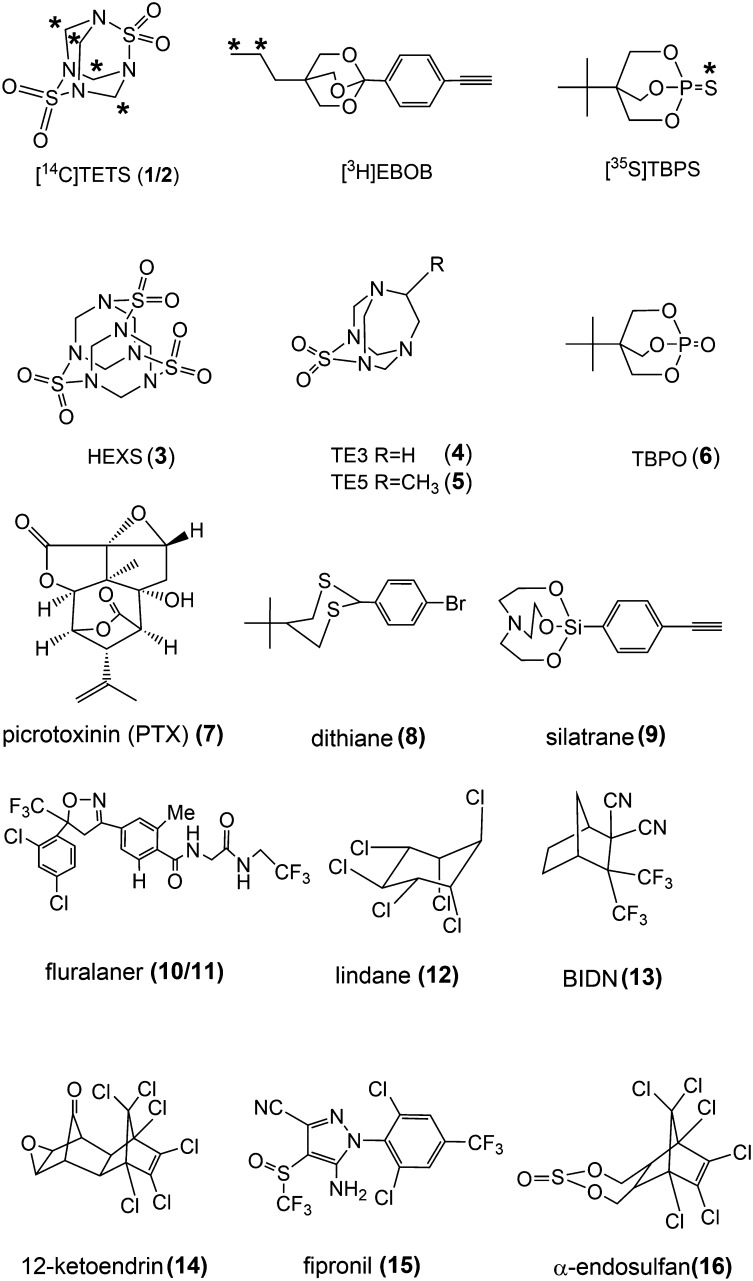
Unlabeled TETS is readily synthesized from sulfamide by reacting with HCHO (37% wt/vol in water) or its equivalent such as s-trioxane or paraformaldehyde in acidic condition (2, 4). However, the low concentration of commercially available H14CHO (1–3% in water) seriously delayed the final ring cyclization step to form [14C]TETS. To overcome this, the procedure was modified by stepwise cyclization (Fig. 2). Reaction conditions were optimized through tests with H13CHO as the H14CHO mimic. H13CHO (20% aqueous solution, 0.25 equivalents relative to sulfamide) and s-trioxane (source of 1.75 equivalents of HCHO as a solid form) ensured that all H13CHO was incorporated into the product owing to the slower release of unlabeled HCHO from s-trioxane. An additional treatment with unlabeled HCHO completed the final cyclization reaction. These conditions were then used to prepare the [14C]TETS as follows. To a chilled solution of sulfamide (1.9 mg, 20 µmol) and s-trioxane (1.1 mg, 12 µmol) in 21 µL of concentrated hydrochloric acid (conc. HCl, 250 µmol) was added 250 µL of H14CHO (5 µmol, 3% in water, 250 µCi, specific activity 50 mCi/mmol, 99% pure by HPLC) at 0 °C. The reaction mixture was slowly warmed to room temperature and stirred for 1 d. After adding acetonitrile (250 μL), the azeotrope was evaporated under a stream of dry air at room temperature. To the remaining reaction mixture were added 21 µL of conc. HCl and 3.8 µL of unlabeled HCHO (37% in water). After 1 h, acetonitrile (100 μL) was added and the azeotrope was again removed under a stream of dry air at room temperature. The remaining solid was dissolved by adding 100 µL of acetone then 500 µL of dichloromethane, yielding a white precipitate. After filtration, the filtrate was evaporated under a stream of dry air and the residual crude TETS was purified by column chromatography on silica gel with an eluent (dichloromethane: n-hexane 3:1, Rf = 0.54). The [14C]TETS was obtained on evaporation as a white solid: 310 µg, 12.9% chemical yield, 7.2% radiochemical yield, specific activity 14 mCi/mmol, and >99% radiochemical purity (SI Appendix, section S2). The product was dissolved in 1 mL acetone and stored in a sealed amber glass ampoule at −20 °C. GC-MS analysis data for the final [13C]TETS and [14C]TETS revealed slightly less label incorporation with [14C]TETS (Table 1).
