Significance
PII, a signal transduction protein involved in nitrogen control in bacteria and plants, and NtcA, the transcriptional nitrogen regulator of cyanobacteria, can form complexes with PII interacting protein X (PipX). We demonstrate by a combination of genetic, transcriptomic, and multivariate analyses that PipX is involved in a much wider interaction network affecting nitrogen assimilation, translation, and photosynthesis. Two groups of genes differentially regulated by pipX provided further insights into the function of NtcA–PipX complexes and an improved definition of the consensus NtcA binding motif. The other four groups suggested the involvement of PipX in NtcA-independent regulatory pathways. Our results pave the way to uncover new regulatory interactions and mechanisms in the control of gene expression in cyanobacteria.
Keywords: nitrogen regulation, transcription, translation, photosynthesis
Abstract
To modulate the expression of genes involved in nitrogen assimilation, the cyanobacterial PII-interacting protein X (PipX) interacts with the global transcriptional regulator NtcA and the signal transduction protein PII, a protein found in all three domains of life as an integrator of signals of the nitrogen and carbon balance. PipX can form alternate complexes with NtcA and PII, and these interactions are stimulated and inhibited, respectively, by 2-oxoglutarate, providing a mechanistic link between PII signaling and NtcA-regulated gene expression. Here, we demonstrate that PipX is involved in a much wider interaction network. The effect of pipX alleles on transcript levels was studied by RNA sequencing of S. elongatus strains grown in the presence of either nitrate or ammonium, followed by multivariate analyses of relevant mutant/control comparisons. As a result of this process, 222 genes were classified into six coherent groups of differentially regulated genes, two of which, containing either NtcA-activated or NtcA-repressed genes, provided further insights into the function of NtcA–PipX complexes. The remaining four groups suggest the involvement of PipX in at least three NtcA-independent regulatory pathways. Our results pave the way to uncover new regulatory interactions and mechanisms in the control of gene expression in cyanobacteria.
Cyanobacteria are phototrophic organisms that perform oxygenic photosynthesis. Autotrophic growth requires the constant assimilation of ammonium via the glutamine synthetase–glutamate synthase cycle, resulting in consumption of the 2-oxoglutarate (2-OG) (1, 2) that accumulates during nitrogen starvation, making this metabolite an excellent indicator of the intracellular carbon-to-nitrogen balance (3, 4). The 2-OG, the signal of nitrogen deficiency, modulates the activity and/or binding properties of three key cyanobacterial nitrogen regulators: the signal transduction protein PII; the transcriptional activator NtcA; and PipX, a regulatory factor that can interact with either NtcA or PII.
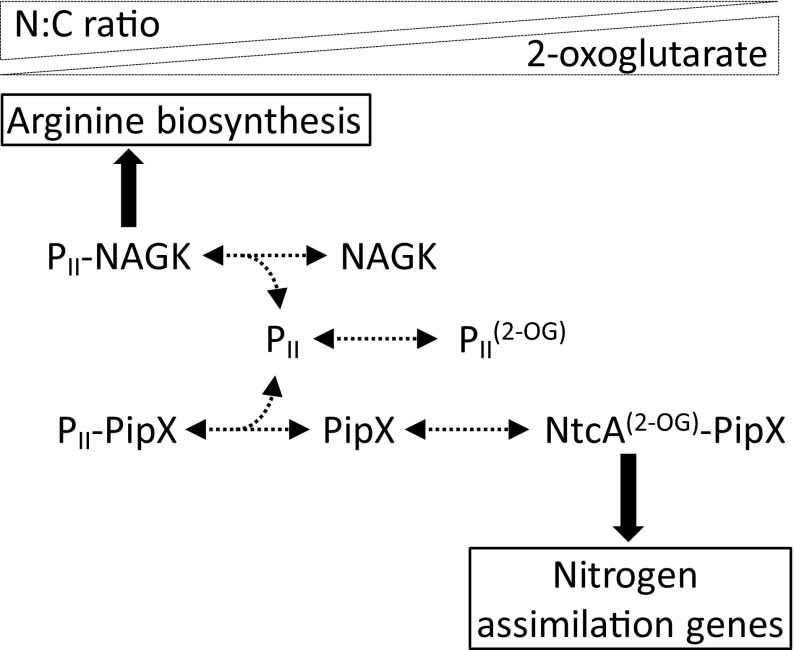
The homotrimeric PII protein, one of the most conserved and widespread signal transduction proteins in nature, plays key roles in nitrogen assimilatory processes (5). PII contains three binding sites (one per subunit) for 2-OG and ATP (6, 7), and it regulates the activity of N-acetyl-glutamate-kinase (NAGK), a key enzyme for biosynthesis of arginine, by direct protein–protein interactions (3, 8, 9). The 2-OG stimulates binding of NtcA to target sites (10), transcription activation in vitro (11), and complex formation between NtcA and PipX (12). The interaction between PipX and NtcA is known to be relevant under nitrogen limitation for activation of NtcA-dependent genes in Synechococcus elongatus and Anabaena sp. PCC 7120 (hereafter, Anabaena) (12–14). The NtcA–PipX complex consists of one active (2-OG–bound) NtcA dimer and two PipX molecules. Each NtcA subunit binds one PipX molecule in such a way that it stabilizes the active NtcA conformation and probably helps recruit RNA polymerase without providing extra DNA contacts (15, 16). The tudor-like domain of PipX provides the contacts for both NtcA–PipX and PII–PipX interactions. When nitrogen is abundant, intracellular levels of 2-OG are low and sequestration of PipX by PII decreases NtcA–PipX complex formation. A summary of the interactions involving NtcA, PipX, or PII is shown in Fig. 1.
Fig. 1.
PipX swapping model and the nitrogen interaction network. The functions and interactions mediated by PipX according to the 2-OG levels are schematized.
The PipX partner-swapping model predicts that, at least under the physiological range of 2-OG levels, pipX mutations specifically impairing PipX–PII complexes would favor formation of NtcA–PipX complexes. Crystal structures of PipX–PII complexes, surface plasmon resonance, PII-stimulated NAGK activity assays, and yeast two-hybrid analysis established the importance of PipX residues Y32 and E4 for interactions with PII proteins and of Y32 for interactions with NtcA (15, 17). Reporter and transcript analyses indicated that both Y32A and E4A mutations had stimulatory effects on the NtcA-activated genes glnB, glnN, and nblA but did not address differences between the in vivo action of PipXE4A and PipXY32A, two proteins with different biochemical properties. Here, we show that the in vivo properties of PipXE4A and PipXY32A are indeed very different and that these differences affect both NtcA-dependent and NtcA-independent genes.
