Significance
This article provides original results on the formative conditions of sheep domestication in the Near East. To our knowledge, none of the results has been published before, and the results are expected to be of wide interest to archaeologists, biologists, and other professionals interested in evolutionary and cultural processes of animal domestication.
Keywords: caprine domestication, zooarchaeology, stabling deposits
Abstract
Aşıklı Höyük is the earliest known preceramic Neolithic mound site in Central Anatolia. The oldest Levels, 4 and 5, spanning 8,200 to approximately 9,000 cal B.C., associate with round-house architecture and arguably represent the birth of the Pre-Pottery Neolithic in the region. Results from upper Level 4, reported here, indicate a broad meat diet that consisted of diverse wild ungulate and small animal species. The meat diet shifted gradually over just a few centuries to an exceptional emphasis on caprines (mainly sheep). Age-sex distributions of the caprines in upper Level 4 indicate selective manipulation by humans by or before 8,200 cal B.C. Primary dung accumulations between the structures demonstrate that ruminants were held captive inside the settlement at this time. Taken together, the zooarchaeological and geoarchaeological evidence demonstrate an emergent process of caprine management that was highly experimental in nature and oriented to quick returns. Stabling was one of the early mechanisms of caprine population isolation, a precondition to domestication.
The Neolithic brought fundamental transformations to human society and humans’ place in natural systems. Early villages reorganized rapidly around new ways of extracting food from the environment, and animal and plant management seem to lie at the heart of many of these changes. The initial conditions of village community evolution are elusive, however, because late forager and forager-producer transition sites are few, in contrast to the abundant records of the later Neolithic. Known exceptions in central Turkey are the early occupations at Aşıklı Höyük in Cappadocia, the ninth millennium occupations at Pınarbaşı and Boncuklu in the Konya Basin (1, 2), and the late Epipaleolithic occupations in Pınarbaşı Rockshelter in Konya and Direkli Cave in Kahramanmaraş (3, 4).
Few of the early Pre-Pottery Neolithic (PPN) communities display precocious histories of animal management, although plant cultivation was widespread. Regional contrasts in the early PPN testify to the volatile and locally variable nature of “neolithization” across the Middle East (1, 5–21). Only later in the PPN were certain staple animal foods truly domesticated, some in energetically powerful combinations with plants that would fuel the expansion of Neolithic systems into other world regions.
Like the opposing faces of Janus, there are two temporal aspects from which one may view Neolithic origins. Comparisons to the more recent have received the bulk of archaeologists’ attention thanks to an abundance of data on the later PPN and historic stock-keeping practices. Looking to the early Neolithic from a deeper past, the Epipaleolithic, is the view less often taken. Comparison data are less easy to come by, but the advantage of this forward-looking view is its consistency with the direction of evolution itself. As revolutionary as neolithization and the emergence of village communities may have been, the Epipaleolithic and PPN are linked by widespread human attempts to optimize the growth of food species and to extend their seasonal availability through storage. Evolution can occur only through existing pathways of opportunity and Epipaleolithic life-ways were exceptionally rich in strategic and cultural variety. The observation of multiple origins in sheep domestication (22), for example, illustrates the need to examine local histories in a ground-upward manner as a complement to interregional comparisons of culture change (8).
Here we examine the formative conditions of the forager-producer transition at Aşıklı Höyük through the lens of animal exploitation. This site preserves a surprisingly detailed record of human–animal interactions in a formative settlement (23). The story of socioeconomic change begins in Level 5, which is not yet excavated. Nonetheless, the cultural deposits of Level 4 reveal important early trade-offs within the meat diet that are accompanied by evidence of human manipulation, or management, of caprine (sheep and goat) survivorship. Although management practices may give rise to domestic taxa after many generations, it is the early elements of these coevolutionary relations that interest us here; hence, we must look to evidence other than morphologic changes. Zooarchaeological analysis provides information about prey choice and ungulate age-sex structures, and geoarchaeological (micromorphology and phytolith) analyses provide information on physical interactions between humans and ungulates within the confines of the settlement.
Background
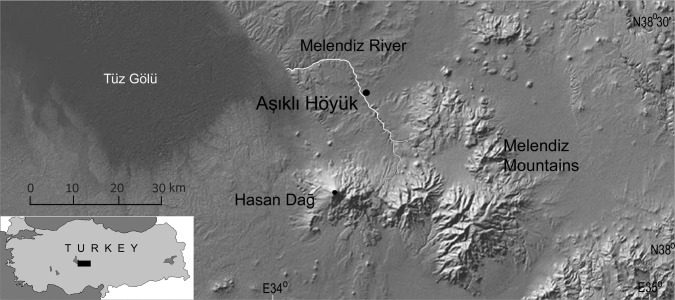
Aşıklı Höyük (AH) sits directly on a floodplain of the Melendiz River (elevation 1,119 m) (Fig. 1) in Cappadocia, a volcanic landscape carved by wind, water, and humans. This short river originates in the Melendiz Mountains (2,963 m) and drains westward into the salt lake known as Tuz Gölü. Beyond the isolated Melendiz highlands lies the great Taurus mountain chain. Rolling plains and upland meadows of the region once supported wild aurochs (Bos primigenius), horse (Equus ferus), onager (Equus hemionus hydruntinus), wild boar (Sus scrofa), deer (Cervus elaphus, Capreolus capreolus, Dama dama), goats (Capra aegagrus), and sheep (Ovis orientalis).
Fig. 1.
Location of Aşıklı Höyük on the Melendiz River in western Cappadocia (Central Anatolia), Turkey.
