Significance
Early-generated subplate cells play important roles in development of the cerebral cortex. Although it has been assumed that most subplate cells are generated in the cortical germinative zone, here we describe an alternative site of neurogenesis, the rostromedial telencephalic wall. The rostromedial telencephalic wall gives rise to both subplate projection neurons and GABAergic interneurons. Both populations migrate tangentially to the cortex and strongly express the Lpar1-eGFP transgene. This unusual extracortical source of subplate projection neurons and interneurons is challenging the currently accepted scheme of cortical development.
Keywords: preplate, cell migration, pallium, projecting cells
Abstract
The subplate layer, the deepest cortical layer in mammals, has important roles in cerebral cortical development. The subplate contains heterogeneous cell populations that are morphologically diverse, with several projection targets. It is currently assumed that these cells are generated in the germinative zone of the earliest cortical neuroepithelium. Here we identify a pallial but extracortical area located in the rostromedial telencephalic wall (RMTW) that gives rise to several cell populations. Postmitotic neurons migrate tangentially from the RMTW toward the cerebral cortex. Most RMTW-derived cells are incorporated into the subplate layer throughout its rostrocaudal extension, with others contributing to the GABAergic interneuron pool of cortical layers V and VI.
The innermost cortical layer, the subplate, is located in apposition to the white matter and contains diverse cell populations of differing morphology, molecular properties, and connections (1–6). Although eventually separated by the cortical plate, the subplate and the Cajal-Retzius cells of the marginal zone are cogenerated at early stages of cortical development (7–9); thus, the subplate is a highly dynamic zone containing glutamatergic and GABAergic neurons and glial cells, as well as some migratory cells during early development (10, 11).
It is widely accepted that the earliest subplate cell populations arise from the cortical ventricular zone (VZ). Other telencephalic structures are formed by various cell types migrating into the telencephalon from different origins (12, 13). Moreover, the other preplate cell population, Cajal-Retzius cells, is generated primarily in a nearby extraneocortical structure, the cortical hem (14). We propose that subplate cells are also of multiple origins.
Here we describe a population of subplate neurons that derives from the germinative zone of the rostromedial telencephalic wall (RMTW). Taken together, our data demonstrate both multiple origins and ontogenetic heterogeneity of the early-born subplate cells.
Results
Three Different Subplate Cell Populations Arise in a Temporally and Spatially Specific Manner.
At embryonic day (E)10, some calretinin (CR) immunoreactive cells (CR+) are radially oriented between the VZ and the pial surface of the cortical neuroepithelium (14). Considering the extracortical origin of Cajal-Retzius cells, and the early stage of neurogenesis, we hypothesize that this CR+ cell population consists of subplate cells generated in the cortical VZ that migrate radially toward the pial surface to settle in the preplate layer.
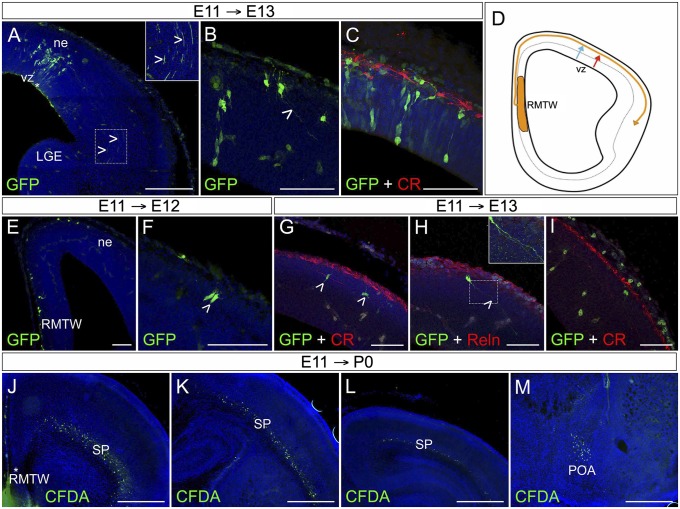
We also performed in utero GFP-retrovirus injections into the mouse pallial VZ at E11 (Fig. 1 A–C). At E13, GFP-labeled cells were radially aligned in an area between the injection site and the pia. Their axons extended throughout the nascent internal capsule (Fig. 1 A and B, open arrowheads), in agreement with previous reports identifying the first subcortically projecting cells in the neuroepithelium as subplate cells (15, 16). Given that those virally labeled, VZ-derived cells are not immunoreactive to CR (CR−) (Fig. 1C), we propose the existence of at least two subplate cell populations in the cortical neuroepithelium, generated at different times: E10 CR+ and E11 CR− subplate cells (Fig. 1D).
Fig. 1.
Both cortical neuroepithelium and RMTW generate cortical subplate cells. (A–C) GFP+ cells in the cortical neuroepithelium (ne) at E13, after in utero ultrasound-guided GFP retrovirus injection at E11 into the VZ (asterisk). Some GFP+ cells extended axons into the nascent internal capsule (open arrowheads in A and B). The GFP+ cell population is distinct from the CR-expressing Cajal-Retzius cells (red in C). (D) Schematic showing the three types of migratory routes followed by different SP cell populations: RMTW-derived cells (yellow line), CR cortical neuroepithelium-derived cells (blue line), and CR+ cortical neuroepithelium-derived cells (red line). (E–I) Ultrasound-guided in utero GFP retrovirus injection into the RMTW at E11 analyzed at E12 (E and F) and at E13 (G–I). Labeled cells (green) take a dorsal pathway (E) and are located in the preplate at E13 (G–I). GFP+ cells display a variety of cell morphologies, including pear-shaped (F, open arrowhead), bipolar, and fusiform morphologies (G, open arrowheads). Some are projecting cells (H, open arrowhead and Inset) that do not express Reln. At E13, the GFP-labeled cells do not express CR (G and I), but some CR+ (presumed non-RMTW origin) cells also have projections (I). (J–M) Ultrasound-guided in utero injection of CFDA into the RMTW at E11, analyzed at birth (P0). The RMTW-derived cells colonize the SP area with a rostrocaudal gradient (J–L), as well as the POA (M). To delineate the slice morphology, 40-µm-thick coronal sections were counterstained with bizbenzimide (blue); midline is left, and dorsal is up. (Scale bars: 25 µm in B, C,G, H, and I; 50 µm in A; 100 µm in E; 500 µm in J–M.)
