Significance
Complex learning and memory are believed to require the weakening or elimination of synapses in the brain, a process mediated by adhesion molecules, which maintain synapse strength and stability. In the present study, we examine in vivo the effects of stabilization of β-catenin, an intracellular protein that is a component of the cadherin adhesion complex. We find that stabilization of β-catenin in the brain prevents normal activity-dependent downscaling of synapse strength, resulting in a striking impairment in cognitive flexibility. These results demonstrate that β-catenin plays an important role in learning and memory and that aberrant increases in synaptic adhesion can have detrimental effects on cognitive function.
Abstract
The cadherin/β-catenin adhesion complex is a key mediator of the bidirectional changes in synapse strength which are believed to underlie complex learning and memory. In the present study, we demonstrate that stabilization of β-catenin in the hippocampus of adult mice results in significant impairments in cognitive flexibility and spatial reversal learning, including impaired extinction during the reversal phase of the Morris water maze and deficits in a delayed nonmatch to place T-maze task. In accordance with these deficits, β-catenin stabilization was found to abolish long-term depression by stabilizing cadherin at the synaptic membrane and impairing AMPA receptor endocytosis, while leaving basal synaptic transmission and long-term potentiation unaffected. These results demonstrate that the β-catenin/cadherin adhesion complex plays an important role in learning and memory and that aberrant increases in synaptic adhesion can have deleterious effects on cognitive function.
Activity-driven increases and decreases in synapse efficacy—termed long-term potentiation (LTP) and long-term depression (LTD), respectively—are believed to underlie learning and memory in the brain. Although LTP has been widely studied as a physiological correlate of learning (1), LTD has emerged as a critical and complementary form of synaptic plasticity in tasks involving the modification or elimination of previously learned information. Pharmacological disruption of LTD impairs reversal learning and behavioral flexibility (2, 3), and it has been suggested that LTD is required to depotentiate synapses from earlier memory traces to allow the storage of new memories in overlapping sets of synapses (2).
Cadherins are homophilic adhesion molecules that play a central role in regulating changes in synapse strength and stability during LTP and LTD (4, 5). The recruitment of cadherin to synapses is essential for the maintenance of LTP and memory consolidation (6–8), whereas patterns of activity that induce LTD have been shown in vitro to cause cadherin internalization (5).
A major regulator of cadherin stability is its intracellular binding partner β-catenin (9). In vitro evidence demonstrates that β-catenin/cadherin interactions are dynamically regulated in response to activity (10, 11), allowing β-catenin to modify cadherin stability during different forms of synaptic plasticity. Enhanced neural activity increases β-catenin/cadherin interaction in dendritic spines, stabilizing cadherin at synapses (10). In contrast, pharmacological manipulations that induce LTD disrupt β-catenin/cadherin interactions (5). Targeted deletion of β-catenin has been shown to block the consolidation of fear memory, indicating that β-catenin is important for long-term memory formation (12). However, the role of β-catenin in regulating cadherin stability during the modification or elimination of memory remains unknown.
Understanding the role of β-catenin in synaptic plasticity and learning is critical in light of reports implicating deregulation of β-catenin in neurological disorders. Significant increases in levels of β-catenin have been reported in the brains of patients with Huntington disease (HD) (13). Alzheimer’s disease (AD)-linked mutations in presenilin-1 (14) and hyperphosphorylation of tau (15) have been shown to increase the stability of β-catenin in cells. Given the importance of β-catenin in regulating adhesion and synaptic plasticity, it is likely that alterations in β-catenin levels have significant ramifications for neuronal function and cognition. In this study, we characterize the effects of β-catenin stabilization on hippocampal synapses and demonstrate that in vivo stabilization of β-catenin impairs behavioral flexibility by aberrantly stabilizing cadherin and AMPA receptors at the synaptic membrane and abolishing LTD.
Results
Conditional Stabilization of β-catenin in Hippocampal Neurons in Vivo.
