Significance
Sensory synapses in the retina are characterized by special structures at the site of neurotransmitter release called synaptic ribbons, which transmit information about both sustained and rapidly changing visual stimuli. Because rapid signaling is important for detecting spatial and temporal contrast, we investigated the mechanism of fast neurotransmitter release at ribbon synapses, using high-resolution imaging of synaptic vesicle turnover and calcium responses. We find that nanodomains of high calcium at ribbons drive exocytosis of membrane-associated synaptic vesicles within milliseconds, accounting for the rapid component of neurotransmitter release, whereas replacement of lost vesicles occurs at membrane-distal sites on the ribbon. The results provide insight into synaptic mechanisms that enhance detection of contrast, a fundamental aspect of visual processing.
Abstract
Ribbon synapses of photoreceptor cells and second-order bipolar neurons in the retina are specialized to transmit graded signals that encode light intensity. Neurotransmitter release at ribbon synapses exhibits two kinetically distinct components, which serve different sensory functions. The faster component is depleted within milliseconds and generates transient postsynaptic responses that emphasize changes in light intensity. Despite the importance of this fast release for processing temporal and spatial contrast in visual signals, the physiological basis for this component is not precisely known. By imaging synaptic vesicle turnover and Ca2+ signals at single ribbons in zebrafish bipolar neurons, we determined the locus of fast release, the speed and site of Ca2+ influx driving rapid release, and the location where new vesicles are recruited to replenish the fast pool after it is depleted. At ribbons, Ca2+ near the membrane rose rapidly during depolarization to levels >10 µM, whereas Ca2+ at nonribbon locations rose more slowly to the lower level observed globally, consistent with selective positioning of Ca2+ channels near ribbons. The local Ca2+ domain drove rapid exocytosis of ribbon-associated synaptic vesicles nearest the plasma membrane, accounting for the fast component of neurotransmitter release. However, new vesicles replacing those lost arrived selectively at the opposite pole of the ribbon, distal to the membrane. Overall, the results suggest a model for fast release in which nanodomain Ca2+ triggers exocytosis of docked vesicles, which are then replaced by more distant ribbon-attached vesicles, creating opportunities for new vesicles to associate with the ribbon at membrane-distal sites.
Sensory systems face the common problem of faithfully encoding the steady intensity of a stimulus over a wide range, while at the same time enhancing the detection of spatial or temporal changes in stimulus intensity. For example, the visual system must simultaneously process information about luminance (that is, steady intensity) and contrast (that is, changes in intensity), and the output synapse of the second-order bipolar neurons of the retina is thought to be an important site for the signaling of these two aspects of visual stimuli to downstream neurons (1). The ribbon synapses (2) of bipolar neurons generate both tonic release and phasic release in response to sustained depolarization (3–12). The phasic component consists of a limited pool of vesicles that can be tapped rapidly upon depolarization but is also rapidly exhausted, generating a transient spike of release, and the tonic component comprises a much larger pool of more slowly released vesicles. The rapid but transient release during the phasic component emphasizes changes in light intensity, whereas the slower but sustained release during the tonic component signals overall luminance (1).
Because ribbon synapses are found in sensory neurons that generate sustained responses to stimuli, such as photoreceptor cells and bipolar neurons in the retina and hair cells in the inner ear, the tonic component of release has received emphasis as the major hallmark of this specialized type of synapse. Indeed, the large reservoir of synaptic vesicles tethered to the ribbon is thought to account for the ability of ribbon synapses to sustain release for long periods, providing the raison d’être for the ribbon itself. However, the rapid, transient component of release is equally important for visual signaling (1), but the basis for rapid release at ribbon-type active zones is incompletely understood. Here, we addressed several questions regarding the generation of fast, transient release at ribbon synapses. Which vesicles associated with ribbons compose the transient component? Where does release occur with respect to the ribbon? After the fast pool is depleted, where are new vesicles recruited to replace those released? Where with respect to the ribbon and how quickly does intracellular Ca2+ rise when voltage-gated Ca2+ channels are activated during brief stimuli? To answer these questions, we exploited the ability to image individual ribbon active zones in the living synapse (13) and used high-resolution fluorescence microscopy to monitor Ca2+ influx and turnover of fluorescently labeled synaptic vesicles at single ribbons.
Results
Kinetic Components of Exocytosis.
The rate of release of the transient and sustained components of the readily releasable pool has not been established in our experimental system, the synaptic terminals of zebrafish retinal bipolar neurons, so we first examined the kinetics of release in these terminals by using capacitance measurements to monitor exocytosis (Fig. 1). For pulse durations of 0.5–10 ms, the capacitance response rose exponentially with a time constant of 2.3 ms to an asymptote of ∼30 fF (Fig. 1B), which reflects fast fusion of a subset of releasable vesicles within the first few milliseconds after onset of depolarization. This subset is the pool—referred to as the “ultrafast” pool in previous work (4)—that supports the rapid, transient component of release. Longer pulse durations (10–500 ms) produced a secondary rise to ∼150 fF with a time constant of 162 ms (Fig. 1B, Inset), which reflects the much slower depletion of the entire readily releasable pool (3, 4) and corresponds to the sustained component of release. Here, we focused on responses to 10-ms depolarization, which is sufficient to release the entire ultrafast pool without appreciably tapping the slower pool.
