Significance
This study presents the first results, to our knowledge, of a whole-genome RNAi screen to identify factors that promote Parkin-mediated mitophagy, which have been validated for conservation across species. Top hits comprise several components of the sterol regulatory element binding transcription factor (SREBF) lipogenesis pathway, and we show that provision of exogenous lipids can promote the mitophagy pathway. Because SREBF1 has been identified as a risk locus for sporadic Parkinson disease (PD) through genome-wide association studies, the identification of SREBF1 in our screen provides evidence for mechanistic insight into the pathogenesis of sporadic PD. Thus, our functional studies provide the first insight, to our knowledge, into why the master regulator for lipogenesis, SREBF1, may contribute to sporadic PD and further underscore the impact of dysfunctional mitophagy on pathogenesis.
Abstract
Genetic analysis of Parkinson disease (PD) has identified several genes whose mutation causes inherited parkinsonism, as well as risk loci for sporadic PD. PTEN-induced kinase 1 (PINK1) and parkin, linked to autosomal recessive PD, act in a common genetic pathway regulating the autophagic degradation of mitochondria, termed mitophagy. We undertook a genome-wide RNAi screen as an unbiased approach to identify genes regulating the PINK1/Parkin pathway. We identified several genes that have a conserved function in promoting mitochondrial translocation of Parkin and subsequent mitophagy, most notably sterol regulatory element binding transcription factor 1 (SREBF1), F-box and WD40 domain protein 7 (FBXW7), and other components of the lipogenesis pathway. The relevance of mechanisms of autosomal recessive parkinsonism to sporadic PD has long been debated. However, with the recent identification of SREBF1 as a risk locus for sporadic PD, our findings suggest a common mechanistic link between autosomal recessive and sporadic PD, and underscore the importance of mitochondrial homeostasis.
Parkinson disease (PD) is an age-related neurodegenerative disease caused principally by the loss of midbrain dopaminergic neurons, resulting in motor disturbances, such as postural instability, resting tremor, and bradykinesia, as well as other nonmotor symptoms. Current therapeutic strategies alleviate symptoms by the replacement of dopamine, with variable efficacy and substantial side effects. However, there are currently no established curative, preventative, or disease-modifying interventions, stemming from a poor understanding of the molecular mechanisms of pathogenesis. Genetic analyses have identified causative mutations for autosomal dominant and recessive forms of familial parkinsonism. Functional studies of these genes have provided great insight into potential pathogenic mechanisms of inherited forms of PD; however, it is unclear how these may relate to the more common sporadic forms of PD. To gain insight into the genetic influence in sporadic PD, genome-wide association studies (GWASs) have revealed several loci that are significantly associated with the occurrence of PD, and so are considered potential risk factors. However, we lack mechanistic insight into how many of the associated risk loci may contribute to pathogenesis.
Mitochondrial dysfunction has long been considered a key contributing factor in the pathogenesis of PD, with evidence from postmortem tissue, epidemiological studies, and genetics. In particular, a body of evidence indicates that two genes linked to recessive parkinsonism, PTEN-induced kinase 1 (PINK1) and parkin, act in a common pathway to regulate mitochondrial turnover, providing an attractive hypothesis for the impact of mitochondrial homeostasis on pathogenesis (1). The process of mitophagy to degrade dysfunctional mitochondria has been extensively studied using uncoupling agents to dissipate the mitochondrial membrane potential (ΔΨm) (2). Following loss of ΔΨm, PINK1 accumulates on the outer mitochondrial membrane (OMM) (3, 4), where its kinase activity stimulates the translocation of Parkin (5, 6) from a diffuse cytoplasmic distribution to accumulate on mitochondria (7). The recruitment of Parkin to mitochondria results in the ubiquitination of multiple proteins (8, 9). These include several proteins on the OMM that regulate mitochondrial dynamics, such as Mfn and Miro. This process serves both to isolate dysfunctional organelles and to mark them for autophagic degradation. Although the downstream events are reasonably understood, the upstream events promoting mitophagy are poorly characterized.
To uncover novel components and pathways promoting PINK1/Parkin-mediated mitophagy, we performed a genome-wide RNAi screen, initially in Drosophila cells and validated in HeLa cells, which identified several genes with a conserved function in promoting Parkin translocation to and degradation of depolarized mitochondria. The strongest effects were found with components of the sterol regulatory element binding protein (SREBP)-lipogenesis pathway, including the master regulator of lipid synthesis SREBF1, indicating a role for lipids in the regulation of mitochondrial homeostasis. Strikingly, SREBF1 has previously been reported to be a risk factor for PD through GWASs of sporadic PD (10). Hence, our findings provide novel insight into the molecular mechanisms regulating mitophagy and also support a common mechanism influencing sporadic PD, further highlighting the important impact of mitochondrial homeostasis in PD.
Results
RNAi Screening Identified 20 Genes with a Conserved Role in Promoting Mitophagy.