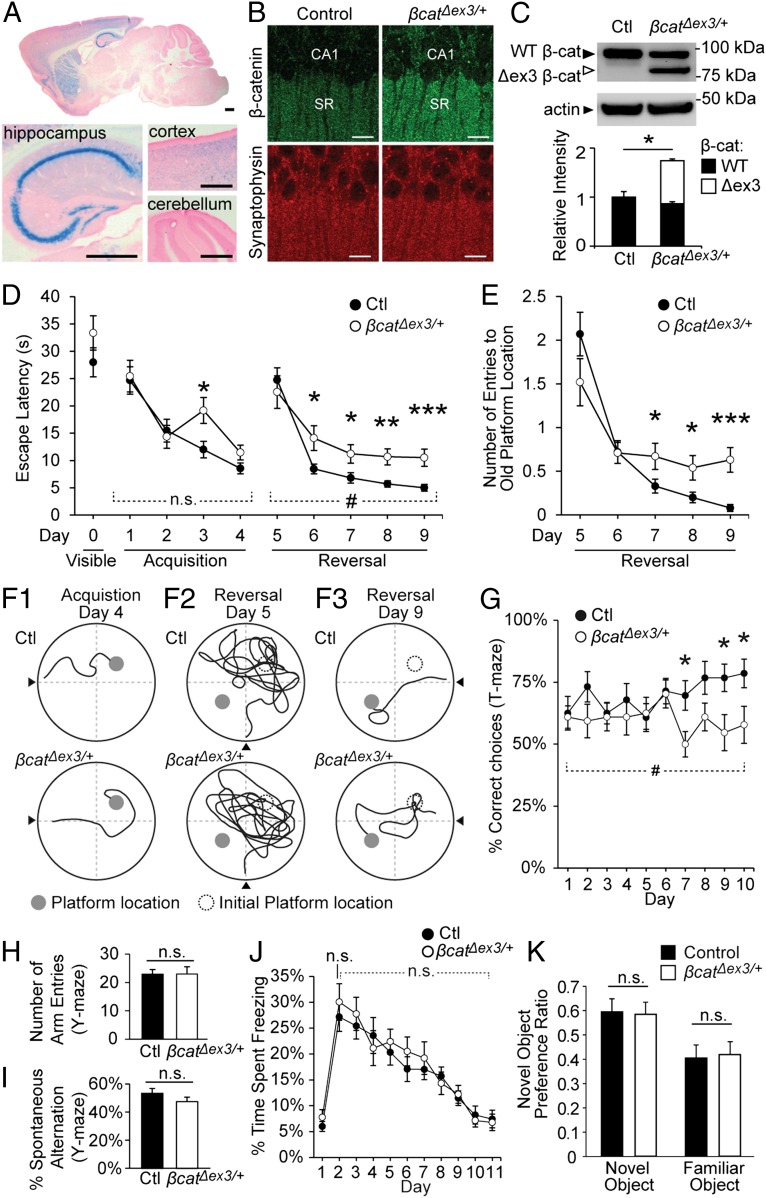
To investigate the effects of increased β-catenin levels on synapses, we generated mice that expressed a stabilized form of β-catenin in a subset of neurons in the brain. β-catenin is degraded following phosphorylation of serine/threonine residues at its N terminus, and mutation of these residues leads to the stabilization of β-catenin (16). By using the Cre/loxP system, β-catenin N-terminal phosphorylation sites can be ablated by excision of exon 3, resulting in a stabilized, active form of β-catenin (17). Importantly, this mutant form of β-catenin still retains all of its binding domains, including its α-catenin binding region (18) and internal armadillo repeats, which mediate binding to cadherin and TCF/LEF transcription factors (19), and is competent for Wnt signaling (20). Thus, this stabilized form of β-catenin can mediate the same functions as wild-type β-catenin and is widely used as a tool to increase overall β-catenin levels in vivo (17, 20, 21). Mice homozygous for this loxP-flanked exon 3 transgene (Ctnnb1lox(ex3)/lox(ex3) mice) (17) were crossed with CaMKIIα:Cre/+ mice (22, 23), generating heterozygous Ctnnb1lox(ex3)/+;CaMKIIα:Cre/+ mice (termed β-catΔex3/+ mice for brevity), as well as Ctnnb1lox(ex3)/+;+/+ littermates, which were used as controls. Cre recombinase is expressed in CaMKIIα:Cre/+ mice from P17, allowing the effects of increased β-catenin levels to be examined in adult animals while leaving earlier neuronal development to progress normally. Cre expression was determined by X-Gal staining (Fig. 1A), which was observed throughout the hippocampus (including the CA1 and CA3 regions and the dentate gyrus), in a subset of cortical and striatal neurons and was absent from the cerebellum. This pattern of Cre expression is consistent with a previous study that reported Cre expression in 99.5% of CA1 neurons, ∼9% of cortical neurons, ∼29% of striatal neurons, and no cerebellar neurons (24). Previous studies have shown that expression of this transgene is restricted to excitatory neurons that express endogenous CamKII (22).
Fig. 1.
Reversal learning, spatial memory extinction, and behavioral flexibility are impaired in β-catΔex3/+ mice. (A) X-Gal staining in sagittal sections of 1-y-old β-catΔex3/+ mice, heterozygous for both CaMKIIα:Cre and the R26R lacZ reporter. X-Gal staining was detected in the CA1 and CA3 regions and dentate gyrus of the hippocampus and a subset of cortical and striatal neurons. Staining was absent in the cerebellum. Counterstaining was with Fast Red. (Scale bars, 0.5 mm.) (B) Confocal images of brain sections from 1-y-old male control and β-catΔex3/+ mice immunostained for β-catenin. SR, stratum radiatum. (Scale bars, 10 µm.) (C) Total levels of β-catenin (sum of wild-type β-catenin and Δexon3 β-catenin) were significantly increased in β-catΔex3/+ mice compared with controls (n = 4 blots from four animals per group; P = 0.017). (D) During initial acquisition of a hidden platform location, escape latencies were similar between β-catΔex3/+ and control mice [repeated-measures (RM) ANOVA, days 1–4, main effect of genotype, P = 0.16]. Following reversal of platform location, β-catΔex3/+ mice showed significantly greater escape latencies compared with controls (P = 0.028; main effect of genotype days 5–9, RM ANOVA). (E) β-catΔex3/+ mice made significantly more entries to the initial platform location over time following learning reversal, indicating impaired spatial memory extinction (RM ANOVA, significant interaction between day and genotype, P = 0.008). (F1–F3) Representative traces of swim paths (n = 15 mice, control; n = 11 mice, β-catΔex3; see also Movie S1). (G) Percent correct choices in a delayed nonmatch to place (DNMTP) T-maze task. Control mice were significantly better than β-catΔex3 mice following 10 d of training (P = 0.026, main effect of genotype days 1–10; RM ANOVA; n = 15 mice, control; n = 16 mice, β-catΔex3/+). (H and I) In the Y-maze task, the number of arm entries (H) and percentage spontaneous alternation (I) were similar between control and β-catΔex3/+ mice (P = 0.98 and P = 0.21, respectively; n = 15 mice, control; n = 16 mice, β-catΔex3/+). (J) No significant difference in acquisition or extinction of context-dependent fear conditioning was observed (day 2, P = 0.70, ANOVA within days; days 2–10, P = 0.65, RM ANOVA; n = 19 mice, control; n = 13 mice, β-catΔex3/+). (K) No difference in novel object preference was observed in β-catΔex3/+ mice (P = 0.86; n = 17 mice, control; n = 14 mice, β-catΔex3/+). Data are shown as mean ± SEM. n.s., not significant; #P < 0.05 (RM ANOVA); *P < 0.05; **P < 0.01; ***P < 0.001 (ANOVA within days).