Fig. 1.
Kinetics of the fast component of synaptic vesicle exocytosis. (A) Ca2+ current (I) recorded from the synaptic terminal of a zebrafish bipolar neuron in response to a voltage-clamp pulse (V) from −60 mV to 0 mV for 10 ms. The sinusoidal voltage stimulus used to monitor membrane capacitance (Cm) and series conductance (Gs) is visible at the beginning and end of the voltage trace. The resulting values of Cm (circles) and Gs (diamonds) before and after activation of Ca2+ current are shown below the traces. (B) The average increase in Cm (± SEM) evoked by voltage-clamp pulses of varying duration in 16 synaptic terminals, measured as demonstrated for a 10-ms pulse in A. The solid line is an exponential rise with a time constant of 2.3 ms. (B, Inset) A similar experiment using longer pulses to measure the release of the slow component (n = 8–17 terminals), reflecting exocytosis of the entire readily releasable pool of synaptic vesicles.
Release of FM4-64 Dye Elicited by Brief Depolarization.
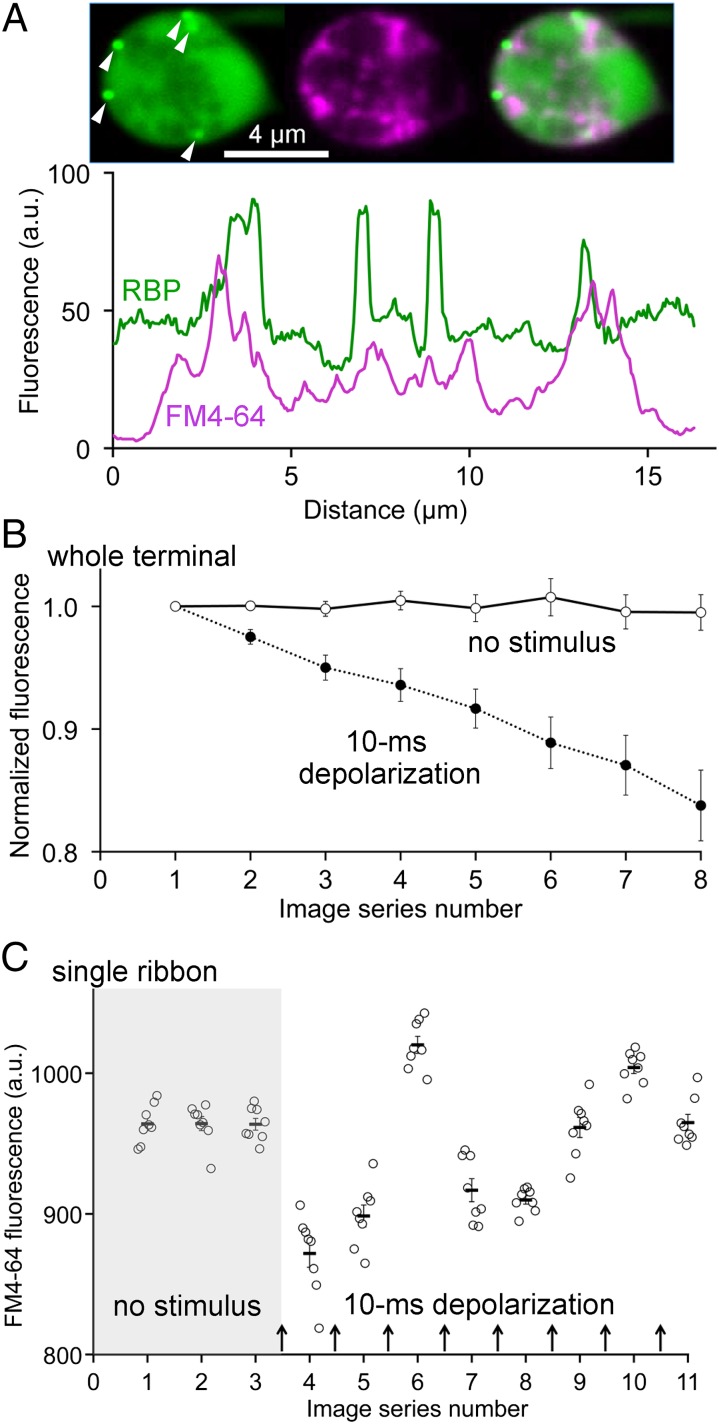
To determine the location of exocytosis during release of the fast pool, we labeled synaptic vesicles by activity-dependent uptake of FM4-64 dye (14–16) and then monitored the subsequent release of dye when labeled vesicles underwent exocytosis in response to activation of Ca2+ channels. Ribbon locations were marked by including Ribeye-binding peptide (RBP; ref. 13) in the whole-cell patch pipette, and as shown in Fig. 2A, FM-labeled vesicles tended to cluster near ribbons, which are the intense green spots marked by arrowheads in the single confocal optical sections at the top of Fig. 2A. Previous FRAP measurements (16) showed that ∼50% of the labeled vesicles not associated with ribbons are highly mobile and support refilling of the releasable pool after it is depleted by stimulation, whereas vesicles associated with ribbons are immobile until depolarization triggers their release. To monitor release of the ultrafast pool, we measured the change in FM4-64 fluorescence averaged across the entire terminal in response to a series of 10-ms depolarizations delivered at intervals of 18 s, which was sufficient to allow refilling of the pool (Fig. S1). Depolarizations produced progressive destaining as vesicles lost their dye upon undergoing fusion (Fig. 2B), but destaining was not observed in the absence of stimulation (Fig. 2B), indicating that it does not reflect spontaneous release or photobleaching. This stimulus-dependent destaining of FM dye represents release of the ultrafast pool at all of the 30–50 ribbons throughout the entire synaptic terminal.