AH is an artificial mound composed of 16 m of anthropogenic deposits (24, 25). Levels 4 through 2 preserve a remarkable history of architectural transitions, from semisubterranean round buildings to densely packed free-standing rectangular buildings (23, 26). The later PPN of Level 2 has occupied nearly two decades of research under the direction of Ufuk Esin and subsequently Nur Balkan-Atlı of Istanbul University. Here, author H.B. (27) found possible evidence for “proto-domestication” (sensu management) of sheep and goats based on age and sex ratios. Domesticated cereal grains were in cultivation at this time (24, 25), although domestic variants are less common in the botanical assemblages than morphologically wild grains and other plant foods (28). Radiocarbon dates for Level 2 range between 8000 and 7500 cal B.C. (29).
Balkan-Atlı and her colleagues concluded their work at AH in 2004 by digging a deep exploratory trench into the northern edge of the mound. This trench passed through the 2-m-thick deposits of Level 3, which contain widely spaced trapezoidal or semioval structures. The trench also penetrated the top of Level 4, the architecture of which is similar to that of Level 3 but with more uniformly oval-round contours. Recent archaeobotanical studies have identified some domestic emmer and einkorn wheat alongside wild morphs in upper Level 4. This occupation is roughly coeval with the early occupations at Pınarbaşı and Boncuklu (8540–8230 cal B.C.) (see refs. 1 and 2). Levels 5 through 4 in AH arguably represent the birth of the PPN in Central Anatolia (30).
In 2010, M.Ö. of Istanbul University began a new excavation at AH with detailed spatial recording of horizontal and stratigraphic exposures, and complete recovery of artifacts, fauna and floral materials via fine screening and flotation techniques (4-mm and 2-mm mesh). The sediments are excavated in a series of shallow units that follow human-defined spaces and vertical units within stratigraphic levels. Only a fraction of the total settlement is visible in the deep (4GH) trench, but the exposed area of approximately 10 × 15 m presents a startling variety of semi-isolated structures, diverse outdoor features, work spaces, and middens. Radiocarbon assays of short-lived burnt plant remains in combustion features indicate that the upper part of Level 4 spans approximately 8400–8100 cal B.C. Level 5 is not yet excavated, but a burned layer exposed by the river in the mound’s western base is reliably dated to 9010 ± 200 cal B.C. The available dates therefore bracket the bulk of the Level 5–4 sequence. As it happens, these dates fall within two separate plateaus in the radiocarbon calibration curve (Figs. S1 and S2 and Table S1), raising the uncertainty associated with the determinations. Radiocarbon dating work continues for the intervening deposits in AH, for which plateau effects are expected to be less problematic. Plant macrofossils and abundant groundstone artifacts in upper Level 4 indicate heavy use of cereal grasses, pulses, hackberry, nuts, and other seeds. It seems likely on these grounds that the site’s placement on the river floodplain was predicated upon an already heavy commitment to plant cultivation.
Results
A wide range of animals were hunted during the occupations of upper Level 4, indicating a diversified meat diet (Table S2). Large prey included goats, aurochs, red deer, boar, horse, onager, roe deer, and fallow deer, but sheep most of all. Among the small prey species hunted, hares (Lepus capensis) and river fish (Cyprinidae carps) were particularly important, but tortoises (Testudo graeca) and pond turtles (Emys/Mauremys sp.), hedgehogs (Erinaceus sp.), bustards (Otis tarda), partridges (Alectoris chukar), and water birds (e.g., ducks, Anatidae) were also eaten. Other large birds, such as common crane (Grus grus), owls (Strigidae), and raptors (Falconiformes) were exploited on occasion, through probably more for raw materials than for food.
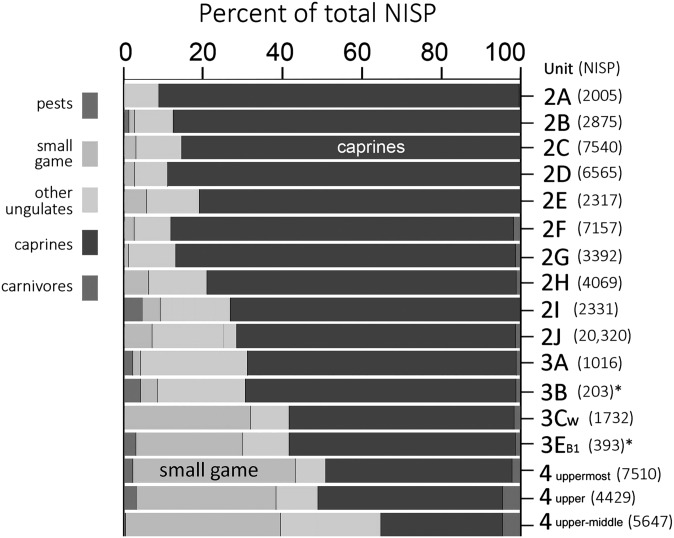
A faunal trend through Levels 4–2 (Fig. 2) reveals a strategic trade-off in the meat diet, from a broad-spectrum strategy that emphasized diverse wild small animals and ungulates to a concerted exploitation of caprines in particular. Caprines constitute less than half of the total number of identified skeletal specimens (NISP) in upper Level 4, but caprines increase gradually to 85–90% by the end of the time series in upper Level 2. The caprines were mainly sheep, which outnumbered goats by a factor of three or more in all periods. The incidence of carnivores hunted by humans and small pests (toads and mice) that entered the site voluntarily changes comparatively little over this span. The contribution of small game declines fastest between Levels 3C and 3B, but this difference is taken up by wild noncaprine ungulates, without interrupting the gradual rise in caprine importance. The replacement of diverse wild taxa with caprines at AH suggests a local, autochthonous evolution of dietary practices. These developments are consistent with faunal patterns observed in early PPN sites in the northern Euphrates region, where caprine- and possibly pig-management practices arose by approximately 8400 cal B.C. (11).
Fig. 2.
Variation in the representation of five major animal categories in the faunal assemblages from Level 2 through upper Level 4 of AH based on NISP. *Note small sample size.
