Significance
Ketamine is an NMDA receptor (NMDAR) antagonist that elicits rapid antidepressant responses in patients with treatment-resistant depression. However, ketamine can also produce adverse side effects, which raised interest in whether the clinically tolerated NMDAR antagonist memantine can elicit similar fast antidepressant action. Rather surprisingly, clinical data have shown that memantine does not trigger rapid antidepressant effects for reasons that have yet to be elucidated. Here, we reconstitute the ketamine and memantine clinical findings in animal models and, combined with the analysis of synaptic function and subsequent intracellular signaling, demonstrate significant differences between the efficacies of ketamine and memantine on NMDAR-mediated neurotransmission and downstream intracellular signaling. These findings suggest a potential mechanism to explain the earlier clinical observations.
Keywords: eEF2, spontaneous neurotransmission
Abstract
Ketamine is an NMDA receptor (NMDAR) antagonist that elicits rapid antidepressant responses in patients with treatment-resistant depression. However, ketamine can also produce psychotomimetic effects that limit its utility as an antidepressant, raising the question of whether the clinically tolerated NMDAR antagonist memantine possesses antidepressant properties. Despite its similar potency to ketamine as an NMDAR antagonist, clinical data suggest that memantine does not exert rapid antidepressant actions for reasons that are poorly understood. In this study, we recapitulate the ketamine and memantine clinical findings in mice, showing that ketamine, but not memantine, has antidepressant-like effects in behavioral models. Using electrophysiology in cultured hippocampal neurons, we show that ketamine and memantine effectively block NMDAR-mediated miniature excitatory postsynaptic currents in the absence of Mg2+. However, in physiological levels of extracellular Mg2+, we identified key functional differences between ketamine and memantine in their ability to block NMDAR function at rest. This differential effect of ketamine and memantine extends to intracellular signaling coupled to NMDAR at rest, in that memantine does not inhibit the phosphorylation of eukaryotic elongation factor 2 or augment subsequent expression of BDNF, which are critical determinants of ketamine-mediated antidepressant efficacy. These results demonstrate significant differences between the efficacies of ketamine and memantine on NMDAR-mediated neurotransmission that have impacts on downstream intracellular signaling, which we hypothesize is the trigger for rapid antidepressant responses. These data provide a novel framework on the necessary functional requirements of NMDAR-mediated neurotransmission as a critical determinant necessary to elicit rapid antidepressant responses.
Ketamine is a noncompetitive glutamate NMDA receptor (NMDAR; also called GluN) antagonist that has been shown to mediate rapid antidepressant efficacy in patients with treatment-resistant major depression (1–3). The antidepressant effects of ketamine are fast-acting, with some patients reporting effects as soon as 30 min to within a few hours following a single i.v. low-dose injection of ketamine. However, ketamine can produce adverse psychotomimetic effects, which may limit its use as an antidepressant. Traditional antidepressant drugs target the monoamine system and typically require several weeks of treatment to mediate a therapeutic effect. There is an urgent need for rapid antidepressant drugs, and the clinical data with ketamine suggest that blocking the NMDAR may be a viable therapeutic target.
Memantine is a noncompetitive NMDAR antagonist that has been approved by the US Food and Drug Administration for the treatment of Alzheimer’s disease. Memantine is a generally well-tolerated drug that lacks the aversive effects (4) observed with ketamine at therapeutic doses. However, attempts to test memantine as an antidepressant in individuals with major depression have yielded mixed results following long-term drug treatment, with no evidence of rapid antidepressant effects (5–7). A better understanding of why ketamine, but not memantine, produces a fast-acting antidepressant response has clinical implications and may provide novel information critical for the development of rapid antidepressant therapeutics based on NMDAR antagonism, with fewer side effects.
There is much interest in identifying the molecular mechanism that underlies the rapid antidepressant response of ketamine. In recent work, we demonstrated that the fast-acting antidepressant effect of ketamine requires deactivation of eukaryotic elongation factor 2 kinase (eEF2K) and subsequent desuppression of BDNF protein translation in the hippocampus (8, 9). We hypothesize that low-dose ketamine mediates its rapid antidepressant response by blockade of spontaneous glutamate release-mediated NMDAR activity. This blockade, in turn, decreases calcium (Ca2+) flow through the receptor, inhibiting eEF2K activity and resulting in decreased levels of phosphorylated eukaryotic elongation factor 2 (eEF2) (10–12) and desuppression of BDNF protein synthesis (8, 9, 13). In this study, we compared ketamine with memantine in their effectiveness to block NMDAR activation during spontaneous neurotransmission, subsequently inhibiting eEF2K and increasing BDNF protein translation. Our results reveal key differences between the effects of ketamine and memantine on resting NMDAR-mediated neurotransmission and subsequent intracellular signaling pathways that may explain the mechanistic differences between these two drugs in eliciting rapid antidepressant effects.
Results
Acute Memantine Treatment Does Not Trigger a Fast-Acting Antidepressant Response.