The 2-OG–dependent partner swapping of PipX between PII and NtcA provides a mechanistic link between PII signaling and NtcA-regulated gene expression but does not exclude the possibility that PipX, either by itself or bound to PII, could participate in additional protein–protein interactions influencing gene expression. To address the question of whether PipX affects S. elongatus gene expression in an NtcA-independent manner, we compared transcript profiles of pipX mutants in cultures grown with either ammonium or nitrate. In these conditions, and to a greater extent in ammonium, the levels of 2-OG are relatively low, NtcA is mainly inactive, and null pipX mutations are not expected to have a significant impact on the NtcA regulon (13). We reasoned that the pipXE4A and pipXY32A mutations provide a means to bypass the need to use ntcA mutants and/or conditions of nitrogen deprivation to identify NtcA target genes. Most relevant to this work, the combined analyses of pipX mutant strains enabled us to identify additional regulatory targets of PipX in a context in which we could further characterize the NtcA regulon.
Results and Discussion
Global Phenotypic Impact of pipX Mutations Under Nitrogen-Rich Conditions.
The involvement of PipX in gene expression and its potential roles in global regulation other than nitrogen control by NtcA were investigated in S. elongatus strains grown in either nitrate or ammonium and carrying either a null (ΔpipX) or point mutation (pipXY32A or pipXE4A) derivative. Under these nitrogen regimes, particularly in ammonium, the concentration of 2-OG is expected to be relatively low and transcriptional regulation by NtcA–PipX complexes would not have widespread importance in WT cells. Because the pipXY32A or pipXE4A allele is associated with a resistance marker (C.S3 cassette) that decreases pipX gene expression (18), a WT derivative with the same insertion in the identical position (strain CS3X) was required to perform isogenic mutant/control comparisons. Therefore, two strains were used as WT controls: WT S. elongatus for mutant strain SA591, which carries the ΔpipX allele, and CS3X for strains CS3XE4A and CS3XY32A, which carry the pipXY32A and pipXE4A alleles, respectively (SI Appendix, Table S1). A total of six global transcriptome comparisons were carried out, considering three mutant/control pairs for each of the two nitrogen regimes: ammonium and nitrate.
Scatter plots of log2 fold changes of pipX mutants vs. their respective controls are represented in Fig. 2. For each mutant/control comparison, subsets of genes that are up-regulated or down-regulated more than fourfold in the mutants only in nitrate, only in ammonium, or in both conditions are highlighted. Interestingly, many genes were differentially regulated in the ΔpipX mutant, and for each condition, there were more genes up-regulated than down-regulated in the absence of an active pipX allele, suggesting that PipX participates more frequently in negative regulation than in positive regulation under the experimental conditions tested (Fig. 2A). Whereas the global impact of pipXY32A on gene expression seemed roughly similar to the effect of the ΔpipX allele, the effect of pipXE4A was restricted to a smaller number of genes and, importantly, most of those genes were up-regulated in both nitrate and ammonium (Fig. 2 B and C).
Fig. 2.
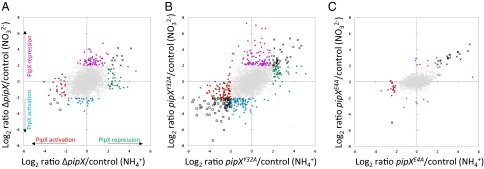
Effect of pipX mutations on nitrate and ammonium transcriptomes. The mutant/control log2 ratios in nitrate vs. ammonium are represented as a scatter plot. (A) ΔpipX/WT with indication of the inferred PipX functions. (B) CS3XY32A/CS3X. (C) CS3XE4A/CS3X. Genes shown in colors and in gray represent above and below, respectively, the cutoff for a log2 ratio >2 (absolute values). Positive values in nitrate (purple), ammonium (green), or both conditions (x) and negative values in nitrate (blue), ammonium (red), or both conditions (□) are shown.
The results supported a role for PipX in both negative and positive regulation of multiple target genes in S. elongatus under conditions of nitrogen sufficiency and demonstrate that the mutations pipXY32A and pipXE4A have very different effects on gene expression. To gain deeper insights into the functions of PipX in vivo while adding robustness to the analysis, we next analyzed combined information from all 10 transcript datasets.
Multivariate Analysis of S. elongatus Transcripts.
To identify groups of genes with discrete expression patterns defined by the ΔpipX, pipXE4A, or pipXY32A alleles, we performed multivariate analysis with standardized residuals from linear regressions of data (log-transformed) from mutant vs. control strains (both CS3XE4A and CS3XY32A vs. CS3X and SA591 vs. WT) cultured in the presence of either ammonium or nitrate. First, we demonstrated that only a small proportion of the transcriptome responded to the nitrogen source and/or to pipX alleles. A total of 1,663 genes with residuals lower than 1.5 were considered as nonresponsive in any of the six mutant/control comparisons, and the distributions of their residuals were fitted well by truncated normal distributions (gray dots in SI Appendix, Fig. S1 and Table S3). For the ammonium and nitrate conditions, 1,958 and 2,052 genes, respectively, were nonresponsive in any of the three mutant/control comparisons. From the group of genes with residuals greater than 1.5, only those with residuals exceeding a threshold value of 2.5 for at least one of the six variables were selected as being differentially regulated. The resulting 282 genes, after excluding pipX itself, were individually analyzed to discard those with reads mapping mainly to the noncoding strand (Dataset S1), bringing the number down to 257 genes.
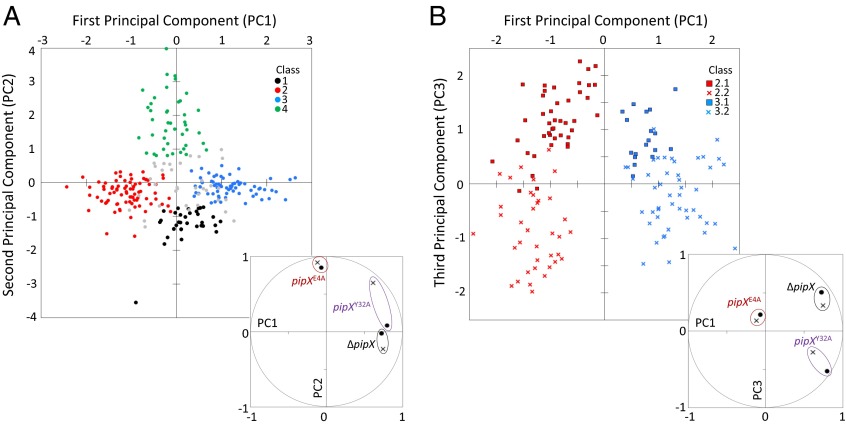
To explore the existence of different expression profiles within the 257 differentially regulated genes further, principal component analysis (PCA) of residuals was used to extract the first two principal components, accounting for about 70% of the total variance. The plot of the data for these two components (Fig. 3A) suggested the classification of the 257 genes into four main groups (classes 1, 2, 3, and 4), defined by using k-means cluster analysis. As shown in Fig. 3A (Inset), changes in expression for ΔpipX in both ammonium and nitrate and for pipXY32A mainly in ammonium were associated with the first axis (PC1). In contrast, changes for pipXE4A in both ammonium and nitrate and, to a lesser extent, for pipXY32A in nitrate, were associated with the second component (PC2).
Fig. 3.