Fig. 2.
Synthesis of [13C]- and [14C]TETS by serial cyclization steps in concentrated hydrochloric acid. The percentages of mono-, di-, tri-, and tetra- labeling are given in Table 1.
Table 1.
Labeling of [13C]TETS and [14C]TETS from 20% aqueous H13CHO and 3% aqueous H14CHO (50 mCi/mmol) and specific activity of [14C]TETS
| No. of labeled carbons | [14C]TETS | |||
| [13C]TETS, %* | %* | mCi/mmol† | Contribution‡ | |
| 0 | 67.3 | 76.2 | 0 | 0 |
| 1 | 25.4 | 19.8 | 50 | 9.9 |
| 2 | 5.7 | 3.4 | 100 | 3.4 |
| 3 | 1.2 | 0.6 | 150 | 0.9 |
| 4 | 0.4 | 0 | 200 | 0 |
| Total | 100 | 100 | 14.2§ | |
Percentage isotopic distribution of TETS determined by GC-MS in full-scan mode for [13C]TETS and selected ion monitoring mode for [14C]TETS (SI Appendix, section S2).
Theoretical specific activity of TETS with the indicated number of 14C-labeled carbon(s).
Contribution of components with one, two, and three 14C-labeled carbon(s) summated to give the total specific activity (mCi/mmol) of the final [14C]TETS.
Experimental specific activity (14.0 mCi/mmol) of the final [14C]TETS was determined by liquid scintillation counting of 1 μL aliquot from 1 mL acetone solution of 310 μg [14C]TETS.
[14C]TETS Binding Parameters.
Neuroreceptor binding assays with a 14C-labeled compound require an ultrasensitive analytical method provided by the use of tandem HPLC and AMS with a typical limit of quantification of 2–20 amol, which proved to be adequate in the present studies.
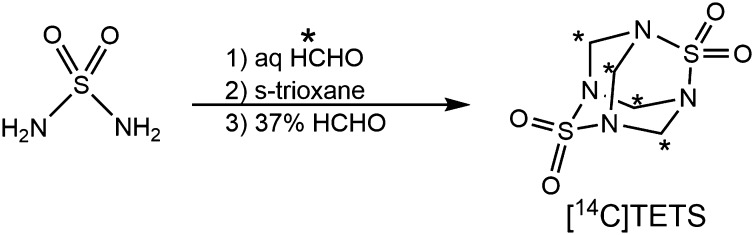
[14C]TETS undergoes specific binding to rat brain membranes at 37 °C with half saturation at 0.08 µM (Fig. 3A). TETS is also a potent inhibitor of [3H]EBOB binding under the same conditions with an IC50 of 0.79 μM (Fig. 3B). Nonspecific binding was determined with unlabeled TETS at 10 μM. Total, nonspecific, and specific binding with [14C]TETS were 1,390, 454, and 1,019 fg TETS/µg protein, respectively (i.e., 67 ± 1% specific binding). The corresponding values with [3H]EBOB were 2,013, 994, and 1,019 dpm/125 µg protein, respectively, corresponding to 50 ± 5% specific binding. GABA at 0.3, 1, and 10 μM inhibited [14C]TETS binding by 15 ± 3, 55 ± 1, and 79 ± 3%, respectively, and the corresponding values for [3H]EBOB were 42 ± 8, 61 ± 4, and 104 ± 2%, respectively. l-glutamic acid did not inhibit binding of either radioligand at 1 μM.
Fig. 3.
TETS target assayed as (A) displacement of [14C]TETS and (B) inhibition of [3H]EBOB binding in rat brain membranes.
Convulsants and Insecticides Compete Similarly for [14C]TETS and [3H]EBOB Binding Sites.