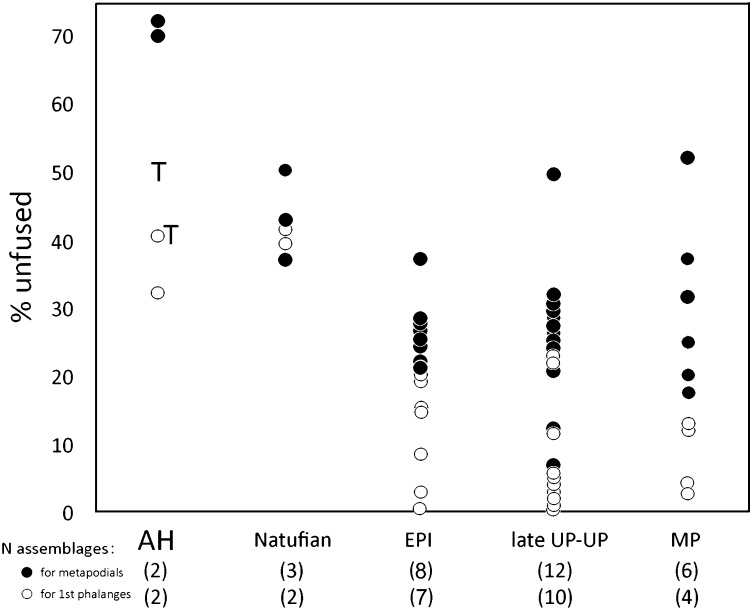
The caprines and aurochs from AH upper Level 4 display a notable bias toward immature individuals (50% and 53%, respectively) based on unfused metapodials or tibias; this bias is not present in the other ungulate species (Tables S3 and S4). Fig. 3 presents a broader, diachronic comparison of the percentages of unfused first-phalanges and distal metapodials (which fuse sequentially) for diverse artiodactyl species, including wild caprines, from eastern Mediterranean and Anatolian sites. Epipaleolithic prey age selection seldom differed from that of the Upper and Middle Paleolithic periods. There are, however, anomalous biases to mountain gazelle fawns and young juveniles (Gazella gazella) during the early Natufian at Hayonim Cave (31), one of the first sedentary communities of the Levant. The same bias is true for the caprines from AH upper Level 4. Because metapodials are relatively uncommon in the AH sample and the element was heavily recruited for bone tools, the fusion rates for the more common distal tibia are also considered; these results are more in line with the Natufian gazelles. Sedentism seems to have exerted a unique impact on the exploitation of the young of staple ungulate species, regardless of whether that species came to be domesticated.
Fig. 3.
Comparison of caprine fusion rates in AH upper Level 4 for first phalanges and distal metapodials to patterns in earlier artiodactyl assemblages (mountain gazelle, roe deer, fallow deer, or caprines). Other cases are from Karain and Öküzini 5 (33), Hallan Çemi, and Üçağızlı Cave I and Üçağızlı Cave II in Turkey, and Hayonim Cave, Hilazon Tachtit, Meged Rockshelter, and Kebara Cave in the southern Levant (31, 40). EPI, Epipaleolithic; MP Middle Paleolithic; T, Distal tibia, which fuses around same time as the metapodials; UP, Upper Paleolithic.
Another interesting feature of the AH upper Level 4 ungulates concerns the proportion of fetal to neonate remains. Neonate remains occur along with adults in the equids, aurochs, and caprines (Table 1). Only the caprine remains also include fetuses. Nearly one-third of lambs/kids in the caprine death assemblage perished while in utero. It is very unlikely that the absence of fetal bone from the samples of Bos and Sus is a result of sampling error alone (Table S5). Because of the small number of equid bones, we cannot be certain that the absence of fetal specimens is not a sampling effect in this case, although there is only a 14.4% chance that this is the reason.
Table 1.
NISP among the infantile ungulate remains in upper Level 4
| Taxon | NISP fetus | NISP neonate | NISP fetus/neonate |
| Equidae | 0 | 10 | 2 |
| Bos | 0 | 18 | 3 |
| Caprines | 30 | 64 | 107 |
| Sus | 0 | 22 | 4 |
| Cervidae | 0 | 0 | 0 |
Differences between other taxa and the caprines are significant at the 0.05 level of probability, except for Equidae. Age determinations are based on aged reference skeletons; not all specimens could be determined (hence the fetus/neonate category).