Fig. 2.
Release of FM4-64 dye from synaptic vesicles in response to depolarizing stimuli. (A) Before voltage-clamp recording, vesicles were labeled by activity-dependent uptake of FM4-64 (magenta), and a labeled synaptic terminal was patch clamped by using a whole-cell pipette filled with a solution containing fluorescent RBP to label synaptic ribbons (green). Images show a single confocal optical section illustrating ribbon labeling (Left; arrowheads indicate ribbons), internalized FM4-64 (Center), and superposition of the two (Right). Traces in the graph show the fluorescence intensity of RBP and FM4-64, measured along a line drawn around the perimeter of the synaptic terminal. (B) Changes in average FM4-64 intensity, measured in a region of interest including the entire synaptic terminal, during a series of 10-ms depolarizations (filled circles; n = 10 terminals; interval between depolarizations = 18 s), or in the absence of stimulation (open circles; n = 9 terminals). For each cell, FM4-64 fluorescence was normalized with respect to the starting level (image series 1). (C) Changes in FM4-64 fluorescence measured in a 1-µm2 square region centered on a single synaptic ribbon in response to a series of 10-ms depolarizations delivered at the arrows. The interval between depolarizations was 18 s. Open circles indicate fluorescence from an individual image in each series of eight images, and the dash indicates the mean (± SEM) for each group of eight images. Significant difference between successive groups of eight was defined as P < 0.05 in a two-tailed t test.
The pattern of stimulus-evoked changes in FM4-64 was quite different, however, when we focused on fluorescence in a 1-µm2 region at a single ribbon (Fig. 2C) instead of measuring the fluorescence of the entire terminal. FM4-64 fluorescence was stable at the ribbon in the absence of stimulation (Fig. 2C, shaded region), consistent with previous work (16) demonstrating that vesicles at ribbons are immobile at rest and do not exchange with cytoplasmic vesicles, but 10-ms depolarizations elicited variable changes in FM4-64 fluorescence, which sometimes decreased, sometimes increased, and sometimes showed no change after a stimulus (Fig. 2C). Under our loading conditions, ∼20% of the vesicles in the synaptic terminal are labeled with FM dye (16), and the total pool of vesicles attached to a ribbon is therefore a mixture of labeled and unlabeled vesicles. Because the fast subgroup tapped by 10-ms depolarization constitutes ∼20% of the total releasable pool (i.e., 30 fF of a total of 150 fF; Fig. 1B), stochastic fluctuations in the outcome are expected, depending on the balance of labeled and unlabeled vesicles among those released and those refilling the pool on a given trial. For example, a decrease in FM-dye fluorescence would occur if more labeled vesicles were released than were replaced on that trial, whereas increases would reflect replacement with more labeled vesicles than were lost on that trial.
Locus of Vesicle Release and Vesicle Replenishment at Ribbons.