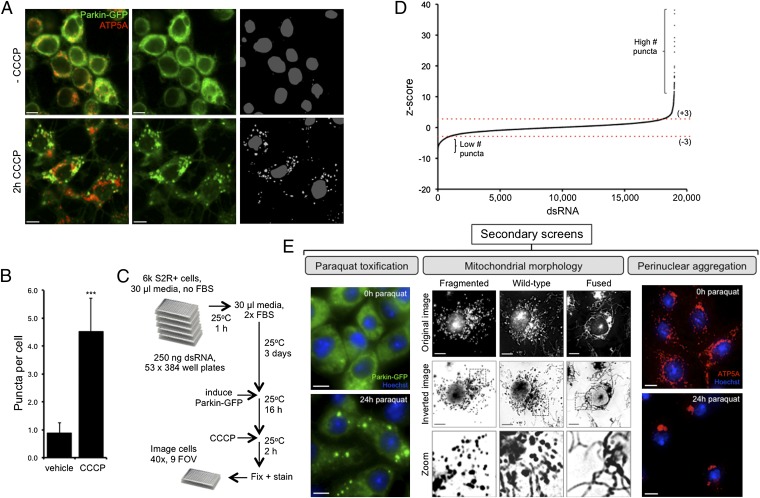
To identify factors that regulate the mitochondrial translocation of Parkin, we sought to perform a genetic screen using Drosophila Schneider 2 receptor plus (S2R+) cells, taking advantage of the genome with lower complexity, thus minimizing the effects of genetic redundancy. Preliminary experiments showed that expression of a Parkin-GFP fusion protein behaved similar to that in mammalian cells, being broadly cytoplasmic under normal conditions but rapidly translocated to colocalize with mitochondrial markers upon ablation of ΔΨm by the protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (Fig. 1 A and B). PINK1 also accumulates on the mitochondrial surface following depolarization (Fig. S1), in agreement with previous reports (3, 4). Hence, cultured Drosophila cells recapitulate established features of PINK1/Parkin cell biology, supporting the potential relevance of screen hits.
Fig. 1.
High-content, genome-wide RNAi screen was performed in Drosophila cells to identify factors required for CCCP-induced Parkin recruitment to mitochondria. (A) Drosophila S2R+ cells stably expressing Parkin-GFP (green) were toxified for 2 h with 10 μM CCCP. Following toxification, Parkin-GFP accumulates on mitochondria (red; anti-ATP5A). The grayscale image shows segmentation output from automated screening software. (B) Quantification of Parkin translocation as the number of Parkin-GFP puncta per cell. The chart represents mean ± SD from five replicate assays. ***P < 0.001, Student t test. (C) Schematic of high-content screen. FOV, field of view. (D) Graphical representation of genome-wide screen output. The z-scores convey the distribution of Parkin-GFP puncta per cell across the entire library. (E) Secondary screens were used to refine the hit list: Parkin-GFP puncta upon paraquat toxification (as for the screen), mitochondrial morphology under basal conditions (imaged live using MitoTracker Red), and mitochondrial perinuclear aggregation following prolonged paraquat exposure (24 h). Mitochondria were visualized with anti-ATP5A (red). (Scale bars: 5 μm.)
We conducted a high-content, genome-wide screen using the Drosophila Heidelberg 2 (HD2) library (11), covering ∼99% of the Drosophila transcriptome. Automated microscopy followed by automated image segmentation analysis allowed the quantification of Parkin-GFP puncta per cell (Fig. 1 A–C). We set initial thresholds for putative hits at −3 or +3 z-scores to identify positive or negative regulators of Parkin translocation, respectively (Fig. 1D). Here, we focused on characterizing positive regulators, although a list of negative regulators, without further validation, is made available (Dataset S1). From the initial cutoff for positive regulators, 115 were deemed robust and reproducible upon rescreening. These confirmed amplicons were subjected to three secondary screen assays assessing (i) Parkin-GFP translocation stimulated by paraquat, a herbicide linked to PD; (ii) mitochondrial morphology, because Parkin translocation leads to mitochondrial fragmentation; and (iii) inhibition of mitochondrial perinuclear aggregation, a process that occurs before lysosomal degradation (12–14) (Fig. 1E). Altogether, this identified 63 hit amplicons corresponding to 60 genes (Table S1).
To assess the functional conservation of our 60 Drosophila genes in a mammalian system, we identified the 76 homologous human genes (Table S1) and analyzed the effect of siRNA-mediated knockdown of these genes on Parkin translocation and mitophagy in human cells. As previously reported, in HeLa cells stably expressing YFP-Parkin transfected with control siRNA, we observed rapid colocalization of Parkin with the mitochondrial network after a short exposure to CCCP, and a pronounced loss of mitochondria upon prolonged depolarization (Fig. S2 A–C). Consistent with the literature, we also found that both of these phenomena are blocked following siRNA targeting of PINK1 (Fig. S2 A–C). A qualitative scale of Parkin translocation and mitophagy was devised (Fig. S3), and the putative hits were screened in triplicate. Although many targets showed little or no impact, siRNA against a subset of genes replicated a robust attenuation of Parkin translocation and mitophagy (Fig. S2 D and E), comparable to PINK1 siRNA. We considered siRNAs whose mean score was more than 3 SDs from the mean of the CCCP-treated negative control for each phenotype as final hits, identifying 20 candidate genes with a conserved effect on both Parkin translocation and mitophagy (Table 1). Linear regression and Pearson’s coefficient analysis showed a strong correlation between the two phenotypes for many of the siRNAs, particularly in the final hit group (Fig. S2 F and G).