β-catΔex3/+ mice exhibited an increase in overall β-catenin levels in the hippocampus, including the stratum radiatum region where CA3 pyramidal neurons synapse onto CA1 neurons (Fig. 1B). Using Western blot analysis, we confirmed the expression of both wild-type and stabilized β-catenin in the hippocampus of 1-y-old β-catΔex3/+ mice, the latter of which was slightly smaller due to the deletion of exon 3 (Fig. 1C). The total amount of β-catenin in hippocampal lysates (sum of wild-type and stabilized β-catenin) was significantly increased in β-catΔex3/+ mice compared with age-matched controls (64.0 ± 6.2% increase). Subcellular fractionation of hippocampal lysates showed that β-catenin levels were increased in the synaptosomal (P2) and soluble (S1) fractions from β-catΔex3/+ mice; however, total β-catenin levels remained unchanged in the crude nuclear fraction (P1) (Fig. S1).
β-catΔex3/+ Mice Exhibit Deficits in Reversal Learning and Spatial Memory Extinction in the Morris Water Maze.
We first tested memory in β-catΔex3/+ mice using the Morris water maze (MWM), a test of spatial learning and reference memory that is dependent on intact hippocampal function (25). During initial visible platform training, we found that β-catΔex3/+ mice exhibited similar escape latency times (Fig. 1D) and average swim speed (control = 13.7 ± 0.67 cm/s; β-catΔex3/+= 14.9 ± 0.22 cm/s; P = 0.12) compared with controls, indicating no gross physical impairments in these mice. Initial acquisition of a spatial memory of a hidden platform location was also similar in control and β-catΔex3/+ mice (Fig. 1D, days 1–4). An impairment in escape latency was observed on day 3 of training in β-catΔex3/+ mice, but by day 4 there were no significant differences compared with controls, indicating effective learning of the initial platform location (see also sample traces in Fig. 1F1). We then sought to determine whether reversal learning was impaired in β-catΔex3/+ mice. On day 5 of MWM testing, we moved the hidden platform to the opposite quadrant of the pool (Fig. 1D, days 5–9). Both control and β-catΔex3/+ mice displayed greatly increased escape latency times after the platform was moved from the initial location (Fig. 1D, day 5; see sample traces in Fig. 1F2). Several days after the platform switch (days 6–9), control mice could quickly locate the new platform (Fig. 1D) and had almost completely eliminated entries to the old platform location (Fig. 1E and Fig. 1F3, Upper). In contrast, β-catΔex3/+ mice showed significantly impaired escape latency times, taking more than twice as long to locate the new platform, even after 5 d of training (Fig. 1D, days 5–9), Remarkably, β-catΔex3/+ mice also showed a striking persistence of entries to the old platform location up to 5 d after the platform location switch, indicating an impairment in the extinction of the initial spatial memory (Fig. 1E; see also sample trace in Fig. 1F3, Lower, and Movie S1).
β-catΔex3/+ Mice Exhibit Impaired Behavioral Flexibility in Delayed Nonmatch to Place T-Maze Task.
We next examined the performance of β-catΔex3/+ mice in a delayed nonmatch to place (DNMTP) version of the T-maze, another hippocampal-dependent spatial task that tests behavioral flexibility. On each trial, both goal arms of the T-maze were baited with reward, and mice were placed in the start arm of the maze. Testing consisted of two phases: a “forced” run, in which one goal arm of the maze was blocked and the open arm contained a reward, and a “choice” run, in which both goal arms were open. To receive a reward, the animal had to choose the previously blocked arm that had not yet been visited (DNMTP). Thus, mice had to learn new spatial information in each trial and use that information to correctly locate the food reward, thereby requiring the suppression or elimination of previously learned spatial information similar to the reversal phase of the MWM. Control animals gradually improved at this task, reaching a plateau of 77.4 ± 3.4% average correct choices for the last 3 d. However, β-catΔex3/+ mice showed significantly impaired performance on this task, frequently making errors in the choice run and reaching a plateau of only 57.8 ± 3.7% average correct choices (Fig. 1G). The deficit we observed in DNMTP T-maze performance was not associated with differences in exploratory behavior when on a similar Y-maze apparatus (Fig. 1 H and I). We also observed no differences in contextual fear conditioning (Fig. 1J) or novel object recognition in β-catΔex3/+ mice (Fig. 1K), two tasks that have been shown to involve hippocampal LTP for acquisition of contextual and object location memory. The lack of impairment in these tasks, together with the normal acquisition of initial platform location in the MWM, indicated that stabilization of β-catenin specifically disrupted cognitive flexibility on hippocampal-dependent spatial memory tasks.