The stochastic behavior of FM-dye fluorescence at single ribbons offers the opportunity to determine the spatial location of dye loss and dye addition with respect to the ribbon by separately analyzing trials showing decreases vs. increases in fluorescence after 10-ms depolarization. To emphasize vesicles stably associated with ribbons and discount any mobile vesicles that transiently entered the region of interest, we collected a series of eight images of FM4-64 fluorescence at a single ribbon before each 10-ms depolarization and eight images 18 s after the stimulus, and then averaged each set of eight images. Images of RBP fluorescence were taken before and after each series to confirm the ribbon location and correct for any drift during the experiment. For trials in which depolarization significantly decreased local fluorescence (e.g., between image series 6 and 7 in Fig. 2C), we subtracted the averaged image after stimulation from the averaged image before stimulation to yield a difference image (ΔF) showing where fluorescence was lost, illustrated for five examples in Fig. 3A. For trials in which fluorescence increased significantly (e.g., between image series 5 and 6 in Fig. 2C), we subtracted the average before-stimulus image from the average after-stimulus image to determine where fluorescence was gained (examples are shown in Fig. 3B). The resulting “decrease” (n = 64) and “increase” (n = 65) images collected from 14 ribbons in 11 cells were then separately averaged to accumulate an overall diffraction-limited picture of the spatial location of ΔF with respect to the ribbon.
Fig. 3.
Vesicle fusion and vesicle replenishment occur at opposite poles of the synaptic ribbon. (A) Examples of individual ΔF images (Upper) and averaged diffraction-limited difference image (Lower) showing where fluorescence was lost in 64 trials in which FM4-64 fluorescence decreased in response to 10-ms depolarization. The amount of fluorescence lost is color coded in magenta. The averaged ribbon image is superimposed in green. The edge of the cell is to the right of the ribbon. (B) Examples of individual ΔF images (Upper) and averaged difference image (Lower) showing where fluorescence was gained in 65 trials in which FM4-64 fluorescence increased in response to 10-ms depolarization. The amount of fluorescence gained is color coded red, and the ribbon image is shown in green. (C) Electronmicrograph of a synaptic ribbon and associated synaptic vesicles in the synaptic terminal of a dissociated zebrafish bipolar neuron in the same approximate orientation as the averaged difference images from confocal images, shown in A and B. The scale bar in A applies to A–C. (D) x-axis profiles of the change in FM4-64 fluorescence (ΔF) in the averaged difference images in A (magenta) and B (red), measured perpendicular to the plasma membrane in horizontal strips 2 pixels high. The lightness of the lines gives the order of the vertical position of the 2-pixel strips, with the darkest at the top of the image. (E) x-axis profiles of ΔF were measured in individual difference images for increase and decrease trials by taking the average of the vertical column of pixels at each x position. The resulting individual profiles were then averaged separately over the 64 decrease trials and the 65 increase trials. The data points show the overall mean ΔF (± SEM) as a function of x-position relative to the center of the ribbon, with positive Δx representing the membrane-proximal side of the ribbon. (F) Plot showing the center of mass of ΔF for each increase (filled circles) and decrease (open circles) trial, relative to the center of mass of the ribbon for each image. Positive Δx is toward the membrane, and positive Δy is toward the top of the image.
Fig. 3A shows the overall ΔF image averaged over decrease trials, color-coded in magenta to indicate the amount of fluorescence decrease, and Fig. 3B shows the ΔF image for increase trials, color-coded in red. In both cases, the corresponding averaged ribbon image is superimposed in green, with the edge of the cell to the right. An electronmicrograph of a ribbon is shown in Fig. 3C on the same spatial scale and orientation to indicate the actual size of ribbons and vesicles. The average decrease in FM4-64 fluorescence—representing vesicle fusion and neurotransmitter release—was largest on the side of the ribbon nearest the plasma membrane (Fig. 3A). However, the average increase in fluorescence—representing the stable addition of new, labeled vesicles—was smaller nearer the membrane and larger on the cytoplasmic side of the ribbon (Fig. 3B). To quantify this differential pattern, we measured the x-axis profiles (i.e., perpendicular to the plasma membrane) of ΔF in horizontal strips 2 pixels high, starting at the top of each averaged image. Fig. 3D shows that the horizontal profiles for decrease and increase images peaked at different x-axis positions, consistent with release and replenishment on opposite poles of the ribbon. The same pattern was observed when we measured the x-axis ΔF profile for each individual image in the two datasets, relative to the center of the ribbon in the corresponding RBP image, and then averaged the individual profiles separately for decrease and increase trials (Fig. 3E). In addition, we measured the x-y coordinates of the center of mass (c.m.) of ΔF in each of the 64 decrease images and 65 increase images, relative to c.m. of the corresponding ribbon. As the plot in Fig. 3F shows, sites of FM-dye increase clustered on the membrane-distal side of the ribbon (i.e., negative values of Δx; mean Δx ± SEM = −64 ± 10 nm), whereas sites of fluorescence decrease clustered on the membrane-proximal side (mean Δx ± SEM = 138 ± 9 nm). On the y axis (parallel to the membrane), 124/129 sites of ΔF clustered within ±150 nm of the c.m. of the ribbon (Fig. 3F), consistent with turnover of ribbon-associated vesicles. Overall, the various analyses of the locus of fluorescence changes indicate that vesicles composing the fast, phasic pool are those associated with the ribbon and docked at the plasma membrane. By contrast, although the membrane-proximal vesicles were released, replenishment occurred preferentially at the opposite pole of the ribbon, instead of where fusion occurred.
