Significance
The physiology of the mammalian retina is governed by a molecular circadian clock. The retina is also the sole photoreceptive tissue for the synchronization of the master circadian clock, the suprachiasmatic nucleus (SCN), to light/dark cycles. The SCN synchronizes the phases of peripheral clocks throughout the body. It is unknown whether the retina is entrained by the SCN, or is directly entrained by light/dark signals. We report that the retinal circadian clock synchronizes directly to light/dark cycles, independent of the SCN phase. Unexpectedly, the retina uses photoreceptors distinct from those used for circadian photoreception or vision for this entrainment.
Keywords: circadian rhythm
Abstract
Synchronization of the mammalian master circadian pacemaker to the daily light/dark cycle is mediated exclusively through retinal photoreceptors. The mammalian retina itself is also a self-sustained circadian oscillator. Here we report that the retinal molecular circadian clock can be entrained by lighting cycles in vitro, but that rods, cones, and melanopsin (Opn4) are not required for this entrainment. In vivo, retinas of Opn4−/−;rd1/rd1 mice synchronize to light/dark cycles regardless of the phase of the master circadian pacemakers of the suprachiasmatic nuclei or the behavior of the animal. These data demonstrate that the retina uses a separate mechanism for local entrainment of its circadian clock than for entrainment of organism-level rhythmicity.
Most mammalian tissues contain autonomous circadian clocks, and these oscillators are synchronized by the “master circadian pacemaker,” which is localized in the suprachiasmatic nucleus (SCN) of the hypothalamus (1). The SCN controls the rhythms of diverse physiological functions, including feeding, temperature cycles, and circulating hormones, which in turn synchronize the clocks of tissues throughout the body (2). Following lesions of the SCN, animal behavior becomes arrhythmic and internal synchrony among tissues throughout the body is lost (3–5). Photic entrainment of the mammalian SCN (and thus the animal’s behavior) is mediated exclusively through photoreception in the retina (6, 7). Although outer retinal (rod/cone)-based and melanopsin-expressing retinal ganglion cell-based photoreceptors are each sufficient for circadian entrainment of behavior, animals lacking rods, cones, and melanopsin (Opn4) cannot entrain to external light/dark cycles (8–10).
The mammalian retina itself is also a self-sustained circadian oscillator (11), and many retinal physiologic functions oscillate with a 24-h period. These include transcription and translation of photoreceptor genes, neurotransmitter synthesis and release, interphotoreceptor coupling, disk shedding in rods, and amplitude of the electroretinogram (ERG) (12–18). More than 1,000 genes in the retina have a significant 24-h diurnal rhythm in transcript abundance (15). When the core circadian clock gene Bmal1 is deleted specifically in the retina (rendering it incapable of free-running rhythmicity), transcriptional and ERG rhythmicity are lost and ERG amplitudes are reduced at all times of day, suggesting that local circadian rhythmicity is required for normal retinal function (15). The circadian clock in the mammalian retina persists in culture, and can be measured ex vivo by neurotransmitter release or by luminescent reporters of circadianly expressed core clock genes (11, 19). Importantly, the retinal rhythm of melatonin synthesis can be synchronized directly by light/dark cycles in culture (11, 20), demonstrating that the SCN is not necessary for synchronization of retinal rhythms. Here we demonstrate that the retinal clock remains entrained to the light/dark cycle regardless of SCN and behavioral phase and that the photoreceptors necessary for local entrainment of the retinal circadian clocks are distinct from those required for behavioral entrainment. In vitro and in vivo, the entrainment of retinal circadian clocks occurs in the absence of rods, cones, and melanopsin.