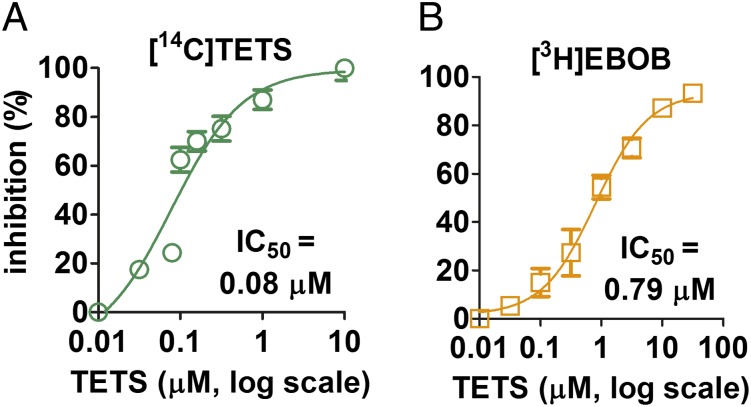
If [14C]TETS and [3H]EBOB bind at the same site in the same way (i.e., are superimposable), they should be similarly inhibited by a series of convulsants and insecticides selected for their widely varied chemotypes and assayed at 1 or 10 μM. The results for 16 compounds or concentrations (Fig. 1) are presented in Fig. 4 and SI Appendix, section S3. Earlier published findings on the [3H]EBOB and [35S]TBPS sites are given in SI Appendix, section S4. TETS with an IC50 of 0.08 μM (Fig. 3A) was considerably more potent than three of its analogs assayed with either radioligand. The TETS-type compounds (1–5) and several insecticides or cage convulsants (6–16) inhibit [14C]TETS and [3H]EBOB binding to a similar extent (r2 = 0.71). TETS, EBOB, and the other convulsants and insecticides therefore compete with each other at comparable binding sites, prompting atomistic, structural examination in the GABAAR pore.
Fig. 4.
Inhibition of [14C]TETS and [3H]EBOB binding in rat brain membranes by convulsants and insecticides at 10 µM (2 and 11) or 1 µM (all other data). Plotted from data in SI Appendix, section S3.
Different Binding Positions for TETS and EBOB.
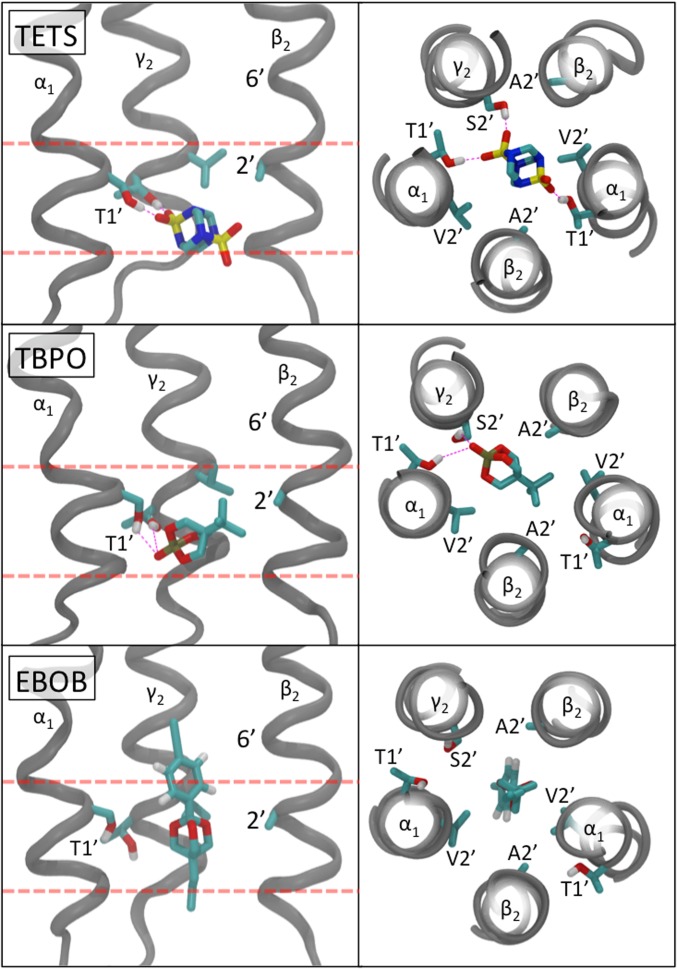
Much is known about the binding sites for EBOB, picrotoxinin (PTX), lindane, 3,3-bis-trifluoromethyl-bicyclo[2,2,1]heptane-2,2-dicarbonitrile, fipronil, and α-endosulfan in the α1β2γ2 GABAAR (33–40). TETS, TBPO, and EBOB were initially molecularly docked in the pore region of the α1β2γ2 GABAAR model. After 40 ns of molecular dynamics (MD) simulations (described in SI Appendix, section S5), their optimized and equilibrated positions (Fig. 5) illustrate that EBOB and TBPO overlap the proposed TETS binding site (located around the 1′2′ region of the pore). MD stimulations of all of the insecticides and convulsants in this study predict a partially overlapping binding site with a common region at the 1′2′ position (the 2′ “contact zone”) (SI Appendix, section S6). However, the interactions with specific residues in the 1′2′ position can differ. TETS and EBOB, for example, both make a substantial proportion of all their calculated contacts to the 1′2′ residues (61% and 45%, respectively). However, whereas EBOB contacts the α/γ subunits at the 1′2′ site 68% of the time (only slightly more than the 60% expected for nonspecific subunit binding), TETS contacts the α/γ subunits at the 1′2′ site 98% of the time with an α1:β2 ratio of 31:1, suggesting a specific α1 interaction. Moreover, 69% of all of the simulated TETS contacts with GABAAR are made to just the α1T1′ and γ2S2′ residues. In contrast, EBOB makes only 6% of its contacts to these residues. Extracting detailed interactions from our simulations posits four ways to interact with the 1′2′ residues (SI Appendix, section S7): specific polar interactions (TETS), both significant polar and hydrophobic interactions (TBPO and TBPS), general hydrophobic interactions (such as EBOB), and nonspecific or nonsignificant interactions that imply that other residues within the GABAAR pore are more important for binding (e.g., PTX interacts with 6′ as a key residue).
