Abstract
Categorization enables listeners to efficiently encode and respond to auditory stimuli. Behavioral evidence for auditory categorization has been well documented across a broad range of human and non-human animal species. Moreover, neural correlates of auditory categorization have been documented in a variety of different brain regions in the ventral auditory pathway, which is thought to underlie auditory-object processing and auditory perception. Here, we review and discuss how neural representations of auditory categories are transformed across different scales of neural organization in the ventral auditory pathway: from across different brain areas to within local microcircuits. We propose different neural transformations across different scales of neural organization in auditory categorization. Along the ascending auditory system in the ventral pathway, there is a progression in the encoding of categories from simple acoustic categories to categories for abstract information. On the other hand, in local microcircuits, different classes of neurons differentially compute categorical information.
Keywords: auditory category, ventral auditory pathway, speech sound, vocalization, pyramidal neuron, interneuron
Introduction
Auditory categorization is a computational process in which sounds are classified and grouped based on their acoustic features and other types of information (e.g., semantic knowledge about the sounds). For example, when we hear the word “Hello” from different speakers, we can categorize the gender of each speaker based on the pitch of the speaker's voice. On the other hand, in order to analyze the linguistic content transmitted by speech sounds, we can ignore the unique pitch, timbre etc. of each speaker and categorize the sound into the distinct word category “Hello.” Thus, auditory categorization enables humans and non-human animals to extract, manipulate, and efficiently respond to sounds (Miller et al., 2002, 2003; Russ et al., 2007; Freedman and Miller, 2008; Miller and Cohen, 2010).
A specific type of categorization is called “categorical perception” (Liberman et al., 1967; Kuhl and Miller, 1975, 1978; Kuhl and Padden, 1982, 1983; Kluender et al., 1987; Pastore et al., 1990; Lotto et al., 1997; Sinnott and Brown, 1997; Holt and Lotto, 2010). The primary characteristic of categorical perception is that the perception of a sound does not smoothly vary with changes in its acoustic features. That is, in certain situations, small changes in the physical properties of an acoustic stimulus can cause large changes in a listener's perception of a sound. In other situations, large changes can cause no change in perception. The stimuli, which cause these large changes in perception, straddle the boundary between categories. For example, when we hear a continuum of smoothly varying speech sounds (i.e., a continuum of morphed stimuli between the phoneme prototypes “ba” and “da”), we experience a discrete change in perception. Specifically, a small change in the features of a sound near the middle of this continuum (i.e., at the category boundary between a listener's perception of “ba” and “da”) will cause a large change in a listener's perceptual report. In contrast, when that same small change occurs at one of the ends of the continuum, there is little effect on the listener's report.
Even though some perceptual categories have sharp boundaries, the locations of the boundary are somewhat malleable. For instance, the perception of a phoneme can be influenced by the phonemes that come before it. When morphed stimuli, which are made from the prototypes “da” and “ga,” are preceded by presentations of “al” or “ar,” the perceptual boundary between the two prototypes shifts (Mann, 1980). Specifically, listeners' reports are biased toward reporting the morphed stimuli as “da” when it is preceded by “ar.” When this morphed stimulus is preceded by “al,” listeners are biased toward reporting the morphed stimulus as “ga.”
Categories are not only formed based on the perceptual features of stimuli but also on more “abstract” types of information. An abstract category is one in which a group of arbitrary stimuli are linked together as a category based on some shared features, a common functional characteristic, semantic information, or acquired knowledge. For instance, a combination of physical characteristics and knowledge about their reproductive processes puts dogs, cats, and killer whales into one category (“mammals”), but birds into a separate category. However, if we use different criteria to form a category of “pets,” dogs, cats, and birds would be members of this “pet” category but not killer whales.
Behavioral responses to auditory communication signals (i.e., species-specific vocalizations) also provide evidence for abstract categorization. One example is the categorization of food-related species-specific vocalizations by rhesus monkeys (Hauser and Marler, 1993a,b; Hauser, 1998; Gifford et al., 2003). In rhesus monkeys, a vocalization called a “harmonic arch” transmits information about the discovery of rare, high-quality food. A different vocalization called a “warble” also transmits the same type of information: the discovery of rare, high-quality food. Importantly, whereas both harmonic arches and warbles transmit the same type of information, they have distinct spectrotemporal properties. Nevertheless, rhesus monkeys' responses to those vocalizations indicate that monkeys categorize these two calls based on their transmitted information and not their acoustic features. In another example, Diana monkeys form abstract-categorical representations for predator-specific alarm calls independent of the species generating the signal. Diana monkeys categorize and respond similarly to alarm calls that signify the presence of a leopard, regardless of whether the alarm calls are elicited from a Diana monkey or a crested guinea fowl (Zuberbuhler and Seyfarth, 1997; Züberbuhler, 2000a,b). Similarly, Diana monkeys show similar categorical-responses to eagle alarm calls that can be elicited from other Diana monkeys or from putty-nose monkeys (Eckardt and Zuberbuhler, 2004).