LTP Is Intact but LTD Abolished in β-catΔex3/+ Mice.
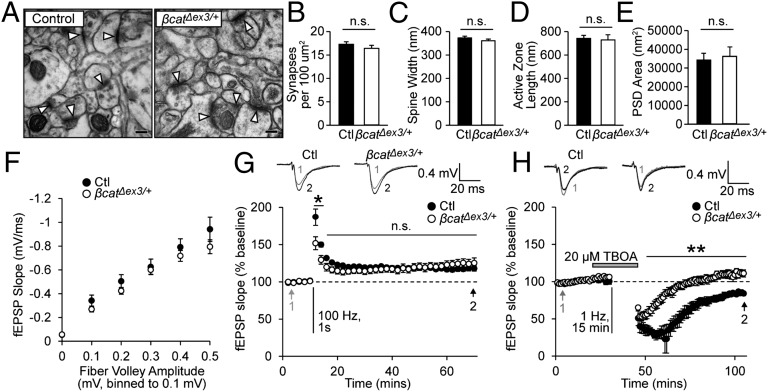
Impairments in spatial learning have frequently been linked to deficits in synaptic plasticity in the hippocampus, so we next determined whether different forms of long-term plasticity were affected by β-catenin stabilization. We first determined whether synapse density and basal synaptic transmission were altered in β-catΔex3/+ mice. We examined synapse ultrastructure in a defined region of the stratum radiatum directly below CA1 pyramidal neurons (Fig. 2A). Because Cre recombinase is expressed in both CA3 and CA1 pyramidal neurons in β-catΔex3/+ mice, β-catenin levels were elevated both presynaptically and postsynaptically at synapses in this region. However, no changes in synapse density (Fig. 2B), dendritic spine head width (Fig. 2C), active zone length (Fig. 2D), or postsynaptic density (PSD) area (Fig. 2E) were detected in β-catΔex3/+ mice. We also observed no difference in the input–output relationship of field excitatory postsynaptic potentials to evoked fiber volley amplitudes at synapses in this region, indicating that basal synaptic transmission was not affected by β-catenin stabilization (Fig. 2F). We observed some deficits in presynaptic responses during repetitive stimulation and an overall increase in synaptic vesicles localized to synapses in β-catΔex3/+ mice (Fig. S2). Because no perturbations in basal synaptic transmission or postsynaptic strength were detected in in β-catΔex3/+ mice, these results suggest that the impaired responses to repetitive stimulation indicate a mild presynaptic impairment in the mobilization, replenishment, or release of synaptic vesicles due to increased levels of β-catenin at the synapse.
Fig. 2.
Basal synaptic transmission and LTP are unchanged, but LTD is abolished in β-catΔex3/+ mice. (A) Electron micrographs of hippocampal synapses. (Scale bar, 100 nm.) Arrowheads indicate synapses. (B–E) No differences in synapse density (B), dendritic spine head width (C), active zone length (D), or PSD area (E) were observed in in β-catΔex3/+ mice (n = 3 mice; >200 synapses). n.s., not significant. (F) Basal synaptic transmission was unaffected in β-catΔex3/+ mice (control, n = 9 slices for five mice; β-catΔex3/+, n = 10 slices for six mice). (G) LTP was intact in β-catΔex3/+ mice (P = 0.60, RM ANOVA; n = 6 slices for six mice, control and β-catΔex3/+). (H) A 1-Hz stimulation induced LTD in controls, but LTD was abolished in β-catΔex3/+ mice (P = 0.012, RM ANOVA; n = 3 slices for three mice, control; n = 5 slices for five mice, β-catΔex3/+). Data are shown as mean ± SEM. #P < 0.05 (RM ANOVA); *P < 0.05, **P < 0.01 (Bonferroni’s test post hoc).
We assayed LTP induced by using brief high-frequency stimulation (HFS; 100 Hz, 1 s). In slices from control and β-catΔex3/+ mice, we observed a persistent increase in synaptic strength up to 60 min after HFS, with no significant differences detected between the two groups (Fig. 2G). The LTP observed (∼20%) was consistent with previous studies showing reduced magnitude of LTP in aged mice (26). After HFS, there was a transient impairment in responses in β-catΔex3/+ mice, but no significant differences in LTP were detected 50–60 min after HFS, indicating that long-term increases in postsynaptic strength were not affected in β-catΔex3/+ mice. We then examined LTD, because disruption of LTD has been shown to produce impairments in reversal learning and behavioral flexibility similar to the deficits observed in β-catΔex3/+ mice (2, 3). We induced LTD by low-frequency stimulation (1 Hz, 900 stimuli) in the presence of 20 µM threo-β-benzylaspartic acid (TBOA), a competitive blocker of glutamate transporters (27). In slices from control mice, we observed a decrease in synaptic strength that persisted >1 h after stimulation, but in β-catΔex3/+ mice this long-lasting depression in synaptic strength was completely abolished (Fig. 2H).
Activity-Dependent Endocytosis of Cadherin and GluA1 Is Impaired in β-catΔex3/+ Mice.