Fig. 5.
The equilibrated positions of TETS (1), TBPO (6), and EBOB following 40 ns of MD simulation in the pore region of the α1β2γ2 GABAAR model, with views from the side (Left, the front two M2 helices have been removed for clarity), and views from the bottom of the pore (Right). The red dashed lines signify the common binding region around the 2′ “contact zone.” The contact zone is the region where a compound can make contacts to 2′, either from above or below the residue. The view down the pore shows that at the 1′2′ region, TETS makes primarily polar interactions to the α and γ subunits (hydrogen bonds shown as dashed magenta lines), TBPO makes both polar and hydrophobic interactions, and EBOB makes nonspecific hydrophobic interactions with this 1′2′ ring of residues.
The predicted TETS and EBOB residue contact differences and binding interactions correspond with the sensitivity and specificity observed in expressed human β3 (hydrophobic at 1′2′) homopentameric GABAARs (33). In agreement with mutation studies that show that changes to the β3 homopentameter 2′ residue from hydrophobic to polar (A→S) decreases the affinity of EBOB for the receptor (36, 37, 39), our binding pattern shows EBOB makes significant hydrophobic interactions at the 1′2′ region during the simulation. Conversely, our simulated TETS makes polar interactions at this 1′2′ region, suggesting that polar residues are needed for TETS binding. In the β3 subunit valine and alanine have replaced the α1T1′ and γ2S2′ residues, abolishing the necessary polar residues that TETS is predicted to bind, and could explain why α1β2γ2 is sensitive to TETS but the homopentamer is not. Thus, a β3 homopentamer 1′V→T or 2′A→S mutant (similar to the α1 or γ2 residues) may show increased affinity for TETS.
Types A and B Toxic Action Relative to Binding Positions.
TETS and EBOB fall into two different types (A and B) on comparing toxicity with target site potency assayed as inhibition of either [35S]TBPS binding in brain membranes or 36Cl uptake in membrane vesicles of the cerebral cortex (20, 32). Type A compounds include EBOB and many insecticides with large substituents or extended structures, and the type B set includes TETS, TBPS, TBPO, and other small compact molecules, some of very high i.p. toxicity to mice (LD50 36 μg/kg for TBPO) (25) (SI Appendix, section S8), although much less toxic to injected houseflies (LD50 90 mg/kg for TETS) (4). The target site mapping studies above suggest a molecular distinction between the binding of type A compounds and Type B cage convulsants (32). Type B antagonists (TETS, TBPO, and TBPS) bind with significant polar interactions, whereas type A antagonists (PTX, lindane, 12-ketoendrin, and EBOB) do not (SI Appendix, section S7). In confirmation, distinct differences appear between types A and B compounds on comparing native, α1β3γ2, and (β3)5 GABAARs (33). Whereas the type A compounds are exceptionally potent on the β3 homopentamer, the type B TBPS acts similarly on all three receptor types and TETS is a poor inhibitor of (β3)5 (33, 34). Considering these relationships, we propose that the type B compact set including TETS and TBPS undergoes significant polar interactions in the 1′2′ ring, whereas the type A elongated compounds such as EBOB do not. Interestingly, the insecticidal activity of the isoxazoline fluralaner (10) seems to result from action at a distinct GABA receptor site (41, 42) not considered here.
TETS Candidate Antidotes.