In order to better understand the mechanisms that underlie auditory categorization, it is essential to examine how neural representations of auditory categories are formed and transformed across different scales of neural organization: from across different brain areas to within local microcircuits. In this review, we discuss the representation of auditory categories in different cortical regions of the ventral auditory pathway; the hierarchical processing of categorical information along the ventral pathway; and the differential role that excitatory pyramidal neurons and inhibitory interneurons (i.e., different neuron classes) contribute to these categorical computations.
The ventral pathway is targeted because neural computations in this pathway are thought to underlie sound perception, which is critically related to auditory categorization and auditory scene analysis (Rauschecker and Scott, 2009; Romanski and Averbeck, 2009; Bizley and Cohen, 2013). The ventral auditory pathway begins in the core auditory cortex (in particular, the primary auditory cortex and the rostral field R) and continues into the anterolateral and middle-lateral belt regions. These belt regions then project either directly or indirectly to the ventral prefrontal cortex (Figure 1) (Hackett et al., 1998; Rauschecker, 1998; Kaas and Hackett, 1999, 2000; Kaas et al., 1999; Romanski et al., 1999a,b; Rauschecker and Tian, 2000; Rauschecker and Scott, 2009; Romanski and Averbeck, 2009; Recanzone and Cohen, 2010; Bizley and Cohen, 2013).
Figure 1.
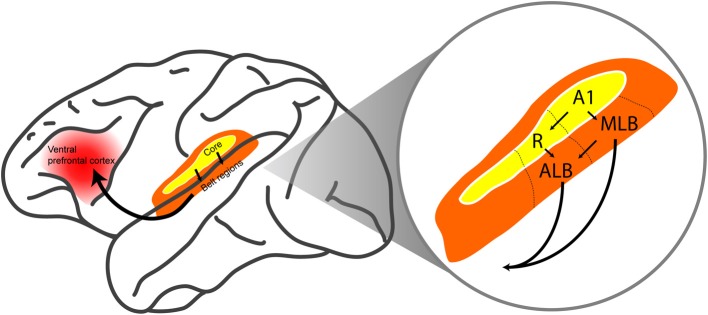
The ventral auditory pathway in the monkey brain. The ventral auditory pathway begins in core auditory cortex (in particular, the primary auditory cortex A1 and the rostral field R). The pathway continues into the middle-lateral (MLB) and anterolateral (ALB) belt regions, which project directly and indirectly to the ventral prefrontal cortex. Arrows indicate feedforward projections. The figure is modified, with permission, from Hackett et al. (1998) and Romanski et al. (1999a).
Neural transformations across cortical areas in the ventral auditory pathway
In this section, we discuss how auditory categories are processed in the ventral auditory pathway. More specifically, we review the representation of auditory categories across different regions in the ventral auditory pathway and then discuss the hierarchical processing of categorical information in the ventral auditory pathway.
Before we continue, it is important to define the concept of a “neural correlate of categorization.” One simple definition is the following: a neural response is “categorical” when the responses are invariant to the stimuli that belong to the same category. In practice, neuroimaging techniques define “categorical” responses as equivalent activations of distinct brain regions by within-category stimuli and the equivalent activation of different brain regions by stimulus exemplars from a second category (Binder et al., 2000; Altmann et al., 2007; Doehrmann et al., 2008; Leaver and Rauschecker, 2010). At the level of single neurons, a neuron is said to be “categorical” if its firing rate is invariant to different members of one category and if it has a second level of (invariant) responsivity to stimulus exemplars from a second category (Freedman et al., 2001; Tsunada et al., 2011). The specific mechanisms that underlie the creation of category sensitive neurons are not known. However, presumably, they rely on the computations that mediate stimulus invariance in neural selectivity and perception (Logothetis and Sheinberg, 1996; Holt and Lotto, 2010; Dicarlo et al., 2012). Moreover, because animals can form a wide range of categories based on individual experiences, a degree of learning and plasticity must be involved in the creation of de-novo category selective responses (Freedman et al., 2001; Freedman and Assad, 2006). Indeed, when monkeys were trained to categorize stimuli with different category boundaries, boundaries for categorical responses in some brain areas (e.g., the prefrontal and parietal cortices) also changed (Freedman et al., 2001; Freedman and Assad, 2006).