We next performed ultrasound-guided in utero injections of carboxyfluorescein diacetate (CFDA) or GFP retroviruses into the RMTW at E11 (Fig. 1 E–M). The RMTW is a medial extracortical pallial structure, dorsal to the septum and rostral to the corticalhem. We studied the distribution of E11-labeled cells at E12 (Fig. 1 E and F), E13 (Fig. 1 G–I), and P0 (Fig. 1 J and M). Cells infected with the GFP retrovirus at E11 were located in the preplate at E12 or in the subplate at E13. These RMTW-derived cells migrate dorsally and caudally to colonize the preplate along the rostrocaudal axis (Fig. 1 E–I). By P0, CFDA-labeled cells were identified in the subplate, throughout the entire rostrocaudal extent of the telencephalon (Fig. 1 J–L). In addition, CFDA-labeled cells were identified in the preoptic area (POA) (Fig. 1M). At E13, some RMTW-derived subplate cells formed long axonal projections extending toward the nascent internal capsule (Fig. 1H, open arrowhead and Inset). RMTW-derived cells in the lower stratum of the preplate did not express CR (Fig. 1G). Most labeled cells were fusiform or bipolar with a tangential orientation (Fig. 1G), characteristic of migrating undifferentiated cells, but pear-shaped cells were observed as well (Fig. 1F).
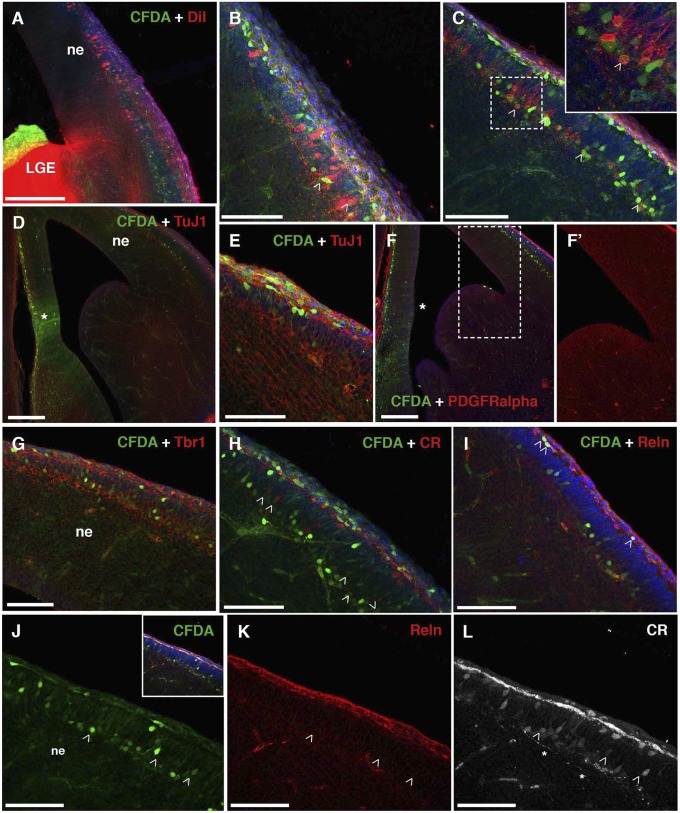
Although the preplate split is not complete at E13, we observed a clear separation of RMTW-derived CFDA+ cells between deeper and superficial strata (Fig. 2). Some cells in the upper stratum were colabeled with Reelin (Reln) and CR (Fig. 2 H–K) and may correspond to Cajal-Retzius cells. At E18, when the preplate split was complete, RMTW-derived cells were located in a band below the cortical plate, in the location of the subplate (Fig. S1), and persisted at P0 (Fig. 1 J–L) and at P8 (Fig. 3 and Fig. S2). At both E18 and P8, some RMTW-derived cells expressed complexin 3 (Cplx3; Figs. S1F and S2A), a synaptic protein and marker of early-born subplate neurons in the mouse (17).
Fig. 2.
Characterization of the RMTW-derived cells at E13. (A–C) E13 brains after in utero CFDA injections into the RMTW at E11. CFDA-labeled, fixed E13 brains were also labeled with DiI placed into the pallial–subpallial border or internal capsule (A). Cells double-labeled with DiI (red) and CFDA (green) in the cortical neuroepithelium (ne) are shown (B and C, open arrowheads and Inset). (D–L) RMTW-derived cells (green) were characterized immunohistochemically in the E13 brain. The CFDA injection area is marked with an asterisk (D and F). CFDA-labeled cells express the neuronal marker TuJ1 (red; D and E) and the pallial marker Tbr1 (red; G) in the cortical neuroepithelium (ne). Expression of the glial marker PDGFRα (red; F and enlargement in F’) is absent from the preplate (F’), and CFDA-labeled, RMTW-derived cells do not coexpress this marker. Some CDFA-labeled, RMTW-derived cells express CR (arrowheads in H and L). CR111+, RMTW-derived cells are located in both the outermost and innermost (L) zone of the neuroephithelium (ne) in regions where the preplate has split. Reln expression is restricted to the outermost layer of the neuroepithelium (I and K), corresponding to the marginal zone. All CFDA-labeled, RMTW-derived cells in the marginal zone are Reln+ at E13 (I). Shwon are 40-µm coronal sections; midline is left, and dorsal is up. Slice morphology is delineated with bisbenzimide (blue). (Scale bars: 25 µm in B, C, E, and H–L; 50 µm in G; 100 µm in A, D, and F.) LGE, lateral ganglionic eminence.