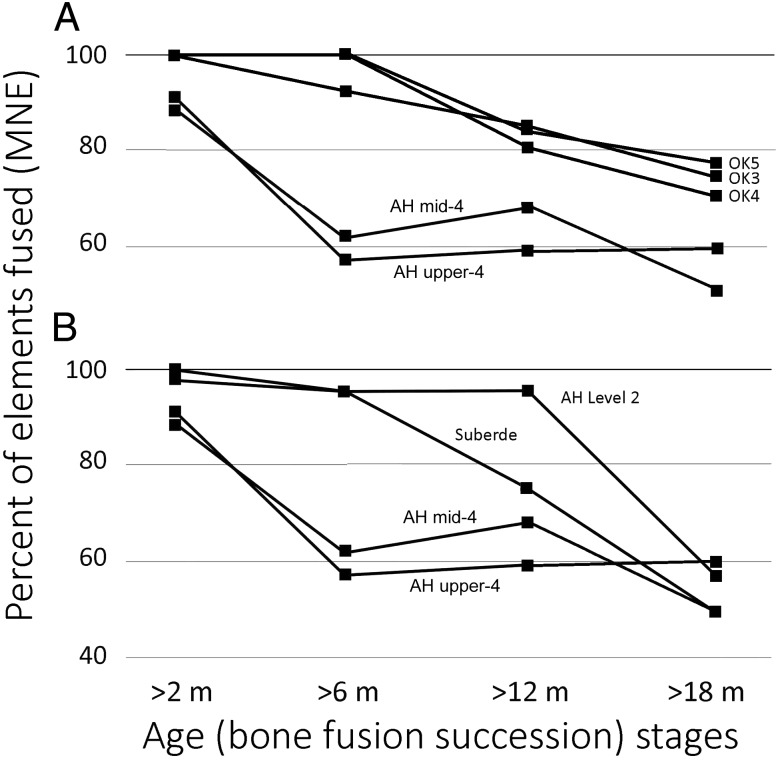
An expanded view of caprine survivorship based on bone fusion over the first 2 y of life sets the AH upper Level 4 assemblages apart from both the Epipaleolithic and other PPN assemblages (Fig. 4). Three states of bone development—neonate, unfused/partly fused, and fully fused—are considered for a series of skeletal elements, ordered according to the average age at which each normally fuses (following ref. 32). The AH upper Level 4 assemblages are subdivided consecutively into uppermost and midupper Level 4 samples. These assemblages are very different to late Epipaleolithic sheep-dominated caprines from Öküzini 3–5 in the western Taurus (33). About 40–50% of the AH caprines died before reaching adulthood, whereas only about 20% of the Epipaleolithic caprines died short of adulthood (Fig. 4A). The AH Level 4 assemblages are also distinct from PPN caprine assemblages (Fig. 4B) from Suberde (34) and the Level 2 occupations at AH (27). The mortality peak after 6 mo of age but before 12 mo most clearly sets the AH Level 4 assemblages apart from all others in this comparison.
Fig. 4.
Comparison of the AH caprine early stages of survivorship in months based on fusion data for assemblages from uppermost Level 4 (MNE = 207) and mid-upper Level 4 (n = 90) to (A) Epipaleolithic sheep-dominated caprine assemblages from Öküzini 3–5 (n = 354, n = 120, n = 124, respectively); and (B) to later PPN caprine assemblages from Level 2 of AH (n = 2,806) and Suberde (n = 95). Unborn individuals from AH are excluded from the comparisons. Sources of comparison data can be found in refs. 16, 33, and 34.
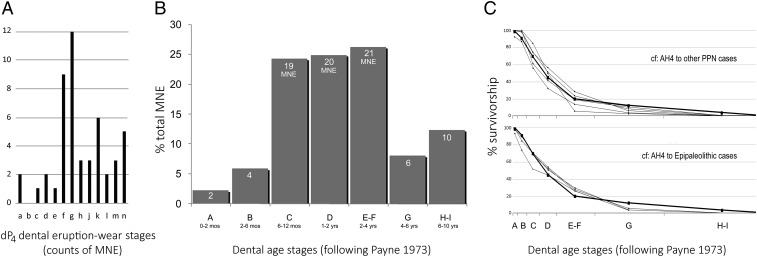
Dental-based mortality patterns are difficult to link directly to the fusion results because of the different parameters involved, but the dental data are important for examining mortality patterns across the full potential lifespan, as well as within the juvenile period. The age stages used in Fig. 5 follow Payne (35), with modifications by Grant (36) for milk teeth. Dental counts (minimum number of element, MNE) combine isolated and articulated mandibular teeth (following ref. 37) (Table S6). Young caprines from AH upper Level 4 were harvested at nearly every age stage over the first 24 mo of life (Fig. 5A), based on eruption-wear data for the deciduous lower fourth premolar (dP4). Culling was generally continuous, but an abrupt peak occurs midway through the functional life of the milk tooth, probably leading into winter. Moderate levels of culling continued thereafter.
Fig. 5.
AH upper Level 4 caprine age structure based on dental eruption-wear stages: (A) detailed juvenile mortality distribution based on counts of dP4 milk teeth according to Grant's (36) sequential stages; (B) percentage MNE across Payne’s (35) age stages for the total lifespan; (C) comparison of AH caprine survivorship (n = 82) to other PPN caprine assemblages from Göbekli Tepe (n = 48), Cafer Höyük (early phase, n = 21; midphase, n = 176), Tell Aswad (n = 97), and Tell Halula (n = 133), and to Epipaleolithic caprines from Karain B 1 and 2 (n = 93, n = 46) and Öküzini 2 and 3 (n = 85, n = 40). Sources of comparison data can be found in refs. 16 and 37–39.
Fig. 5B shows that most adult deaths (across stages E–I) occurred between 2 and 4 y of age, or in stages E–F. Fig. 5C compares AH caprine survivorship to other early to mid-PPN and Epipaleolithic caprine assemblages using a Kolmogorov–Smirnov test. In all cases the D statistic was less than the 0.05 rejection threshold (Table S7), indicating no statistically significant differences in the distribution among the seven age categories of the AH4 caprine age structure and those from PPN Göbekli Tepe (16), Cafer Höyük (38), Tell Aswad (39), and Tell Halula [Sana Segui (37)]. Moreover, the AH4 caprine survivorship pattern is not statistically different from the early Epipaleolithic caprines of Karain B 1 and 2 (17,800–17,000 cal B.C.) or suitably large samples from the later Epipaleolithic occupations at Öküzini 2 (16,000–15,300 cal B.C.) and Öküzini 3 (16,000–15,300 cal B.C.).
Caprines can live 10 y or longer in the wild and in captivity, yet the AH upper Level 4 assemblage is poorer than expected in older adults, especially 4–6 y olds (Fig. 5B). Interpretation of the mortality data are complicated by the strong likelihood that caprines were being harvested both from wild and captive populations. The juvenile culling pattern is explained largely by management, but the adult distribution almost certainly represents a mixture of managed and wild sources. Although Epipaleolithic assemblages are not poor in adult prey as a rule, some Mediterranean cases are exceptionally poor in older adults, such as in Karain and Öküzini (33) and certain other Epipaleolithic cases in the Mediterranean Basin (40). These cases may reflect situations in which heavy hunting squeezed wild-prey populations into a perpetual growth mode (40). This information is not enough to isolate incipient management practices.