Table 1.
Human homologs of conserved genes that play a role in promoting Parkin translocation and mitophagy, listed in order of phenotypic strength
| Gene | Gene function (UniProt/NCBI Gene database) |
| SREBF1 | Transcriptional activator required for lipid homeostasis (mainly fatty acids) |
| FBXW7 | Substrate recognition component of a SKP1–CUL1–F-box E3 ubiquitin ligase complex |
| SLC17A9 | Vesicular storage and exocytosis of ATP |
| USP36 | Deubiquitinating enzyme with a role in selective autophagy |
| WBP11 | Pre-mRNA splicing activator |
| ZBTB17 | Antiapoptotic role in early lymphocyte development |
| RUVBL1 | Component of the NuA4 histone acetyltransferase complex |
| RPL28 | Component of the ribosomal 60S subunit |
| U2AF1 | Pre-mRNA splicing factor |
| ACTL6A | Component of the NuA4 histone acetyltransferase complex |
| SREBF2 | Transcriptional activator required for lipid homeostasis (mainly cholesterol) |
| GSK3A | Multifunctional serine/threonine protein kinase |
| ZDHHC8 | Palmitoyltransferase involved in glutamatergic transmission |
| U2AF2 | Pre-mRNA splicing factor represses the splicing of MAPT/Tau |
| POLR3A | Catalytic component of RNA polymerase III |
| FZD5 | Receptor for the Wnt5A ligand, involved in β-catenin pathway induction |
| CSNK2A2 | Catalytic subunit of a constitutively active serine/threonine protein kinase complex |
| CTSK | Lysosomal cysteine proteinase involved in bone remodelling and resorption |
| WDR75 | Nuclear protein with unknown function contains nine WD repeats |
| KAT2A | Histone acetyltransferase |
CUL1, cullin 1; MAPT, microtubule-associated protein Tau; NCBI, National Center for Biotechnology Information; SKP1, S-phase kinase-associated protein 1; WD, tryptophan-aspartate.
SREBF1, FBXW7, and GSK3A, Components of the Lipogenesis Pathway, Promote Mitophagy.
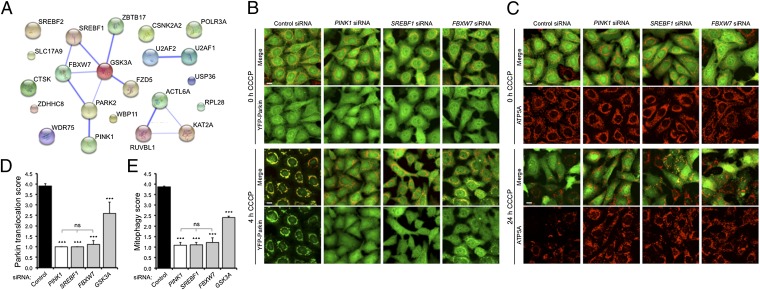
Bioinformatic network analysis of the hit list revealed three known interaction pathways, which included histone acetyltransferase transcriptional activation, pre-mRNA splicing, and lipid synthesis (Fig. 2A). SREBF1 and its close homolog SREBF2 encode the SREBPs, transcription factors that act as master regulators of lipid synthesis (15–17). Two additional components of this pathway, FBXW7 (18, 19) and GSK3A (19, 20), were also hits in our screen. Notably, SREBF1 has been reported as a risk locus in GWASs for sporadic PD (10). In addition, FBXW7 has been shown to function directly with Parkin, promoting neuronal survival by regulating the levels of cyclin E and the mitochondrial prosurvival factor Mcl-1 (21, 22). Therefore, follow-up analysis assessed the relationship between PINK1, Parkin, and components of the SREBP lipogenesis pathway.
Fig. 2.
SREBF1 pathway components are required for Parkin translocation and mitophagy. (A) Schematic representation of known and predicted protein interactions between the 20 final human hits and PINK1 and parkin (STRING 9.0). The thickness of the lines connecting the nodes represents the strength of evidence supporting an interaction. (B) Low-throughput analysis of CCCP-induced YFP-Parkin (green) translocation to mitochondria (anti-ATP5A; red) transfected with control, PINK1, SREBF1, or FBXW7 siRNAs. (C) Low-throughput analysis of CCCP-induced mitophagy in HeLa cells expressing YFP-Parkin transfected with control, PINK1, SREBF1, or FBXW7 siRNAs. (D) Quantification of analysis in B. (E) Quantification of analysis in C. Additional controls and expression analysis are shown in Fig. S4. Data represent mean ± SD from triplicate experiments. ***P < 0.001 (one-way ANOVA with Bonferroni correction compared with the CCCP-treated control siRNA unless otherwise indicated); ns, nonsignificant. (Scale bars: 10 μm.)