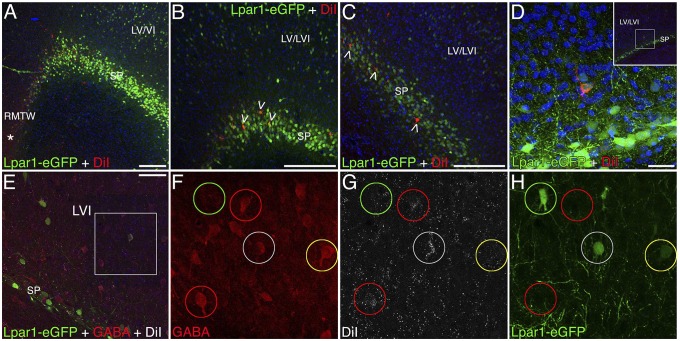
Fig. 3.
Characterization of the RMTW-derived cells at P8. In utero DiI injection at E11 into the RMTW of Lpar1-eGFP mouse brains labels cells in the SP and in layers V/VI at P8 (red in A–C; white in E–H). The distribution of Lpar1-eGFP+ cells (green) in the transgenic mouse mimics that of RMTW-derived DiI+ cells at P8. (A–C) Lpar1-GFP+ cells are located in the SP and in the infragranular layers at P8. In A, an asterisk denotes the DiI injection area. Some of the RMTW-derived cells express Lpar1-eGFP in the SP (open arrowheads in B and C) and in cortical layers V and VI (D). (E–H) Some of the RMTW-derived, DiI-labeled cells (white) express GABA (red) and Lpar1-eGFP (green) in infragranular layers (white circles). Other RMTW-derived cells express GABA, but not Lpar1-eGFP (red circles). Some cells express Lpar1-eGFP and GABA but are not labeled with DiI (yellow circles), and some cells are labeled only with Lpar1-eGFP. Cortical cytoarchitecture is revealed by the counterstain bisbenzimide (blue). F–H are enlarged views of the boxed area in E. Coronal sections are 40 µm thick; midline is left, and dorsal is up. (Scale bars: 100 µm in A; 50 µm in B and C; 25 µm in D and E.)
The RMTW region also gave rise to cells eventually residing in the postnatal cortical layers V and VI (Fig. 3 and Fig. S2), as assessed with E11 GFP retroviral, CFDA or 1,1′-dioctadecyl-3,3,3′,3′-tetra-methyl-indocarbocyanine perchlorate (DiI) tracing, as well as the POA (Fig. 1M).
RMTW-Derived Subplate Cells Are Generated at E10 and E11.
Subplate neurons are generated at E11 and E12 (6), with a small proportion of CR+ subplate cells generated at E10 (14). BrdU birthdating in combination with ultrasound-guided in utero injections of cell trackers into the RMTW region (Fig. S3) demonstrated that some RMTW-derived cells in the subplate area were generated at E11 (44 ± 13% analyzed at P0 and 43 ± 10% analyzed at P8). No RMTW-derived cells were generated at or beyond E12. RMTW-derived cells subsequently located in cortical layers V and VI were cogenerated with the subplate cells at E11 (54 ± 13%).
Regions Adjacent to RMTW Generate Nonsubplate Cells.
To identify the boundaries of the specific RMTW region that gives rise to subplate cells, and to avoid contamination from adjacent proliferative compartments, we performed focal CFDA injections at E11 into several structures adjacent or close to the RMTW: septum, POA, and medial, lateral, and caudal ganglionic eminences (Fig. S4). Distribution of CFDA-labeled cells was analyzed at E13, as was done for RMTW-derived cells. Neither the VZ of the septum nor the medial ganglionic eminence give rise to any cells that reached the cerebral cortex by E13 (Fig. S4 A and B). Slightly later in development, the ganglionic eminences will give rise to interneurons that reach the cortical neuroepithelium by tangential migration (18). In contrast, the lateral and caudal ganglionic eminences and POA gave rise to a small number of cells that colonized the neocortical mantle (Fig. S4 C and D), but their distribution was limited to places next to the pallial–subpallial boundary (Fig. S4 C and D, dashed lines, and enlargements in Fig. S4 C′ and D′).
RMTW-Derived Subplate Cells Are Projection Neurons.
Because glial cells can be derived from medial areas of the telencephalic wall (19–21), we performed immunohistochemistry for the neuronal marker TuJ1 and the glial marker PDGF receptor alpha (PDGFRα) at E13 to confirm that RMTW-derived cells are neurons. Tracer injections into RMTW were carried out at E11. All CFDA-labeled cells were positive for TuJ1 (Fig. 2 D and E), whereas no PDGFRα immunoreactive cells were seen in the cortical neuroepithelium (Fig. 2 F and F′). At E13, some CFDA+ subplate cells in the innermost stratum of the splitting preplate (Fig. 2 H and L) were colabeled with CR. By E18, none of the CR+ cells in the subplate were labeled after electroporation of a GFP-vector into the E11 RMTW (Fig. S3I). In contrast, GFP retrovirally labeled, RMTW-derived cells in the E18 subplate colocalized with the subplate marker complexin 3 (Cplx3) (Fig. S1F). Furthermore, at P8, some RMTW-derived, CFDA-labeled cells located in the subplate were colabeled by CTGF and Nurr1 and expressed Cplx3 (Fig. S2, solid arrowheads).