Multivariate analysis and clustering of differentially expressed genes. (A) Scores for the two first principal components in the PCA of standardized residuals from mutant/control comparisons for the 257 genes with residuals larger than 2.5 in at least one comparison. Classified and nonclassified genes are colored and gray, respectively. (Inset) Scatter of mutant/control comparisons plotted as the correlation coefficients between them and the first two principal components in the unit circle. Nitrate (x) and ammonium (●) are shown. (B) Same as in A, but the first and third principal components in the PCA are represented only for genes classified originally in classes 2 and 3. Different symbols and colors identify genes from the final classes (2.1, 2.2, 3.1, and 3.2). (Inset) Same as in A, but PC2 is replaced by PC3.
The relatively large sizes of the groups and the wide distribution of the dataset, particularly in classes 2 and 3, prompted us to use additional classification criteria to obtain better-defined groups with smaller numbers of similarly regulated genes. This was done by defining an additional four groups according to independent clustering by hierarchical Ward’s minimum variance and fuzzy c-means methods. Only those genes that were coherently grouped into the same class with the three clustering methods were retained. The cluster dendrogram from Ward’s method was then cut to produce six coherent groups comprising a total of 222 genes.
As a result of this classification process, the four original groups became six groups. The 35 genes that fell outside these groups (Fig. 3A, gray dots, and Dataset S2) included the genes with the lowest expression levels (among the 257 differentially regulated genes), as well as genes with rather unique expression patterns. Only class 4 (43 genes) was left untouched, although class 1 lost some members (32 genes remained) and classes 2 and 3 (losing 10 and 17 genes, respectively) were each split into two new classes: 2.1 (37 genes), 2.2 (43 genes), 3.1 (18 genes), and 3.2 (49 genes) (Datasets S3–S8). Differences between the new classes originating from a common class, that is, 2.1 vs. 2.2 and 3.1 vs. 3.2, were largely related to the third principal component (Fig. 3B, PC3 axis), which accounted for an additional 13% of the total variance and was mainly correlated with differences in expression provided by ΔpipX and pipXY32A alleles, with the ammonium conditions providing the greatest differences in these mutant control comparisons. Hereafter, each of the six classes was treated and analyzed as a distinct class.
NtcA-Activated Genes and NtcA Binding Motifs in S. elongatus.
The distinctive feature of class 4 transcripts was their up-regulation in CS3XE4A in both nitrate and ammonium and in CS3XY32A only in nitrate (Figs. 3A and 4A). Notably, a rather small, but still significant, down-regulation in the ΔpipX strain was observed in nitrate. Class 4 comprised most of the paradigmatic nitrogen assimilation genes or operons from S. elongatus, that is, gene targets known or predicted to be activated by NtcA–PipX complexes under conditions of nitrogen deficiency (SI Appendix, Table S2). They include ntcA itself, genes encoding the key glutamine synthetase enzyme glnA, ammonium transporters amtB and amt1, components of the nitrate transport and assimilation system nirA/nrtABCD/narB, and components of the cyanate transport and assimilation system cynABD and cynS. The transcriptional response in all five genetic backgrounds and two nitrogen regimes is illustrated in Fig. 4B for two paradigmatic NtcA target genes: ntcA, which is autogenously regulated, and nirA, which encodes nitrite reductase. Both genes were similarly affected by the mutant alleles ΔpipX, pipXY32A, and pipXE4A under each of the nitrogen regimes tested.
Fig. 4.
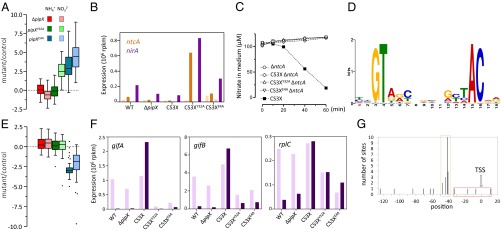
Gain-of-function mutations pipXE4A and pipXY32A target NtcA-regulated genes. (A) Box plot representation (median, box from first to third quartile and Tukey whiskers) of the within-group variance of mutant/control comparisons in class 4. (B) Expression pattern of the ntcA and nirA genes in ammonium (light-colored bars) and nitrate (dark-colored bars). (C) Effect of ntcA inactivation on nitrate uptake. Nitrate was added at time 0 to cell suspensions containing 20 μg of chlorophyll a per milliliter. A representative experiment is shown. (D) WebLogo based on 30 NtcA binding motifs identified with MEME upstream of sequences from class 4 genes. (E) Box plot representation of class 1 expression patterns. Outliers are represented by dots. (F) Expression patterns of class 1 genes gifA, gifB, and rplC. Light and dark bars are used for ammonium and nitrate, respectively. (G) Positioning of 29 NtcA boxes (SI Appendix, Table S2) relative to the TSS with activation (green) and repression (red) functions (details are provided in SI Appendix, Fig. S3 A and B).
Class 4 expression patterns suggest that WT PipX can weakly activate some NtcA target genes in nitrate but not in ammonium-grown cells. In contrast, PipXY32A and, to a greater extent, PipXE4A appear to be competent to interact with NtcA and coactivate target promoters at low concentrations of 2-OG. Our interpretation of the effects of pipXY32A and pipXE4A alleles on the expression of NtcA gene targets is that the lower affinity of PipXE4A or PipXY32A (compared with PipX) for PII would result in increased concentration of the mutant NtcA–PipX complexes, and consequent activation of NtcA targets in nitrate-grown cells. In addition, the lower affinity of PipXY32A (compared with PipXE4A) for NtcA would account for the nitrogen regulation observed in CS3XY32A (i.e., the differences between nitrate and ammonium cultures). This result suggests that the mutant protein PipXY32A, despite its reduced affinity for PII, is still engaged in partner swapping between NtcA and PII. Strong support for the idea that the two mutant proteins still interact with PII in vivo comes from the finding that the toxicity conferred by pipXY32A and pipXE4A alleles is counteracted by PII (17, 19).
To provide functional evidence that the mutant proteins PipXY32A and PipXE4A exerted their effect on class 4 genes by interacting with NtcA rather than an NtcA-independent mechanism, we tested two functions that require NtcA in S. elongatus: growth on nitrate as a nitrogen source and nitrate transport. The prediction was that the ntcA null allele should be epistatic to the pipXY32A and pipXE4A alleles.
When we attempted to inactivate ntcA by homologous recombination with the ntcA::aphII null allele, kanamycin-resistant clones carrying the inactive allele were obtained for CS3X, CS3XE4A, or CS3XY32A when transformants were selected on plates containing ammonium but not on nitrate. Furthermore, the ammonium-selected clones failed to grow on nitrate and to transport it (Fig. 4C). This therefore implies that the increased expression of the nitrate transport and assimilation systems in the CS3XE4A and CS3XY32A strains requires NtcA. Thus, the results support the activation of class 4 by mutant NtcA–PipX complexes.