Sex-Biased Harvesting.
One of the most important zooarchaeological tests for animal management is sex-biased culling (21, 41, 42). Here we target the pelvis because it is well represented in the AH faunas of upper Level 4, fuses early (approximately, 6–7 mo), and the sex of the animal usually can be determined from the structure of the pubic-acetabulum region, regardless of whether it has fused. Fusion and sex determinations for 52 caprine innominates indicate that only 11% of females died before the age of 6–7 mo, whereas 58% of males died before this age (Fisher’s exact test, P = 0.0004). The age-sex bias in the caprines poses a strong contrast to hunting patterns for various ungulate species during the Upper and Epipaleolithic in the Near East and southern Europe (40). Returning to the fusion-based survivorship results in Fig. 4, the first interval of high mortality must relate to preferential culling of young males. Most females and a subset of the males lived beyond this age, although few if any of them enjoyed a long adult life. The pattern of sex-biased culling distinguishes the upper Level 4 AH caprines from the Epipaleolithic comparators. Instead, this pattern allies with early PPN comparators classified by Helmer et al. (37) as “Meat-focused Type A” culling. If milk exploitation occurred, such as from goats (the minority caprine at AH), this view of the data is not yet sufficient to expose it.
Geoarchaeological Evidence for Stabling.
A critical geoarchaeological signature of ungulate management is in situ or primary dung deposits (sensu stabling) within the confines of a settlement. Ungulate dung may enter a Neolithic site as material collected by humans from the surrounding landscape for use as fuel and construction material or via on-site defecation by captive animals. The AH people did use dung as temper in mudbricks and as a binding agent for mortar and mud plasters. They also used dung in their fireplaces. These uses are not sufficient, however, to explain the volumes and distributions of dung residues among the buildings in upper Level 4 or in the overlying deposits.
There is also considerable, diagnostic variation in the character of the dung residues. Stabling deposits, or primary dung accumulations, are distinguished in the field by visible laminations and a striking orange sediment color (Fig. S3). Stabling deposits are confirmed in the laboratory from their microscopic characteristics (43, 44), including the presence of abundant calcareous spherulites interbedded with siliceous phytoliths, along with lesser amounts of secondary phosphorus and calcium oxalate crystals. A well-developed microlaminated undulating structure is also typical of intact remnants of stabling deposits (45). Such deposits have been identified in upper Level 4 as well in later occupations of the mound, both within the midden areas and in some outdoor spaces where other types of activities, such as knapping and butchery, were also conducted periodically (Table S8).
Phytoliths provide corroborating evidence of significant dung inputs and contents in upper Level 4. Phytolith remains from deposits that were identified as dung based on micromorphology are attributable to dicot plants, wild grasses, cereals, riverine sedges, rushes, and reeds. Among this diverse range of plant taxa, wild grass phytoliths occur in the largest quantities by far. Some of the wild grass remains in the site more generally may represent food processing debris, but the majority of the grass phytoliths were introduced into the site as animal dung. The clear dominance of grass over dicot leaf phytoliths in the ruminant dung is consistent, moreover, with that produced by grazers (sheep or cattle) rather than browsing goats (46).
Concluding Remarks
The faunal and geoarchaeological evidence suggest a rapidly evolving predator–prey relationship at AH. A meat diet consisting of diverse ungulate and small prey species in upper Level 4 shifted smoothly over just a few centuries to one that overwhelmingly emphasized caprines. Human–caprine interactions in upper Level 4 were neither classically Epipaleolithic nor classically PPN in habit. Disproportionate culling of young males indicates deliberate manipulation of caprine survivorship by humans by or before 8200 cal B.C. Young caprines were killed throughout the year, but harvesting of juveniles rose sharply as winter approached and continued at moderate levels thereafter. Unfortunately, any interpretation of adult mortality in the caprines is complicated by the likely mixing of managed and hunted caprines in the archaeofaunas. Only the early mortality peak in the caprine assemblage, which is mostly composed of young males, clearly signals management by humans.
The distinctive caprine age-sex structures in upper Level 4 associate with primary dung deposits inside the settlement. More work is needed to understand the full scope and contexts of dung inputs in this level, but it is clear that ruminants were held captive between the buildings and occasionally in some of the work areas. The dung was produced foremost by grazing rather than browsing ungulates, and the zooarchaeological and phytolith evidence narrow the candidate species primarily to sheep. The caprines of upper Level 4 certainly were not domesticated (they retained wild morphology), but there is no doubt that small captive populations were being manipulated selectively by humans.
The gradual shift in species importance from upper Level 4 through Levels 3 and 2 suggests a local evolution of caprine management in this Central Anatolian site. The management methods appear primitive in that they were oriented to returns over shorter intervals than is typical in other PPN systems (37), including the methods of the AH Level 2 occupants. The Level 4 folk may have culled fattened young caprines very heavily in late autumn and early winter because they did not want to risk overwintering them when food supplies would be limited, risks of loss high, and foddering necessary. Even if this early system was relatively inefficient or unstable by later standards, considerable productivity could still be gained from captive juvenile and young adult stock. Sheep and goats depend on their mothers until 10 mo of age, but females can reach reproductive maturity as early as 18 mo, bearing their first young at 2 y. The goals of management during the occupations of upper Level 4 were geared principally or exclusively to meat production and multimonth (rather than long-term) live storage (37). The wide developmental spread of fetal-neonate losses seems to underscore the high risk and loosely experimental nature of early attempts at caprine management at AH. The fact that about one-third of all infantile caprine specimens are from fetuses could reflect indiscriminant culling of young pregnant females or spontaneously aborted lambs. Either way, the pattern implies a steep learning curve in the early evolution of management practices.