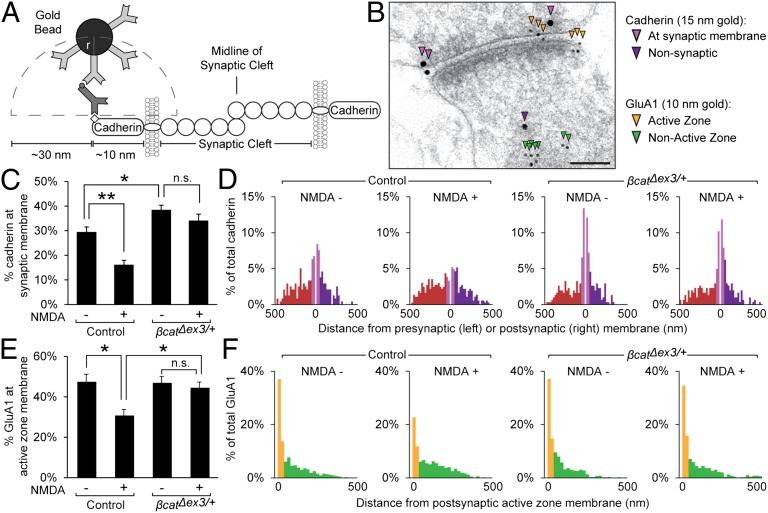
To elucidate the mechanism underlying deficits in LTD and cognitive flexibility in β-catΔex3/+ mice, we sought to determine whether enhanced β-catenin stabilization could be impairing the activity-induced internalization of cadherin and associated AMPA receptors. We treated acute hippocampal slices with 20 μM NMDA for 3 min (chemical LTD)—which has been shown to induce widespread downscaling of synapse strength (28) and endocytosis of AMPA receptors (5, 29)—and then used immunogold electron microscopy to quantify the resulting changes in the distribution of cadherin and GluA1 subunits in these slices. Immunogold labeling allows for extremely precise spatial resolution of targets at the synapse; empirical studies have shown that antibody-conjugated immunogold particles are localized to within ∼30 nm of epitopes identified (30). Consequently, to analyze the amount of GluA1 situated to respond to presynaptic neurotransmitter release, we quantified the proportion of immunogold-labeled GluA1 (10-nm beads) within only 30 nm of the postsynaptic active zone membrane. Similarly, because the C-terminal tail of cadherin could be located up to 10 nm from the synaptic membrane, we have considered immungold particles (15-nm beads) within 40 nm from the synaptic membrane to represent cadherin molecules that are situated to participate in transsynaptic adhesion (Fig. 3 A and B).
Fig. 3.
Cadherin and GluA1 endocytosis following NMDA treatment is significantly impaired in β-catΔex3/+ mice. (A) Estimated sizes of immunogold reagents (adapted from ref. 30). (B) Electron micrograph of hippocampal synapse from control mice showing immunogold-labeled cadherin and GluA1. (Scale bar, 100 nm.) (C) The percentage of cadherin at the synaptic membrane was significantly decreased in control mice following NMDA treatment, but not β-catΔex3/+ mice, indicating stabilization of cadherin at synapses (P < 0.0001, ANOVA; n = 3 mice, four sections per condition, and >100 synapses per group). (D) Histograms of immungold-labeled cadherin distances from synaptic membranes. (E) The percentage of GluA1 localized to the postsynaptic active zone membrane was significantly decreased in control mice following NMDA treatment, but not β-catΔex3/+ mice. (P = 0.079, ANOVA; n = 3 mice, four sections per condition, >100 synapses per group). (F) Histograms of immungold-labeled GluA1 distances from postsynaptic membrane. Data are shown as mean ± SEM. n.s., not significant; *P < 0.05; **P < 0.01 (Tukey’s test post hoc).
Although we observed no difference in total amount of cadherin and GluA1, the proportion of cadherin localized to the synaptic membrane under basal conditions (within 40 nm) was significantly increased in β-catΔex3/+ (Fig. 3 C and D; control, 29.3 ± 2.23%; β-catΔex3/+, 38.3 ± 2.03%), whereas the proportion of GluA1 localized to the active zone was similar in control and β-catΔex3/+mice (control, 47.2 ± 5.1%; β-catΔex3/+, 46.7 ± 2.4%). The lack of change in GluA1 under basal conditions was consistent with data showing similar postsynaptic strength under basal conditions in control and β-catΔex3/+ mice (Fig. 2A). Following NMDA treatment, there was a striking reduction in the proportion of immunogold-labeled cadherin at the synaptic membrane (Fig. 3 C and D) and GluA1 at the active zone (Fig. 3 E and F) in acute hippocampal slices from control mice, consistent with the expected endocytosis of AMPA receptors and cadherin (5). In contrast, in β-catΔex3/+ mice, we observed no significant change in the proportion of immunolabeled cadherin at the synaptic membrane following NMDA treatment (Fig. 3 C and D) or GluA1 at the active zone (Fig. 3 E and F). Analysis of immunogold particle localization by frequency distribution (Fig. 3 D and F) further demonstrated that, following NMDA treatment in control mice, there was a redistribution of cadherin and GluA1 to the nonsynaptic or “recycling” population, indicating activity-dependent endocytosis, but that this redistribution was impaired in β-catΔex3/+ mice.
Cadherin/β-catenin Interactions at Synapses Are Increased in β-catΔex3/+ Mice.