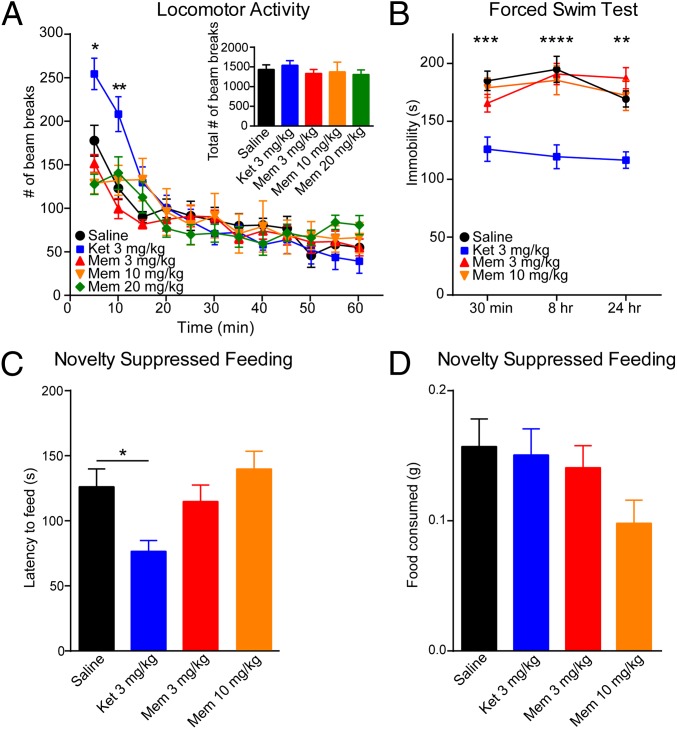
We assessed whether memantine affects locomotor activity immediately following drug treatment. In all experiments, we included a ketamine group as a direct comparison, which has previously been shown to elicit an antidepressant response in mice 30 min after administration without effects on locomotor activity at this time point (8, 9, 14, 15). To examine a range of doses for the effects of this drug in vivo, we injected memantine at 20 mg/kg, a dose reported to have neuroprotective effects in rodents (16); 10 mg/kg, a dose that blocks morphine dependence (17); and 3 mg/kg, a dose that prevents estrogen-dependent tolerance to morphine. We found that a 3-, 10-, or 20-mg/kg dose of memantine did not have any effect on total locomotor activity in comparison to the saline-treated mice during the 60-min testing period (Fig. 1A, Inset). We examined the data in 5-min epochs and also did not find any significant differences between mice treated with memantine compared with saline (Fig. 1A). Ketamine caused an initial increase in locomotor activity during the first 10 min following drug administration; however, there were no significant differences in activity during the remaining 50 min of the test, specifically at the 30-min time point when antidepressant responses are measured (Fig. 1A).
Fig. 1.
Memantine (Mem) treatment does not cause a fast-acting antidepressant effect. (A) Ketamine (Ket) causes a significant increase in locomotor activity at the 5- and 10-min intervals [two-way ANOVA interaction: F44,420 = 1.904, P = 0.0007; time: F11,420 = 25.87, P < 0.0001; treatment effects: F4,420 = 2.784, P = 0.0264; Tukey’s post hoc analysis for Ket: 5 min vs. saline, *P = 0.01; 10 min vs. saline, **P = 0.001 (n = 8 per group)]. (Inset) There were no significant differences in the total number of beam breaks over 1 h. (B) Ket treatment caused a significant decrease in immobility in the FST compared with the saline control group at 30 min, 8 h, and 24 h following injection, whereas Mem (3 mg/kg and 10 mg/kg) did not cause a significant decrease in immobility time [two-way ANOVA: F3,107 = 28.96, P < 0.0001; Tukey’s post hoc analysis for Mem: 30 min: saline vs. Ket, ***P = 0.0002; saline vs. 3 mg/kg Mem, P = 0.501; saline vs. 10 mg/kg Mem, P = 0.972 (n = 9–10 per group); 8 h: saline vs. Ket, ****P < 0.0001, saline vs. 3 mg/kg Mem, P = 0.991; saline vs. 10 mg/kg Mem, P = 0.904; 24 h: saline vs. Ket, **P = 0.0017, saline vs. 3 mg/kg Mem, P = 0.584; saline vs. 10 mg/kg Mem, P = 0.996]. (C) Single dose of Mem treatment (30 min: 3 mg/kg or 10 mg/kg) did not have an impact on latency to feed in the NSF test compared with the saline-treated group. However, acute Ket treatment (3 mg/kg) results in a significant decrease in latency to feed compared with saline [ANOVA: F3,35 = 4.97, *P = 0.0056; Bonferroni’s post hoc comparison: saline vs. 3 mg/kg Mem, P > 0.9999; saline vs. 10 mg/kg Mem, P > 0.999; saline vs. Ket, P = 0.0465 (n = 9–10 per group)]. (D) Appetite posttest following the NSF test indicates that the total amount of food consumed is indistinguishable across groups (ANOVA: F3,35 = 1.999, P = 0.1321).
We next examined whether memantine produces rapid antidepressant effects in the forced swim test (FST). In agreement with previous data, ketamine significantly reduced the immobility time at 30 min following injection, suggestive of an antidepressant response (8, 9) (Fig. 1B). In contrast, a single injection of memantine at 3 or 10 mg/kg did not significantly alter immobility time in the FST at 30 min (Fig. 1B). Using separate cohorts of mice, we found that ketamine caused a significant decrease in immobility time at 8 or 24 h following acute administration in the FST that was not observed with either 3 or 10 mg/kg of memantine (Fig. 1B). Rather unexpectedly, mice treated with 20 mg/kg of memantine had severe adverse effects in the FST, including general motor instability and drowning; thus, to avoid potential complications and undue stress to the animals, we did not use this dose in vivo in subsequent experiments.