First, we confirmed the effects of siRNA knockdown of pathway components on PINK1/Parkin function in a low-throughput setting. HeLa cells stably expressing YFP-Parkin were transfected with siRNAs targeting SREBF1, FBXW7, and GSK3A, and Parkin translocation and mitophagy were assessed (Fig. 2 B–E and Fig. S4). Consistent with the effect seen in the high-throughput screen, SREBF1 and FBXW7 knockdown blocked both Parkin translocation and mitophagy comparable to the loss of PINK1, whereas GSK3A knockdown reduced Parkin translocation and mitophagy, but to a lesser extent. As further support for a direct role of the SREBP pathway in mitophagy, we also saw a dose-dependent reduction in CCCP-induced Parkin translocation by the inhibitor of SREBP1 activation, genistein (23, 24) (Fig. S5).
To address the potential confounding issue of off-target effects, we took a number of measures. First, we analyzed individual siRNAs from the SMARTpool (Dharmacon). In each case, at least two independent siRNAs reproduced the phenotypes seen, with at least one siRNA causing an effect equivalent to the pooled siRNAs (Fig. S6). Next, siRNA-resistant expression constructs were generated against the strongest single siRNA. Upon expression of the siRNA-resistant construct, Parkin translocation was significantly restored for SREBF1, FBXW7, and GSK3A (Fig. S7). Taken together, these data provide support that the observed effects are not due to off-target effects.
Several indirect effects could account for a reduction in Parkin translocation following CCCP treatment, which we next sought to eliminate. First, Parkin translocation would also be prevented if PINK1 expression levels were substantially reduced. However, neither SREBF1 nor FBXW7 knockdown interfered with PINK1 expression levels (Fig. S4E). Second, ΔΨm could be restored by the reversal of ATP synthase, as was recently reported for ATPase inhibitory factor 1 (ATPIF1) (25). However, analysis using a potentiometric sensitive dye confirmed that ΔΨm was ablated in SREBF1 and FBXW7 knockdown cells (Fig. S8). Finally, we addressed the specificity of the block on mitophagy by assessing the effect of SREBF1 and FBXW7 knockdown on general autophagic flux. Monitoring the production of the autophagosome marker light chain 3-II (LC3-II) upon nutrient starvation, no change in the induction or flux of bulk autophagy was observed in cells deficient for SREBF1 or FBXW7 (Fig. S8). Together, these results support a direct role for SREBF1 and FBXW7 in mitophagy.
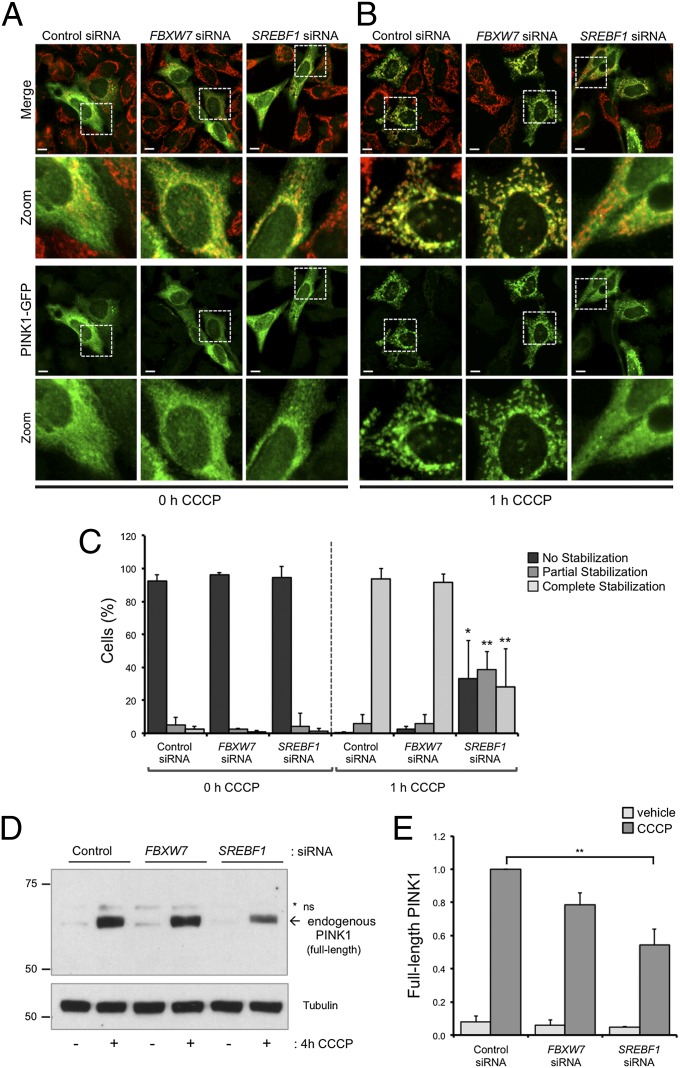
To understand further how SREBF1 and FBXW7 have an impact on the PINK1/Parkin pathway, we next analyzed the effect of their attenuation on the OMM stabilization of PINK1 following CCCP depolarization. Consistent with previous reports, we observe that expression of a PINK1-GFP reporter construct is broadly diffuse under basal conditions (Fig. 3A) but becomes accumulated on mitochondria following a short exposure to CCCP (Fig. 3B). Interestingly, although FBXW7 knockdown did not affect PINK1 stabilization, loss of SREBF1 significantly reduced the propensity of PINK1 to become stabilized (Fig. 3 B and C). In addition, immunoblotting for endogenous full-length PINK1 under the same conditions gave equivalent results (Fig. 3 D and E).