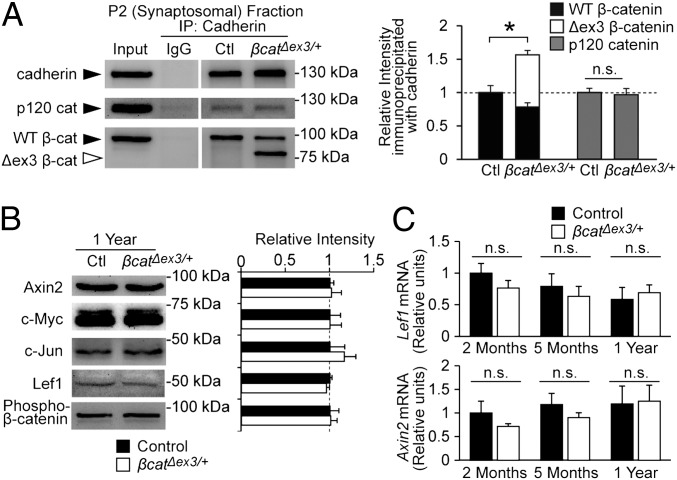
To confirm the mechanism by which stabilization of β-catenin was responsible for the impairment in cadherin and AMPA receptor endocytosis observed in β-catΔex3/+ mice, we next examined β-catenin/cadherin interactions directly by immunoprecipitation. Synaptosomal fractions from hippocampal lysates were immunoprecipitated with anticadherin antibodies and analyzed by Western blot. Both wild-type and stabilized forms of β-catenin immunoprecipitated with cadherin, and a significant increase in total β-catenin associated with cadherin was observed in β-catΔex3/+ mice (Fig. 4A; 56.5 ± 13.0% increase), indicating enhanced β-catenin/cadherin association at synapses. Increasing β-catenin levels in the brain did not significantly impact cadherin’s association with p120 catenin, another component of the cadherin adhesion complex (Fig. 4A). There were also no differences in expression of components of the cadherin adhesion complex (cadherin, p120 catenin), postsynaptic proteins (PSD-95, GluR2, and NR1), or synaptic vesicle proteins (synaptophysin, synaptotagmin, and synapsin I) (Fig. S3). Thus, stabilization of β-catenin enhances β-catenin's association with cadherin at synapses but does not perturb overall synaptic protein expression in the hippocampus.
Fig. 4.
β-catenin/cadherin interaction is significantly increased, and expression of Wnt pathway targets is unchanged in β-catΔex3/+ mice. (A) Representative immunoblots and quantification of synaptosomal fractions from hippocampal lysates immunoprecipitated with anti–pan-cadherin. There was an increase in total cadherin-associated β-catenin (wild-type β-catenin plus Δex3 β-catenin; P = 0.029), but not p120 catenin. (B) Representative immunoblots and quantification for Wnt pathway targets in hippocampal lysates from 1-y β-catΔex3/+ mice and controls (n = 3 blots; three separate mice for each condition). No significant differences in Wnt target expression or N-terminal phosphorylation (Ser-33/37, Thr-41) of wild-type β-catenin was observed in β-catΔex3/+ mice. (C) RT-PCR analysis of relative Lef1 and Axin2 mRNA levels from the hippocampus of 2-mo-old, 5-mo-old, and 1-y-old mice. Expression is reported in relative units normalized to GAPDH expression (2 mo: n = 4 for β-catΔex3/+ and control; 5 mo: n = 4 for β-catΔex3/+ and control; 1 y: n = 3 for β-catΔex3/+and 4 for control). See also Fig. S4. Data are shown as mean ± SEM. n.s., not significant; *P < 0.05.
Wnt Signaling Is Not Affected in β-catΔex3/+ Mice.
We then determined whether any changes in Wnt signaling may have contributed to the impairments observed in β-catΔex3/+ mice. We examined the expression of several known Wnt targets by Western blot analysis (Fig. 4B) and RT-PCR (Fig. 4C) but found no changes in Wnt target expression in the hippocampus of β-catΔex3/+ mice. We also examined Wnt target expression by immunohistochemistry, but saw no changes in expression in the dentate gyrus or other subregions of the hippocampus (Fig. S4). These findings were in accord with the observations that β-catenin levels in the P1 crude nuclear fraction were similar between β-catΔex3/+ mice and controls and expression of synaptic proteins was unchanged (Figs. S1 and S3). We therefore concluded that Wnt signaling was not augmented in β-catΔex3/+ mice, providing further support that impaired cadherin and AMPA receptor endocytosis following β-catenin stabilization was responsible for the deficits in LTD and cognitive flexibility observed in β-catΔex3/+ mice.
Discussion
In the present study, we demonstrate that stabilization of β-catenin in the adult hippocampus is sufficient to cause significant disruption of synaptic plasticity and cognitive flexibility. The results from this study support three main conclusions. First, increased levels of β-catenin in the hippocampus leads to significant deficits in spatial memory flexibility and reversal learning. Second, β-catenin stabilization is sufficient to abolish LTD at hippocampal synapses, while leaving basal synapse function and LTP intact. Third, increased β-catenin/cadherin interaction at synapses results in impaired activity-dependent endocytosis of cadherin and AMPA receptors at synapses, which appears to be the primary mechanism responsible for these synaptic and cognitive impairments because Wnt signaling was not perturbed in β-catΔex3/+ mice. Together, these results indicate that aberrant increases in the stability of synaptic adhesion molecules can have a negative effect on synaptic plasticity and cognitive function.