TETS was the first and because of many poisoning cases is now the best known of the small-cage convulsants, but some bicyclophosphorus compounds are much more toxic and probably act in the same way (19, 25). After a half century of search, there are still no adequate antidotes for TETS-induced poisoning, either accidental or intentional. The candidates have come from anticonvulsants used to counteract convulsant action, trials in rats and mice, and mechanism studies in animals, cells, and in vitro systems (SI Appendix, section S1). Cell and nerve studies confirm action on GABA-induced signals and chloride flux. Diazepam and Na phenobarbital increase the mouse i.p. LD50 of TETS by severalfold (19) and diazepam and midazolam inhibit [3H]EBOB and [35S]TBPS binding (SI Appendix, sections S3 and S4). The highest inhibitory effect among the benzodiazepines and barbiturates examined at 1 or 10 µM was 30–40% for midazolam and flurazepam (SI Appendix, section S3). Several GABAAR modulators that alter [35S]TBPS binding (30) are also allosteric inhibitors of [14C]TETS or [3H]EBOB binding. Allopregnanolone is known to be active in [35S]TBPS binding assays (30) and alleviating TETS toxicity (11, 27, 28). NaDMPS, a chelating agent normally used for treating heavy metal poisoning, is effective as a TETS antidote in rodent models and human poisonings proposed to be due to elevating GABA levels rather than as a chelator (SI Appendix, section S1). GABA levels are elevated by TETS poisoning in rats and GABA administration relieves the convulsions (SI Appendix, section S1). The seizures induced by acute and repeated exposure to TETS are characterized as actions at both GABA and NMDA receptors (28, 29). TETS inhibition of NMDA-induced Ca2+ signaling in cultured hippocampal neurons is partially reversed by either, or both, NaDMPS and allopregnanolone (28). Binding of [14C]TETS or [3H]EBOB, or both, is inhibited by avermectin at 1 µM and by preganolone, isoguvacine, NMDA, propofol, and pyridoxine but not by NaDMPS at 1 or 10 µM, whereas bicuculline at 1 µM stimulated [14C]TETS and [3H]EBOB binding by 59–68% (SI Appendix, section S3). Baclofen at 1 µM and ethanol at 300 mM had apparently somewhat different effects with the two radioligands (SI Appendix, section S3). However, TETS poisoning cases in humans have been treated with diazepam, allopregnanolone, and NaDMPS with little or no benefit (9–15).
The GABAAR is the target of many toxicants for mammals (TETS) and insects (insecticides) and exists in a multiplicity of subunit and interface combinations (43, 44), allowing high toxicity that reaches its extreme for mammals with TETS and some other small-cage convulsants. In the search for antidotes the GABAAR in vitro assays described here may provide a rapid means of limiting the number of compounds for animal experimentation and ultimate testing in cases of human poisoning. Further test of this hypothesis requires a larger dataset for inhibition of [14C]TETS and [3H]EBOB binding versus toxicity.
Materials and Methods
Chemicals and Chromatography.
H14CHO (1 mCi/mL, 250 µCi) was purchased from American Radiolabeled Chemicals Inc. H13CHO (99 atom % 13C, 20% aqueous solution) was obtained from Sigma-Aldrich. [3H]EBOB (26 Ci/mmol) was from Perkin-Elmer Inc.. All other reagents and solvents were obtained from commercial suppliers and used without further purification. The synthesized products were characterized by TLC comparisons on Merck silica gel 60 F254 plates detected for unlabeled and [13C]TETS by potassium permanganate and for [14C]TETS by radio TLC using a Bioscan System 200 Imaging Scanner. Purification involved column chromatography using Spe-ed SPE Cartridges (Super Spe-ed silica gel, 5101; Applied Separations). Radioactivity was determined by liquid scintillation analysis using a Tri-Carb 2810 TR. GC-MS data were recorded on a HP 6890 GC with the 5973 MS instrument.
GABAAR Membrane Preparation.
The preparation method was modified from that of Squires et al. (30). Whole rat brains from Pel-Freez Biologicals stored at −80 °C were thawed and homogenized in 50 volumes of ice-cold 1 mM EDTA using a Brinkmann Polytron Homogenizer. The homogenate was centrifuged at 1,000 × g for 10 min, and the supernatant was then centrifuged at 25,000 × g for 30 min. The resulting pellets were suspended in 50 volumes of 1 mM EDTA, packed into cellophane tubing, and dialyzed against distilled/deionized water in an ice-bath (1–2 L, three times for 2 h). The dialyzed suspension was then centrifuged at 25,000 × g for 30 min and the pellets were stored at −80 °C.
Binding Assays.
The rat brain membrane pellets from storage at −80 °C were suspended in ice-cold buffer B [10 mM phosphate buffer (pH 7.5) containing 300 mM NaCl]. Incubation mixtures consisted of membranes (125 µg protein) (45) and 0.5 nM [3H]EBOB or 1.5 nM [14C]TETS in 1.0 mL of buffer B. After incubation with shaking for 90 min at 37 °C, the mixtures were filtered through GF/C filters and rapidly rinsed three times with 5 mL of cold buffer B using a Brandel M-24 cell harvester. Tritium from bound [3H]EBOB was quantitated by liquid scintillation counting (31). Rabiocarbon from [14C]TETS was analyzed by AMS. The filter papers were collected, put in Eppendorf tubes, and held up to 4 wk at 4 °C. Then, each filter loaded with protein was placed with 1 µL tributyrin carbon carrier in a quartz tube (∼6 × 30 mm, 4 mm i.d.) nested inside two borosilicate glass culture tubes (10 × 75 mm in 12 × 100 mm) and dried overnight in a vacuum centrifuge. An excess of CuO (∼40 mg) was added and the inner quartz vials were transferred to quartz combustion tubes, evacuated, and sealed with a torch. The samples were combusted at 900 °C for 3.5 h to oxidize all organic carbon to CO2 and then reduced to filamentous carbon as previously described (46). Carbon samples were packed into aluminum sample holders, and carbon isotope ratios were measured on the compact 1-MV AMS spectrometer at the Lawrence Livermore National Laboratory. Typical AMS measurement times were 3–5 min per sample, with a counting precision of 0.6–1.4% and a SD among 3–10 measurements of 1–3%. The 14C/13C ratios of the protein samples were normalized to measurements of four identically prepared standards of known isotope concentration (IAEA C-6, also known as ANU sucrose) and converted to units of femtograms TETS per microgram protein (47). Each experiment was performed in triplicate and repeated three times in determining the mean and SEs. Curve fitting used the nonlinear (Fig. 3) or linear (Fig. 4) regression program with Prism Software Version 5.0 (GraphPad Software Inc.).