How do different cortical areas in the ventral auditory pathway similarly or differentially represent categorical information?
It is well known that neurons become increasingly sensitive to more complex stimuli and abstract information between the beginning stages of the ventral auditory pathway (i.e., the core) and the latter stages (e.g., the ventral prefrontal cortex). For example, neurons in the core auditory cortex are more sharply tuned for tone bursts than neurons in the lateral belt (Rauschecker et al., 1995), whereas lateral-belt neurons are more sensitive to the spectrotemporal properties of complex sounds, such as vocalizations (Rauschecker et al., 1995; Tian and Rauschecker, 2004). Furthermore, beyond the auditory cortex, the ventral prefrontal cortex not only encodes complex sounds (Averbeck and Romanski, 2004; Cohen et al., 2007; Russ et al., 2008a; Miller and Cohen, 2010) but also has a critical role for attention and memory-related cognitive functions (e.g., memory retrieval) which are critical for abstract categorization (Goldman-Rakic, 1995; Miller, 2000; Miller and Cohen, 2001; Miller et al., 2002, 2003; Gold and Shadlen, 2007; Osada et al., 2008; Cohen et al., 2009; Plakke et al., 2013a,b,c; Poremba et al., 2013).
These observations are consistent with the idea that there is a progression of category-information processing along the ventral auditory pathway: brain regions become increasingly sensitive to more complex types of categories. More specifically, it appears that neurons in core auditory cortex may encode categories for simple sounds, whereas neurons in the belt regions and the ventral prefrontal cortex may encode categories for more complex sounds and abstract information.
Indeed, neural correlates of auditory categorization can be seen in the core auditory cortex for simple frequency contours (Ohl et al., 2001; Selezneva et al., 2006). For example, in a study by Selezneva and colleagues, monkeys categorized the direction of a frequency contour of tone-burst sequences as either “increasing” or “decreasing” while neural activity was recorded from the primary auditory cortex. Selezneva et al. found that these core neurons encoded the sequence direction independent of its specific frequency content: that is, a core neuron responded similarly to a decreasing sequence from 1 to 0.5 kHz as it did to a decreasing sequence from 6 to 3 kHz. In a second study, Ohl et al. demonstrated that categorical representations need not be represented in the firing rates of single neurons but, instead, can be encoded in the dynamic firing patterns of a neural population. Thus, even in the earliest stage of the ventral auditory pathway, there is evidence for neural categorization.
Although the core auditory cortex processes categorical information for simple auditory stimuli (e.g., the direction of frequency changes of pure tones), studies using more complex sounds, such as human-speech sounds, have shown that core neurons primarily encode the acoustic features that compose these complex sounds but do not encode their category membership (Liebenthal et al., 2005; Steinschneider et al., 2005; Obleser et al., 2007; Engineer et al., 2008, 2013; Mesgarani et al., 2008, 2014; Nourski et al., 2009; Steinschneider, 2013). That is, the categorization of complex sounds requires not only analyses at the level of the acoustic feature but also subsequent computations that integrate the analyzed features into a perceptual representation, which is then subject to a categorization process. For example, distributed and temporally dynamic neural responses in individual core neurons can represent different acoustic features of speech sounds (Schreiner, 1998; Steinschneider et al., 2003; Engineer et al., 2008; Mesgarani et al., 2008, 2014), but the categorization of the speech sounds requires classifying the activation pattern across the entire population of core neurons.
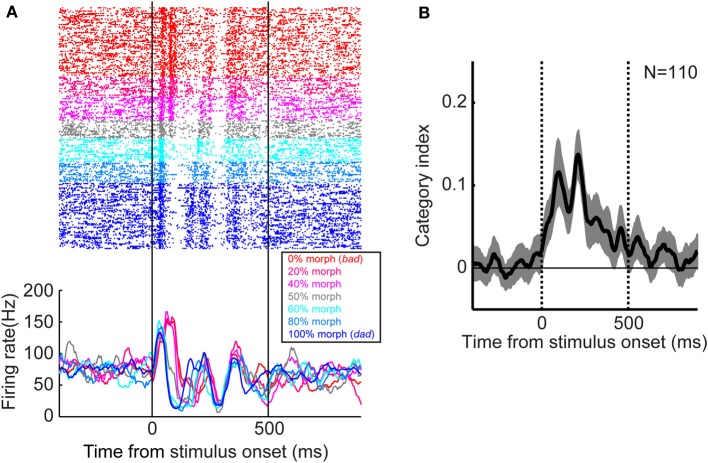
Categorical representations of speech sounds at the level of the single neuron or local populations of neurons appear to occur at the next stage of auditory processing in the ventral auditory pathway, the lateral-belt regions. Several recent studies have noted that neural activity in the monkey lateral-belt and human superior temporal gyrus encodes speech-sound categories (Chang et al., 2010; Steinschneider et al., 2011; Tsunada et al., 2011; Steinschneider, 2013). For example, our group found that, when monkeys categorized two prototypes of speech sounds (“bad” and “dad”) and their morphed versions, neural activity in the lateral belt discretely changed at the category boundary, suggesting that these neurons encoded the auditory category rather than smoothly varying acoustic features (Figure 2).