Because subplate cells are the first cortical neurons to form subcortical projections, specifically to the mid-internal capsule at E14 in the rat (16), we further analyzed whether the RMTW-derived subplate cells project beyond the cortex in the mouse. As before, tracer injections into RMTW were made at E11, and projections were analyzed at E13. DiI crystals were placed into the pallial–subpallial boundary of fixed brains. The presence of CFDA+DiI+ double-labeled subplate cells (Fig. 2 A–C) suggests that some RMTW-derived cells extend processes into or through the pallial–subpallial boundary by E13.
RMTW-Derived Cells Are Not Restricted to the Subplate.
RMTW-derived cells colonize the preplate and are present in both the marginal zone and the subplate after they split. Some CFDA-labeled, RMTW-derived cells in the marginal zone of the E13 cortex expressed Reln (Fig. 2 I and K), and some of these cells also expressed CR (Fig. 2 H and L), suggesting that they are Cajal-Retzius cells.
Survival until E13 does not allow identification of the contribution of RMTW-derived cells to the cortical plate, because the marginal zone, cortical plate, and subplate distinctions are not apparent at these early stages. For this purpose, plasmids pPB-Ubc-EGFP and mPBase (transposase) were coelectroporated in utero into the RMTW at E11. Brains were examined at E18 to evaluate whether the RMTW contributes to nonpreplate cells in the neocortex (Fig. S1). Whereas most GFP+ cells were confined to the subplate area, with some in the marginal zone, an additional RMTW-derived cell population was dispersed in the infragranular layers (Fig. S1 F and I). We established the neuronal character of these GFP+ cells by immunohistochemistry for NeuN, Tbr1, and TuJ1 (Fig. S1 A–D). GFP+ cells expressed neither the glial marker PDGFRα (Fig. S1E) nor calbindin (Fig. S1 G and H). The marginal zone contained Reln+GFP+ cells (Fig. S1 K–M) and CR+GFP+ cells (Fig. S1 J and M), some of which were triple-labeled (GFP+CR+Reln+; Fig. S1M).
Non-subplate, RMTW-derived cells in the infragranular layers did not coexpress the subplate markers CTGF or Cplx3 at P8 (Fig. S2 A and B). In the lateral cortex, Nurr1+ cells were not restricted to the subplate but are also distributed throughout the deep layers; however, Nurr1+ cells outside of the subplate were not CFDA-labeled (Fig. S2C).
RMTW-Derived Cells Spatially Overlap the Distribution of Lpar1-eGFP Cells.
The distribution of Lpar1-eGFP positive cells in the Tg(Lpar1-EGFP)GX193Gsat transgenic mouse (6) resembles that of CFDA-labeled, RMTW-derived cells in the neocortex. Lpar1-eGFP labels cells in the embryonic preplate or its derivative structures the subplate and marginal zone, as well as a population of GABAergic interneurons in cortical layers V and VI. By P8, strong GFP labeling is restricted to subplate cells, with fainter GFP+ cells present in the deep cortical layers (6). In addition, Lpar1-eGFP subplate neurons are generated preferentially on E11 (6), and some GFP+ cells can be identified in the VZ of the RMTW-area at E11.
DiI was injected (in utero) into the RMTW of Lpar1-eGFP embryos at E11. At P8, DiI-labeled cells were restricted to the subplate and cortical layers V and VI (Fig. 3; POA not analyzed). Colocalization of Lpar1-eGFP and DiI-labeling suggests the existence of RMTW-derived, Lpar1-eGFP+ cells (Fig. 3 A–D). Approximately one-half of the nonsubplate Lpar1-eGFP+ cells are immunoreactive for GABA (6). GABA immunohistochemistry revealed colocalization of GABA and DiI (Fig. 3 E–H, red and white circles) in the infragranular layers, but not in the subplate (Fig. 3E). Some GABAergic, RMTW-derived interneurons in cortical layers V and VI also expressed Lpar1-eGFP (Fig. 3 E–H, white circles). As reported previously, not all Lpar1-eGFP+ cells in layers V and VI were GABA+ (Fig. 3 E–H, green circles), and not all Lpar1-eGFP+GABA+ cells were DiI+ (Fig. 3 E–H, yellow circles).
Discussion
We have demonstrated that the subplate is a cell population of diverse telencephalic origins. We have identified the RMTW as a further pallial but extracortical (22) origin of subplate cells and also confirmed that the VZ of the cortical neuroepithelium is an area of subplate cell generation. In addition, we have identified differences in marker expression among cortical VZ-derived subplate cells. Some RMTW-derived subplate cells are projection neurons and coexpress the subplate markers CTGF, Cplx3, Nurr1, and Lpar1-eGFP. The RMTW also gives rise to cells in the marginal zone and in cortical layers V and VI; some of the latter cells are GABAergic, along with previously described cells destined for the olfactory cortex (12). All RMTW-derived cells, including the projection neurons, reach their final destination by tangential migration.
Extracortical Origin of Murine Subplate Cells.
The neocortical subplate is thought to be derived largely from the cortical VZ, although the vast majority of brain structures are formed by cells from multiple origins. In fact, the germinative zone of cortical interneurons is situated in the subpallium (18, 23, 24), whereas the olfactory cortex receives cell contributions from multiple telencephalic structures (12, 25). The diverse cell populations that contribute to a given structure reach their final targets by both radial migration and tangential migration. Tangential migration allows cell movements for longer distances, even across compartmental boundaries, as in the case of ganglionic eminence-derived cortical interneurons. In this sense, the longest tangential migratory route described to date is from the hypothalamic nucleus to the telencephalic amygdaloid nuclei, which involves long trajectories across two embryonic vesicles, diencephalon to telencephalon (13). These examples emphasize the importance of cellular ontogenetic heterogeneity in the formation of different cerebral structures.