Modeling the GABAAR Binding Sites.
The GABAAR α1β2γ2 homology model was built with a GluCl template (PDB ID code 3RHW) (48) using previously published protocols (36, 37). Small molecules were parameterized with the PRODRG server (49) and docked into the pore using VinaLC (50). The protein–ligand system was embedded in a lipid bilayer and solvated. Atomistic simulations were performed using GROMACS (51). For more details, see SI Appendix, section S5.
Supplementary Material
Acknowledgments
C.Z. thanks Prof. Lihong Qiu (China Agricultural University) for academic counsel and Berkeley laboratory colleagues Amanda Ly, Breanna Ford, and Madhur Garg for assistance in manuscript preparation. S.H.H. and B.D.H. thank Jai Woong Seo for the [14C]TETS radio-TLC analysis. We thank the Livermore Computing Grand Challenge for computer time. This work was supported in part by State Scholarship Fund 2011635139 provided by the China Scholarship Council (to C.Z.), National Institutes of Health Office of the Director and the CounterACT Program National Institute of Neurological Disorders and Stroke Grant U54 NS079202 (to S.H.H. and B.D.H.), National Institute of General Medical Sciences Grant 8P41GM103483 (to B.A.B.), and Laboratory Directed Research and Development Grant 13-LW-085 (to T.S.C. and F.C.L.). Portions of this work were performed under the auspices of the US Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344, Release LLNL-JRNL-649601.
Footnotes
The authors declare no conflict of interest.
*Zolkowska D, et al., American Epilepsy Society Annual Meeting, December 2–6, 2011, Baltimore, abstr 3.069.
This paper contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407379111/-/DCSupplemental.
References
- 1.Wood FC, Battye AE. The condensation of sulphamide, dimethylsulphamide, and aniline-p-sulphonamide with formaldehyde. J Soc Chem Ind. 1933;56:346–349. [Google Scholar]
- 2.Hecht G, Henecka H. Uber ein hochtoxisches kondensationsprodukt von sulfamid und formaldehyde. Angew Chem. 1949;61:365–366. [Google Scholar]
- 3.Hagen J. Schwere vergiftungen in liner polstermöbelfabrik durch einen neuartigen hoch toxischen giftstoff (tetramethylendisulfotetramin) Dtsch Med Wochenschr. 1950;75:183–184. [Google Scholar]
- 4.Esser T, Karu AE, Toia RF, Casida JE. Recognition of tetramethylenedisulfotetramine and related sulfamides by the brain GABA-gated chloride channel and a cyclodiene-sensitive monoclonal antibody. Chem Res Toxicol. 1991;4(2):162–167. doi: 10.1021/tx00020a007. [DOI] [PubMed] [Google Scholar]
- 5.Radwan M. Translocation and metabolism of C14-labeled tetramine by douglas-fir, orchard grass, and blackberry. For Sci. 1967;13:265–273. [Google Scholar]
- 6.Owens J, Hok S, Alcaraz A, Koester C. Quantitative analysis of tetramethylenedisulfotetramine (tetramine) spiked into beverages by liquid chromatography-tandem mass spectrometry with validation by gas chromatography-mass spectrometry. J Agric Food Chem. 2009;57(10):4058–4067. doi: 10.1021/jf900271z. [DOI] [PubMed] [Google Scholar]
- 7.Shan G, et al. Isotope-labeled immunoassays without radiation waste. Proc Natl Acad Sci USA. 2000;97(6):2445–2449. doi: 10.1073/pnas.040575997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht G, Henecka H, Meisenheimer H. 1949. US Patent 2,650,186.