To provide additional evidence that the PipXY32A and PipXE4A exerted their effect on class 4 genes by interacting with NtcA, and to gain further insights into the NtcA regulon in S. elongatus, we looked for NtcA binding motifs in promoter regions. The canonical NtcA-activated promoter is composed of an NtcA binding box, traditionally described with the consensus GTAN8TAC, centered at ∼41.5 nt upstream from the transcription start site (TSS), and separated 22–23 nt from a −10 box conforming to the consensus TAN3T (20). In addition to this orthodox promoter structure, which matches that of the Escherichia coli class II Crp-dependent promoters, NtcA can activate from positions further upstream or from sequences not matching the reported consensus (21).
NtcA binding motifs were found in a few genes or operons experimentally characterized in S. elongatus (22–24) and in others predicted to be controlled by NtcA in the cyanobacterium S. elongatus PCC 6301 (25), which is almost identical to S. elongatus. Remarkably, NtcA binding motifs were found in association with 29 of the 30 transcription units in class 4 (Fig. 5 and SI Appendix, Table S2), a result indicating that class 4 contains almost exclusively operons directly activated by NtcA.
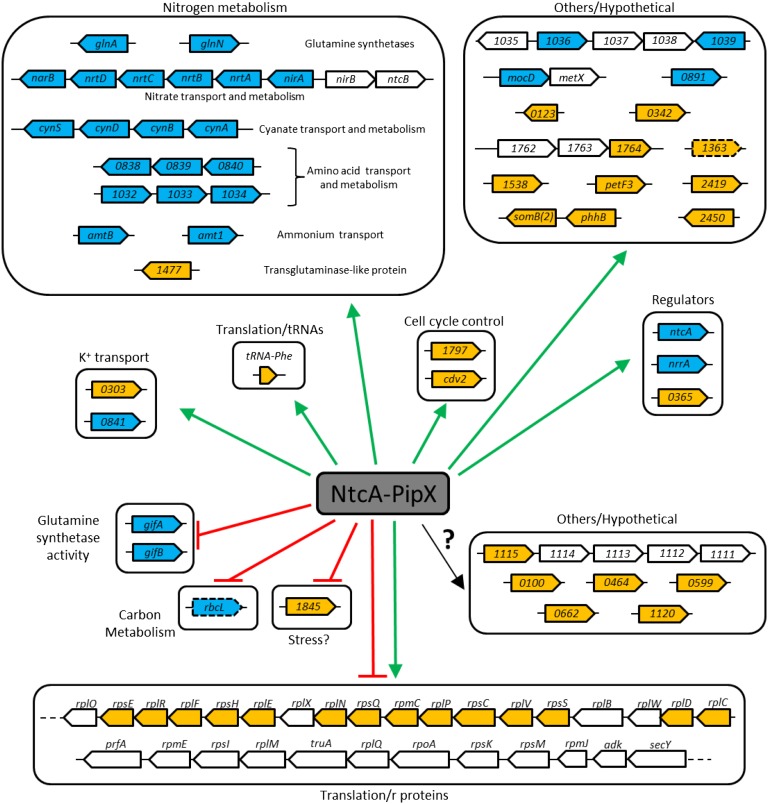
Fig. 5.
Genomic organization of the S. elongatus NtcA–PipX regulon. Transcriptional units identified from the multivariate analysis as targets of NtcA–PipX complexes are shown in separate panels according to functional categories. ORFs and their orientation on the chromosome are shown as wide arrows in blue or orange, respectively, when there is or is not experimental or in silico evidence (22–25). Dashed arrows indicate no recognizable NtcA boxes. Green arrows and red blunt lines refer to activation and repression roles, respectively, inferred for NtcA–PipX. The question mark refers to genes with NtcA boxes of unknown function.
The extended NtcA binding consensus derived from the in silico analysis of class 4 genes (Fig. 4D) included the experimentally characterized binding site at the glnN promoter, previously referred to as atypical (23, 26). The high levels of transcripts found in strain CS3XY32A in nitrate and in strain CS3XE4A in both ammonium and nitrate enabled us to map putative TSSs roughly from the RNA-sequencing read data (SI Appendix, Table S2 and Fig. S2). This, together with the predicted −10 elements, helped position the putative NtcA boxes. According to our analysis, most NtcA boxes appear to be centered at the canonical −41.5 position (Fig. 4G and SI Appendix, Fig. S3A).
Taking into account both the presence and positions of NtcA boxes and the expression patterns, only two class 4 genes were atypical: Synpcc7942_1363 (the only gene in this class without recognizable NtcA boxes) and tRNA-Phe. Although direct regulation and binding in the absence of predictable NtcA binding sites has recently been suggested (27, 28), the rather attenuated NtcA-dependent pattern of Synpcc7942_1363 could be explained by indirect activation. On the other hand, tRNA-Phe was up-regulated in strains CS3XE4A and CS3XY32A in nitrate and its promoter contains an NtcA binding site. However, in contrast to all other class 4 genes, which exhibit higher expression in nitrate, tRNA-Phe was induced in ammonium (Dataset S9). A precedent for a rather atypical regulation of a translation-related factor by NtcA is gltX, encoding the glutamyl-tRNA synthetase, which is expressed in both nitrate and ammonium in an NtcA-dependent manner (29).
In summary, transcriptomic analysis from a panel of five S. elongatus strains and two nitrogen conditions, followed by multivariate analyses, resulted in the identification of a coherent group of genes activated by the NtcA–PipX complex. These assignments, when considered alongside previously known NtcA target genes, identified new members of the NtcA regulon. In silico analysis allowed us to expand the consensus NtcA binding motif and to map NtcA boxes in promoter regions. The results provide further insights into the 2-OG–dependent partner swapping of PipX between NtcA and PII, as well as the properties of the PipXY32A and PipXE4A proteins.
NtcA-Repressed Genes.
As happens with other members of the CRP family of transcriptional activators (30), NtcA can also mediate repression when its binding sites overlap the RNA polymerase binding region located between −40 and +20 nt relative to the TSS. Paradigms of this type of control are the Synechocystis sp. PCC 6803 genes gifA and gifB, encoding the glutamine synthetase-inactivating factors IF7 and IF17 (31).
If repression of target genes is also favored by mutations increasing NtcA–PipX complex formation, the prediction is that pipX mutations would have the opposite effect on NtcA-repressed and NtcA-activated transcripts. As shown in Fig. 3A, classes 1 and 4 occupy opposite positions along the PC2 axis, suggesting that NtcA may negatively control class 1 genes. Furthermore, the box plot of class 1 (Fig. 4E) can be regarded as a roughly inverted version of the class 4 box plot, with the main difference being exhibited by the pipXY32A allele.
The extended consensus from Fig. 4D was then used to search for NtcA boxes outside class 4, for which the complete set of 257 differentially regulated genes was used. Only 10 new hits (associated with nine genes) were found, four of which corresponded to three class 1 genes and included gifA and gifB, with NtcA boxes located at −32.5 and −16.5, respectively (SI Appendix, Fig. S3B and Table S2). Their expression pattern in WT S. elongatus (Fig. 4F) is consistent with the regulatory importance of IF7 and IF17 in the nitrogen metabolism of cyanobacteria (32, 33). Although PipX does not seem to be required for nitrate repression, the pipXE4A and pipXY32A alleles confer repression in nitrate and, to a lesser extent, also in ammonium. Neither gifA or gifB showed ammonium induction in strain CS3X, a phenomenon that remains to be investigated.