Results and Discussion
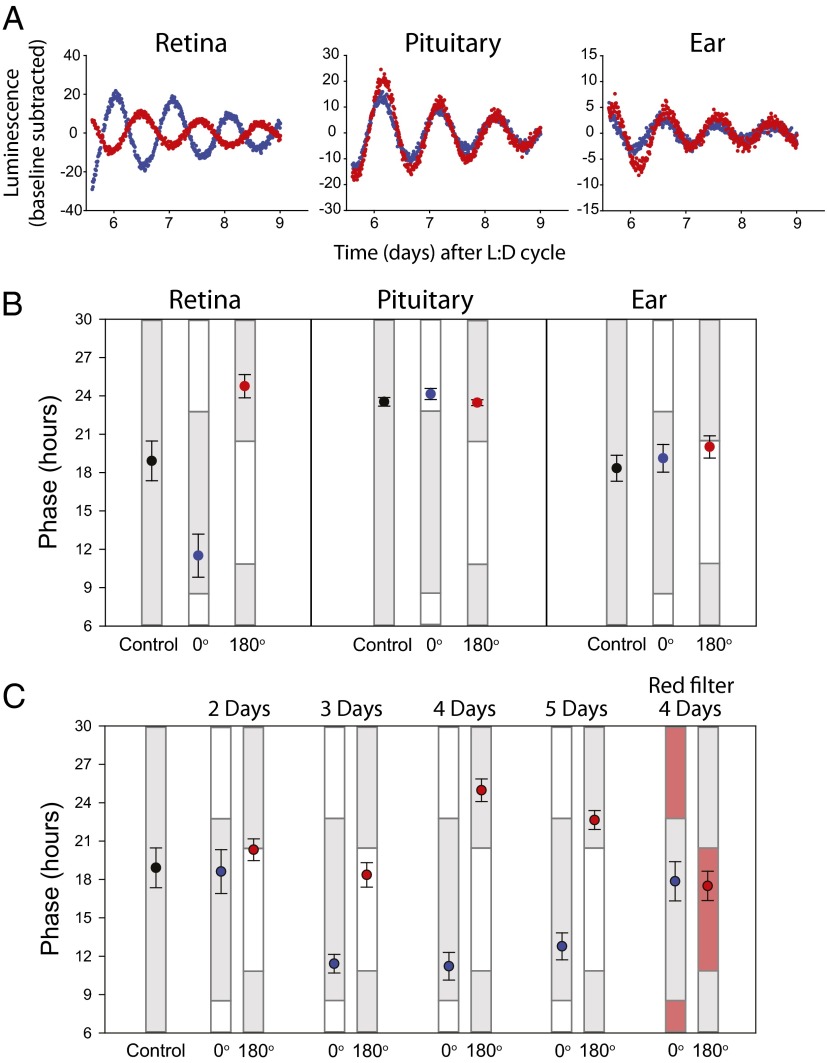
To test the ability of light to entrain the core molecular clock of murine retinas, pairs of retinas from mice carrying the Per2Luciferase reporter (5) were exposed to antiphasic white light/dark cycles in vitro by using a shutter that ensured constant temperature throughout the cycle (Fig. S1). The peak light intensity was 4 W/m2. After 4 d of exposure to light/dark cycles consisting of 9 h of light and 15 h of darkness, the Per2Luciferase bioluminescence rhythms of retina pairs became oppositely phased from each other when monitored in total darkness (Fig. 1 A and B). The phases of the retinas exposed to the oppositely phased light/dark cycles were significantly different from each other as well as from untreated control cultures (one-way ANOVA, P < 0.001). The light-entrained retinas adopted a phase with peak luciferase activity occurring during the early- to middle-dark phase. Retina pairs required a minimum of 4 d exposure to light/dark cycles to stably reach these phases in advance and delay directions (Fig. 1C). To determine if this effect is specific to the retina, cultures of pituitary glands [whose rhythms are highly sensitive to small temperature variations (21)] and ear pinnae (which are naturally exposed to light/dark cycles) of Per2Luciferase mice were exposed to the same 4 d light/dark paradigm. Neither demonstrated phase entrainment (one-way ANOVA, P > 0.05; Fig. 1B). We also confirmed previous studies demonstrating that cultures of SCN are not entrained by light/dark cycles in vitro (22). Photic entrainment was not observed when cultures were covered with aluminum foil or red acetate (Fig. 1C). Thus, entrainment in vitro to light/dark cycles is specific to the retina among the tissues tested, and unlikely an artifact of temperature or nonspecific photic effects.
Fig. 1.
The retina is entrained by light/dark cycles in vitro. (A) Per2Luciferase luminescence traces with background luminescence subtracted by using a polynomial fit line with an order of 1. Red and blue traces represent two separate cultures of the specified tissue from the same animal. Traces are recorded in constant darkness after 4 d of light/dark treatment in the 0° (blue) or 180° (red) position in a light/dark clock apparatus. (B) Average time of peak Per2Luciferase luminescence on the first day of constant darkness following a 4-d light/dark treatment (error bars represent 1 SEM) in the 0° (blue) or 180° (red) position. Gray bars indicate times of day when tissues previously experienced dark, and white boxes indicate times of exposure to 5 W/m2 light from LEDs ranging from 417 to 530 nm. Black points represent the average phase (±SEM) of untreated tissues on the fifth day in culture. Retina: control, n = 8; 0°, n = 6; and 180°, n = 6. Pituitary: control, n = 5; 0°, n = 5; and 180°, n = 5. Ear: control, n = 5; 0°, n = 6; and 180°, n = 6. (C) Average time (±SEM) of luminescence as shown in B for retina pairs exposed to 2 d (n = 5), 3 d (n = 5), 4 d (n = 6), or 5 d (n = 5) of a light/dark cycle. (Right) Phases of retinas in clock apparatus but with individual culture dishes covered with a layer of red acetate (n = 5).
Circadian systems that are entrainable to environmental cycles are often also susceptible to acute pulses of that same entraining signal (23). Cultured WT retinas were exposed to 3-h blue light pulses (3 × 1015 photons per square meter per second of 475 nm light) and exhibited phase shifts, which showed phase delays in what was previously the early dark phase and phase advances in the late dark phase (Fig S2A), characteristic of a “type 1” phase response curve. Associated with this are acute inductions of Per1 and Per2 during light pulses occurring when Per levels would be expected to be at low levels (Fig. S2B). The induction of the Per1 and Per2 transcripts is similar, though not identical, to light-induced Per expression in the rodent SCN and Xenopus retina (24–26).