- 9.Croddy E. Rat poison and food security in the People’s Republic of China: Focus on tetramethylene disulfotetramine (tetramine) Arch Toxicol. 2004;78(1):1–6. doi: 10.1007/s00204-003-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SL, Ding MB. Diagnosis and treatment of tetramine poisoning. Chin J Ind Med. 2001;14:163–165. [Google Scholar]
- 11.Whitlow KS, Belson M, Barrueto F, Nelson L, Henderson AK. Tetramethylenedisulfotetramine: Old agent and new terror. Ann Emerg Med. 2005;45(6):609–613. doi: 10.1016/j.annemergmed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Jett DA, Yeung DT. The CounterACT Research Network: Basic mechanisms and practical applications. Proc Am Thorac Soc. 2010;7(4):254–256. doi: 10.1513/pats.201001-003SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Su M, Tian DP. Tetramine poisoning: A case report and review of the literature. Forensic Sci Int. 2011;204(1-3):e24–e27. doi: 10.1016/j.forsciint.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Li JM, Gan J, Zeng TF, Sander JW, Zhou D. Tetramethylenedisulfotetramine intoxication presenting with de novo Status Epilepticus: A case series. Neurotoxicology. 2012;33(2):207–211. doi: 10.1016/j.neuro.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JS, Xiang P, Zhuo XY, Shen M. Acute poisoning types and prevalence in Shanghai, China, from January 2010 to August 2011. J Forensic Sci. 2014;59(2):441–446. doi: 10.1111/1556-4029.12334. [DOI] [PubMed] [Google Scholar]
- 16.Voss E, Haskell AR, Gartenberg L. Reduction of tetramine toxicity by sedatives and anticonvulsants. J Pharm Sci. 1961;50:858–860. doi: 10.1002/jps.2600501014. [DOI] [PubMed] [Google Scholar]
- 17.Bowery NG, Brown DA, Collins JF. Tetramethylenedisulphotetramine: An inhibitor of γ-aminobutyric acid induced depolarization of the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1975;53(3):422–424. doi: 10.1111/j.1476-5381.1975.tb07379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dray A. Tetramethylenedisulphotetramine and amino acid inhibition in the rat brain. Neuropharmacology. 1975;14(9):703–705. doi: 10.1016/0028-3908(75)90094-5. [DOI] [PubMed] [Google Scholar]
- 19.Casida JE, et al. Structure-toxicity relationships of 2,6,7-trioxabicyclo(2.2.2)octanes and related compounds. Toxicol Appl Pharmacol. 1976;36(2):261–279. doi: 10.1016/0041-008x(76)90006-5. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Casida JE. Characterization of [3H]ethynylbicycloorthobenzoate ([3H]EBOB) binding and the action of insecticides on the γ-aminobutyric acid-gated chloride channel in cultured cerebellar granule neurons. J Pharmacol Exp Ther. 1996;279(3):1191–1196. [PubMed] [Google Scholar]
- 21.Qiu Z, Lan H, Zhang S, Xia Y, Huang S. [Antidotal effects of vitamin B(6) and sodium dimercaptopropane sulfonate on acute poisoning with tetramethylene disulphotetramine in animals] Zhonghua Nei Ke Za Zhi. 2002;41(3):186–188. Chinese. [PubMed] [Google Scholar]
- 22.Chen ZK, Lu ZQ. Sodium dimercaptopropane sulfonate as antidote against non-metallic pesticides. Acta Pharmacol Sin. 2004;25(4):534–544. [PubMed] [Google Scholar]
- 23.Wright DW, et al. 2007. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med 49(4):391–402.
- 24.Xie H, et al. The therapeutic effects of combination of γ-aminobutyric acid, sodium dimercaptopropane sultanate and vitamin B6 in large doses on liver and heart in rats with acute tetramine intoxication. Chin J Emergency Med. 2010;19:703–707. [Google Scholar]
- 25.Milbrath DS, Engel JL, Verkade JG, Casida JE. Structure—toxicity relationships of 1-substituted-4-alkyl-2,6,7-trioxabicyclo[2.2.2.]octanes. Toxicol Appl Pharmacol. 1979;47(2):287–293. doi: 10.1016/0041-008x(79)90323-5. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Luo Y, Wang YA. Treatment drugs against tetramine-induced seizure: Research advances. Int J Pharm Res. 2011;38:284–287. [Google Scholar]
- 27.Zolkowska D, et al. Characterization of seizures induced by acute and repeated exposure to tetramethylenedisulfotetramine. J Pharmacol Exp Ther. 2012;341(2):435–446. doi: 10.1124/jpet.111.190579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Z, et al. Tetramethylenedisulfotetramine alters Ca²⁺ dynamics in cultured hippocampal neurons: Mitigation by NMDA receptor blockade and GABA(A) receptor-positive modulation. Toxicol Sci. 2012;130(2):362–372. doi: 10.1093/toxsci/kfs244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shakarjian MP, Velíšková J, Stanton PK, Velíšek L. Differential antagonism of tetramethylenedisulfotetramine-induced seizures by agents acting at NMDA and GABA(A) receptors. Toxicol Appl Pharmacol. 2012;265(1):113–121. doi: 10.1016/j.taap.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squires RF, Casida JE, Richardson M, Saederup E. [35S]t-Butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to γ-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983;23(2):326–336. [PubMed] [Google Scholar]