A different cohort of C57BL/6 mice was used to assess whether memantine triggers a rapid antidepressant effect in the novelty suppressed feeding (NSF) test. In agreement with previous data, a single low-dose injection of ketamine administered 30 min before testing significantly reduced the time required to initiate eating in the mice, suggestive of an antidepressant response (8, 9) (Fig. 1C). However, a single injection of memantine at either 3 or 10 mg/kg did not reduce the time required for the mice to initiate feeding compared with saline. We also found that neither ketamine nor memantine treatment had any significant effect on the mice’s appetite, ruling out a possible confound to the NSF test (Fig. 1D).
Memantine Exhibits Reduced NMDAR Blockade in Physiological Magnesium.
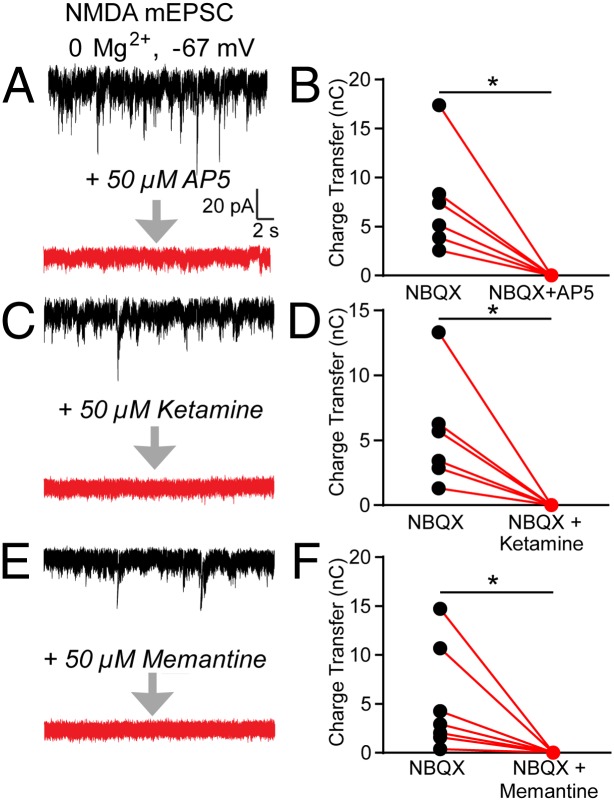
We examined ketamine, memantine, and the commonly used NMDAR antagonist R-2-amino-5-phosphonopentanoate (AP5) regarding their ability to block NMDA-mediated miniature excitatory postsynaptic currents (mEPSCs) in cultured hippocampal neurons. To record NMDA-mEPSCs, the extracellular recording solution did not include the endogenous NMDAR pore blocker, magnesium (Mg2+); however, the AMPA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) was added. To measure the total decrease in charge transfer conferred by the NMDAR antagonists, baseline NMDAR-mEPSCs were recorded for 4 min. Each of the individual NMDAR antagonists, AP5 (Fig. 2A), ketamine (Fig. 2C), or memantine (Fig. 2E), was then perfused into the bath, and recordings were continued for an additional 4 min. Analysis before and after drug application by an observer blinded to the treatment revealed that perfusion of AP5, ketamine, and memantine resulted in a significant and similar reduction in charge transfer of NMDAR-mEPSCs (Fig. 2 B, D, and F). This finding is in agreement with recent results demonstrating equal efficacy of ketamine and memantine in blockade of NMDAR-mediated responses (16).
Fig. 2.
AP5, ketamine, and memantine block NMDAR-mEPSCs in the absence of Mg2+. (Left) Example traces recorded before and after incubation of the neurons with AP5 (A), ketamine (C), or memantine (E). (Right) Quantification of the charge transfer in a 4-min period before and after applying AP5 (B), ketamine (D), or memantine (F). The application of AP5, ketamine, and memantine caused a significant decrease in charge transfer (Student’s paired t test: AP5: *P = 0.019, n = 6 coverslips; ketamine: *P = 0.025, n = 6 coverslips; memantine: *P = 0.043, n = 7 coverslips).