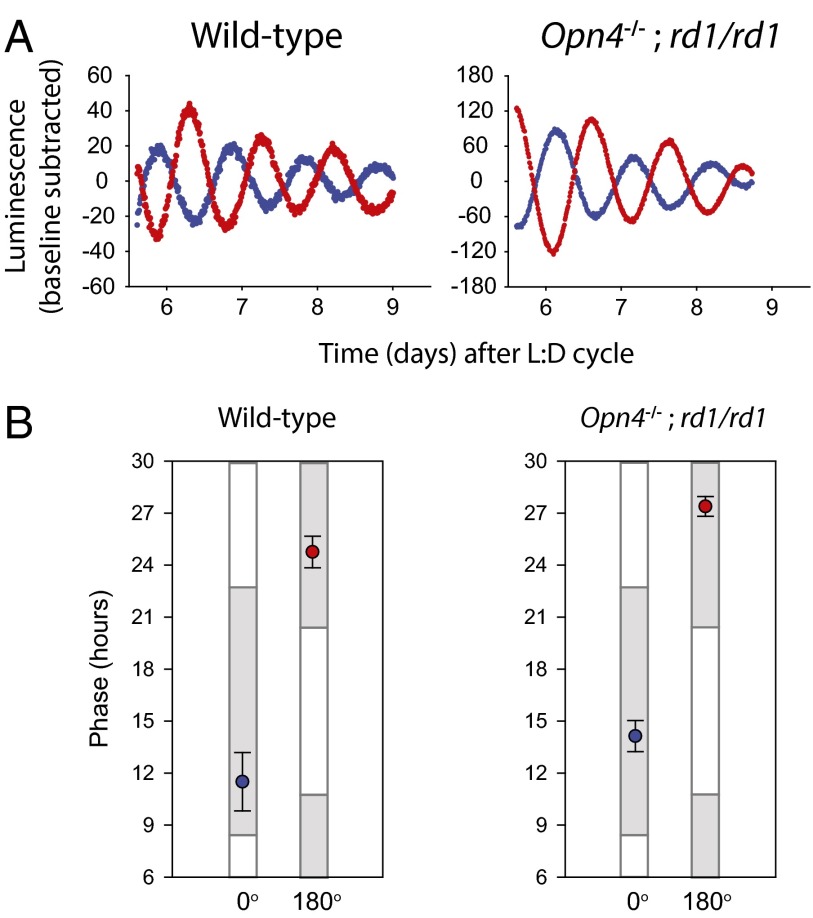
To ascertain if the photoreceptors that mediate behavioral entrainment to light are also required for the photic entrainment of local clocks in the retina, retinas of mice lacking melanopsin, rods, and cones (i.e., Opn4−/−;rd1/rd1 mice) were tested for photic entrainment. Melanopsin alone or the rod/cone pathways are sufficient to entrain behavioral rhythms, but animals lacking all systems cannot entrain behavioral rhythms to light/dark cycles (8, 10). Free-running behavioral rhythmicity in the presence of a light/dark cycle of Opn4−/−;rd1/rd1 mice used in these experiments was confirmed (see Fig. 3 A and B). As reported previously (10), Opn4−/−;rd1/rd1 mice also lacked pupillary light responses even to bright (<20 W/m2) white light (Fig. S3). To confirm retinal degeneration, we analyzed the transcripts of opsin genes in fresh and cultured retinas. Opn1-SW was undetectable in all cultured retinas (Fig. S4). Rhodopsin (Rho) was detectible at high levels in fresh and cultured retinas but was found at 0.04% of this level in fresh Opn4−/−;rd1/rd1 retinas, consistent with reported expression in Müller cells (27). Other putative “nonclassical” opsins that are expressed in the retina were stably expressed in all retinas even after extended days in culture (Fig. S4) (28). Surprisingly, retinas from Opn4−/−;rd1/rd1 mice were readily entrained to light/dark cycles in vitro (Fig. 2 and Fig. S5). The entrained phases of retinas from Opn4−/−;rd1/rd1 mice were approximately 2.5 h delayed compared with the WT groups; however, this difference was not statistically significant (one-way ANOVA, Tukey post hoc test, P = 0.353 for 0° and P = 0.349 for 180°). Other parameters of the circadian oscillations of Per2Luciferase expression including free-running period and rhythm amplitude were not affected in Opn4−/−;rd1/rd1 retinas, confirming previous work suggesting that the majority of retinal rhythmicity resides in the inner retina (29). In sum, these data demonstrate the photoreceptors necessary for the entrainment of the retina to light/dark cycles in vitro are independent of the known visual and nonvisual photoreceptors.