Interestingly, 16 ribosomal genes were found in class 1. Two NtcA binding sites were found upstream of the coding region of rplC, the first gene of the main ribosome cluster. The site centered at −41.5, suggesting activation, is at odds with the inclusion of this gene in class 1. However, the second NtcA site at +10.5 is easier to reconcile with the negative effect of the gain-of-function alleles on transcript levels (Fig. 4 E and F). It is worth noting that the ribosomal genes that make a major contribution to the class 1 box plot were not down-regulated by CS3XY32A in nitrate. Taken together, these results suggest complex NtcA-dependent regulation at the ribosomal gene cluster and an intriguing connection between the nitrogen signaling system and the gene expression machinery.
No recognizable NtcA boxes were found at the remaining 16 genes found in class 1. These include rbcL, reported to be NtcA-repressed in Anabaena (34). Here, it is tempting to propose the involvement of the two orphan response regulators from class 4: NrrA, reported to be part of the NtcA regulatory cascade in Anabaena (35, 36), and the Synpcc7942_0365 gene product, either or both of which may account for indirect NtcA-dependent repression of some class 1 genes.
A simple interpretation of the results presented so far on classes 4 and 1 (Fig. 4 and SI Appendix, Fig. S3 and Table S2) is that whereas positive regulation tends to be directly exerted by NtcA, negative regulation would tend to be exerted indirectly, via an NtcA-activated repressor.
NtcA Targets Outside Classes 1 and 4.
The only gene outside class 1 matching functional and structural criteria for NtcA repression was Synpcc7942_1845 from class 2.1 (SI Appendix, Fig. S4). Its expression differed significantly from the representative pattern of class 2.1 (Fig. 6). As expected for NtcA-repressed genes, it was down-regulated in CS3XE4A and, to a greater extent, in CS3XY32A. It also contains an NtcA binding site centered at −2.5 (SI Appendix, Fig. S3B). However, the expression pattern of Synpcc7942_1845 differed between the two control strains, being higher in the CS3X background, a result suggesting that additional regulatory mechanisms may prevent transcript accumulation in ammonium in the WT. Recent data indicate that Synpcc7942_1845 acts as a general stress protein in S. elongatus (37), suggesting that it may be subjected to multiple regulatory controls.
Fig. 6.
Box plot representation of the within-group variance of the mutant/control comparisons in classes 2.1, 2.2, 3.1, and 3.2. Details are as described in Fig. 4A.
Only six genes, belonging to class 2.2 (one gene), class 3.2 (three genes), and the group of nonclassified genes (two genes), had NtcA boxes for which we could not infer a particular function. Their atypical expression patterns may be due to the presence of cryptic NtcA sites or to the confluence of additional regulatory systems.
Recent transcriptomic and ChIP studies of the Anabaena NtcA regulon allowed the identification of large numbers of NtcA targets in this heterocyst-forming cyanobacterium (28, 38). In this context, it is worth noting that our multivariate analysis did not aim to provide a comprehensive study of NtcA targets but, instead, was designed to identify genes with paradigmatic and simple regulation by PipX, which may be representative of interactions with NtcA or with other transcriptional regulators. In this context, several NtcA target genes previously characterized in S. elongatus did not score above the cutoff levels. Notably, glnB, also expressed from a strong NtcA-independent promoter (39), gltX, subjected to both positive and negative regulation by NtcA (29) and nblA, controlled by several additional regulators (40–42), were not detected in our analysis.
NtcA-Independent Expression Patterns and Functions of PipX Regulons.
The effects of mutations on the expression patterns of classes 2.1, 2.2, 3.1, and 3.2 are illustrated in Figs. 3B and 6. The distinctive feature of class 2.1 transcripts was their down-regulation by ΔpipX and pipXY32A alleles in both nitrate and ammonium. Exactly the opposite effect was found for class 3.2 genes: up-regulation by ΔpipX and pipXY32A alleles in both nitrate and ammonium. Class 2.2 was characterized by significant down-regulation by pipXY32A, especially in ammonium, and class 3.1 was characterized by up-regulation by ΔpipX in ammonium.
Most of the mutant/control changes detected in our analysis were similar in nitrate and ammonium cultures. The finding that the ΔpipX allele affected class 2.1 and class 3.2 genes similarly in nitrate and ammonium cultures suggests that PipX plays the same role in both nitrogen-rich conditions. On the other hand, only ΔpipX in class 3.1 and pipXY32A in class 4 affected gene expression specifically in one condition. Although the molecular basis of PipX regulation of class 3.1 genes remains elusive, the 2-OG–dependent partner swapping of PipX between NtcA and PII (12) provides the background to interpret the class 4 expression pattern, further indicating that 2-OG was limiting for NtcA–PipXY32A complex formation but not for NtcA–PipXE4A complex formation in our ammonium cultures.
The effect of ΔpipX, pipXE4A, and pipXY32A alleles on the transcript levels of classes 2.1, 2.2, 3.1, and 3.2 cannot be reconciled with the involvement of NtcA–PipX complexes in the regulation of their corresponding genes. In particular, the drastic impact of pipX inactivation at classes 2.1, 3.1 (specifically in ammonium), and 3.2 and the lack of effect of the alleles pipXE4A (in classes 2.2 and 3.1) and pipXY32A (in class 3.1) do not support the involvement of NtcA. Furthermore, with very few exceptions (in classes 2.2 and 3.2; SI Appendix, Table S2), NtcA boxes were absent from the genes or transcription units involved. Because both the response to the ΔpipX, pipXE4A, and pipXY32A alleles and the promoter structure (discussed above) argued against the involvement of NtcA–PipX complexes in regulation of class 2.1, 2.2, 3.1, and 3.2 genes, our working hypothesis is that each of these four groups constitutes regulons influenced by PipX in an NtcA-independent manner.
To investigate further the internal coherence of the groups of genes obtained by multivariate analyses, which was based on the ability of S. elongatus gene transcripts to respond similar to pipX alleles, we followed the cluster of orthologous genes (COG) classification system to assign functions within groups.
The distribution of both COG categories and genes of unknown function differed greatly across the six groups (SI Appendix, Figs. S5 and S6). Genes of unknown function made similar contributions to the complete S. elongatus genome (ca. 37%) and to the 222 genes included in the six groups analyzed here (ca. 34%) but were especially abundant in class 2.1 (24 of 37 genes) and rare in class 1 (four of 32 genes). Translation was overrepresented (14% vs. 7% in the complete S. elongatus genome), but ribosomal proteins were found exclusively in class 1 (18 of 32 genes) and tRNAs were found almost exclusively in class 3.2 (12 of 13 genes), thus revealing a strong connection between PipX and translation. The second most abundant category was energy production and conversion, which was particularly abundant in class 3.1 (9 of 18 genes were photosynthesis-related genes). Inorganic ion transport and metabolism and amino acid transport and metabolism were found almost exclusively in class 4 (21 of 43 genes in total), in close agreement with their role in nitrogen assimilation. Carbohydrate transport and metabolism was relatively well represented in class 2.2 (5 of 43 genes).