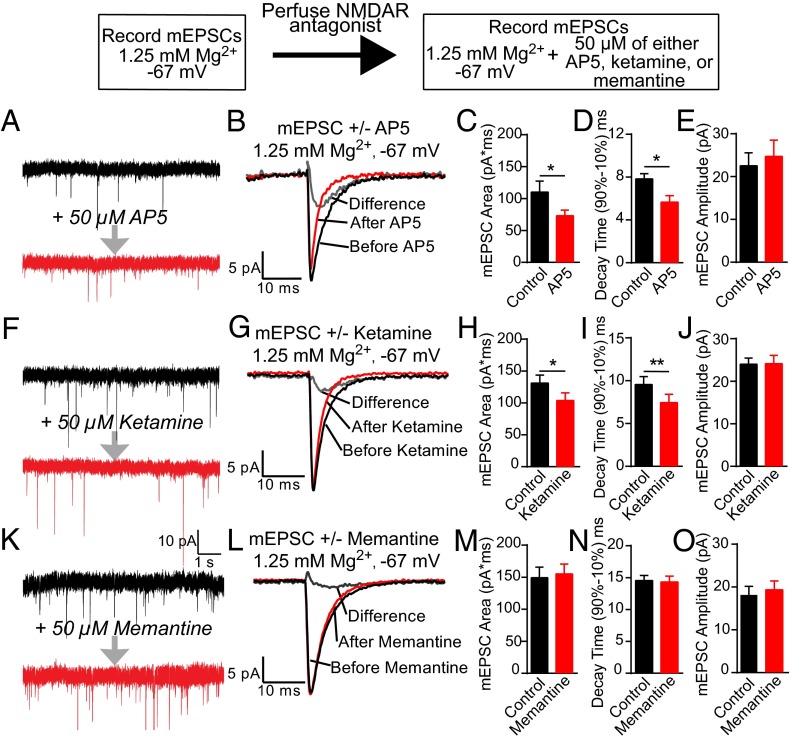
Next, we evaluated whether ketamine and memantine show similar efficacy in blockade of spontaneous glutamate release-mediated NMDAR responses under physiological levels of Mg2+ blockade. Previous work from our group has shown that acute treatment with AP5 under physiological conditions, 1.25 mM Mg2+, and −67 mV holding potential causes a significant decrease in decay time and charge transfer of mEPSCs (18), suggesting a significant contribution of NMDARs to glutamatergic neurotransmission at rest. Using whole-cell patch-clamp methods in the presence of 1.25 mM Mg2+, dual-component mEPSCs were recorded from dissociated hippocampal neurons before and subsequent to treatment with AP5 (Fig. 3A), ketamine (Fig. 3F), or memantine (Fig. 3K). As previously reported, AP5 perfusion caused a significant reduction in the area and decay time of mEPSCs (18) (Fig. 3 C and D). We found that ketamine treatment also significantly decreased mEPSC area and decay time, indicative of blocking the NMDAR component of the mEPSC (Fig. 3 H and I). In contrast, memantine treatment caused no significant change in the mEPSC area or decay time under physiological conditions (Fig. 3 M and N). We also found that none of the NMDAR antagonists examined caused a change in the average amplitude of mEPSCs (Fig. 3 E, J, and O).
Fig. 3.
Memantine does not block the NMDAR component of mEPSCs when physiological concentrations of Mg2+ are present. (Left) Representative traces before and after applying AP5 (A), ketamine (F), or memantine (K); AMPA receptor mEPSCS are still present following NMDAR antagonist incubation. (Center) Average traces of 100 mEPSCS before (black trace) and after (red trace) perfusion of AP5 (B), ketamine (G), or memantine (L), with the calculated difference trace (gray). (C, H, and M) Both AP5 and ketamine caused a significant decrease in mEPSC area, whereas no significant differences were measured with memantine (Student’s paired t test: AP5: *P = 0.031, n = 7 coverslips; ketamine: *P = 0.039, n = 8 coverslips; memantine: P = 0.357, n = 15 coverslips). Application of AP5 (D) and ketamine (I) caused a significant decrease in mEPSC decay time compared with control groups, with no changes detected with memantine (N) (Student’s paired t test: AP5: *P = 0.013, n = 7 coverslips; ketamine: **P = 0.003, n = 8 coverslips; memantine: P = 0.695, n = 15 coverslips). mEPSC amplitude was not affected by AP5 (E), ketamine (J), or memantine (O) application (Student’s paired t test: AP5: P = 0.244, n = 7 coverslips; ketamine: P = 0.926, n = 8 coverslips; memantine: P = 0.153, n = 15 coverslips).
To test our model that blockade of the NMDAR at rest mediates a fast-acting antidepressant response, we assessed the ability of another noncompetitive NMDAR antagonist, Dizocilpine (MK-801), to block NMDAR-mEPSCs in the absence of Mg2+ and the NMDAR component of mEPSCs in the presence of physiological concentrations of Mg2+. We chose MK-801 because we had previously demonstrated that acute treatment with MK-801 causes a significant decrease in immobility time in the FST 30 min after drug administration (8). Similar to AP5, ketamine, and memantine, perfusion of MK-801 caused a substantial inhibition of NMDAR-mEPSCs (Fig. S1 A and B), indicating that it is able to block the NMDAR at rest when no Mg2+ is included in the recording solution. MK-801 treatment also caused a significant decrease in the decay time of mEPSCs, a strong trend toward a decrease in the area of mEPSCs in the presence of 1.25 mM Mg2+, and no change in mEPSC amplitude, which closely mimics the effects of AP5 and ketamine (Fig. S1 C–F).
Ketamine and Memantine Have Differing Intracellular Signaling Effects.
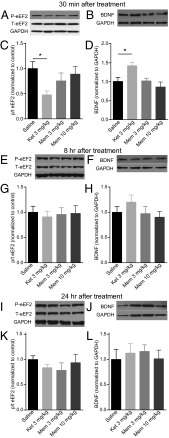
The fast-acting antidepressant effect of ketamine is dependent on protein translation (8). The increase in protein translation following administration of ketamine is hypothesized to be mediated through blockade of NMDARs at rest, which inhibits eEF2K, resulting in decreased phosphorylation of eEF2 followed by desuppression of BDNF protein translation. We examined whether memantine treatment affects eEF2 phosphorylation and BDNF expression in the hippocampus by Western blot analysis. In agreement with previous data, ketamine treatment triggered a significant decrease in phosphorylation of eEF2 (Fig. 4 A and C) and a significant increase in BDNF protein levels compared with vehicle 30 min following injection (8, 9) (Fig. 4 B and D). In contrast, memantine did not alter the phosphorylation level of eEF2 or total eEF2 (Fig. 4 A and C) and did not have any significant effect on BDNF protein levels (Fig. 4 B and D).