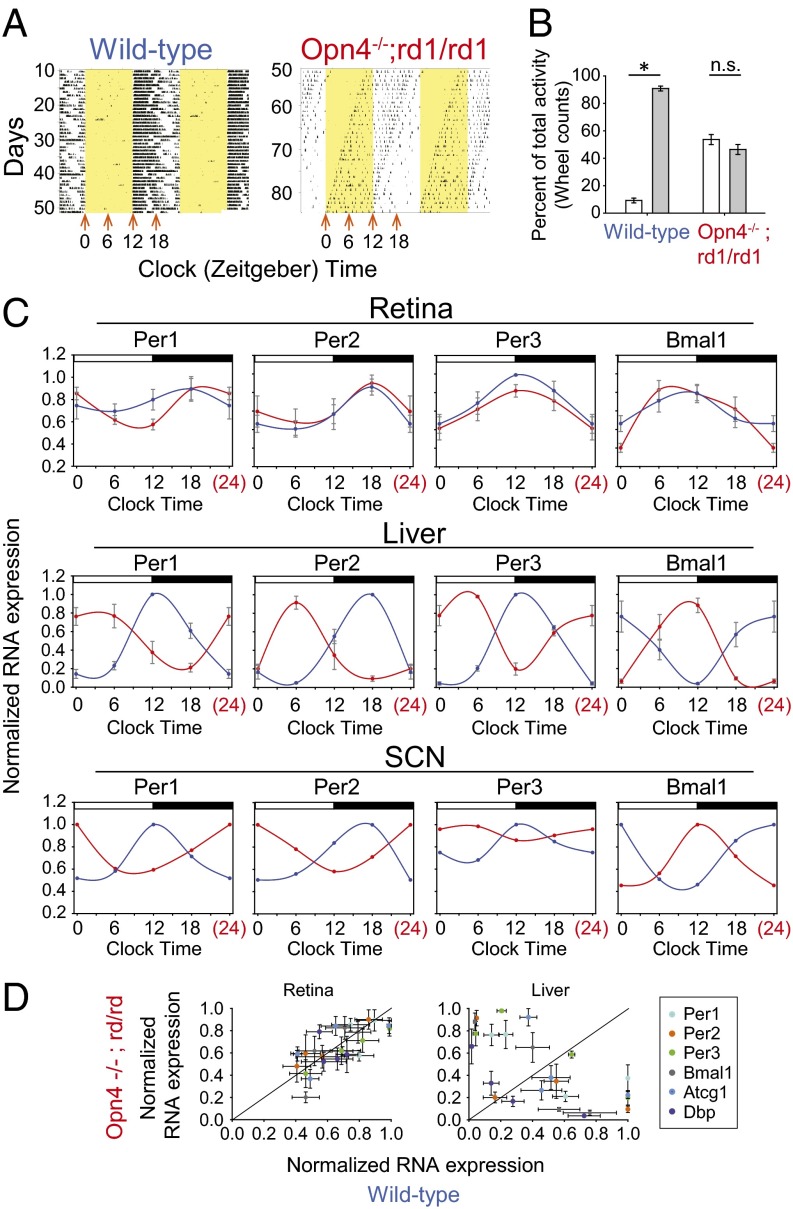
Fig. 3.
Retina clocks are entrained to light/dark cycles in vivo. (A) Representative actograms of wheel-running behavior of a WT (Left) and an Opn4−/−;rd1/rd1 (Right) mouse. Black indicates time of day when the mouse was running in the wheel, and yellow indicates times of day when 5 W/m2 lights were on. On the day at which the Opn4−/−;rd1/rd1 mice were active beginning at lights-on, tissues were collected at times indicated by orange arrows. (B) Wheel-running activity in the light (white bars) and dark (gray bars) is plotted as percentage of total activity for WT (n = 16) and Opn4−/−;rd1/rd1 (n = 16) mice (two-way ANOVA, P < 0.05; *Tukey post hoc test, P < 0.05). (C) Quantitative PCR on cDNA reverse transcribed from total RNA from WT (blue) or Opn4−/−;rd1/rd1 (red) tissues . Standard curve quantitative PCR was performed by using SYBR Green, and a standard curve was generated by using individual amplicons cloned into TOPO cloning plasmids. Values shown were normalized to the peak values for each group (n = 4 each). Error bars represent 1 SEM. The data shown as clock time 24 is double-plotted from clock time 0. (D) Normalized expression of RT-PCR with values from the same time point of WT (x axis) and Opn4−/−;rd1/rd1 (y axis) plotted for each gene.
Fig. 2.
Opn4−/−;rd1/rd1 retinas are entrained by light/dark cycles. (A) Background-subtracted Per2Luciferase luminescence traces of retinas from WT and Opn4−/−;rd1/rd1 mice recorded in constant darkness after being exposed to 4 d of light/dark cycle in 0° (blue) or 180° (red) positions in a light/dark clock apparatus. (B) Average time of peak Per2Luciferase luminescence on the first day of constant darkness following a 4 d light/dark treatment (error bars represent 1 SEM) in the 0° (blue) or 180° (red) position. Gray bars indicate times of day when tissues previously experienced dark. WT, n = 6 each; Opn4−/−;rd1/rd1, n = 6.