The behavioral deficits we observed in β-catΔex3/+ mice are all consistent with a specific impairment in hippocampal LTD and a subsequent impairment in spatial memory plasticity. Behavioral tasks that have been shown to involve hippocampal LTP or nonhippocampal brain regions were largely unaffected in β-catΔex3/+ mice, including contextual fear conditioning, novel object recognition, and initial acquisition of spatial memory in the MWM. Our data support the hypothesis that LTD acts to depotentiate synapses to facilitate the acquisition of novel information (2); we observed that β-catΔex3/+ mice exhibited impairments in reversal learning in the MWM and behavioral flexibility on the DNMTP T-maze—two tasks that involve the modification or elimination of spatial memory rather than simply memory acquisition. The most intriguing result from the present study was the persistence of β-catΔex3/+ mice in entries to the old learned platform location in the reversal phase of the MWM, indicating that the deficit in acquiring novel spatial information was due to an inability to eliminate previously learned spatial information. This finding suggests that transient changes in β-catenin/cadherin– mediated adhesion and stability are critical for the restructuring of synapses underlying cognitive and behavioral flexibility. Indeed, previous studies have shown that following enhanced activity a transient disruption of β-catenin association with cadherin precedes increased association of the two proteins (12), suggesting a window of structural plasticity exists that facilitates normal experience-induced changes in synapse strength.
Our data indicate that the impairments in LTD observed in β-catΔex3/+ mice were due to increased association of β-catenin with cadherin at hippocampal synapses, resulting in impaired activity-dependent endocytosis of both cadherin and associated AMPA receptors. Cadherins form both direct and indirect associations with AMPA receptors (31–33), and stabilizing β-catenin/cadherin interactions in vitro has been shown to prevent the internalization of both cadherin and AMPA receptors during conditions that induce LTD (5). Interestingly, mGluR-dependent LTD has also been shown to require interaction between N-cadherin and the GluR2 subunit of AMPA receptors, providing further evidence of an important functional relationship between cadherin and AMPA receptors at synapses (33). Because LTD is achieved through removal of AMPA receptors from the postsynaptic membrane (34), the physiological impairment in LTD observed in β-catΔex3/+ mice is consistent with the deficits in AMPA receptor endocytosis observed by immunogold electron microscopy. This model of synaptic dysfunction is also consistent with the lack of impairment in hippocampal LTP in β-catΔex3/+ mice; enhanced cadherin stability at the synaptic membrane is unlikely to affect the insertion of additional AMPA receptors to the synaptic membrane, which is the primary mechanism responsible for LTP (34). Interestingly, basal synaptic transmission and postsynaptic strength were unaffected by the stabilization of cadherin in β-catΔex3/+ mice, and previous studies have shown that knockdown of N-cadherin (35, 36) or β-catenin (23) in vivo also did not significantly impact synapse number or basal synaptic transmission. These findings suggest that, although cadherin is important for activity-dependent plasticity, other mechanisms play a more dominant role in determining basal synapse density and synaptic strength. Furthermore, ablation of N-cadherin in vivo has been shown to impair spine enlargement and LTP, but does not affect LTD (36), compared with our study in which cadherin stabilization results in intact LTP but impaired LTD. Together, these studies suggest a consistent model where N-cadherin must be present to stabilize synapses and AMPA receptors to mediate LTP, but must be transiently destabilized or removed from synapses to facilitate LTD.
We observed no difference in Wnt target expression in β-catΔex3/+ mice, indicating that changes in Wnt signaling did not contribute to the physiological and behavioral changes observed in these animals, although the stabilized form of β-catenin lacking exon 3 is competent for Wnt signaling (17, 20). Why was Wnt signaling unaffected in the hippocampus of β-catΔex3/+ mice? β-catenin stabilization was also restricted to adult, differentiated neurons, which are less sensitive to changes in β-catenin levels (37), and much of the available β-catenin was localized to synapses due to its association with cadherin. Finally, nuclear transport of β-catenin may also be more carefully regulated in neurons (38), and levels of Lef1 are relatively low in the hippocampus compared with other brain regions (39), reducing the sensitivity of Wnt signaling to changes in levels of cytoplasmic β-catenin (39).
The findings of the present study indicate that changes in β-catenin and cadherin stability can have pronounced effects on synapse function and, more generally, demonstrate that increases in synaptic adhesion can have a detrimental effect on normal synaptic plasticity and cognition. Increased levels of β-catenin have been reported in a wide variety of neurological disorders, including AD (14, 15), HD (13), and alcoholism (40), but the contribution of β-catenin to these disorders remains unclear. In HD, β-catenin levels are significantly increased in the brains of HD patients as well as in mouse and Drosophila models of HD, and targeted reduction of β-catenin was shown to have a therapeutic effects (13). Intriguingly, deficits in reversal learning due to perseveration similar to those observed in β-catΔex3/+ mice have been reported in both mouse models of HD and in HD patients (41). Together with these studies, our work supports the hypothesis that enhanced levels of β-catenin may contribute to the pathology of HD. Also, lithium, a widely used antipsychotic, is known to inhibit GSK3β, stabilizing β-catenin in neurons (42). Increased Wnt signaling is believed to exert a therapeutic effect in these cases, but data from the present study lead us to speculate that enhanced structural stability at synapses may also contribute. Because alterations in β-catenin levels can have significant effects on synaptic plasticity and memory, understanding the contribution of β-catenin and synaptic adhesion in individual neurological disorders may provide important insights into disease pathology and therapeutic approaches.