What sort of conceptual background was cradle to these innovations? In some regards, animal management was not a radical departure from complex strategies of the Epipaleolithic. Foragers of the earlier period frequently insulated themselves from risk and unpredictable food supplies by broadening the diet and by intensifying their use of certain resources. Both strategy sets generally entailed more work, and new kinds of work and social contracts, for some members of the group, but they are excellent solutions to reduced mobility from any cause. Intensification is largely a matter of squeezing more benefits out of every food package or patch that consumers could reliably control. Storage, grease rendering, and processing rich seeds and nuts are all examples of intensifying strategies. Diversification and intensification are two sides of the same coin: each is a common response to constrained food supplies or loss of mobility, and they often co-occur in complex hunter-gatherers (47). The strategic balance between the two modes tilted more toward intensified exploitation of core plant and animal species with the early Neolithic, at the expense of a diversified meat diet. The challenge for archaeologists is to better understand why such state shifts occurred.
One of the more striking energetic outcomes of the evolving predator–prey relationship at AH was the resurgence of large prey as the principle meat source. Taken at face value, this departure from an Epipaleolithic background of broad-spectrum hunting would seem a major reversal in humans’ place in the local food web. However, the ratio of meat to plant energy sources in the early PPN diet may not have increased at all. Indeed the opposite is generally indicated by the many demographic pulses of the Neolithic (48), implying further “downward” shifts in humans’ position in the food web as cereals and pulses became the main sources of carbohydrates. Groundstone artifacts and remains of cereal grasses, pulses, hackberry, nuts, and other seeds are widespread in upper AH Level 4 and testify to their great economic importance. Whatever humans’ larger motives for manipulating caprine subpopulations, the changes in exploitation at AH were geared to maintaining ever-more reliable access to this meat source.
Caprine management at AH may not have been about creating a better prey animal but rather individual human solutions to an impending “tragedy of the commons” (49). The location of AH on the Melendiz floodplain and the rich botanical record suggest a heavy commitment to plant cultivation with the founding of the community. Permanent settlement on the floodplain would have led to local depression of meat sources, irrespective of whether climate was benevolent or harsh. Hunters might respond by searching farther afield, but not without incurring significant travel costs and time away from gardens and family. Sedentism, reduced prey encounter rates, and scheduling conflicts between long-distance hunting and plant cultivation may have induced AH residents to innovate rapidly to accommodate the latter.
One early pathway to stock-keeping could have been the timeless hunter-gatherer habit of bringing home young animals as pets. Ethnographic sources show, however, that such pets seldom survive for long because of neglect, abuse, or becoming yet another meal. At AH, a significant number of the captive sheep and goats lived many months, until their “stored” meat value could be realized. Another pathway to stock-keeping with much stronger incentives for care and vigilance comes from Epipaleolithic traditions of food storage and plant tending. If reasonably well cared for, captive animals are a means for both growing and live-storing meat and fat. The food transport costs are reduced because the animals are not hunted in remote places. Feeding herbivorous animals is work that must be done instead, but such work would already be supported by or embedded within the historical precedent of plant cultivation. Incipient animal tending might be especially viable for communities located on the edge—neither within or very remote to—highland hunting grounds. Failed experiments could be restarted or enriched on occasion by new expeditions into remote areas (see ref. 50 for related discussions). Sequestered live storage meanwhile would have reduced losses to competitors (leopards, wolves, and other people) and deflected the need for quick consumption.
If sequestered live storage improved meat security at AH, it must also have introduced new social strains. Some of the meat no longer came from a wild, common pool. Meanwhile the per capita consumption of meat may have gradually reduced as the caloric contributions of plant seeds overtook other foods. Keeping animals required new kinds of work and spatial accommodations. These developments surely had consequences for individual and cooperative labor over the long run, and for the social institutions that bind community members and affect their access to critical resources.
The trajectory of change at AH roughly parallels trends observed in certain other regions of the Middle East. Selective manipulation of caprines or pigs is evidenced at sites in the upper Euphrates region by 8500–8400 cal B.C., including Nevali Çori, Cafer Höyük, and probably Çayönü (11, 16, 38, 51), and on the island of Cyprus (20). As radiocarbon-dating efforts continue at AH, the bulk of the Level 4 sequence will likely fall across this temporal range, confirming AH’s place in a larger web of socioeconomic and ideological innovations. AH is but one of several emergent cases of low-level stock production. Taken together, these cases demonstrate the futility of looking for a single point of origin in animal domestication. Experiments were geographically widespread, highly variable, and couched within a continuing tradition of hunting practices (11, 20).
Supplementary Material
Acknowledgments
We thank Britt Starkovich for unpublished data on Hallan Çemi; Joris Peters for his contributions to data acquisition and discussion; three anonymous PNAS reviewers for their many constructive criticisms of the first draft; the generosity of the members of the Kızılkaya village community; and our colleagues and University of Istanbul students for their unfailing dedication to the Aşıklı Höyük project, without which this research would have been impossible. This research was funded by Ministry of Culture and Tourism-Turkey, Istanbul University Project 24030 (to M.Ö.); Archaeology Program Grant BCS 0912148 from the National Science Foundation of the United States (to M.C.S.); the German Research Foundation Grants PE 424/10-1 and 10-2 (to N.P. and J. Peters); and the German Academic Exchange Service (to S.M.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322723111/-/DCSupplemental.
References
- 1.Baird D, Fairbairn A, Martin L, Middleton C. In: The Neolithic in Turkey, New Excavations and New Research, Central Turkey. Özdoğan M, Başgelen N, Kuniholm P, editors. İstanbul: Arkeoloji ve Sanat; 2012. pp. 219–244. [Google Scholar]
- 2.Thissen L. 2002. in The Neolithic of Central Anatolia, eds Gerard F, Thissen L (Proc Internat CANeW Table Ronde, November 2001, EGE Yayınları, Istanbul), pp 13–26.