Fig. 4.
Differential effects of ketamine and memantine on eEF2 phosphorylation and BDNF protein expression at three different time points following treatment. (A and C) Densitometric analysis of phosphorylated eEF2 (P-eEF2) levels revealed ketamine caused a significant decrease in the ratio of P-eEF2/total eEF2 (T-eEF2) at 30 min, whereas memantine (3 mg/kg and 10 mg/kg) did not have an impact on phosphorylation of eEF2. [ANOVA: F3,44= 3.579, *P = 0.021; Tukey’s post hoc analysis: saline vs. ketamine, *P = 0.018; saline vs. 3 mg/kg memantine, P = 0.569; saline vs. 10 mg/kg memantine, P = 0.930 (n = 10–13 per group)]. (B and D) Densitometric analysis of BDNF levels revealed that memantine did not have a significant effect on BDNF protein expression 30 min after injection; however, ketamine treatment caused a significant increase in BDNF protein [ANOVA: F3,49 = 5.893, P = 0.0016; Tukey’s post hoc analysis: saline vs. ketamine, *P = 0.02; saline vs. 3 mg/kg memantine, P = 0.999; saline vs. 10 mg/kg memantine, P = 0.745 (n = 12–15 per group)]. (E and G) Neither ketamine nor memantine caused a significant change in the levels of P-eEF2 8 h after injection, as shown by densitometric analysis (ANOVA: F3,21 = 0.0828, P = 0.969). (F and H) There was no change in the amount of BDNF protein in the hippocampus 8 h following injection with ketamine or memantine (ANOVA: F3,24 = 1.006, P = 0.407). (I and K) Densitometric analysis of P-eEF2 levels showed no difference in P-eEF2/T-eEF2 between saline and ketamine or memantine treatment 24 h after injection (ANOVA: F3,16 = 0.731, P = 0.552). (J and L) Densitometric analysis of BDNF protein showed no difference between saline, ketamine, or memantine treatment 24 h following injection (ANOVA: F3,25 = 0.206, P = 0.891). p/t, phospho/total.
We previously demonstrated that ketamine-mediated effects on eEF2 phosphorylation and BDNF protein abundance are transient and disappear by 24 h postinjection (8). However, to determine whether memantine may mediate effects on eEF2 phosphorylation and BDNF protein levels at later time points, we examined these protein levels 8 or 24 h after acute injection. As with previous data, ketamine treatment did not cause any significant changes in eEF2 phosphorylation at 8 h (Fig. 4 E and G) or 24 h (Fig. 4 I and K). Additionally, there was no change in BDNF protein at 8 h (Fig. 4 F and H) or 24 h (Fig. 4 J and L) following ketamine injection. Similarly, memantine treatment did not cause any changes in eEF2 phosphorylation or BDNF protein levels 8 h (Fig. 4 E–H) or 24 h (Fig. 4 I–L) after drug administration.
Discussion
In this study, we used behavioral, electrophysiological, and biochemical approaches to compare the actions of ketamine and memantine on antidepressant-like effects in behavioral models, spontaneous NMDAR-mEPSCs, and downstream signaling in the hippocampus to work out a mechanistic explanation for why ketamine, but not memantine, is able to exert rapid antidepressant actions. In this way, we recapitulated the clinical findings of ketamine and memantine in mice, showing that ketamine, but not memantine, has antidepressant-like effects in behavioral models. Electrophysiological analysis revealed that ketamine and memantine antagonize the NMDAR at rest when Mg2+ is absent. However, only ketamine blocks the NMDAR at rest when physiological concentrations of Mg2+ are included in the external solution, providing a key functional difference between ketamine and memantine in their ability to block NMDAR function at rest. The differential functional effects of ketamine and memantine on NMDAR-mEPSCs extend to intracellular signaling coupled to NMDARs at rest. We found that memantine does not inhibit the phosphorylation of eEF2 or augment subsequent BDNF protein expression, which are critical determinants of ketamine-mediated antidepressant efficacy. Collectively, these results identify key functional differences between ketamine and memantine in their ability to suppress NMDAR function at rest, and thus inhibit the eEF2K signaling pathway, providing insight into the mechanistic basis for NMDAR antagonism and rapid antidepressant action.