Fig. 3.
Loss of SREBF1 inhibits CCCP-induced PINK1 stabilization. (A and B) HeLa cells were treated with control, FBXW7, or SREBF1 siRNA for 3 d before the transient transfection of PINK1-GFP (green). Cells were subjected to either vehicle (A) or CCCP (B) (10 μM for 1 h) before being assessed for PINK1-GFP stabilization on the mitochondrial network (anti-ATP5A; red). (Scale bars: 10 μm.) (C) Quantification of PINK1 stabilization scored in A and B. The chart represents mean ± SD of triplicate assays. *P < 0.05; **P < 0.01 (one-way ANOVA with Bonferroni correction compared with the equivalent control siRNA condition). (D and E) HeLa cells were treated with control, FBXW7, or SREBF1 siRNA for 3 d before treatment with either vehicle or CCCP (10 μM for 4 h). (D) Alterations in endogenous full-length PINK1 levels were assessed by immunoblotting. Tubulin was used as a loading control. (E) Quantification of full-length endogenous PINK1 levels. The chart represents mean ± SEM of triplicate assays. **P < 0.01 (one-way ANOVA with Bonferroni correction, applied across corresponding depolarizing conditions).
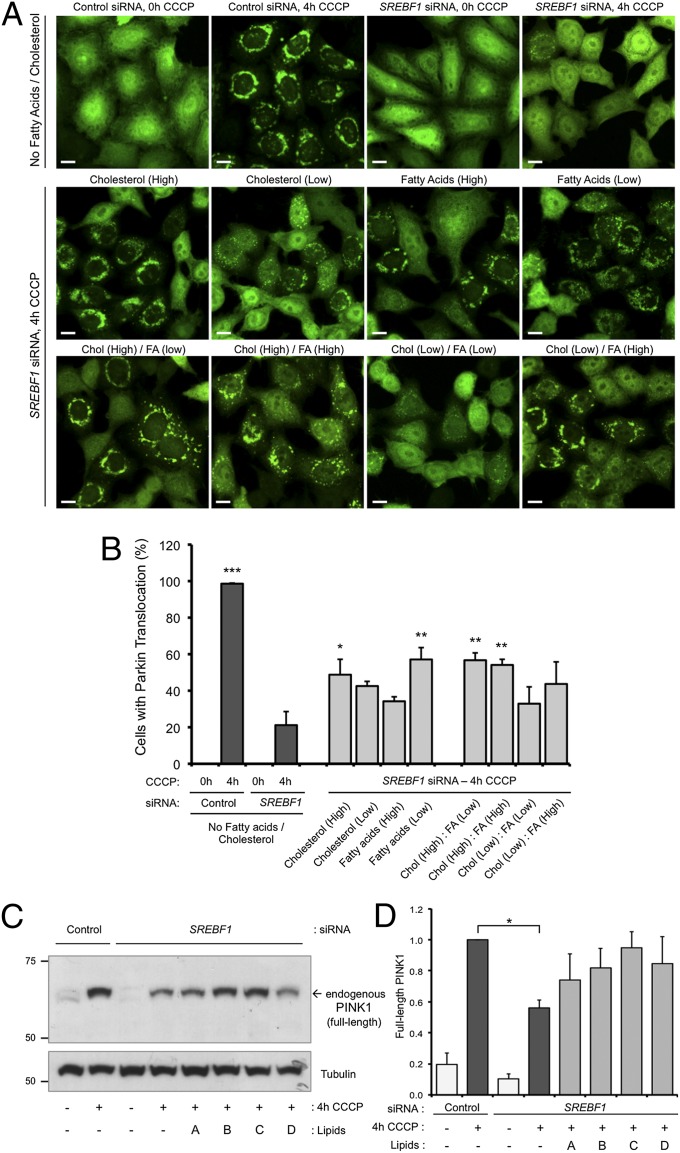
Having found that several components of the SREBP pathway regulate the proper execution of Parkin-mediated mitophagy, this suggested that lipid synthesis might be required to trigger mitophagy. Thus, we hypothesized that in the absence of an active SREBP pathway, the availability of lipids may be a limiting factor in stimulating Parkin translocation. SREBPs primarily regulate fatty acid and cholesterol metabolism, so we surmised that providing excess fatty acid or cholesterol might restore Parkin translocation in the absence of SREBF1. HeLa cells expressing YFP-Parkin were transfected with SREBF1 or control siRNAs and treated with high or low amounts of fatty acid or cholesterol. We found that addition of high amounts of cholesterol significantly restored CCCP-induced Parkin translocation, whereas for fatty acids, low but not high amounts rescued Parkin translocation (Fig. 4 A and B and Fig. S9). Addition of both cholesterol and fatty acids in different amounts did not provide greater rescue, and only restored Parkin translocation if cholesterol was high (Fig. 4 A and B). Interestingly, exogenous lipids also appeared to promote PINK1 stabilization in SREBF1 knockdown cells (Fig. 4 C and D), although further work is needed to clarify this effect. Notably, exogenous cholesterol or fatty acids cannot restore Parkin translocation in the absence of PINK1 (Fig. S9). These data demonstrate that excess lipids can partially restore Parkin translocation in the absence of a key regulator of lipid synthesis, however, this process still requires PINK1. Together, these findings further support our genetic results that the SREBP lipogenesis pathway plays a role in regulating mitophagy.