- 3.Arbuckle BS, Erek CM. 2012. Late Epipaleolithic hunters of the Central Taurus: Faunal remains from Direkli Cave, Kahramanmaraş, Turkey. Internat J Osteoarchaeology 22(6):694–707.
- 4.Kartal M. Epi-Paleolitik Dönem Türkiye’de Son Avcı Toplayıcılar: Konar-Göçerlikten Yerleşik Yaşama Geçiş. Istanbul: Arkeoloji ve Sanat Yayınları; 2009. [Google Scholar]
- 5.Bar-Yosef O, Belfer-Cohen A. 2002. in The Dawn of Farming in the Near East, eds Cappers RTJ, Bottema S (Ex oriente, Berlin), pp 55–66.
- 6.Hauptmann H. 2002. in The Neolithic of Central Anatolia, eds Gerard F, Thissen L (Proc Internat CANeW Table Ronde, November 2001, EGE Yayınları, Istanbul), pp 263–271.
- 7.Matthews R. 2002. in The Neolithic of Central Anatolia, eds Gerard F, Thissen L (Proc Internat CANeW Table Ronde, November 2001, EGE Yayınları, Istanbul), pp 91–103.
- 8.Özbaşaran M, Duru G. 2014. The early sedentary community of Cappadocia: Aşıklı Höyük, Troisièmes Rencontres d'Archéologie de l’IFEA. Cappadocia, in press.
- 9.Özdoğan M. The beginning of Neolithic economies in southeastern Europe: An Anatolian perspective. J European Archaeol. 1997;5:1–33. [Google Scholar]
- 10.Willcox G. The distribution, natural habitats and availability of wild cereals in relation to their domestication in the Near East: Multiple events, multiple centres. Veg Hist Archaeobot. 2005;14(4):534–541. [Google Scholar]
- 11.Conolly J, et al. Meta-analysis of zooarchaeological data from SW Asia and SE Europe provides insight into the origins and spread of animal husbandry. J Archaeol Sci. 2011;38(3):538–545. [Google Scholar]
- 12.Colledge S, Conolly J. Reassessing the evidence for the cultivation of wild crops during the Younger Dryas at Tell Abu Hureyra, Syria. Environ Archaeol. 2010;15(2):124–138. [Google Scholar]
- 13.Fuller DQ. Contrasting patterns in crop domestication and domestication rates: Recent archaeobotanical insights from the Old World. Ann Bot (Lond) 2007;100(5):903–924. doi: 10.1093/aob/mcm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hongo H, Meadow R, Öksüz B, Ilgezdi G. 2002. in Archaeozoology of the Near East V, eds Al-Shiyab AH, Choyke AM, Buitenhuis H (ARC, Groningen, The Netherlands), pp 153–165.
- 15.Horwitz LK. Temporal and spatial variation in Neolithic caprine exploitation strategies: A case study of fauna from the site of Yiftah'el, (Israel) Paléorient. 2003;29(1):19–58. [Google Scholar]
- 16.Peters J, Buitenhuis H, Grupe G, Schmidt K, Pöllath N. In: The Origins and Spread of Domestic Animals in Southwest Asia and Europe. Colledge S, et al., editors. Walnut Creek, CA: Left Coast Press; 2013. pp. 83–114. [Google Scholar]
- 17.Redding RW. In: The First Steps of Animal Domestication: New Archaeological Approaches. Vigne J-D, Peters J, Helmer D, editors. Oxford: Oxbow Books; 2005. pp. 41–48. [Google Scholar]
- 18.Savard M, Nesbitt M, Jones MK. The role of wild grasses in subsistence and sedentism: New evidence from the northern Fertile Crescent. World Archaeol. 2006;38(2):179–196. [Google Scholar]
- 19.Starkovich B, Stiner MC. Hallan Çemi Tepesi: High-ranked game exploitation alongside intensive seed processing at the Epipaleolithic-Neolithic transition in Southeastern Turkey. Anthropozoologica. 2009;44(1):41–61. [Google Scholar]
- 20.Vigne J-D, et al. First wave of cultivators spread to Cyprus at least 10,600 y ago. Proc Natl Acad Sci USA. 2012;109(22):8445–8449. doi: 10.1073/pnas.1201693109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeder MA, Hesse B. The initial domestication of goats (Capra hircus) in the Zagros mountains 10,000 years ago. Science. 2000;287(5461):2254–2257. doi: 10.1126/science.287.5461.2254. [DOI] [PubMed] [Google Scholar]
- 22.Pedrosa S, et al. Evidence of three maternal lineages in Near Eastern sheep supporting multiple domestication events. Proc Biol Sci. 2005;272(1577):2211–2217. doi: 10.1098/rspb.2005.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Özdoğan M, Başgelen N, Kuniholm P, editors. The Neolithic in Turkey, New Excavations and New Research, Central Turkey. Vol 3. Istanbul: Arkeoloji ve Sanat Yayınları; 2012. pp. 135–158. [Google Scholar]
- 24.Esin U, Harmankaya S. Aşıklı, Neolithic in Turkey. İstanbul: Arkeoloji ve Sanat; 1999. [Google Scholar]
- 25.Esin U, Harmankaya S. 1999. in Neolithic in Turkey, eds Özdoğan M, Başgelen N (Arkeoloji ve Sanat, Istanbul), pp 115–132.