Recent clinical findings demonstrating that ketamine shows rapid antidepressant effects in patients with major depression (1–3) have triggered a great deal of interest in the field of depression research. However, even the low-dose ketamine used in the depression studies causes psychotomimetic effects in some patients, with the potential for abuse (19). To circumvent these potential liabilities associated with ketamine, there has been interest in investigating whether memantine possesses the antidepressant properties of ketamine. However, in two recent clinical trials, chronic memantine did not elicit an antidepressant response in depressed patients compared with patients given placebo (5, 7). In a separate open-label trial, depressed patients treated with chronic memantine did show some clinical improvement in ratings of their depression symptoms 1 wk after starting daily dosing and continuing for at least 12 wk; however, this study did not contain a placebo control group (6). Ketamine has faster pharmacokinetics following in vivo administration than memantine, and it is likely to reach peak concentration in brain much faster than memantine. In addition, in vitro studies suggest that ketamine has slightly higher potency than memantine. However, given that the clinical studies used chronic memantine treatment yet memantine lacked antidepressant properties, it seems unlikely that the clinical differences between these two drugs are due to differences in pharmacokinetics and/or affinity. The clinical findings demonstrating differences between ketamine and memantine in triggering rapid antidepressant responses are rather surprising, because both drugs are noncompetitive NMDAR antagonists that block the receptor when it is in an open configuration (16, 20). Importantly, the two compounds do not show significant differences in their ability to block NMDAR-mediated synaptic or extrasynaptic currents in the absence of physiological Mg2+ (16). Although our findings agree with this earlier work, we could detect a biologically significant difference in their differential ability to block the NMDAR component of mEPSCs in the presence of physiological levels of Mg2+. Therefore, we propose that the disparity in rapid antidepressant responses between these compounds is likely due to differences in how they affect NMDAR function at rest under physiological conditions.
Previous work has shown that ketamine and memantine block the NMDAR by binding inside of the ion channel in an area overlapping the binding site for Mg2+, which blocks the channel in a voltage-dependent manner (21). In the absence of Mg2+, ketamine is more potent than memantine in the blockade of the two most highly expressed GluN2 subunits in the hippocampus, GluN2A and GluN2B, as evidenced by lower IC50 values (GluN1/GluN2A: memantine, ∼0.80 μM, vs. ketamine, ∼0.33 μM; GluN1/GluN2B: memantine, ∼0.57 μM, vs. ketamine, ∼0.31 μM) (22), which may indicate a higher affinity for the receptor. Under more physiological conditions in the presence of Mg2+, ketamine (IC50: GluN1/GluN2A, ∼5.35 μM; GluN1/GluN2B, ∼5.08 μM) is still more potent than memantine (IC50: GluN1/Glu2A, ∼13.4 μM; GluN1/GluN2B, ∼10.4 μM) (22). However, the difference seen between the ability of memantine and ketamine to block GluN2A and GluN2B containing NMDARs is not extended to GluN2C and GluN2D containing NMDARs without Mg2+ present (GluN1/GluN2C: memantine, ∼0.52 μM, vs. ketamine, ∼0.51 μM; GluN1/GluN2D: memantine, ∼0.54 μM, vs. ketamine, ∼0.83 μM) (22). There also appears to be no significant difference in blockade of GluN2C and GluN2D containing NMDARs by memantine (GluN1/GluN2C: ∼1.61 μM, GluN1/GluN2D: ∼1.76 μM) or ketamine (GluN1/GluN2C: ∼1.18 μM, GluN1/GluN2D: ∼2.95 μM) when Mg2+ is included in the external solution (22). Additionally, it seems unlikely that any differences found between ketamine and memantine would be mediated by GluN3 containing NMDARs, because they do not show significant sensitivity to blockade by Mg2+ or use dependent blockers (23, 24). Because our experiments specifically assess the ability of memantine to block NMDAR-mediated mEPSCs in the presence of Mg2+ in cultured hippocampal neurons, the twofold difference between the abilities of ketamine and memantine to block NMDAR-mediated transmission in the presence of Mg2+ could partly explain our observations. Recent electrophysiological studies have shown that blockade of the NMDAR by Mg2+ is incomplete, even at physiological resting potentials, allowing for a significant amount of current to flow through the NMDAR (9, 18, 25). These results are in agreement with classical studies that assessed the degree of NMDAR blockade by Mg2+ at near-resting membrane potentials (26–29). Therefore, our current findings demonstrating that ketamine, but not memantine, blocks the NMDAR-mediated component of mEPSCs when Mg2+ is present may provide a functional explanation for how these two compounds have differing effects as fast-acting antidepressants.
The importance of blockade of NMDAR-mEPSCs as a key determinant in the rapid antidepressant action of ketamine extends to intracellular signaling coupled to NMDAR at rest. Blockade of NMDAR-mEPSCs has been linked to the control of dendritic protein translation by decreasing calcium influx through the NMDAR into the synapse, leading to decreased phosphorylation of eEF2 by eEF2K and desuppression of protein translation (10, 12), thus providing a potential mechanism for ketamine’s rapid antidepressant effects. The rapid antidepressant effects of ketamine have also been suggested to be mediated by mammalian target of rapamycin (mTOR)-dependent synapse formation, although it remains unclear how blockade of the NMDAR activates mTOR (30). BDNF is a potent activator of mTOR; thus, the blockade of NMDAR-mEPSCs and inactivation of eEF2K, leading to decreased phosphorylation of eEF2 and, ultimately, up-regulation of BDNF protein expression, may well explain the mTOR findings (31). In this study, we found that memantine does not inhibit the phosphorylation of eEF2 or augment subsequent expression of BDNF, which are necessary requirements for ketamine-mediated antidepressant efficacy (8, 9, 13). The differential actions of ketamine and memantine on NMDAR function at rest, coupled with differential effects on downstream intracellular signaling pathways coupled to these receptors, further corroborate the functional requirements of NMDAR-mediated neurotransmission at rest as a necessary determinant of rapid antidepressant responses.