Fig. 4.
Inhibition of Parkin translocation by SREBF1 knockdown is rescued by exogenous lipids. (A and B) HeLa cells expressing YFP-Parkin were transfected with control or SREBF1 siRNA before the addition of vehicle or high/low concentrations of cholesterol (Chol), fatty acids (FA), or their combination, as indicated. Cells were then treated with either vehicle or CCCP (10 μM for 4 h). (Scale bars: 10 μm.) (B) Quantification of Parkin translocation shown in A. The chart represents mean ± SEM from triplicate experiments. *P < 0.05; **P < 0.01; ***P < 0.001 (one-way ANOVA with Bonferroni correction compared with “SREBF1 siRNA, 4-h CCCP” samples). Additional controls are shown in Fig. S9. (C and D) HeLa cells were treated with siRNA, lipids, and CCCP as in A, where A = cholesterol (high); B = fatty acids (low); C = cholesterol (high), fatty acids (low); and D = cholesterol (high), fatty acids (high). (C) Levels of full-length endogenous PINK1 were assessed by immunoblotting. Tubulin was used as a loading control. (D) Quantification of full-length endogenous PINK1 levels. The chart represents mean ± SEM of triplicate assays. *P < 0.05 (one-way ANOVA with Bonferroni correction, compared with “control siRNA, 4-h CCCP” samples).
Discussion
Using a genome-wide RNAi screening approach initially in Drosophila cells with subsequent validation in human cells, we have identified 20 genes that have a conserved function in promoting Parkin translocation and mitophagy. Among these are several components of the SREBP lipogenesis pathway. We have shown that loss of SREBF1, FBXW7, and GSK3A inhibits Parkin translocation and mitophagy following mitochondrial depolarization, and that provision of exogenous lipids circumvents this block. Interestingly, loss of SREBF1 but not FBXW7 also attenuates the stabilization of PINK1 on the OMM upon ablation of ΔΨm, suggesting a complex regulation of this process by the SREBP pathway. Currently, it is not known whether or how lipids may influence PINK1 stabilization, but our results indicate that addition of exogenous lipids may also restore PINK1 stabilization. Overall, our data suggest a key role for lipid metabolism in the regulation of mitophagy following loss of membrane potential.
Exactly how lipids may regulate mitophagy remains to be determined; however, lipid metabolism has an intimate relationship with mitochondria, with the tricarboxylic acid cycle providing raw materials for lipid production under nutrient-rich conditions and initiating fatty acid oxidation under nutrient-poor conditions. In addition, synthesis of a number of phospholipids requires the exchange of intermediates between the endoplasmic reticulum (ER) and mitochondrial membranes, likely at ER-mitochondria contact sites. Autophagic turnover of mitochondria needs to be balanced with biogenesis, which will, of course, rely upon SREBP pathway targets for membrane synthesis; thus, it is rational that the two processes could be coregulated. Furthermore, recent studies have revealed that during mitophagy, cardiolipin, a mitochondria-specific phospholipid, becomes externalized to the OMM, promoting mitochondrial degradation via a putative interaction with LC3 (26), reinforcing a role for lipids in this process.
The role of FBXW7 in regulating mitophagy presents a complex picture. First, its function in the SREBP pathway is to promote the rapid turnover of SREBP1 following activation, thereby ensuring a tight on/off regulation of lipogenesis. However, loss of this negative regulator would be predicted to have the opposite outcome from loss of SREBF1. One interpretation would be that inactivation of the SREBP pathway may be as crucial as activation to get a fine balance of lipid synthesis; thus, disruption of both processes inhibits mitophagy. On the other hand, it may be that the relevant function of FBXW7 is SREBP-independent. FBXW7 is expressed as three isoforms, with FBXW7α and FBXW7γ regulating cyclin E degradation in the nucleus (27), whereas cytoplasmic FBXW7β promotes the degradation of the mitochondrial prosurvival factor Mcl-1 (21). In response to oxidative stress, Parkin targets the proteasomal degradation of FBXW7β to allow Mcl-1 stabilization and promote neuron survival. However, even in this context, loss of FBXW7β would be predicted to have the opposite effect as loss of Parkin, counterintuitive to our present findings. Clearly, more work is needed to understand the precise role of FBXW7 in regulating mitophagy, and its interaction with Parkin.
Thus, the regulation of mitophagy by SREBP pathway components and lipids is complex, with SREBF1, FBXW7, and lipids promoting Parkin translocation and mitophagy. Moreover, the regulation of FBXW7 by Parkin suggests a putative feedback loop providing regulatory countermeasures. Although further work will be required to elucidate these mechanisms, additional evidence supports a role for Parkin in regulating lipid metabolism; Parkin has been reported to ubiquitinate and stabilize both the SREBP target FASN and the fatty acid transporter CD36 (9, 28). Finally, the identification of SREBF1 as a GWAS risk locus for sporadic PD (10), coupled with our findings that this pathway regulates mitophagy, provides further support that the causes of sporadic and autosomal recessive PD may share some mechanistic overlap.