The RMTW is also known as the pallial septum (26), which becomes the anterior commissural plate in adults. We previously described this structure as the origin of two different cell populations that follow tangential but divergent migrations, dorsal and ventral (12), to colonize the olfactory cortex at the preplate stage. In that earlier study we used in toto embryo cultures, in which cell migration can be followed for only up to two days. Thus, it was not possible to identify the dorsal migratory route as giving rise to subplate neurons. Here, using in utero ultrasound-guided injections of cell tracers (CFDA, DiI) or viral GFP constructs, we could follow labeled cells into the postnatal period, allowing us to identify subplate and deep cortical plate cell populations derived from the RMTW.
At early developmental stages, the RMTW is extremely close to the septal areas and POAs, areas that have been reported to generate cells that migrate toward the cortical epithelium. Our data show that neocortical RMTW-derived cells follow only a dorsal migratory pathway, whereas injections into the septum label ventral migratory streams. Pax-6 mutant mice exhibit loss of the dorsal migratory pathway for cells derived from the medial telencephalic wall (27). Perhaps the lack of this dorsal migratory stream explains the smaller subplate cell population in Pax-6 mutants (28) and, consequently, a defect in the formation of thalamocortical and corticothalamic connections (29).
Although subplate cells are reportedly generated between E10 and E12 in mice (6), we observed that the generation of the RMTW-derived subset was restricted to E10/E11. We demonstrated that approximately one-half of the RMTW-derived subplate cells underwent their final cell division at E11, and, in combination with the absence of any dorsal migration on or after E12, we can extrapolate that the remaining one-half of RMTW-derived subplate cells should be generated before E11. After delivery of a BrdU pulse at E11, we also observed RMTW-derived cells in cortical layers V and VI. Thus, we propose that these cortical plate cells are cogenerated with the RMTW-derived subplate cells.
According to some previously reported data, subplate cells disappear in the first postnatal week (30); however, others have reported the presence of cells in the subplate at later ages (6, 31), and many of the molecular labels of the subplate layer persist in a band of cells between the white matter and cortical layer VI into adulthood (5). We provide evidence of the continued presence of a population of cells in the subplate layer derived from the RMTW until at least P8. Our results are in complete agreement with a preferential survival of early born subplate cells that are Lpar1-eGFP+ (6), and further strengthen the link between RMTW-derived and Lpar1-eGFP+ cells.
Subplate Phenotypic Heterogeneity Correlates with Ontogenic Heterogeneity.
The subplate is a heterogeneous cell population (6, 17, 32, 33), with the wide variety of cell types suggesting multiple origins (34). Indeed, we have shown that different subplate cell populations are generated in both cortical and extracortical areas.
The RMTW Germinal Zone Gives Rise to Distinct Cell Populations.
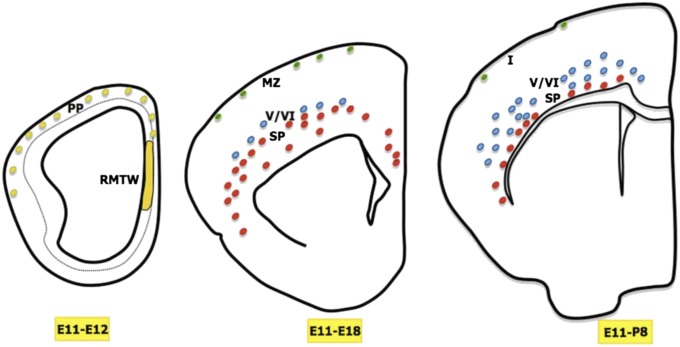
To date, five distinct cell populations arising from the RMTW region have been described: a small population of Cajal-Retzius cells, two highly distinct populations that colonize subplate and infragranular layers (Fig. 4), POA cells, and the cells in the olfactory cortex (12). On a technical note, the Cajal-Retzius cells located in the marginal zone could have been labeled by tracer spread to the rostral cortical hem; however, Cajal-Retzius cells generated in that region are expected to colonize only the rostral part of the telencephalon (27), whereas we observed labeled Cajal-Retzius cells throughout the entire rostrocaudal extent of the neocortex.
Fig. 4.
Summary schematic, representing coronal hemisections at three developmental stages (E12, E18, and P8) when different cell populations arise from the RMTW at E11. At E12, the RMTW-derived cells populate the preplate (PP) along its entire extension. At E18, after the preplate split, three different cell populations are evident: SP cells (red circles), infragranular layer cells (blue circles), and Cajal-Retzius cells (green circles) in the marginal zone (MZ). At P8, there is an increase in the number of cells in cortical layers V and VI and a reduction in the number of cells in the marginal zone.
Pallial Origin of Cortical GABAergic Cells.
Telencephalic cells are generated at various distinct locations and reach their final destinations through radial and tangential migrations (12). It is generally accepted that all cortical GABAergic interneurons are generated in the subpallial ganglionic eminences and reach the cortical mantle by tangential migration (35), whereas cortical projecting cells are generated in the VZ of the neuroepithelium during the same period and reach their final strata by radial migration (34). Our results indicate that RMTW is a unique area that gives rise to both projection neurons and interneurons, both of which subsequently migrate tangentially. This is not the first time that the existence of a pallial-derived cortical interneuron population has been proposed based on in vitro studies, however (36).
Our observations may be further supported by recent descriptions of GABAergic cells in the cerebral mantle early in the postnatal period (37–39). These interneurons are generated at early embryonic stages, reach the cortex by tangential migration, and, after some waiting period in the deep layers of the cortex, migrate radially upward to reach their final laminar destination. In agreement with this idea, we observed an increase in the number of nonsubplate cells in the cortical infragranular layers at postnatal stages, despite evidence suggesting these cells are generated in the RMTW at early developmental stages (Fig. 4).
Tangential Migration of the RMTW-Derived Projecting Cells.