In the present study, our data strengthen and extend our previous findings that decreased eEF2 phosphorylation triggered by ketamine-mediated blockade of NMDAR-mEPSCs is critical for the rapid antidepressant effect (8, 9, 13). These findings provide a mechanistic explanation for why ketamine, but not memantine, is able to exert rapid antidepressant actions, which provides important information for the development of more effective antidepressants based on NMDAR antagonism with fewer side effects.
Materials and Methods
Mice and Drug Treatments.
Male C57BL/6 mice aged 6–8 wk old were habituated to the animal facility for 1 wk before testing. Mice were kept on a 12/12-h light/dark cycle and allowed ad libitum access to food and water, except where indicated. Mice were injected i.p. to mimic the route of administration in humans more closely. Mice were injected with drug 30 min, 8 h, or 24 h before testing or euthanasia to assess behavior and molecular events at the time of initial antidepressant responses, with the exception of the studies examining locomotor activity, in which mice were injected and immediately placed in the boxes to assess drug effects with time. Memantine hydrochloride (3.0, 10, or 20 mg/kg; Sigma) and ketamine (2–3 mg/kg; Fort Dodge Animal Health) were dissolved in saline. Experiments were conducted by an observer blinded to drug treatment. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Locomotor Activity.
Following a 1-h habituation period in the testing room, mice were injected with saline, ketamine, or memantine; they were then placed under red light into standard cages, and locomotor activity was measured for 60 min by photocell beams linked to computer acquisition software (San Diego Instruments).
FST.
The FST was performed according to published protocols (8). Briefly, mice were video-recorded in a 4-L glass beaker containing 3 L of 24 ± 1 °C water for 6 min. The last 5 min of each 6-min trial were scored by a blinded observer to determine the time spent immobile.
NSF Test.
The NSF test was performed according to published protocols (8). Mice were food-deprived for 24 h before the test and then habituated to the behavioral room for 1 h before testing. A mouse was placed into a 42 × 42-cm open field with a food pellet placed in the center and allowed to explore for up to 5 min, with the time required to initiate eating the food pellet determined. To assess differences in appetite, the amount of food consumed in a 5-min period for each mouse in its home cage was measured.
Cell Culture.
Dissociated hippocampal cultures were prepared as previously described (8). Whole hippocampi were dissected from postnatal day 0–3 (P0–P3) C57BL/6 mice; they were then trypsinized (∼10 mg/mL trypsin; Invitrogen), mechanically dissociated, and plated on Matrigel (BD Biosciences)-coated coverslips. At 1 d in vitro (DIV), 4 μM cytosine arabinoside (ARAC; Sigma) was added; at 4 DIV, the ARAC concentration was reduced to 2 μM. All experiments were done on 14- to 21-DIV cultures.
Electrophysiology.
Whole-cell patch-clamp recordings were performed on hippocampal pyramidal neurons. Data were acquired using a MultiClamp 700B amplifier and Clampex 10.0 software (Molecular Devices). Recordings were sampled at 100 μs and filtered at 2 kHz, with a gain of 5. The external Tyrode’s solution contained 150 mM NaCl, 4 mM KCl, 2 mM CaCl2, 10 mM glucose, and 10 mM Hepes (pH 7.4) at ∼300 mOsm. The external MgCl2 concentration was either 0 mM or 1.25 mM, depending on the experiment. The pipette internal solution contained 110 mM K-gluconate, 20 mM KCl, 10 mM NaCl, 10 mM Hepes, 0.6 mM EGTA, 4 mM Mg-ATP, 0.3 mM Na-GTP, and 10 mM lidocaine N-ethyl bromide (QX-314) (pH 7.3) at ∼300 mOsm. Pipettes had a resistance between 3 and 6 MΩ. The junction potential between the external solution and internal solution was ∼12 mV, and it was subtracted from all recordings. NMDAR-mediated mEPSCs were recorded in the presence 1 μM tetrodotoxin (TTX; EMD Millipore), 50 μM picrotoxin (PTX; Sigma), and 10 μM NBQX (Sigma) for 4 min before and 4 min after the application of 50 μM AP5, 50 μM ketamine, 50 μM memantine, or 50 μM MK-801. Dual-component mEPSCS were recorded at −67 mV in the presence 1 μM TTX and 50 μM PTX for 4 min before and 4 min after the application of 50 μM AP5, 50 μM ketamine, 50 μM memantine, or 50 μM MK-801.
Data Analysis.
To isolate mEPSCs recorded in the absence or presence of the NMDAR antagonists, events were selected using a template search in pClamp 10.0. The experimenter was blinded to drug condition for time shift analysis and averaging of mEPSCs. For comparison of mEPSCs in each cell before and after drug application, 100 mEPSCs under steady-state conditions (∼2 min into the recording or after solution exchange) were averaged. Charge transfer calculations were performed for before and after comparisons of NMDAR-mEPSCs on the entire 4-min recording.
Protein Quantification.