Locus of Calcium Influx.
The concentration of Ca2+ at the ribbon active zone should rise rapidly to high levels to drive fusion of synaptic vesicles at the speed achieved during the ultrafast component. A variety of lines of evidence indicate that Ca2+ channels cluster in the plasma membrane at ribbon locations in bipolar cell synaptic terminals (17–21), and Ca2+ influx has been shown to occur preferentially at ribbons (19, 22). Therefore, nanodomains of high Ca2+ at the ribbon are likely to be involved in triggering vesicle fusion (19, 22–25), a conclusion also supported by blockade of the fast component by 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA) but not EGTA (4). However, no measurements of Ca2+ changes elicited by opening voltage-gated Ca2+ channels have yet been made at ribbons on the temporal scale of the fast, transient component of release detected by capacitance measurements (Fig. 1). To provide this information, bipolar-cell terminals were filled via a whole-cell patch pipette with red-fluorescent RBP to mark ribbons and the calcium indicator Fluo-2LA to monitor changes in [Ca2+]i using rapid x-t line scans at ribbon and nonribbon locations, as illustrated in Fig. 4A. A scan line was defined perpendicular to the plasma membrane at a ribbon or at a nearby nonribbon location, extending from the extracellular space to the cytoplasmic region beyond the ribbon (Fig. 4A, Right), and the excitation laser was scanned along the line at rate of 1–2 ms per line. Under these conditions, the spatial resolution of Fluo-2LA fluorescence measurements is limited by the point-spread function of the microscope (FWHM ∼ 260 nm, determined by fitting a 2D Gaussian to images of single 27-nm fluorescent beads; see Fig. S2).
Fig. 4.
Time course of changes in Ca2+ elicited by 10-ms depolarization at a ribbon or at a nearby nonribbon location. (A) Overview of locations for rapid one-dimensional line scans at ribbon and nonribbon positions in the synaptic terminal of a zebrafish retinal bipolar neuron. (A, Left) A single confocal optical section through a terminal filled with red-fluorescent RBP to mark ribbon locations (spots). (A, Right) Close-up of region indicated by box on the left, showing the orientation of lines scanned repetitively to monitor fluorescence of Fluo-2LA at ribbon and nonribbon locations. (B) x-t plots showing the fluorescence intensity of Fluo-2LA along the lines indicated in A, as a function of distance (horizontal axis) and time (vertical axis). The extracellular space is the darker region at the right edge of each plot. For the ribbon location (right plot), a similar set of line scans of RBP fluorescence is shown at the bottom to indicate the position of the ribbon along the scanned line. Black trace in the middle shows timing of depolarization. (C) Spatially averaged Fluo-2LA fluorescence as a function of time at ribbon (filled circles) and nonribbon (open circles) locations. Data points show the average intensity (± SEM) in each horizontal row of pixels for three 10-ms depolarizations at ribbon and nonribbon locations. Fluorescence intensity is normalized with respect to the baseline fluorescence before stimulation, averaged over all pixels (i.e., over space and time). The arrow indicates the onset of the 10-ms depolarizing stimulus. The plateau level observed after termination of depolarization at both ribbon and nonribbon locations declined slowly to baseline, with an exponential time constant of 560 ± 47 ms (n = 6). (C, Inset) Average Ca2+ current (lower trace) elicited by 10-ms depolarization from −60 to 0 mV (upper trace).
Fig. 4B shows individual examples of Fluo-2LA fluorescence intensity vs. time (vertical axis) and distance (horizontal axis) at ribbon and nonribbon locations (defined in Fig. 4A), together with RBP fluorescence to indicate the position of the ribbon along the scanned line. Data points in Fig. 4C are the spatially averaged intensity in each scan line (i.e., horizontal row of pixels) across three 10-ms depolarizations (onset at arrow) at ribbon and nonribbon locations, and the inset shows the average Ca2+ current evoked by the depolarization. During the stimulus, fluorescence rose more rapidly and to a higher level at the ribbon than at the nonribbon location, indicating that the locus of Ca2+ influx is at the ribbon. After depolarization terminated, Ca2+ current rapidly deactivated (Fig. 4C, Inset), and Fluo-2LA fluorescence at the ribbon quickly collapsed to the same plateau level as at the nonribbon location, as expected given that after influx ceased, both positions should reflect the global level of Ca2+. These results provide evidence for a local domain of high Ca2+ driving rapid vesicle fusion specifically at the ribbon location.
Speed and Amplitude of Calcium Responses at Ribbons.