Materials and Methods
Cell Culture, Plasmids, and Transfection.
Drosophila S2R+ cells were grown in Schneider’s medium (SM; Gibco) containing 10% (vol/vol) FBS and 1% penicillin/streptomycin (Sigma). An S2R+ cell line stably expressing pMK33-Parkin-GFP (Parkin-GFP.S2R+) was maintained in growth medium containing 300 μg/mL hygromycin B (Gibco). A HeLa cell line stably expressing pLVX-Puro-YFP-Parkin (YFP-Parkin.HeLa) was a kind gift from Jon Lane (University of Bristol, Bristol, United Kingdom). Plasmids expressing pAct-Mito-GFP and pMK33-Parkin-GFP were described previously (29). pMK33-PINK1-Myc was produced using full-length Drosophila PINK1 cloned with a C-terminal 6× Myc epitope tag. pMK33 contains a metallothionein promoter, which was induced by 500 μM copper sulfate solution. Plasmid transfection was performed using Effectene reagent (Qiagen) according to the manufacturer’s instructions (additional information is provided in SI Materials and Methods).
Gene Silencing.
Drosophila dsRNA probes were acquired from the Sheffield RNAi Screening Facility. Drosophila gene silencing involved bathing cells in FBS-free SM containing dsRNA (∼4 μg/mL) for 1 h, followed by the addition of equal volumes of 2× FBS-containing SM. Cells were routinely incubated for 3–4 d before analysis. Human siRNA was acquired from Dharmacon and used at a concentration of 25–100 nM. siRNA was delivered using Dharmafect 1 reagent (Dharmacon) or Lipofectamine 2000 (Life Technologies) according to each manufacturer’s instructions. Cells were routinely incubated for 2–4 d before analysis.
High-Content Screening.
Drosophila genome-wide primary screening used the HD2.0 second-generation dsRNA library (30). The dsRNA amplicons were seeded into 384-well plates at 250 ng per well. Six thousand Parkin-GFP.S2R+ cells per well were dispensed in FBS-free SM and incubated for 1 h. Following this, equal volumes of 2× FBS-containing SM were added and plates were incubated for a further 3 d. Copper sulfate induced the expression of pMK33-Parkin-GFP for 16 h before toxification with CCCP-containing SM for 2 h. Samples were fixed with 3.7% formaldehyde solution (Sigma) containing Hoechst dye before imaging using an ImageXpress Micro wide-field, high-content screening microscope (Molecular Devices). Nine images were acquired per well using a CFI S Plan Fluor N.A. 0.60 40× objective (Nikon). Microscopy images were analyzed automatically using an automated data capture application called “Transfluor” (Molecular Devices). Parameters were optimized to enable detection of Parkin-GFP puncta between 0.6 and 1 μm in size and 60 gray levels above local background intensity. Values were averaged across the nine images, producing a single “puncta per cell” value.
Statistical Analyses.
Drosophila genome-wide screen data were normalized using the z-score method, incorporating the median absolute deviation (11). The “hit” cutoff was defined as −3 or lower z-scores. All follow-up Drosophila screens used nonnormalized data and a cutoff of 2 SDs, and HeLa screening used nonnormalized data and a cutoff of 3 SDs. The linear regression line equation, coefficient of determination (R2), and significance of positive data correlation (P) were calculated in Microsoft Excel (Microsoft Corp.) and Prism 6 (GraphPad).
Bioinformatics.
The “DRSC Interactive Ortholog Prediction Tool” was used to identify the human orthologs of Drosophila genes (31). Known and predicted protein interactions were assessed using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING 9.0) (32). Gene symbol, name, and function were acquired from a number of sources, including the FlyBase, UniProt, and the National Center for Biotechnology Information Gene databases.
Assessing PINK1 Stabilization.
HeLa cells were transfected with 25 nM siRNA as indicated and incubated for 2 d. PINK1-GFP was transiently transfected using Effectene transfection reagent (Qiagen), and samples were incubated for a further 16 h before treatment with vehicle or CCCP (10 μM) for 1 h. Samples were fixed and processed for imaging. Immunoblotting was performed by standard procedures, using anti-PINK1 (Novus).
Lipid Supplementation.
Synthetic cholesterol (SyntheChol; Sigma) and Fatty Acid Supplement (Sigma) were applied in varying concentrations [SyntheChol: 1:100 (very high), 1:250 (high), 1:500 (low); fatty acid supplement: 1:2,000 (high), 1:4,000 (low)] 24 h before the application of vehicle or CCCP solution, also containing the indicated lipids. Images were analyzed manually, with a minimum of 200 cells per condition per assay. Cells containing two or more YFP-Parkin puncta signified “Parkin translocation.”