- 26.Duru G. 2013. Tarihöncesinde insan-mekan, topluluk-yerleşme ilişkisi: MÖ 9. bin sonu -7. bin başı, Aşıklı ve Akarçay Tepe. PhD dissertation, (Department of Prehistory, Istanbul Univ, Istanbul)
- 27.Buitenhuis H. Aşıklı Höyük: A protodomestication site. Anthropozoologica. 1997;25–26:655–662. [Google Scholar]
- 28.Van Zeist W, Jan De Roller G. 2003. in Reports on Archaeobotanical Studies in the Old World, ed Van Zeist W (Groningen, The Netherlands), pp 115–142. [Google Scholar]
- 29.Esin U. The Aceramic site of Aşıklı and its ecological conditions based on its floral and faunal remains. TÜBA-AR. 1998;1:95–103. [Google Scholar]
- 30.Aurenche O, Galet P, Regagnon-Carolin E, Évin J. Proto-Neolithic and Neolithic cultures in the Middle East—The birth of agriculture, livestock raising and ceramics: A calibrated 14C chronology 12,500-5500 cal BC. Radiocarbon. 2001;43(3):1191–1202. [Google Scholar]
- 31.Munro ND. Zooarchaeological measures of hunting pressure and occupation intensity in the Natufian. Curr Anthropol. 2004;45(Suppl 4):S5–S33. [Google Scholar]
- 32.Zeder MA. In: Recent Advances in Ageing and Sexing Animal Bones. Ruscillo D, editor. Chippenham: Oxbow Books; 2006. pp. 87–118. [Google Scholar]
- 33.Atıcı L. Implications of age structures for Epipaleolithic hunting strategies in the Western Taurus Mountains, southwest Turkey. Anthropozoologica. 2009;44(1):13–39. [Google Scholar]
- 34.Arbuckle BS. Revisiting Neolithic caprine exploitation at Suberde, Turkey. J Field Archaeol. 2008;33(2):219–236. [Google Scholar]
- 35.Payne S. Kill-off patterns in sheep and goats: The mandibles from Asvan Kale. Anatolian Studies. 1973;23:281–303. [Google Scholar]
- 36.Grant A. In: Aging and Sexing Animal Bones from Archaeological Sites. Wilson B, Grigson C, Payne S, editors. Oxford: British Archaeological Reports; 1982. pp. 91–108. [Google Scholar]
- 37.Helmer D, Gourichon L, Vila E. The development of the exploitation of products from Capra and Ovis (meat, milk, and fleece) from the PPNB to the Early Bronze Age in the northern Near East (8700–9200 cal BP) Anthropozoologica. 2007;42(2):41–69. [Google Scholar]
- 38.Helmer D. 2008. in Archaeozoology of the Near East VIII: Proceedings of the 8th International Symposium on the Archaeozoology of Southwestern Asia and Adjacent Areas, eds Vila E, et al. (Maison de l’Orient et de la Méditerranée, Lyon), pp 169–195.
- 39.Helmer D, Gourichon L. 2008. in Archaeozoology of the Near East VIII: Proceedings of the 8th International Symposium on the Archaeozoology of Southwestern Asia and Adjacent Areas, eds Vila E, et al. (Maison de l’Orient et de la Méditerranée, Lyon), pp 120–151.
- 40.Stiner MC. The Faunas of Hayonim Cave, Israel: A 200,000-Year Record of Paleolithic Diet, Demography, and Society. Cambridge, MA: Peabody Museum Press; 2005. [Google Scholar]
- 41.Ducos P. 1978. in Approaches to Faunal Analysis in the Middle East, eds Meadow RH, Zeder MA (Peabody Museum, Harvard Univ, Cambridge, MA), Bull 2, pp 53–56.
- 42.Grigson C. 1989. in The Beginnings of Agriculture, eds Milles AD, Williams D, Gardner N (BAR International Series, Oxford), Vol 496, pp 77–109.
- 43.Canti MG. The micromorphological identification of faecal spherulites from archaeological and modern materials. J Archaeol Sci. 1998;25(5):435–444. [Google Scholar]
- 44.Shahack-Gross R. Herbiverous livestock dung: Formation, taphonomy, methods for identification, and archaeological significance. J Archaeol Sci. 2011;38(2):205–218. [Google Scholar]
- 45.Shahack-Gross R, Finkelstein I. Subsistence practices in an arid environment: A geoarchaeological investigation in an Iron Age site, the Negev Highlands, Israel. J Archaeol Sci. 2008;35(4):965–982. [Google Scholar]
- 46.Tsartsidou G, Lev-Yadun S, Efstratiou N, Weiner S. Use of space in a Neolithic village in Greece (Makri): Phytolith analysis and comparison of phytolith assemblages from an ethnographic setting in the same area. J Archaeol Sci. 2009;36(10):2342–2352. [Google Scholar]
- 47.Kuhn SL, Stiner MC. 2001. in Hunter-Gatherers: Interdisciplinary Perspectives, eds Panter-Brick C, Layton RH, Rowley Conwy RA (Cambridge Univ Press, Cambridge, UK), pp 99–142.
- 48.Bocquet-Appel J-P. When the world’s population took off: The springboard of the Neolithic Demographic Transition. Science. 2011;333(6042):560–561. doi: 10.1126/science.1208880. [DOI] [PubMed] [Google Scholar]
- 49.Ostrom E. How types of goods and property rights jointly affect collective action. J Theor Polit. 2003;15(3):239–270. [Google Scholar]
- 50.Vigne J-D, Carrère I, Briois F, Guilaine J. The early process of mammal domestication in the Near East: New evidence from the Pre-Neolithic and Pre-Pottery Neolithic in Cyprus. Curr Anthropol. 2011;52(S4):S255–S271. [Google Scholar]
- 51.Ervynck A, Dobney K, Hongo H, Meadow R. Born Free? New evidence for the status of Sus scrofa at Neolithic Çayönü Tepesi (southeastern Anatolia, Turkey) Paléorient. 2001;27(2):47–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.