Although activation of Ca2+ current lasting 10 ms is sufficient to deplete the fast pool of vesicles, briefer depolarizations also trigger significant release (Fig. 1B). Therefore, we also examined Ca2+ responses triggered at ribbons by depolarizing pulses 1–5 ms in duration, corresponding to the rising phase of the fast capacitance response. x-t plots of individual responses (Fig. 5A) showed that Ca2+ rose abruptly in a narrow region just at the plasma membrane during a 1-ms depolarization, and with increasing duration of depolarization, the region of Ca2+ elevation spread distally from the membrane. However, the fluorescence of Fluo-2LA near the membrane appeared to reach a plateau with pulse durations of 3–5 ms, without further increase with longer influx. To examine this plateau in more detail, we measured the change in Fluo-2LA fluorescence within a region 254 nm wide (approximately the FWHM of the PSF; Fig. S2) at the membrane-proximal side of the ribbon, as an index of the change in Ca2+ in the region where vesicles composing the fast pool reside. As shown in Fig. 5B, the maximum value of ΔF/Frest achieved at the membrane in response to depolarization (dotted line) did not change with durations of 3, 5, and 10 ms, although the peak response with a 1-ms stimulus was smaller. In seven similar experiments, peak ΔF/Frest at the membrane, expressed relative to that for 10-ms depolarization, was 0.48 ± 0.05 for 1-ms pulses, 0.87 ± 0.08 for 3-ms pulses, and 0.98 ± 0.19 for 5-ms pulses (mean ± 95% confidence limit). This behavior suggests that activation of Ca2+ channels raises [Ca2+]i sufficiently to locally saturate Fluo-2LA at the site of influx within 3 ms after onset of depolarization.
Fig. 5.
Ca2+ responses elicited by brief depolarization at a synaptic ribbon. (A) Line scans were performed at a ribbon location in the manner shown in Fig. 4. x-t plots show Fluo-2LA fluorescence as a function of distance along the scanned line (vertical) and time (horizontal). Depolarizing voltage-clamp pulses of the indicated durations evoked Ca2+ influx, causing an increase in Fluo-2LA fluorescence. (B) Changes in Fluo-2LA fluorescence in proximity to the plasma membrane in response to depolarizations lasting 1, 3, 5, or 10 ms, color coded as indicated. Fluorescence for each line scan was measured in a region 9 pixels wide (254 nm) at the membrane, defined as the transition point between the lower fluorescence in the extracellular space and the higher fluorescence of the cytoplasm containing Fluo-2LA. Data are expressed as the change in fluorescence (ΔF) from baseline before stimulation divided by the baseline fluorescence (Frest). The dotted line indicates the plateau level of ΔF/Frest reached by all depolarizations longer than 3 ms.
To estimate how high [Ca2+]i rises during these saturated responses, we determined the half-saturating Ca2+ concentration for Fluo-2LA by measuring its fluorescence in buffered Ca2+ solutions (Fig. S3). Fitting the Hill equation to the results yielded an estimate of 5.6 µM for K1/2, which is reasonably consistent with the value of 6.7 µM provided by the manufacturer. Because depolarization for 3 ms was sufficient to nearly saturate Fluo-2LA, we conclude that [Ca2+]i likely exceeded 10 µM in the nanodomain of high Ca2+ at the ribbon within 3 ms after the onset of depolarization. One caveat here is that binding of Ca2+ indicators to cellular constituents can alter dye properties (26), so the estimate of [Ca2+]i derived from in vitro calibration should be regarded as tentative. Nevertheless, a high level of local [Ca2+]i would explain the rapid rate of fusion observed for the pool of vesicles underlying the fast, transient component of release at bipolar-cell ribbon synapses, and the localization of FM dye destaining at the membrane-proximal edge of the synaptic ribbon.
Discussion
We were able to image vesicle turnover and the underlying changes in Ca2+ at a single synaptic active zone with millisecond temporal precision and with spatial resolution limited by diffraction. The key feature that enables our experimental approach is the ability to mark the location of the active zone in a living ribbon synapse, while the presynaptic terminal is under voltage-clamp control. The latter allowed us to precisely control the activation of Ca2+ channels that drive release, and the former informed us where to apply high-resolution fluorescence imaging to monitor vesicle turnover and local Ca2+ responses. By looking at destaining of vesicles labeled with FM dye at an individual active zone rather than from the terminal as a whole, we uncovered stochastic fluctuations in the local population of labeled vs. unlabeled vesicles that, in turn, allowed us to separately determine the loci of vesicle fusion and vesicle replenishment at single ribbons.
We exploited these experimental advantages to determine the basis for the fast, transient component of neurotransmitter release, which is essential for the ability of retinal ribbon synapses to transmit information about contrast in visual stimuli (1). In particular, we showed that FM-dye destaining—reflecting vesicle fusion and neurotransmitter release—occurred preferentially at the membrane-proximal side of the synaptic ribbon during brief stimuli sufficient to exhaust the fast component of release. This pattern is consistent with selective release of the subgroup of ribbon-attached vesicles nearest the plasma membrane. By contrast, the addition of new vesicles to replace those lost during release of the fast component occurred preferentially on the side of the ribbon distal to the membrane. Overall, the spatial separation of release and refilling suggests a model in which vesicles that fuse at the base of the ribbon, near the membrane, are replaced by vesicles at more distal sites on the ribbon, freeing “slots” at those more distal sites for recruitment of vesicles to refill the total pool. In other words, the results are consistent with a “conveyor-belt” model (2, 27–29) for how the synaptic ribbon operates during neurotransmitter release.