Subplate cells are one of the earliest-generated cell populations in the telencephalon (7), with important roles in cortical development and the establishment of cortical projections, both intracortical (40, 41) and subcortical, toward the thalamus (15). In fact, a subpopulation of this type of cells was described as the first efferent projection from the developing cortical neuroepithelium (16) and the majority of subplate cells are thought to be projecting cells (15, 16). RMTW is a previously unidentified source of subplate cells, and we demonstrate that RMTW-derived cells extend processes to or across the pallial–subpallial boundary by E13. Whereas the pallial origin of these projecting subplate cells was an expected finding, we were surprised to find that they reach the subplate by tangential migration, given that classically projecting cells are thought to migrate radially.
Our findings in this study emphasizesthe importance of tangential migration for the first cohorts of neurons of the cerebral cortex and open up new directions for the study of early cortical circuit formation. Moreover, in disease models of disorders of tangential migration, the integrity of the subplate merits a closer look.
Methods
Animals.
Embryos (n = 184) were obtained from WT C57/Bl6 mice and transgenic Lpar1-GFP [Tg(Lpar1-EGFP)GX193Gsat, with a NIHS background] pregnant mice (n = 25; Table S1), raised at the in-house colony at Instituto Cajal. The day of detection of the vaginal plug was considered embryonic day 0 (E0), and the day of birth was considered E19 or postnatal day 0 (P0). All animal handling and experimental protocols were in compliance with Spanish legislation (R.D. 1201/2005 and Law 32/2007) and the Guidelines of the European Union Council (2003/65/CE) for the care and use of experimental animals, and were approved by the Animal Care and Use Committee of Instituto Cajal.
Intrauterine Experiments.
For labeling newly generated cells in specific regions of the developing forebrain, embryos were injected in utero with cell tracers, aided by an ultrasound device (VeVo 770; VisualSonics). In brief, E11–E13 pregnant mice were anesthetized with isoflurane (Isova Vet, 240055; Centauro), and their uterine horns were exposed and covered with prewarmed ultrasound gel (Parker Laboratories). A total volume of 23 nL of CFDA, GFP retrovirus, or DiI solution was injected into the desired areas of each embryo. Alternatively, 1–2 µL of pPB-Ubc-EGFP and mPBase (transposase), kindly provided by A. Bradley (Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK) (42), was injected into the lateral ventricle, followed by electroporation accomplished by 5 pulses (50 ms each) discharging a 500-µF capacitor charged to 25 V with a sequencing power supply. The voltage pulse was discharged across a pair of platinum round plates (5 mm diameter; CUY650P5; Nepagene) positioned on either side of the head of each embryo within the uterus. Uterine horns were returned into the abdominal cavity, and the antibiotic enrofloxacin (Baytril 5 mg/kg; Bayer) and the anti-inflammatory ketorolac (Droal, 300 μg/kg; Vita Laboratories) were administered to the dams. At the appropriate embryonic age (E13 or E18), the pregnant mice were anesthetized with Equithesin (3 mL/kg body weight), and the embryos were removed by cesarean section. Table S1 summarizes the injection times, injection sites, tracers used, and postinjection survival times.
Tracers.
The following tracers were used: tiny crystals or a 2.5% solution in DMSO of DiI (Molecular Probes), 10 mM solution of carboxy-fluorescein diacetate succinimidyl ester (CFDA SE, 557 molecular weight; Molecular Probes) in DMSO, and GFP retrovirus.
Retrovirus Synthesis.
To stably transfect dividing cells with enhanced green fluorescent protein (eGFP), Moloney murine leukemia-derived retroviral vectors were used with the pCL-eco packaging vector and pBabe IRES-eGFP (donated by Susana Gonzalo, Saint Louis University, St. Louis, MO) (43). Viruses were produced by transient cotransfection of the two plasmids into the 293-T-cell line in Gibco Opti-MEM reduced serum medium (Life Technologies) and using the FuGene HD transfection reagent (Roche; 04 709 705 001) at a ratio of 8 μL of FuGene HD reagent to 2 μg of each vector. The supernatant from transfected 293-T cells containing the retrovirus was collected after 12 and 24 h, concentrated twice at 45,000 × g for 2 h at 4 °C, and then stored at −80 °C in PBS with 10% BSA. The viral titer was determined at 3 d after infection of 3T3 cells by flow cytometry, based on eGFP expression. The transducing units obtained ranged from 0.4 × 108 to 2.6 × 108/mL.
Immunohistochemistry.
Single and double immunofluorescence was performed to characterize tracer-labeled cells in 4% paraformaldehyde-fixed brains. Primary antibodies were diluted in 0.1 M PBS with Tween containing 5% normal goat serumand 0.1% BSA, and sections were incubated with primary antibody overnight at 4 °C. After rinsing with PBS with Tween, sections were incubated with the secondary antibodies diluted in the same blocking solution for 2 h at room temperature. Sections were counterstained with bizbenzimide. For all antibodies, a series of control sections were stained with the primary antibody omitted, and no nonspecific staining of secondary antibodies was observed.
The following primary antibodies were used: rabbit anti-PDGFRα (Sc388, 1:300 dilution; Santa Cruz Biotechnology), mouse anti-NeuN (MAB377, 1:300; Chemicon), rabbit anti-Complexin3 (Cplx3; 16949-1-AP, 1:100; ProteinTech), rabbit anti-Cplx3 (122-302; Synaptic Systems), rabbit anti-GABA (A-2052, 1:1,000; Sigma-Aldrich), mouse anti-Reln (MAB5364; clone G10, 1:1,000; Chemicon), rabbit anti–Calbindin-D28K (CB38, 1:10,000; Swant); rabbit anti-CR antiserum (7699/4, 1:2,000; Swant); mouse anti–β-tubulin class III (TuJ1; MAB1637, 1:1,000; Chemicon); rabbit anti-Tbr1 (AB9616, 1:1,000; Chemicon); rat anti-GFP (04404-84, 1:2,000, Nacalai Tesque); mouse anti-BrdU (G3G4 IgG1, kappa light chain isotype, developed by S. J. Kaufman and obtained from the Developmental Studies Hybridoma Bank; 1:2,000). The subplate markers CTGF (goat anti-CTGF; sc-14939, 1:500; Santa Cruz Biotechnology) and Nurr1 (goat anti-Nurr1, AF2156, 1:100; R&D Systems) were also labeled for, but because the blocking solution used contained 5% normal donkey serum instead of goat serum, no BSA and Triton X-100 were used (1% for CTGF; 0.2% for Nurr1) instead of Tween. Donkey anti-goat secondaries antibodies were used, with sections counterstained with DAPI.