To determine if the phase of retinal circadian clocks in vivo is primarily set by light or by SCN-dependent internal timing cues, we exploited the observation that Opn4−/−;rd1/rd1 mice do not entrain their behavior to light/dark cycles (10) even though retinas from these mice show photic entrainment in vitro. Thus, the behavior of the mice free-runs while still being exposed to a stable light/dark cycle (Fig. 3A). On certain days, these mice will be active during the light phase and rest during the dark, corresponding to the opposite behavioral phase of a WT nocturnal mouse entrained to a light/dark cycle. We examined the phases of molecular clocks within tissues of mice with this temporary behavioral phase inversion. WT and Opn4−/−;rd1/rd1 mice were exposed to light/dark cycles of 12 h of 5 W/m2 white light and 12 h of darkness. The light was from LEDs ranging from 417 to 530 nm as shown in Fig. S1. WT mice entrained their running-wheel behavior to the lighting cycle, consolidating the majority of their wheel-running activity in the dark phase, but Opn4−/−;rd1/rd1 mice were active equally in light and dark phases (Fig. 3B). After >10 d in the light/dark cycle, the behavior of the Opn4−/−;rd1/rd1 mice was monitored for the day at which the onset of activity coincided with the transition from darkness to light. On this day, the mice were euthanized at one of four time points across the day, and their retinas, livers, and hypothalamic slices containing the SCN were removed for RNA isolation (Materials and Methods). Similarly, the retinas, livers, and brains were collected from WT mice at the same time points.
RT-PCR analysis of total RNA revealed the transcripts of clock genes Per1, Per2, Per3, and Bmal1 in the liver to be oppositely phased between the Opn4−/−;rd1/rd1 and WT animals, demonstrating that liver gene expression was synchronized with behavior (Fig. 3C). Similar to liver, RT-PCR analysis of hypothalamic sections containing the SCN revealed the clock genes to be in opposite phases and correlated in each case with behavior (Fig. 3C). However, the phase of these same transcripts in the retina remained identical between day-active Opn4−/−;rd1/rd1 and night-active WT animals. The phases observed from the retinas in vivo were similar to those observed from in vitro photo-entrainment (Figs. 1 and 2). Correlation analysis revealed liver gene expression followed behavioral phase, wheras retinal rhythm phase was dependent on lighting phase (Fig. 3D).
Taken together, these results demonstrate that the retina is capable of direct photic entrainment in vitro and in vivo, and that in vivo light signaling provides a stronger synchronizing stimulus than any SCN-dependent signal or the animal’s behavior. SCN-dependent cues are neither necessary nor sufficient to synchronize the retinal clock in the setting of external light/dark cycles. Thus, the retina appears to be similar to the liver clock which can synchronize to environmental/systemic cues despite the phase of the SCN (30). Additionally, as the retina is capable of entrainment under conditions in which it cannot entrain the SCN (i.e., in Opn4−/−;rd1/rd1 mice), it appears that the mechanisms for local entrainment of the retinal clock are distinct from the mechanisms by which the retina entrains the SCN and organismal rhythmicity.
The present studies have not identified the loci for rhythmicity in the retina, the location of the local entraining photopigment, or mechanism of local retinal entrainment, but do provide important constraints on each. Rhythmic expression of core clock genes has been reported in the outer nuclear layer, inner nuclear layer, and ganglion cell layer (15, 19, 29, 31–33). Expression patterns of clock genes in the photoreceptor layer are unique compared with other described circadian patterns with Bmal1 and Per genes peaking simultaneously only in the outer nuclear layer (34). It has been suggested that core clock genes in the photoreceptor layer stop cycling in constant darkness, whereas the whole retinal rhythmicity continues (35). We observed no statistical difference in any circadian parameters (free-running period, amplitude, or phase angle of entrainment) between retinas from WT mice and rd1/rd1 mice. Although we cannot exclude circadian clock gene expression in the photoreceptor layer, we conclude that the Per2-dependent circadian clock we are measuring is primarily located within the inner nuclear layer and ganglion cell layer, similar to what was observed by Ruan et al. (29). It should be noted that prolonged culture also causes loss of some ganglion cells, presumably from the complete axotomy at dissection, and the cells of the inner nuclear layer are the most preserved (36). The outer retinal layer is also required for rhythmic expression of melatonin from the retina (20). The strains of mice we used in the present study (C57BL/6J and 129S1/Sv) lack melatonin, and, in the case of the rd1 mutation, lack the outer nuclear layer (37–40). Thus, our observation of robust circadian rhythmicity in rd1/rd1 retinas supports the conclusion that neither melatonin nor the outer photoreceptor layer is necessary for retinal circadian gene expression or its local photic entrainment (15, 19). It is possible that the photoreception we observe here is autonomous within Müller glia cells, amacrine cells, or horizontal cells.