We found that the fast subgroup of vesicles is released with a time constant of 2.3 ms after onset of depolarization, which implies that Ca2+ rises rapidly to high levels to drive this rapid vesicle fusion. To directly measure Ca2+ responses selectively at the active zone, we once again made use of the ability to label the ribbon active zone in living synapses to guide high-speed fluorescence measurements by using a low-affinity calcium indicator dye, Fluo-2LA (in vitro K1/2 ∼ 5.6 µM). During brief depolarization, Ca2+ rose more rapidly and to higher levels at ribbon locations compared with nearby regions lacking a ribbon, demonstrating that Ca2+ channels cluster near the ribbon. This finding is consistent with other types of evidence showing a high density of Ca2+ channels near ribbon-type active zones (18–25). Furthermore, even brief depolarization (3 ms) was sufficient to locally saturate the indicator dye in a diffraction-limited region near the plasma membrane. The diffraction limit prevents us from resolving the Ca2+ response within tens of nanometers of the membrane, which is the domain where docked vesicles reside, but because Ca2+ influx saturated the dye in a much larger region, the same would be true in the nanodomain near open Ca2+ channels. Because [Ca2+] in the range of 10–100 µM is necessary to saturate Fluo-2LA when measured in vitro, we conclude that [Ca2+]i near the membrane likely exceeded 10 µM—probably by a substantial amount—within 3 ms after opening Ca2+ channels.
Ribbon synapses mediate both fast, transient neurotransmission and slower, sustained release, and these distinct kinetic components are responsible for different aspects of their sensory function (1, 2). Here, we have demonstrated the basis for the fast component of release, the locus of vesicle replenishment to refill the fast pool, and the speed and amplitude of local Ca2+ changes that drive fast release at ribbon active zones.
Materials and Methods
Cell Isolation.
The procedure for isolating bipolar neurons from zebrafish retina was similar to that described (30). Briefly, retinas were dissected from eyecups, treated for 25 min with hyaluronidase (500 units/mL; Worthington Biochemical), transferred to saline containing 2.7 mM DL-cysteine HCl (EMD Biosciences), cut into small pieces, and incubated 15–40 min in saline containing DL-cysteine and 15–30 units/mL papain (Sigma) at 25 °C. A piece of retina was triturated via a flame-polished Pasteur pipette, and dissociated cells were plated onto glass-bottomed dishes for patch-clamp recording. Large-terminal bipolar neurons were identified by their characteristic morphology.
Patch Clamping and Imaging.
Pipette and bath solutions were similar to those used for goldfish and mouse (16, 30). The bipolar cell terminal was patch-clamped, and pipette solution containing fluorescently labeled ribbon binding peptide (RBP) was dialyzed into the cell to mark synaptic ribbons (13). Bipolar cells with leak current >20 pA were excluded. In some experiments, the pipette solution also contained the low-affinity version of the calcium indicator dye, Fluo-2 (Fluo-2LA; Teflabs). Activity-dependent labeling with FM4-64 dye (Biotium) was as described (16). All fluorescence images were acquired by using an Olympus FV-1000 laser-scanning confocal microscope controlled by FluoView software (Olympus). Both line scans with scan duration of 1–2 ms and XY clips with longer scan times were used. For the latter, square regions of interest of ∼1 μm × 1 μm were positioned at ribbons, which were identified by using RBP staining. Image acquisition was synchronized with patch-clamp data by exchange of transistor-transistor logic pulses between the imaging and patch-clamp computers. Timing was confirmed by using PatchMaster software (HEKA) to digitize the horizontal-scan synch pulse generated by the hardware of the confocal microscope, in parallel with the voltage-clamp data. Data analysis was initially performed in FluoView and PatchMaster, and data were then exported to Igor Pro, Excel, or ImageJ. Individual ΔF images for the data sets described in Fig. 3 were normalized to the maximum value of ΔF for that image before generating averaged images. Statistical significance was assessed by using two-tailed, unpaired t tests with unequal variance.
Supplementary Material
Acknowledgments
We thank Diane Henry-Vanisko and Wendy Akmentin (Stony Brook University) for expert technical assistance. This work was supported by National Institutes of Health Grant R01EY003821 (to G.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323962111/-/DCSupplemental.