The following secondary antibodies were used: Alexa Fluor 568 goat anti-rabbit IgG (A11011, 1:2,000; Molecular Probes), Alexa Fluor 488 goat anti-rabbit IgG (A11008, 1:2,000; Molecular Probes); Alexa Fluor 568 anti-mouse IgG (A11004, 1:2,000; Molecular Probes); Alexa Fluor 488 goat anti-mouse IgG (A11001, 1:2,000; Molecular Probes); Alexa Fluor 488 anti-rat (A11034, 1:2,000: Molecular Probes); Alexa Fluor 568 donkey anti-rabbit (A11057, 1:500; Molecular Probes); Alexa Fluor 633 streptavidin (1:2,000; Molecular Probes), biotinylated goat anti-rabbit IgG (BA-1000, 1:2,000; Vector Laboratories); biotinylated goat anti-mouse IgG (BA-9200; 1:2,000; Vector Laboratories;).
Birthdating Studies.
Timed-pregnant mice (E11, E12, and E13) were injected with a single i.p. injection (50 mg/kg body weight) of 5-bromo-2′-deoxyuridine-5′-monophosphate solution dissolved in sterile 0.1 M Tris⋅HCl buffer (BrdU; Boehringer Mannhein). Postnatal animals of the desired age (P0 or P8) were transcardially perfused with 4% paraformaldehyde and postfixed overnight. Then 40-µm-thick coronal sections were cut on a vibratome, and the DNA was denatured with 2N HCl for 60 min at 37 °C and then for 10 min in 0.1 M borate buffer (pH 8.5 at room temperature) to neutralize the residual acid. Anti-BrdU immunohistochemistry was performed as described above, using mouse monoclonal anti-BrdU (0.25 μg/mL; Roche Applied Science).
Image Acquisition.
Whole brain fluorescent images were acquired using a fluorescent dissecting microscope (Leica MZFL-III) and either a fluorescent microscope (Nikon Eclipse E600) equipped with a digital camera (Nikon DMX 1200F) or a spectral confocal microscope (Leica TCS 4D). All photographs were adjusted equally for contrast and brightness using Adobe Photoshop CS7.
Colocalization Analysis.
The percentage of cells colabeled with different markers was quantified (n = 4 animals) in serial confocal images obtained every 2 μm from the 40-μm-thick sections (representative of the rostral, medial, and caudal zones) of each animal, using the Cell Calculator plugin (University of Sheffield) for ImageJ software.
Supplementary Material
Acknowledgments
We thank Ray Guillery and Laura López-Mascaraque for their valuable coments on the manuscript, and Sandra Rodriguez and Raul Nuñez for their excellent technical assistance. This work was supported by the Spanish Ministerio de Ciencia e Innovación (Grant BFU2010-21377) and by the Medical Research Council of the United Kingdom (Grant G00900901).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8325.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323816111/-/DCSupplemental.
References
- 1.Liu N, Baker H. Activity-dependent Nurr1 and NGFI-B gene expression in adult mouse olfactory bulb. Neuroreport. 1999;10(4):747–751. doi: 10.1097/00001756-199903170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Arimatsu Y, Ishida M, Kaneko T, Ichinose S, Omori A. Organization and development of corticocortical associative neurons expressing the orphan nuclear receptor Nurr1. J Comp Neurol. 2003;466(2):180–196. doi: 10.1002/cne.10875. [DOI] [PubMed] [Google Scholar]
- 3.Heuer H, et al. Connective tissue growth factor: A novel marker of layer VII neurons in the rat cerebral cortex. Neuroscience. 2003;119(1):43–52. doi: 10.1016/s0306-4522(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 4.Schneider S, Gulacsi A, Hatten ME. Lrp12/Mig13a reveals changing patterns of preplate neuronal polarity during corticogenesis that are absent in reeler mutant mice. Cereb Cortex. 2011;21(1):134–144. doi: 10.1093/cercor/bhq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoerder-Suabedissen A, et al. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex. 2009;19(8):1738–1750. doi: 10.1093/cercor/bhn195. [DOI] [PubMed] [Google Scholar]
- 6.Hoerder-Suabedissen A, Molnár Z. Molecular diversity of early-born subplate neurons. Cereb Cortex. 2013;23(6):1473–1483. doi: 10.1093/cercor/bhs137. [DOI] [PubMed] [Google Scholar]
- 7.Luskin MB, Shatz CJ. Neurogenesis of the cat’s primary visual cortex. J Comp Neurol. 1985;242(4):611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- 8.Chun JJ, Shatz CJ. The earliest-generated neurons of the cat cerebral cortex: characterization by MAP2 and neurotransmitter immunohistochemistry during fetal life. J Neurosci. 1989;9(5):1648–1667. doi: 10.1523/JNEUROSCI.09-05-01648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Exp Neurol. 1990;107(1):48–62. doi: 10.1016/0014-4886(90)90062-w. [DOI] [PubMed] [Google Scholar]
- 10.Hevner RF, Zecevic N. In: Development and Plasticity in Sensory Thalamus and Cortex. Erzurumlu RS, Guido W, Molnár Z, editors. New York: Springer; 2006. pp. 1–17. [Google Scholar]
- 11.Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- 12.García-Moreno F, López-Mascaraque L, de Carlos JA. Early telencephalic migration topographically converging in the olfactory cortex. Cereb Cortex. 2008;18(6):1239–1252. doi: 10.1093/cercor/bhm154. [DOI] [PubMed] [Google Scholar]
- 13.García-Moreno F, et al. A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei. Nat Neurosci. 2010;13(6):680–689. doi: 10.1038/nn.2556. [DOI] [PubMed] [Google Scholar]
- 14.García-Moreno F, López-Mascaraque L, De Carlos JA. Origins and migratory routes of murine Cajal-Retzius cells. J Comp Neurol. 2007;500(3):419–432. doi: 10.1002/cne.21128. [DOI] [PubMed] [Google Scholar]