PipX Modulon, a Working Model.
The results presented in this work provided important insights into the S. elongatus NtcA regulon while revealing unexpected functions of PipX (Fig. 7). The finding that the six groups of genes that emerged from the multivariate analysis showed very good internal coherence gave credit to the hypothesis that PipX is involved in processes other than coactivation/correpression of NtcA targets, which are mainly related to nitrogen assimilation (class 4 and some class 1 genes). In this context, the finding that the double-mutant ntcA pipX is less viable than the ntcA single mutant, as inferred by failure to segregate the ΔpipX allele in the ntcA null mutant (SI Appendix, Fig. S7), supports the involvement of PipX in NtcA-independent functions.
Fig. 7.
PipX regulatory functions inferred from this work. Positive regulation and negative regulation are depicted by arrowheads and blunt lines, respectively.
The finding that regulation by PipX could be observed in both or just one of the nitrogen conditions used (ammonium in class 3.1) supports the idea that signals other than 2-OG affect PipX interactions. The identification as members of the PipX modulon of highly expressed genes for ribosomal proteins (class 1), tRNAs (class 3.2), and photosynthesis (class 3.1) further suggests that NtcA-independent regulons participate in the adaptation of the cyanobacterial machineries for translation and photosynthesis to nutrient or other environmental changes. The emerging picture is that of PipX as a multifunctional protein involved in fine-tuning of different gene expression programs in response to different signals.
The effect of pipX inactivation on transcript levels indicated a positive role for PipX in class 2.1 and a negative role in classes 3.1 and 3.2. On the other hand, the impact of pipXY32A on class 2.2 transcripts indicated that PipX has the potential to act as a negative regulator, probably under environmental conditions not tested in this work. How PipX exerts the different roles inferred here is still a matter of speculation. It may function by interacting with transcriptional, signal transduction, or even posttranscriptional regulators.
To account for the six groups of genes identified here with common expression patterns, we propose the involvement of PipX in a minimum of four types of regulatory complexes, of which only one (NtcA–PipX complex) is presently characterized. The rather symmetrical effects of pipX mutations in the expression patterns of classes 1 and 4, reflecting the involvement of NtcA–PipX complexes in both groups, have a parallel in classes 2.1 and 3.2, which gave remarkably symmetrical box plots; thus, it is tempting to speculate that the same regulatory complex could be involved in activating and repressing these genes. Different complexes would be involved in repression of class 2.2 and 3.1 genes.
The intracellular availability of PipX and its specific interactions in particular environmental conditions or genetic backgrounds are probably determined by a network of interactions involving NtcA, PII, their binding proteins and effectors (Fig. 1), and perhaps PII-modifying enzymes (43, 44). This complexity makes it difficult to infer a potential role for PII in the proposed PipX complexes. Notably, PII proteins acting in complexes with DNA-binding transcriptional regulators have been reported in other systems (45–47).
The crucial impairment of binding to PII caused by the PipX substitution Y32A argues against the involvement of PII in the regulation of class 2.2 genes, where the pipXY32A allele causes gain of function (as a repressor). Direct involvement of the tudor-like domain, and therefore of PII, also appears unlikely in class 3.1 regulatory complexes, because pipXY32A and pipXE4A alleles had no effect on the repressive function of PipX; here, it is tempting to propose a role for the C-terminal α-helices of PipX in repression. Finally, although the (complete) loss of function conferred by pipXY32A would support the involvement of the tudor-like domain in regulation of class 2.1 and 3.2 genes, the silent effect of the pipXE4A allele argues against direct involvement of PII.
Whatever mechanisms are involved, our results suggest that PipX participates in at least three novel regulatory scenarios, different from the known NtcA–PipX complexes, to modulate gene expression.
Materials and Methods
Strains, Growth Conditions, and Nitrate Transport Assays.
S. elongatus strains and plasmids are listed in SI Appendix, Table S1. Growth, genetic manipulations and nitrate uptake assays were as described (13, 17).
Preparation and RNA Analysis.
For RNA preparations, 100-mL cultures of each strain were grown in BG11 or BG11A until a OD750 nm of ∼0.9 was attained. RNA was purified with a Qiagen RNeasy Protect Bacteria Mini Kit and on-column RNase-free DNase I digestion. Samples were assayed for RNA integrity using an Agilent 2100 Bioanalyzer and quantified with a Qubit fluorometer (Life Technologies). Removal of 16S and 23S rRNA from total RNA was performed using a MicrobExpress Bacterial mRNA Purification Kit (Ambion) or treatment with a Ribo-Zero Magnetic Kit (Epicenter). RNA samples were divided into multiple aliquots of ≤5 μg of RNA, and separately enriched mRNA samples were pooled, run on the 2100 Bioanalzyer to confirm reduction of 16S and 23S rRNA before preparation of cDNA fragment libraries with a ScriptSeq v2 RNA-Seq Library Preparation Kit, and sequenced (Illumina HiSeq2000; Macrogene).
Computational Methods.
Ten datasets of unique mappable reads covered each nucleotide strand specifically for an average of ∼120 to 500 times for the chromosome and 1.5 to 190 times for the plasmid. Read alignments were performed using Bowtie2 (48) against an S. elongatus reference chromosome and endogenous plasmid (GenBank accession nos. CP000100 and CP000101, respectively). Gene expression, represented as reads per kilobase, was determined by Samtools (49), the Artemis Genome Browser (Wellcome Trust Sanger Institute) (50), and homemade Perl scripts. The data were normalized by quantiles (51). Statistical analysis was performed using the DESeq package (52) and R software (www.r-project.org/). Normalized reads per kilobase per million data are provided in Dataset S9.
NtcA motifs were first identified with MEME (53) (150 nt upstream of the TSSs or, when unpredicted, of initiation codons and a background consisting of a fourth-order Markov model of the entire genome) and were used to search for palindromic motifs between 6 and 20 bp. FIMO was used (54) to identify NtcA boxes outside class 4. The position-specific probability matrix for the motif was derived from the 30 matches provided by MEME. Searches were performed in sequences comprising 250 nt upstream and 50 nt downstream of the TSSs or 250 nt upstream of the initiation codon. The hits were filtered using two criteria: P value <0.0001 and q-value <0.7.
A COG list was downloaded from Cyanobase (http://genome.microbedb.jp/cyanobase/SYNPCC7942), with manual assignation where relevant.
Multivariate analyses were carried out with SPSS (IBM) and R. PCA was applied on the correlation matrix, with varimax rotation of the first two principal components. Package cluster (55) was used for fuzzy c-means clustering (56).
Supplementary Material
Acknowledgments
We thank J. I. Labella for carrying out MEME and FIMO analysis and Circos Perl programming, A. Obrebska and S. Burillo for generation of mutants, K. Forchhammer for plasmid pNTCA-KAN, and V. Rubio for critically reading the manuscript. This work was supported by the Spanish Ministry of Economy and Competitivity (Grants BFU2009-07371, BFU2012-33364, and BFU2011-26608) and the European Seventh Framework Program (Grants 289326/FP7-KBBE-2011-5 and 282004/FP7-HEALTH-2011-2.3.1-2).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404097111/-/DCSupplemental.