References
- 1.Oesch NW, Diamond JS. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci. 2011;14(12):1555–1561. doi: 10.1038/nn.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci. 2010;11(12):812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Gersdorff H, Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994;367(6465):735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]
- 4.Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17(6):1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 5.von Gersdorff H, Matthews G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J Neurosci. 1997;17(6):1919–1927. doi: 10.1523/JNEUROSCI.17-06-01919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21(5):1177–1188. doi: 10.1016/s0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- 7.Burrone J, Lagnado L. Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J Neurosci. 2000;20(2):568–578. doi: 10.1523/JNEUROSCI.20-02-00568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrone J, Neves G, Gomis A, Cooke A, Lagnado L. Endogenous calcium buffers regulate fast exocytosis in the synaptic terminal of retinal bipolar cells. Neuron. 2002;33(1):101–112. doi: 10.1016/s0896-6273(01)00565-7. [DOI] [PubMed] [Google Scholar]
- 9.Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23(34):10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95(5):3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- 11.Snellman J, Zenisek D, Nawy S. Switching between transient and sustained signalling at the rod bipolar-AII amacrine cell synapse of the mouse retina. J Physiol. 2009;587(Pt 11):2443–2455. doi: 10.1113/jphysiol.2008.165241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snellman J, et al. Acute destruction of the synaptic ribbon reveals a role for the ribbon in vesicle priming. Nat Neurosci. 2011;14(9):1135–1141. doi: 10.1038/nn.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenisek D, Horst NK, Merrifield C, Sterling P, Matthews G. Visualizing synaptic ribbons in the living cell. J Neurosci. 2004;24(44):9752–9759. doi: 10.1523/JNEUROSCI.2886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouze NC, Schwartz EA. Continuous and transient vesicle cycling at a ribbon synapse. J Neurosci. 1998;18(21):8614–8624. doi: 10.1523/JNEUROSCI.18-21-08614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Alfonso T, Ryan TA. The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron. 2004;41(6):943–953. doi: 10.1016/s0896-6273(04)00113-8. [DOI] [PubMed] [Google Scholar]
- 16.LoGiudice L, Sterling P, Matthews G. Mobility and turnover of vesicles at the synaptic ribbon. J Neurosci. 2008;28(12):3150–3158. doi: 10.1523/JNEUROSCI.5753-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raviola E, Raviola G. Structure of the synaptic membranes in the inner plexiform layer of the retina: A freeze-fracture study in monkeys and rabbits. J Comp Neurol. 1982;209(3):233–248. doi: 10.1002/cne.902090303. [DOI] [PubMed] [Google Scholar]
- 18.Llobet A, Cooke A, Lagnado L. Exocytosis at the ribbon synapse of retinal bipolar cells studied in patches of presynaptic membrane. J Neurosci. 2003;23(7):2706–2714. doi: 10.1523/JNEUROSCI.23-07-02706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaumont V, Llobet A, Lagnado L. Expansion of calcium microdomains regulates fast exocytosis at a ribbon synapse. Proc Natl Acad Sci USA. 2005;102(30):10700–10705. doi: 10.1073/pnas.0501961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv C, Gould TJ, Bewersdorf J, Zenisek D. High-resolution optical imaging of zebrafish larval ribbon synapse protein RIBEYE, RIM2, and CaV 1.4 by stimulation emission depletion microscopy. Microsc Microanal. 2012;18(4):745–752. doi: 10.1017/S1431927612000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thoreson WB, Mercer AJ, Cork KM, Szalewski RJ. Lateral mobility of L-type calcium channels in synaptic terminals of retinal bipolar cells. Mol Vis. 2013;19:16–24. [PMC free article] [PubMed] [Google Scholar]
- 22.Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23(7):2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci. 2010;30(36):11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graydon CW, Cho S, Li GL, Kachar B, von Gersdorff H. Sharp Ca2+ nanodomains beneath the ribbon promote highly synchronous multivesicular release at hair cell synapses. J Neurosci. 2011;31(46):16637–16650. doi: 10.1523/JNEUROSCI.1866-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer JH, Lassová L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7(8):826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- 26.Baylor SM, Hollingworth S. Calcium indicators and calcium signalling in skeletal muscle fibres during excitation-contraction coupling. Prog Biophys Mol Biol. 2011;105(3):162–179. doi: 10.1016/j.pbiomolbio.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenzi D, von Gersdorff H. Structure suggests function: The case for synaptic ribbons as exocytotic nanomachines. BioEssays. 2001;23(9):831–840. doi: 10.1002/bies.1118. [DOI] [PubMed] [Google Scholar]
- 28.Parsons TD, Sterling P. Synaptic ribbon. Conveyor belt or safety belt? Neuron. 2003;37(3):379–382. doi: 10.1016/s0896-6273(03)00062-x. [DOI] [PubMed] [Google Scholar]
- 29.LoGiudice L, Matthews G. The role of ribbons at sensory synapses. Neuroscientist. 2009;15(4):380–391. doi: 10.1177/1073858408331373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidelberger R, Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol. 1992;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.