- 31.Cole LM, Casida JE. GABA-gated chloride channel: Binding site for 4′-ethynyl-4-n-[2,3-3H2]propylbicycloorthobenzoate ([3H]EBOB) in vertebrate brain and insect head. Pestic Biochem Physiol. 1992;44:1–8. [Google Scholar]
- 32.Palmer CJ, Casida JE. Two types of cage convulsant action at the GABA-gated chloride channel. Toxicol Lett. 1988;42(2):117–122. doi: 10.1016/0378-4274(88)90068-9. [DOI] [PubMed] [Google Scholar]
- 33.Ratra GS, Kamita SG, Casida JE. Role of human GABA(A) receptor β3 subunit in insecticide toxicity. Toxicol Appl Pharmacol. 2001;172(3):233–240. doi: 10.1006/taap.2001.9154. [DOI] [PubMed] [Google Scholar]
- 34.Ratra GS, Casida JE. GABA receptor subunit composition relative to insecticide potency and selectivity. Toxicol Lett. 2001;122(3):215–222. doi: 10.1016/s0378-4274(01)00366-6. [DOI] [PubMed] [Google Scholar]
- 35.Law RJ, Lightstone FC. Gaba receptor insecticide non-competitive antagonists may bind at allosteric modulator sites. Int J Neurosci. 2008;118(5):705–734. doi: 10.1080/00207450701750216. [DOI] [PubMed] [Google Scholar]
- 36.Carpenter TS, Lau EY, Lightstone FC. A role for loop F in modulating GABA binding affinity in the GABA(A) receptor. J Mol Biol. 2012;422(2):310–323. doi: 10.1016/j.jmb.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter TS, Lau EY, Lightstone FC. Identification of a possible secondary picrotoxin-binding site on the GABA(A) receptor. Chem Res Toxicol. 2013;26(10):1444–1454. doi: 10.1021/tx400167b. [DOI] [PubMed] [Google Scholar]
- 38.Hisano K, Ozoe F, Huang J, Kong X, Ozoe Y. The channel-lining 6′ amino acid in the second membrane-spanning region of ionotropic GABA receptors has more profound effects on 4′-ethynyl-4-n-propylbicycloorthobenzoate binding than the 2′ amino acid. Invert Neurosci. 2007;7(1):39–46. doi: 10.1007/s10158-006-0035-x. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Durkin KA, Casida JE. Structural model for γ-aminobutyric acid receptor noncompetitive antagonist binding: Widely diverse structures fit the same site. Proc Natl Acad Sci USA. 2006;103(13):5185–5190. doi: 10.1073/pnas.0600370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charon S, Taly A, Rodrigo J, Perret P, Goeldner M. Binding modes of noncompetitive GABA-channel blockers revisited using engineered affinity-labeling reactions combined with new docking studies. J Agric Food Chem. 2011;59(7):2803–2807. doi: 10.1021/jf102468n. [DOI] [PubMed] [Google Scholar]
- 41.Ozoe Y, Asahi M, Ozoe F, Nakahira K, Mita T. The antiparasitic isoxazoline A1443 is a potent blocker of insect ligand-gated chloride channels. Biochem Biophys Res Commun. 2010;391(1):744–749. doi: 10.1016/j.bbrc.2009.11.131. [DOI] [PubMed] [Google Scholar]
- 42.García-Reynaga P, Zhao C, Sarpong R, Casida JE. New GABA/glutamate receptor target for [³H]isoxazoline insecticide. Chem Res Toxicol. 2013;26(4):514–516. doi: 10.1021/tx400055p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozoe Y. γ-Aminobutyrate- and glutamate-gated chloride channels as targets of insecticides. Adv Insect Physiol. 2013;44:211–286. [Google Scholar]
- 45.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 46.Ognibene TJ, Bench G, Vogel JS, Peaslee GF, Murov S. A high-throughput method for the conversion of CO2 obtained from biochemical samples to graphite in septa-sealed vials for quantification of 14C via accelerator mass spectrometry. Anal Chem. 2003;75(9):2192–2196. doi: 10.1021/ac026334j. [DOI] [PubMed] [Google Scholar]
- 47.Vogel JS, Love AH. Quantitating isotopic molecular labels with accelerator mass spectrometry. Methods Enzymol. 2005;402:402–422. doi: 10.1016/S0076-6879(05)02013-6. [DOI] [PubMed] [Google Scholar]
- 48.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474(7349):54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schüttelkopf AW, van Aalten DM. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 8):1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Wong SE, Lightstone FC. Message passing interface and multithreading hybrid for parallel molecular docking of large databases on petascale high performance computing machines. J Comput Chem. 2013;34(11):915–927. doi: 10.1002/jcc.23214. [DOI] [PubMed] [Google Scholar]
- 51.Van Der Spoel D, et al. GROMACS: Fast, flexible, and free. J Comput Chem. 2005;26(16):1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.