Materials and Methods
See SI Materials and Methods for a detailed description of transgenic mice, behavioral testing immunohistochemistry, immunoblot analysis, subcellular fractionation, coimmunoprecipitation, immunogold electron microscopy, NMDA-evoked endocytosis assay, electrophysiology, RNA real-time PCR, and statistical analysis.
Supplementary Material
Acknowledgments
This work was supported by Canadian Institutes of Health Research Grants MOP-81158 (to S.X.B.) and MOP-38090 (to Y.T.W.), American Alzheimer’s Association Grant NIRG-07-58917 (to S.X.B.), the Alzheimer Society of Canada (S.X.B.), and Polish Ministry of Science and Higher Education Grants 4245/B/P01/2010/38 (to M.B.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404670111/-/DCSupplemental.
References
- 1.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls RE, et al. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58(1):104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Dong Z, et al. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 2013;64:65–73. doi: 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Brigidi GS, Bamji SX. Cadherin-catenin adhesion complexes at the synapse. Curr Opin Neurobiol. 2011;21(2):208–214. doi: 10.1016/j.conb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54(5):771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28(1):245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 7.Tang L, Hung CP, Schuman EM. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20(6):1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 8.Schrick C, et al. N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron. 2007;55(5):786–798. doi: 10.1016/j.neuron.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem. 2001;276(15):12301–12309. doi: 10.1074/jbc.M010377200. [DOI] [PubMed] [Google Scholar]
- 10.Murase S, Mosser E, Schuman EM. Depolarization drives beta-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35(1):91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- 11.Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174(2):289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11(11):1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godin JD, Poizat G, Hickey MA, Maschat F, Humbert S. Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington’s disease. EMBO J. 2010;29(14):2433–2445. doi: 10.1038/emboj.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano S, et al. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001;152(4):785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li HL, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci USA. 2007;104(9):3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18(21):5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aberle H, Schwartz H, Hoschuetzky H, Kemler R. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J Biol Chem. 1996;271(3):1520–1526. doi: 10.1074/jbc.271.3.1520. [DOI] [PubMed] [Google Scholar]
- 19.Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105(3):391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 20.Li C, et al. Stabilized beta-catenin in lung epithelial cells changes cell fate and leads to tracheal and bronchial polyposis. Dev Biol. 2009;334(1):97–108. doi: 10.1016/j.ydbio.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiser PW, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135(4):1288–1300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu B, et al. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: Modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20(18):6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamji SX, et al. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40(4):719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsien JZ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87(7):1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 25.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 27.Massey PV, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24(36):7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21(5):1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 29.Beattie EC, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3(12):1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 30.Mathiisen T, et al. Postembedding Immunogold cytochemistry of membrane molecules and amino acid transmitters in the central nervous system. In: Zaborszky L, Wouterlood F, Lanciego J, editors. Neuroanatomical Tract-Tracing 3. New York: Springer; 2006. pp. 72–108. [Google Scholar]
- 31.Nuriya M, Huganir RL. Regulation of AMPA receptor trafficking by N-cadherin. J Neurochem. 2006;97(3):652–661. doi: 10.1111/j.1471-4159.2006.03740.x. [DOI] [PubMed] [Google Scholar]
- 32.Saglietti L, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54(3):461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Hu J, Passafaro M, Xie W, Jia Z. GluA2 (GluR2) regulates metabotropic glutamate receptor-dependent long-term depression through N-cadherin-dependent and cofilin-mediated actin reorganization. J Neurosci. 2011;31(3):819–833. doi: 10.1523/JNEUROSCI.3869-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5(12):952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 35.Jüngling K, et al. N-cadherin transsynaptically regulates short-term plasticity at glutamatergic synapses in embryonic stem cell-derived neurons. J Neurosci. 2006;26(26):6968–6978. doi: 10.1523/JNEUROSCI.1013-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozdagi O, et al. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci. 2010;30(30):9984–9989. doi: 10.1523/JNEUROSCI.1223-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kratz JE, et al. Expression of stabilized beta-catenin in differentiated neurons of transgenic mice does not result in tumor formation. BMC Cancer. 2002;2:33. doi: 10.1186/1471-2407-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmeisser MJ, Grabrucker AM, Bockmann J, Boeckers TM. Synaptic cross-talk between N-methyl-D-aspartate receptors and LAPSER1-beta-catenin at excitatory synapses. J Biol Chem. 2009;284(42):29146–29157. doi: 10.1074/jbc.M109.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wisniewska MB, et al. LEF1/beta-catenin complex regulates transcription of the Cav3.1 calcium channel gene (Cacna1g) in thalamic neurons of the adult brain. J Neurosci. 2010;30(14):4957–4969. doi: 10.1523/JNEUROSCI.1425-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Housseini AM, et al. Upregulation of beta-catenin levels in superior frontal cortex of chronic alcoholics. Alcohol Clin Exp Res. 2008;32(6):1080–1090. doi: 10.1111/j.1530-0277.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 41.Lione LA, et al. Selective discrimination learning impairments in mice expressing the human Huntington’s disease mutation. J Neurosci. 1999;19(23):10428–10437. doi: 10.1523/JNEUROSCI.19-23-10428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29(1):32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]