Supplementary Material
Acknowledgments
We thank A. Singleton, J. Hardy, and N. Wood for communicating results of GWAS meta-analysis prior to publication. We thank D. Strutt and M. Zeidler for stimulating discussions and critical reading of the manuscript, J. Lane for the gift of the YFP-Parkin.HeLa cells, and E. Wilson and H. Mortiboys for technical assistance. We thank the Sheffield RNAi Screening Facility, supported by the Wellcome Trust (Grant 084757), for providing RNAi libraries, laboratory space, bioinformatics tools, and other support for the screen. The work was funded by Wellcome/Medical Research Council (MRC) Parkinson’s Disease Consortium Grant WT089698 to University College London/Institute of Neurology, the University of Sheffield, and the MRC Protein Phosphorylation Unit at the University of Dundee; the European Project on Mendelian Forms of Parkinson’s Disease (MeFoPa) project funded through the European Union FP7 research program; and European Research Council Starting Grant 309742. R.M.I. is funded by an MRC PhD studentship (Grant G070091). The Wellcome Trust is acknowledged for support of the Sheffield Light Microscopy Facility (Grant GR077544AIA). The MRC Centre for Developmental and Biomedical Genetics is supported by Grant G070091.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321207111/-/DCSupplemental.
References
- 1.Grenier K, McLelland GL, Fon EA. Parkin- and PINK1-Dependent Mitophagy in Neurons: Will the Real Pathway Please Stand Up? Front Neurol. 2013;4:100. doi: 10.3389/fneur.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuda N, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189(2):211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okatsu K, et al. PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat Commun. 2012;3:1016. doi: 10.1038/ncomms2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiba-Fukushima K, et al. PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci Rep. 2012;2:1002. doi: 10.1038/srep01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan NC, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20(9):1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarraf SA, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496(7445):372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Do CB, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 2011;7(6):e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher KH, Wright VM, Taylor A, Zeidler MP, Brown S. Advances in genome-wide RNAi cellular screens: A case study using the Drosophila JAK/STAT pathway. BMC Genomics. 2012;13:506. doi: 10.1186/1471-2164-13-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okatsu K, et al. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells. 2010;15(8):887–900. doi: 10.1111/j.1365-2443.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vives-Bauza C, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010;107(1):378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189(4):671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimano H. Sterol regulatory element-binding proteins (SREBPs): Transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001;40(6):439–452. doi: 10.1016/s0163-7827(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 16.Horton JD, Goldstein JL, Brown MS. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bengoechea-Alonso MT, Ericsson J. SREBP in signal transduction: Cholesterol metabolism and beyond. Curr Opin Cell Biol. 2007;19(2):215–222. doi: 10.1016/j.ceb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Punga T, Bengoechea-Alonso MT, Ericsson J. Phosphorylation and ubiquitination of the transcription factor sterol regulatory element-binding protein-1 in response to DNA binding. J Biol Chem. 2006;281(35):25278–25286. doi: 10.1074/jbc.M604983200. [DOI] [PubMed] [Google Scholar]
- 19.Sundqvist A, et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7) Cell Metab. 2005;1(6):379–391. doi: 10.1016/j.cmet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, et al. Regulatory role of glycogen synthase kinase 3 for transcriptional activity of ADD1/SREBP1c. J Biol Chem. 2004;279(50):51999–52006. doi: 10.1074/jbc.M405522200. [DOI] [PubMed] [Google Scholar]
- 21.Ekholm-Reed S, Goldberg MS, Schlossmacher MG, Reed SI. Parkin-dependent degradation of the F-box protein Fbw7β promotes neuronal survival in response to oxidative stress by stabilizing Mcl-1. Mol Cell Biol. 2013;33(18):3627–3643. doi: 10.1128/MCB.00535-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staropoli JF, et al. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron. 2003;37(5):735–749. doi: 10.1016/s0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 23.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96(20):11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin ES, et al. Genistein downregulates SREBP-1 regulated gene expression by inhibiting site-1 protease expression in HepG2 cells. J Nutr. 2007;137(5):1127–1131. doi: 10.1093/jn/137.5.1127. [DOI] [PubMed] [Google Scholar]
- 25.Lefebvre V, et al. Genome-wide RNAi screen identifies ATPase inhibitory factor 1 (ATPIF1) as essential for PARK2 recruitment and mitophagy. Autophagy. 2013;9(11):1770–1779. doi: 10.4161/auto.25413. [DOI] [PubMed] [Google Scholar]
- 26.Chu CT, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15(10):1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhaskaran N, et al. Fbw7α and Fbw7γ collaborate to shuttle cyclin E1 into the nucleolus for multiubiquitylation. Mol Cell Biol. 2013;33(1):85–97. doi: 10.1128/MCB.00288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KY, et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J Clin Invest. 2011;121(9):3701–3712. doi: 10.1172/JCI44736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci USA. 2010;107(11):5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn T, Sandmann T, Boutros M. Design and evaluation of genome-wide libraries for RNA interference screens. Genome Biol. 2010;11(6):R61. doi: 10.1186/gb-2010-11-6-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, et al. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics. 2011;12:357. doi: 10.1186/1471-2105-12-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen LJ, et al. STRING 8—A global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37(Database issue):D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.