Anterior hippocampal slices (two to three per mouse, ∼1-mm thick) were dissected and flash-frozen from mice 30 min following drug injection. Hippocampal tissue was lysed in a buffer containing phosphatase and protease inhibitors (Roche). Protein concentration was quantified with Bradford analysis. Approximately 30 μg of protein was run on SDS/PAGE gels and then transferred to PVDF membranes activated in methanol. Primary antibodies were used at the following dilutions: BDNF (ab108319; Abcam), 1:1,000; GAPDH (2118s; Cell Signaling), 1:50,000; and phosphorylated eEF2 (Thr56) and total eEF2 (2331s and 2332, respectively; Cell Signaling), 1:2,000. Total and phospho-eEF2 primary antibody dilutions included 5% (vol/vol) BSA. After washing, the membranes were incubated with anti-rabbit secondary antibodies: BDNF, 1:5,000; GAPDH, 1:10,000; and phospho-eEF2 and total eEF2, 1:5,000. Protein bands were detected using ECL and exposed to film. Following development of phospho-eEF2 bands, membranes were stripped in buffer [25 mM glycine, 1% SDS (pH 2)] before blocking and reprobing with total eEF2 primary antibody. The films were analyzed using ImageJ (National Institutes of Health). Phospho-eEF2 intensity and total eEF2 intensity were measured as a ratio normalized to GAPDH. BDNF protein was normalized to GAPDH.
Statistical Analysis.
Data are reported as mean ± SEM. Statistical differences were assessed using the unpaired or paired two-tailed Student’s t test, one-way ANOVA, or two-way ANOVA when appropriate. Tukey and Bonferroni post hoc tests were used when appropriate. Statistical significance was defined as P < 0.05.
Supplementary Material
Acknowledgments
We thank M. Adachi, D. Crawford, M. Mahgoub, and K. Szabla for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health Grants MH070727 (to L.M.M.), MH066198 (to E.T.K.), and T32 NS069562-04 (to E.S.G.), as well as awards from the Brain and Behavior Research Foundation (to E.T.K. and L.M.M.) and the International Mental Health Research Organization (to L.M.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323920111/-/DCSupplemental.
References
- 1.Zarate CA, Jr, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 2.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 3.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist—A review of preclinical data. Neuropharmacology. 1999;38(6):735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 5.Lenze EJ, et al. Memantine for late-life depression and apathy after a disabling medical event: a 12-week, double-blind placebo-controlled pilot study. Int J Geriatr Psychiatry. 2012;27(9):974–980. doi: 10.1002/gps.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson JM, Shingleton RN. An open-label, flexible-dose study of memantine in major depressive disorder. Clin Neuropharmacol. 2007;30(3):136–144. doi: 10.1097/WNF.0b013e3180314ae7. [DOI] [PubMed] [Google Scholar]
- 7.Zarate CA, Jr, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163(1):153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 8.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosyreva E, et al. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33(16):6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304(5679):1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- 11.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55(4):648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Sutton MA, et al. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125(4):785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169(11):1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 14.Maeng S, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani M, Pértile R, Calixto JB, Santos AR, Rodrigues AL. Melatonin exerts an antidepressant-like effect in the tail suspension test in mice: Evidence for involvement of N-methyl-D-aspartate receptors and the L-arginine-nitric oxide pathway. Neurosci Lett. 2003;343(1):1–4. doi: 10.1016/s0304-3940(03)00306-9. [DOI] [PubMed] [Google Scholar]
- 16.Emnett CM, et al. Indistinguishable synaptic pharmacodynamics of the NMDAR channel blockers memantine and ketamine. Mol Pharmacol. 2013;84(6):935–947. doi: 10.1124/mol.113.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popik P, Skolnick P. The NMDA antagonist memantine blocks the expression and maintenance of morphine dependence. Pharmacol Biochem Behav. 1996;53(4):791–797. doi: 10.1016/0091-3057(95)02163-9. [DOI] [PubMed] [Google Scholar]
- 18.Espinosa F, Kavalali ET. NMDA receptor activation by spontaneous glutamatergic neurotransmission. J Neurophysiol. 2009;101(5):2290–2296. doi: 10.1152/jn.90754.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvadore G, Singh JB. Ketamine as a fast acting antidepressant: Current knowledge and open questions. CNS Neurosci Ther. 2013;19(6):428–436. doi: 10.1111/cns.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotermanski SE, Wood JT, Johnson JW. Memantine binding to a superficial site on NMDA receptors contributes to partial trapping. J Physiol. 2009;587(Pt 19):4589–4604. doi: 10.1113/jphysiol.2009.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiwagi K, et al. Channel blockers acting at N-methyl-D-aspartate receptors: Differential effects of mutations in the vestibule and ion channel pore. Mol Pharmacol. 2002;61(3):533–545. doi: 10.1124/mol.61.3.533. [DOI] [PubMed] [Google Scholar]
- 22.Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29(9):2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterton JE, et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415(6873):793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- 24.Smothers CT, Woodward JJ. Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-D-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther. 2007;322(2):739–748. doi: 10.1124/jpet.107.123836. [DOI] [PubMed] [Google Scholar]
- 25.Povysheva NV, Johnson JW. Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons. J Neurophysiol. 2012;107(8):2232–2243. doi: 10.1152/jn.01017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990;10(9):3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahr CE, Stevens CF. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990;10(6):1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collingridge GL, Herron CE, Lester RA. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collingridge GL, Herron CE, Lester RA. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteggia LM, Gideons E, Kavalali ET. The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry. 2013;73(12):1199–1203. doi: 10.1016/j.biopsych.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.