Figure 2.
Categorical neural activity in the monkey lateral belt during categorization of speech sounds. (A) An example of the activity of a lateral belt neuron. The speech sounds were two human-speech sounds (“bad” and “dad”) and their morphs. Neural activity is color-coded by morphing percentage of the stimulus as shown in the legend. The raster plots and histograms are aligned relative to onset of the stimulus. (B) Temporal dynamics of the category index at the population level. Category-index values >0 indicate that neurons categorically represent speech sounds (Freedman et al., 2001; Tsunada et al., 2011). The thick line represents the mean value and the shaded area represents the bootstrapped 95%-confidence intervals of the mean. The two vertical lines indicate stimulus onset and offset, respectively, whereas the horizontal line indicates a category-index value of 0. The figure is adopted, with permission, from Tsunada et al. (2011).
Human-neuroimaging studies have also found that the superior temporal sulcus is categorically activated by speech sounds, relative to other sounds (Binder et al., 2000; Leaver and Rauschecker, 2010). Specifically, the superior temporal sulcus was activated more by speech sounds than by frequency-modulated tones (Binder et al., 2000) or by other sounds including bird songs and animal vocalizations (Leaver and Rauschecker, 2010). Furthermore, activity in the superior temporal sulcus did not simply reflect the acoustic properties of speech sounds but, instead, represented the perception of speech (Mottonen et al., 2006; Desai et al., 2008).
Additionally, studies with other complex stimuli provide further evidence for the categorical encoding of complex sounds in the human non-primary auditory cortex, including superior temporal gyrus and sulcus, but not in the core auditory cortex (Altmann et al., 2007; Doehrmann et al., 2008; Leaver and Rauschecker, 2010). These studies found that complex sound categories were represented in spatially distinct and widely distributed sub-regions within the superior temporal gyrus and sulcus (Obleser et al., 2006, 2010; Engel et al., 2009; Staeren et al., 2009; Chang et al., 2010; Leaver and Rauschecker, 2010; Giordano et al., 2013). For example, distinct regions of the superior temporal gyrus and sulcus are selectively activated by musical-instrument sounds (Leaver and Rauschecker, 2010), tool sounds (Doehrmann et al., 2008), and human-speech sounds (Belin et al., 2000; Binder et al., 2000; Warren et al., 2006); whereas the anterior part of the superior temporal gyrus and sulcus is preferentially activated by the passive listening of conspecific vocalizations than other vocalizations (Fecteau et al., 2004). Similar findings for con-specific vocalizations have been obtained in the monkey auditory cortex (Petkov et al., 2008; Perrodin et al., 2011). Consistent with these findings, neuropsychological studies have shown that human patients with damage in the temporal cortex have deficits in voice recognition and discrimination (i.e., phonagnosia Van Lancker and Canter, 1982; Van Lancker et al., 1988; Goll et al., 2010). Thus, hierarchically higher regions in the auditory cortex encode complex-sound categories in spatially distinct (i.e., modular) and widely distributed sub-regions.
Moreover, recent studies posit that the sub-regions in the non-primary auditory cortex process categorical information in a hierarchical manner (Warren et al., 2006). A recent meta-analysis of human speech-processing studies suggests that a hierarchical organization of speech processing exists within the superior temporal gyrus: the middle superior temporal gyrus is sensitive to phonemes; anterior superior temporal gyrus to words; and the most anterior locations to short phrases (Dewitt and Rauschecker, 2012; Rauschecker, 2012). Additionally, a different hierarchical processing of speech sounds in the superior temporal sulcus has also been articulated: the posterior superior temporal sulcus is preferentially sensitive for newly acquired sound categories, whereas the middle and anterior superior temporal sulci are more responsive to familiar sound categories (Liebenthal et al., 2005, 2010). Thus, within different areas of the non-primary auditory cortex, multiple and parallel processing may progress during auditory categorization.