Perhaps the most surprising finding in the present work is that the photic entrainment of the circadian clock within the retina does not require rods, cones, or melanopsin. This leads us to conclude that another photoreceptor must be present in the rd1/rd1 retina. Although it is possible that residual rod or cone function from incomplete outer retinal degeneration may underlie this photoreception, this is unlikely, as the tested animals were unable to entrain behaviorally to even bright light/dark cycles, display the pupillary light reflex, and did not show significant levels of opsin transcripts. Because retinal circadian entrainment persists in the Opn4−/−;rd1/rd1 model, the responsible pigment is likely localized in the inner nuclear layer or ganglion cell layer where the strongest circadian signals are observed (29). Several orphan opsins have been described in recent years [i.e., Opn3 (41), Opn5 (42), RGR opsin (43)], which may be candidates, as might nonopsin photopigments such as the cryptochrome family (44).
Materials and Methods
In Vitro Light/Dark Cycles.
We wished to expose organotypic tissue cultures to light/dark cycles without turning lights on and off to avoid temperature changes. We constructed an apparatus in which the motor from a 24-h wall clock was used to rotate a disk with a period of 24 h. The disk used in the present study was opaque except for a transparent wedge-shaped window that allowed light to pass through to a given spatial coordinate for 9 h every 24-h cycle. Culture dishes (35 mm) were placed on opposite sides of a larger opaque dish under the motor-driven disk. Light-impermeable baffles were also used in the larger dish to control the passage of light between the two halves of the dish. This allowed for light exposure to one 35-mm dish while the other was in darkness and created antiphasic light/dark cycles for the two dishes. The two positions used in this study are arbitrarily designated 0° and 180°.
The light source used was an array of LEDs of various wavelengths. The wavelengths of the peak intensity for the three types of LEDs used were 417 nm, 475 nm, and 530 nm. They were left on continuously for in vitro light/dark cycles. Because the transparent window is moved continuously, the cultures experience a gradual transition between light (“sunrise”) and dark (“sunset”; Fig. S1). The LED array and the clock apparatuses are contained in a 37 °C incubator. Radiometric measurements were made by using a Macam Q203 quantum radiometer. Spectral irradiance was measured by using a SpectroCal spectral radiometer (Cambridge Research Systems). Temperature was monitored by placing an EL-USB-1 temperature data logger (Lascar Electronics) inside of a light cycle apparatus for 4 d. The temperature was collected every 5 min and stored on the logger until retrieval at the end of the experiment.
Phase Response Curve.
Tissue cultures were transferred from the Lumicycle into a small insulated box, which was kept inside or near the Lumicycle apparatus at the same temperature. The cultures in the open box were then exposed to 475 nm LEDs at 3 × 1015 photons per square millimeter per second for 3 h, and then returned to the Lumicycle. Phase shifts were calculated as the difference in the phase of best-fit sine waves to the 3 d after the pulse and the 3 d before the pulse as described previously (21).
Bioluminescence Recording from Per2Luciferase Reporter Mice.
C57Bl6/J Per2Luciferase mice (5) were euthanized by CO2 asphyxiation 2-3 h before lights off (zeitgeber time 9–11) and tissues were immediately removed into cold HBSS (Gibco). Pituitary and pinnae cultures were cultured on cell culture inserts (PICMORG50; Millipore) in sealed dishes containing DMEM containing B-27 supplement (Life Technologies), 352.5 μg/mL sodium bicarbonate, 10 mM Hepes (Life Technologies), 25 U/mL penicillin, 25 μg/mL streptomycin (Life Technologies), and 0.1 mM luciferin potassium salt (Biosynth). Retinas were cultured on cell culture inserts in Neurobasal A medium (Cellgro) containing B-27 serum-free supplement (Life Technologies), 25 U/mL penicillin, 25 μg/mL streptomycin (Life Technologies), and 2 mM l-glutamine in 5% CO2 for ∼16 h. Retinas were then transferred to sealed dishes containing DMEM/luciferin media as described earlier.
Tissue pairs from the same animal were placed on opposite sides of the clock apparatus described earlier for 4 d. At the end of 4 d, they were placed in the dark into a Lumicycle luminometer machine (Actimetrics), in which bioluminescence was measured continuously by photomultiplier tubes.
Lumicycle Analysis software was used to detrend steady reductions in background bioluminescence with a polynomial fit line with an order of 1. Period of oscillations was determined by using best-fit sine wave analysis contained within the Lumicycle Analysis software. The time of the peak of luminescence on the first day of constant conditions (first day in Lumicycle) was determined as the time of peak of the sine wave on that day. One-way ANOVA tests with Tukey post hoc analyses were used to determine statistical differences between groups within each tissue type.
Animals.
All animal experiments were carried out after approval of protocols by the institutional animal care and use committee at the University of Washington. Opn4−/− mice were genotyped as described previously (10), and were of the strain C57BL/6J outcrossed from 129S1/Sv at least seven generations. Mice with a mutation of the Pde6b gene (rd1) on the background C57BL/6J were obtained from Jackson Laboratory and were genotyped by PCR analysis according to instructions from Jackson Laboratory.
Monitoring Wheel-Running Behavior.