References
- 1.Muro-Pastor MI, Reyes JC, Florencio FJ. Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. J Biol Chem. 2001;276(41):38320–38328. doi: 10.1074/jbc.M105297200. [DOI] [PubMed] [Google Scholar]
- 2.Muro-Pastor MI, Reyes JC, Florencio FJ. Ammonium assimilation in cyanobacteria. Photosynth Res. 2005;83(2):135–150. doi: 10.1007/s11120-004-2082-7. [DOI] [PubMed] [Google Scholar]
- 3.Forchhammer K. Global carbon/nitrogen control by PII signal transduction in cyanobacteria: From signals to targets. FEMS Microbiol Rev. 2004;28(3):319–333. doi: 10.1016/j.femsre.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, et al. Nonmetabolizable analogue of 2-oxoglutarate elicits heterocyst differentiation under repressive conditions in Anabaena sp. PCC 7120. Proc Natl Acad Sci USA. 2005;102(28):9907–9912. doi: 10.1073/pnas.0502337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leigh JA, Dodsworth JA. Nitrogen regulation in bacteria and archaea. Annu Rev Microbiol. 2007;61:349–377. doi: 10.1146/annurev.micro.61.080706.093409. [DOI] [PubMed] [Google Scholar]
- 6.Fokina O, Chellamuthu VR, Forchhammer K, Zeth K. Mechanism of 2-oxoglutarate signaling by the Synechococcus elongatus PII signal transduction protein. Proc Natl Acad Sci USA. 2010;107(46):19760–19765. doi: 10.1073/pnas.1007653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truan D, et al. A new P(II) protein structure identifies the 2-oxoglutarate binding site. J Mol Biol. 2010;400(3):531–539. doi: 10.1016/j.jmb.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Burillo S, Luque I, Fuentes I, Contreras A. Interactions between the nitrogen signal transduction protein PII and N-acetyl glutamate kinase in organisms that perform oxygenic photosynthesis. J Bacteriol. 2004;186(11):3346–3354. doi: 10.1128/JB.186.11.3346-3354.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llácer JL, et al. The crystal structure of the complex of PII and acetylglutamate kinase reveals how PII controls the storage of nitrogen as arginine. Proc Natl Acad Sci USA. 2007;104(45):17644–17649. doi: 10.1073/pnas.0705987104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vázquez-Bermúdez MF, Herrero A, Flores E. 2-Oxoglutarate increases the binding affinity of the NtcA (nitrogen control) transcription factor for the Synechococcus glnA promoter. FEBS Lett. 2002;512(1-3):71–74. doi: 10.1016/s0014-5793(02)02219-6. [DOI] [PubMed] [Google Scholar]
- 11.Tanigawa R, et al. Transcriptional activation of NtcA-dependent promoters of Synechococcus sp. PCC 7942 by 2-oxoglutarate in vitro. Proc Natl Acad Sci USA. 2002;99(7):4251–4255. doi: 10.1073/pnas.072587199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinosa J, Forchhammer K, Burillo S, Contreras A. Interaction network in cyanobacterial nitrogen regulation: PipX, a protein that interacts in a 2-oxoglutarate dependent manner with PII and NtcA. Mol Microbiol. 2006;61(2):457–469. doi: 10.1111/j.1365-2958.2006.05231.x. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa J, Forchhammer K, Contreras A. Role of the Synechococcus PCC 7942 nitrogen regulator protein PipX in NtcA-controlled processes. Microbiology. 2007;153(Pt 3):711–718. doi: 10.1099/mic.0.2006/003574-0. [DOI] [PubMed] [Google Scholar]
- 14.Valladares A, et al. Specific role of the cyanobacterial PipX factor in the heterocysts of Anabaena sp. strain PCC 7120. J Bacteriol. 2011;193(5):1172–1182. doi: 10.1128/JB.01202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llácer JL, et al. Structural basis for the regulation of NtcA-dependent transcription by proteins PipX and PII. Proc Natl Acad Sci USA. 2010;107(35):15397–15402. doi: 10.1073/pnas.1007015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao MX, et al. Structural basis for the allosteric control of the global transcription factor NtcA by the nitrogen starvation signal 2-oxoglutarate. Proc Natl Acad Sci USA. 2010;107(28):12487–12492. doi: 10.1073/pnas.1001556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laichoubi KB, Espinosa J, Castells MA, Contreras A. Mutational analysis of the cyanobacterial nitrogen regulator PipX. PLoS ONE. 2012;7(4):e35845. doi: 10.1371/journal.pone.0035845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espinosa J, Castells MA, Laichoubi KB, Forchhammer K, Contreras A. Effects of spontaneous mutations in PipX functions and regulatory complexes on the cyanobacterium Synechococcus elongatus strain PCC 7942. Microbiology. 2010;156(Pt 5):1517–1526. doi: 10.1099/mic.0.037309-0. [DOI] [PubMed] [Google Scholar]
- 19.Espinosa J, Castells MA, Laichoubi KB, Contreras A. Mutations at pipX suppress lethality of PII-deficient mutants of Synechococcus elongatus PCC 7942. J Bacteriol. 2009;191(15):4863–4869. doi: 10.1128/JB.00557-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrero A, Muro-Pastor AM, Flores E. Nitrogen control in cyanobacteria. J Bacteriol. 2001;183(2):411–425. doi: 10.1128/JB.183.2.411-425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luque I, Forchhammer K. Nitrogen assimilation and C/N balance sensing. In: Herrero EFA, editor. The Cyanobacteria: Molecular Biology, Genetics and Evolution. Norwich, UK: Caister Academic; 2008. pp. 335–382. [Google Scholar]
- 22.Luque I, Flores E, Herrero A. Molecular mechanism for the operation of nitrogen control in cyanobacteria. EMBO J. 1994;13(23):5794. doi: 10.1002/j.1460-2075.1994.tb06920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauer J, Dirmeier U, Forchhammer K. The Synechococcus strain PCC 7942 glnN product (glutamine synthetase III) helps recovery from prolonged nitrogen chlorosis. J Bacteriol. 2000;182(19):5615–5619. doi: 10.1128/jb.182.19.5615-5619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vázquez-Bermúdez MF, Paz-Yepes J, Herrero A, Flores E. The NtcA-activated amt1 gene encodes a permease required for uptake of low concentrations of ammonium in the cyanobacterium Synechococcus sp. PCC 7942. Microbiology. 2002;148(Pt 3):861–869. doi: 10.1099/00221287-148-3-861. [DOI] [PubMed] [Google Scholar]