- 15.McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245(4921):978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- 16.De Carlos JA, O’Leary DDM. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992;12(4):1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoerder-Suabedissen A, et al. Expression profiling of mouse subplate reveals a dynamic gene network and disease association with autism and schizophrenia. Proc Natl Acad Sci USA. 2013;110(9):3555–3560. doi: 10.1073/pnas.1218510110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Carlos JA, López-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16(19):6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivier C, et al. Monofocal origin of telencephalic oligodendrocytes in the anterior entopeduncular area of the chick embryo. Development. 2001;128(10):1757–1769. doi: 10.1242/dev.128.10.1757. [DOI] [PubMed] [Google Scholar]
- 20.He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21(22):8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Lopez R, Martínez S. Oligodendrocyte precursors originate in the parabasal band of the basal plate in prosomere 1 and migrate into the alar prosencephalon during chick development. Glia. 2010;58(12):1437–1450. doi: 10.1002/glia.21019. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Liu FC. Genetic patterning of the mammalian telencephalon by morphogenetic molecules and transcription factors. Birth Defects Res C Embryo Today. 2006;78(3):256–266. doi: 10.1002/bdrc.20077. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278(5337):474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 24.Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17(21):8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceci ML, Pedraza M, de Carlos JA. The embryonic septum and ventral pallium, new sources of olfactory cortex cells. PLoS ONE. 2012;7(9):e44716. doi: 10.1371/journal.pone.0044716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixit R, et al. Ascl1 participates in Cajal-Retzius cell development in the neocortex. Cereb Cortex. 2011;21(11):2599–2611. doi: 10.1093/cercor/bhr046. [DOI] [PubMed] [Google Scholar]
- 27.Ceci ML, López-Mascaraque L, de Carlos JA. The influence of the environment on Cajal-Retzius cell migration. Cereb Cortex. 2010;20(10):2348–2360. doi: 10.1093/cercor/bhp305. [DOI] [PubMed] [Google Scholar]
- 28.Stoykova A, Hatano O, Gruss P, Götz M. Increase in reelin-positive cells in the marginal zone of Pax6 mutant mouse cortex. Cereb Cortex. 2003;13(6):560–571. doi: 10.1093/cercor/13.6.560. [DOI] [PubMed] [Google Scholar]
- 29.Molnár Z, Higashi S, López-Bendito G. Choreography of early thalamocortical development. Cereb Cortex. 2003;13(6):661–669. doi: 10.1093/cercor/13.6.661. [DOI] [PubMed] [Google Scholar]
- 30.Price DJ, Aslam S, Tasker L, Gillies K. Fates of the earliest generated cells in the developing murine neocortex. J Comp Neurol. 1997;377(3):414–422. [PubMed] [Google Scholar]
- 31.Valverde F, López-Mascaraque L, Santacana M, De Carlos JA. Persistence of early-generated neurons in the rodent subplate: Assessment of cell death in neocortex during the early postnatal period. J Neurosci. 1995;15(7 Pt 1):5014–5024. doi: 10.1523/JNEUROSCI.15-07-05014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: Its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 33.Oeschger FM, et al. Gene expression analysis of the embryonic subplate. Cereb Cortex. 2012;22(6):1343–1359. doi: 10.1093/cercor/bhr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Carlos JA, García-Moreno F. In: From Development to Degeneration and Regeneration of the Nervous System. Ribak CE, Aramburo C, Jones EG, Larriva JA, Swanson L, editors. New York: Oxford Univ Press; 2009. pp. 19–44. [Google Scholar]
- 35.Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 36.Cai Y, Zhang Y, Shen Q, Rubenstein JL, Yang Z. A subpopulation of individual neural progenitors in the mammalian dorsal pallium generates both projection neurons and interneurons in vitro. Stem Cells. 2013;31(6):1193–1201. doi: 10.1002/stem.1363. [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21(4):845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Magueresse C, et al. Subventricular zone-derived neuroblasts use vasculature as a scaffold to migrate radially to the cortex in neonatal mice. Cereb Cortex. 2012;22(10):2285–2296. doi: 10.1093/cercor/bhr302. [DOI] [PubMed] [Google Scholar]
- 39.Inta D, et al. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci USA. 2008;105(52):20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myakhar O, Unichenko P, Kirischuk S. GABAergic projections from the subplate to Cajal-Retzius cells in the neocortex. Neuroreport. 2011;22(11):525–529. doi: 10.1097/WNR.0b013e32834888a4. [DOI] [PubMed] [Google Scholar]
- 41.Viswanathan S, Bandyopadhyay S, Kao JPY, Kanold PO. Changing microcircuits in the subplate of the developing cortex. J Neurosci. 2012;32(5):1589–1601. doi: 10.1523/JNEUROSCI.4748-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009;6(5):363–369. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgenstern JP, Land HA. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990;18(4):1068–1073. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.