Mice were housed in individual cages equipped with running wheels under 12 h/12 h light/dark conditions. Wheel activity and timing of light changes were collected by using a Clock Lab data collection system (Actimetrics). An LED array identical to the one used for in vitro light/dark cycles was used for the in vivo experiments. Lights were given as a square wave 12 h/12 h light/dark cycle with an irradiance of 5 W/m2 during the light phase.
Quantitative RT-PCR.
Mice were euthanized under dim red light by cervical dislocation. Retinas were dissected from the eyes in ice cold HBSS under dim red light and were placed in RNAlater (Qiagen). Brains were immediately frozen on dry ice. Livers were dissected directly into RNAlater. Sections containing the SCN were later removed from frozen brains by slicing a coronal section by using a hot razor blade on either side of the optic chiasm followed by finer SCN removal by dissection on dry ice, and then transferred to RNAlater. The four SCN samples collected for each time point were pooled before RNA extraction. RNA was extracted from tissues by using an RNeasy RNA isolation kit (Qiagen) according to the manufacturer’s protocol. cDNA was reverse transcribed from 200 ng of RNA by using SuperScript VILO cDNA synthesis kit (Invitrogen), which uses random hexamer and 3′end polyA tail priming.
Amplicons for each gene were cloned into the pCR 2.1 TOPO cloning vector (Invitrogen) after being amplified from cDNA reverse transcribed from RNA from mouse retina. Inserts were sequenced to confirm integrity of expected sequence. Standard curves were generated starting with 1 × 109 copies of the plasmid with appropriate amplicon and creating 1: 100 dilutions down to 1 × 102 copies. Quantitative PCR was performed by using Absolute Blue SYBR Low Rox master mix (Thermo Scientific) and an Applied Biosystems 7500 Real Time PCR system. Melt curves and gel electrophoresis of PCR products were used to confirm correct amplification of targets. Gapdh was used as a noncycling endogenous control. For opsin genes, the delta-delta cT method was used for relative abundance of RNA. This compared the cT of the target gene to the cT of Gapdh in the same sample and then compared that level to delta cT (i.e., opsin − Gapdh) of a liver sample.
Primer sequences.
Per1 forward, 5′-CCCAGCTTTACCTGCAGAAG-3′;
Per1 reverse, 5′-ATGGTCGAAAGGAAGCCTCT-3′ (91-bp amplicon);
Per2 forward, 5′-CCAACACAGACGACAGCATC-3′;
Per2 reverse, 5′-TCTCGCAGTAAACACAGCCT-3′ (216-bp amplicon);
Per3 forward, 5′-CACTTTGTCGACCTGCTTGC-3′;
Per3 reverse, 5′-ATGAACCAAATAGGGGAGGAT-3′ (210-bp amplicon);
Bmal1 forward, 5′-GACATTTCCTCAACCATCAGCG-3′;
Bmal1 reverse, 5′-GCATTCTTGATCCTTCCTTGGT-3′ (200-bp amplicon);
Atcg1 forward, 5′-TGGATCTCTGTGAGCACCAC-3′;
Atcg1 reverse, 5′-AGGCAACTAACAACCGATGG-3′ (203-bp amplicon);
Dbp forward, 5′-CGAAGAACGTCATGATGCAG-3′;
Dbp reverse, 5′-GGTTCCCCAACATGCTAAGA-3′ (118-bp amplicon);
Gapdh forward, 5′-GACTTCAACAGCAACTCCCA-3′;
Gapdh reverse, 5′ -ATTGTGAGGGAGATGCTCAGT-3′ (129-bp amplicon);
Opn1-SW forward, 5′-TCACGGATACTTCCTCTTTGGTC-3′;
Opn1-SW reverse, 5′-GGCCAACTTTGCTAGAAGAGAC-3′ (600-bp amplicon);
Rho forward, 5′-CTTTGCCACACTTGGAGGTGA-3′;
Rho reverse, 5′-TGATCCAGGTGAAGACCACAC-3′ (200-bp amplicon);
Opn4 forward, 5′-TCTGTTAGCCCCACGACATC-3′;
Opn4 reverse, 5′-TGAACATGTTTGCTGGTGTCC-3′ (180-bp amplicon);
Opn3 forward, 5′ -CTGTTCGGAGTCACCTTCAC-3′;
Opn3 reverse, 5′-GTATGTCTAGGATGTACCTGTTC-3′ (227-bp amplicon);
Opn5 forward, 5′ -AGCTTTTGGAAGGCCAGAC-3′;
Opn5 reverse, 5′ -CAGCACAGCAGAAGACTTC-3′ (212-bp amplicon);
RGR forward, 5′ -TGTACACTGGACTATTCGAGG-3′; and
RGR reverse, 5′ -GGTTTTGGCGATGAGAGCAG-3′ (285-bp amplicon).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants F32EY21104 (to E.D.B.) and P30EY01730, the Burroughs–Wellcome Clinical Scientist Award in Translational Science (to R.N.V.G.), and an unrestricted award from Research to Prevent Blindness.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323350111/-/DCSupplemental.