- 25.Su Z, Olman V, Mao F, Xu Y. Comparative genomics analysis of NtcA regulons in cyanobacteria: Regulation of nitrogen assimilation and its coupling to photosynthesis. Nucleic Acids Res. 2005;33(16):5156–5171. doi: 10.1093/nar/gki817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aldehni MF, Forchhammer K. Analysis of a non-canonical NtcA-dependent promoter in Synechococcus elongatus and its regulation by NtcA and PII. Arch Microbiol. 2006;184(6):378–386. doi: 10.1007/s00203-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 27.Camargo S, Valladares A, Flores E, Herrero A. Transcription activation by NtcA in the absence of consensus NtcA-binding sites in an anabaena heterocyst differentiation gene promoter. J Bacteriol. 2012;194(11):2939–2948. doi: 10.1128/JB.05994-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picossi S, Flores E, Herrero A. ChIP analysis unravels an exceptionally wide distribution of DNA binding sites for the NtcA transcription factor in a heterocyst-forming cyanobacterium. BMC Genomics. 2014;15(1):22. doi: 10.1186/1471-2164-15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luque I, Contreras A, Zabulon G, Herrero A, Houmard J. Expression of the glutamyl-tRNA synthetase gene from the cyanobacterium Synechococcus sp PCC 7942 depends on nitrogen availability and the global regulator NtcA. Mol Microbiol. 2002;46(4):1157–1167. doi: 10.1046/j.1365-2958.2002.03236.x. [DOI] [PubMed] [Google Scholar]
- 30.Kolb A, Busby S, Buc H, Garges S, Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- 31.García-Domínguez M, Reyes JC, Florencio FJ. NtcA represses transcription of gifA and gifB, genes that encode inhibitors of glutamine synthetase type I from Synechocystis sp. PCC 6803. Mol Microbiol. 2000;35(5):1192–1201. doi: 10.1046/j.1365-2958.2000.01789.x. [DOI] [PubMed] [Google Scholar]
- 32.Galmozzi CV, Fernández-Avila MJ, Reyes JC, Florencio FJ, Muro-Pastor MI. The ammonium-inactivated cyanobacterial glutamine synthetase I is reactivated in vivo by a mechanism involving proteolytic removal of its inactivating factors. Mol Microbiol. 2007;65(1):166–179. doi: 10.1111/j.1365-2958.2007.05773.x. [DOI] [PubMed] [Google Scholar]
- 33.García-Domínguez M, Reyes JC, Florencio FJ. Glutamine synthetase inactivation by protein-protein interaction. Proc Natl Acad Sci USA. 1999;96(13):7161–7166. doi: 10.1073/pnas.96.13.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramasubramanian TS, Wei TF, Golden JW. Two Anabaena sp. strain PCC 7120 DNA-binding factors interact with vegetative cell- and heterocyst-specific genes. J Bacteriol. 1994;176(5):1214–1223. doi: 10.1128/jb.176.5.1214-1223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehira S, Ohmori M. NrrA directly regulates expression of hetR during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 2006;188(24):8520–8525. doi: 10.1128/JB.01314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehira S, Ohmori M. NrrA, a nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol Microbiol. 2006;59(6):1692–1703. doi: 10.1111/j.1365-2958.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 37.Ruffing AM. RNA-Seq analysis and targeted mutagenesis for improved free fatty acid production in an engineered cyanobacterium. Biotechnol Biofuels. 2013;6(1):113. doi: 10.1186/1754-6834-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitschke J, Vioque A, Haas F, Hess WR, Muro-Pastor AM. Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc Natl Acad Sci USA. 2011;108(50):20130–20135. doi: 10.1073/pnas.1112724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HM, Vázquez-Bermúdez MF, de Marsac NT. The global nitrogen regulator NtcA regulates transcription of the signal transducer PII (GlnB) and influences its phosphorylation level in response to nitrogen and carbon supplies in the Cyanobacterium synechococcus sp. strain PCC 7942. J Bacteriol. 1999;181(9):2697–2702. doi: 10.1128/jb.181.9.2697-2702.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Redondo ML, et al. Environmental control of phosphorylation pathways in a branched two-component system. Mol Microbiol. 2010;78(2):475–489. doi: 10.1111/j.1365-2958.2010.07348.x. [DOI] [PubMed] [Google Scholar]
- 41.Luque I, Zabulon G, Contreras A, Houmard J. Convergence of two global transcriptional regulators on nitrogen induction of the stress-acclimation gene nblA in the cyanobacterium Synechococcus sp. PCC 7942. Mol Microbiol. 2001;41(4):937–947. doi: 10.1046/j.1365-2958.2001.02566.x. [DOI] [PubMed] [Google Scholar]
- 42.Salinas P, et al. The regulatory factor SipA provides a link between NblS and NblR signal transduction pathways in the cyanobacterium Synechococcus sp. PCC 7942. Mol Microbiol. 2007;66(6):1607–1619. doi: 10.1111/j.1365-2958.2007.06035.x. [DOI] [PubMed] [Google Scholar]
- 43.Chellamuthu VR, Alva V, Forchhammer K. From cyanobacteria to plants: Conservation of PII functions during plastid evolution. Planta. 2013;237(2):451–462. doi: 10.1007/s00425-012-1801-0. [DOI] [PubMed] [Google Scholar]
- 44.Forchhammer K. The network of P(II) signalling protein interactions in unicellular cyanobacteria. Adv Exp Med Biol. 2010;675:71–90. doi: 10.1007/978-1-4419-1528-3_5. [DOI] [PubMed] [Google Scholar]
- 45.Castellen P, Rego FG, Portugal ME, Benelli EM. The Streptococcus mutans GlnR protein exhibits an increased affinity for the glnRA operon promoter when bound to GlnK. Braz J Med Biol Res. 2011;44(12):1202–1208. doi: 10.1590/s0100-879x2011007500138. [DOI] [PubMed] [Google Scholar]
- 46.Kayumov A, Heinrich A, Fedorova K, Ilinskaya O, Forchhammer K. Interaction of the general transcription factor TnrA with the PII-like protein GlnK and glutamine synthetase in Bacillus subtilis. FEBS J. 2011;278(10):1779–1789. doi: 10.1111/j.1742-4658.2011.08102.x. [DOI] [PubMed] [Google Scholar]
- 47.Oliveira MA, et al. Interaction of GlnK with the GAF domain of Herbaspirillum seropedicae NifA mediates NH4+- regulation. Biochimie. 2012;94(4):1041–1047. doi: 10.1016/j.biochi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, et al. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutherford K, et al. Artemis:Sequence visualization and annotation. Bioinformatics. 2000;16(10):944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 51.Bullard JH, Purdom E, Hansen KD, Dudoit S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinformatics. 2010;11:94. doi: 10.1186/1471-2105-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey TL, et al. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant CE, Bailey TL, Noble WS. FIMO: Scanning for occurrences of a given motif. Bioinformatics. 2011;27(7):1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. 2014. Cluster Analysis Basics and Extensions. R package version 1.15.2. Available at http://cran.stat.ucla.edu/web/packages/cluster/index.html. Accessed April 20, 2014. [Google Scholar]
- 56.Kaufman L, Rousseeuw PJ. Finding Groups in Data: An Introduction to Cluster Analysis. New York: Wiley; 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.