References
- 1.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 2.Brown SA, Azzi A. Peripheral circadian oscillators in mammals. Handb Exp Pharmacol. 2013;217:45–66. doi: 10.1007/978-3-642-25950-0_3. [DOI] [PubMed] [Google Scholar]
- 3.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 4.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo SH, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101(15):5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146(1):1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 7.Nelson R, Zucker I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp Biochem Physiol A. 1981;69:145–148. [Google Scholar]
- 8.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Güler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301(5632):525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 11.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272(5260):419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 12.Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59(5):790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grace MS, Wang LM, Pickard GE, Besharse JC, Menaker M. The tau mutation shortens the period of rhythmic photoreceptor outer segment disk shedding in the hamster. Brain Res. 1996;735(1):93–100. doi: 10.1016/0006-8993(96)00600-2. [DOI] [PubMed] [Google Scholar]
- 14.LaVail MM, Ward PA. Studies on the hormonal control of circadian outer segment disc shedding in the rat retina. Invest Ophthalmol Vis Sci. 1978;17(12):1189–1193. [PubMed] [Google Scholar]
- 15.Storch KF, et al. Intrinsic circadian clock of the mammalian retina: Importance for retinal processing of visual information. Cell. 2007;130(4):730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandenburg J, Bobbert AC, Eggelmeyer F. Evidence for the existence of a retino-hypothalamo-retinal loop in rabbits. Int J Chronobiol. 1981;8(1):13–29. [PubMed] [Google Scholar]
- 17.Cameron MA, et al. Electroretinography of wild-type and Cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J Biol Rhythms. 2008;23(6):489–501. doi: 10.1177/0748730408325874. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji T, et al. Circadian proteomics of the mouse retina. Proteomics. 2007;7(19):3500–3508. doi: 10.1002/pmic.200700272. [DOI] [PubMed] [Google Scholar]
- 19.Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci USA. 2006;103(25):9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tosini G, Menaker M. The clock in the mouse retina: Melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789(2):221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 21.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzog ED, Huckfeldt RM. Circadian entrainment to temperature, but not light, in the isolated suprachiasmatic nucleus. J Neurophysiol. 2003;90(2):763–770. doi: 10.1152/jn.00129.2003. [DOI] [PubMed] [Google Scholar]
- 23.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents II. The variability of phase response curves. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1976;106:253–266. [Google Scholar]
- 24.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: Differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20(6):1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 25.Vitaterna MH, et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci USA. 2006;103(24):9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steenhard BM, Besharse JC. Phase shifting the retinal circadian clock: xPer2 mRNA induction by light and dopamine. J Neurosci. 2000;20(23):8572–8577. doi: 10.1523/JNEUROSCI.20-23-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel M, Dhingra NK. Müller glia express rhodopsin in a mouse model of inherited retinal degeneration. Neuroscience. 2012;225:152–161. doi: 10.1016/j.neuroscience.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 28.Kumbalasiri T, Provencio I. Melanopsin and other novel mammalian opsins. Exp Eye Res. 2005;81(4):368–375. doi: 10.1016/j.exer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6(10):e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21(14):3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dkhissi-Benyahya O, et al. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci. 2013;70(18):3435–3447. doi: 10.1007/s00018-013-1338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witkovsky P, et al. Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J Neurosci. 2003;23(20):7670–7676. doi: 10.1523/JNEUROSCI.23-20-07670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider K, et al. Unique clockwork in photoreceptor of rat. J Neurochem. 2010;115(3):585–594. doi: 10.1111/j.1471-4159.2010.06953.x. [DOI] [PubMed] [Google Scholar]
- 35.Sandu C, Hicks D, Felder-Schmittbuhl MP. Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci. 2011;34(3):507–516. doi: 10.1111/j.1460-9568.2011.07772.x. [DOI] [PubMed] [Google Scholar]
- 36.Ogilvie JM, Speck JD, Lett JM, Fleming TT. A reliable method for organ culture of neonatal mouse retina with long-term survival. J Neurosci Methods. 1999;87(1):57–65. doi: 10.1016/s0165-0270(98)00157-5. [DOI] [PubMed] [Google Scholar]
- 37.Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231(4737):491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- 38.Roseboom PH, et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: Rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63(1):189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 39.Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7(2):195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 40.Chang B, et al. Retinal degeneration mutants in the mouse. Vision Res. 2002;42(4):517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 41.Blackshaw S, Snyder SH. Encephalopsin: A novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19(10):3681–3690. doi: 10.1523/JNEUROSCI.19-10-03681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kojima D, et al. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS ONE. 2011;6(10):e26388. doi: 10.1371/journal.pone.0026388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang M, Pandey S, Fong HK. An opsin homologue in the retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34(13):3669–3678. [PubMed] [Google Scholar]
- 44.Oztürk N, et al. Structure and function of animal cryptochromes. Cold Spring Harb Symp Quant Biol. 2007;72:119–131. doi: 10.1101/sqb.2007.72.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.