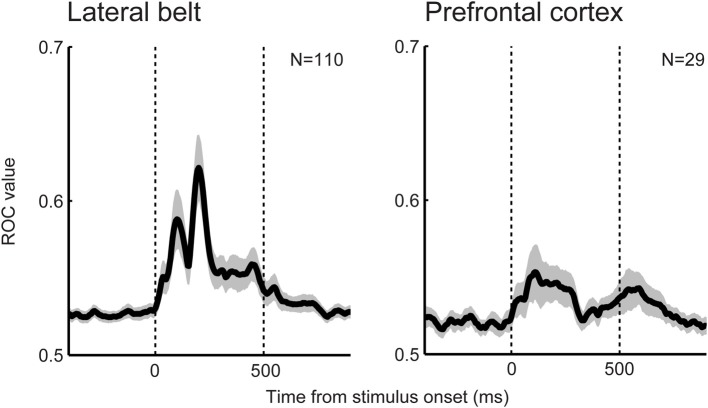
Beyond the auditory cortex, do latter processing stages (e.g., the monkey ventral prefrontal cortex and human inferior frontal cortex) process categories for even more complex sounds? A re-examination of previous findings from our lab (Russ et al., 2008b; Tsunada et al., 2011) indicated important differences in neural categorization between the lateral belt and the ventral prefrontal cortex (Figure 3). We found that, at the population level, the category sensitivity for speech sounds in the prefrontal cortex was weaker than that in the lateral belt although neural activity in the prefrontal cortex transmitted a significant amount of categorical information. Consistent with this finding, a human-neuroimaging study also found that neural activity in the superior temporal gyrus is better correlated with a listener's ability to discriminate between speech sounds than the activity in the inferior prefrontal cortex (Binder et al., 2004). Because complex sounds, including speech sounds, are substantially processed in the non-primary auditory cortex as discussed above, the prefrontal cortex may not represent, relative to the auditory cortex, a higher level of auditory perceptual-feature categorization.
Figure 3.
Category sensitivity for speech sounds in the prefrontal cortex (right) is weaker than that in the lateral belt (left). Temporal dynamics of the category sensitivity at the population level are shown. Category sensitivity was calculated using a receiver-operating-characteristic (ROC) analysis (Green and Swets, 1966; Tsunada et al., 2012). Larger ROC values indicate better differentiation between the two categories.
Instead, the prefrontal cortex may be more sensitive to categories that are formed based on the abstract information that is transmitted by sounds. For example, the human inferior prefrontal cortex may encode categories for abstract information like emotional valence of a speaker's voice (Fecteau et al., 2005). Furthermore, human electroencephalography and neuroimaging studies have also revealed that the inferior prefrontal cortex plays a key role in the categorization of semantic information of multisensory stimuli (Werner and Noppeney, 2010; Joassin et al., 2011; Hu et al., 2012): Joassin et al. showed that the inferior prefrontal cortex contains multisensory category representations of gender that is derived from a speaker's voice and from visual images of a person's face.
Similarly, the monkey ventral prefrontal cortex encodes abstract categories. We have found that neurons in the ventral prefrontal cortex represent categories for food-related calls based on the transmitted information (e.g., high quality food vs. low quality food) (Gifford et al., 2005; Cohen et al., 2006). A more recent study found that neural activity in the monkey prefrontal cortex categorically represents the number of auditory stimuli (Nieder, 2012). Thus, along the ascending auditory system in the ventral auditory pathway, cortical areas encode categories for more complex stimuli and more abstract information.
Neural transformations within local microcircuits
In this section, we discuss how the categorical information represented in each cortical area of the ventral auditory pathway is computed within local microcircuits. First, we briefly review the cortical microcircuit. Next, we focus on the role that two main cell classes of neurons in cortical microcircuits (i.e., excitatory pyramidal neurons and inhibitory interneurons) and discuss how different classes of neurons process categorical information.
How do different classes of neurons in local microcircuits process categorical information?
A cortical microcircuit can be defined as a functional unit that processes inputs and generates outputs by dynamic and local interactions of excitatory pyramidal neurons and inhibitory interneurons (Merchant et al., 2012). Consequently, pyramidal neurons and interneurons are considered to be the main elements of microcircuits. Pyramidal neurons, which consist ~70–90% of cortical neurons, provide excitatory-outputs locally (i.e., within a cortical area) and across brain areas (Markham et al., 2004). On the other hand, interneurons, which consist small portion of cortical neurons (~10–30%), provide mainly inhibitory-outputs to surrounding pyramidal neurons and other interneurons (Markham et al., 2004).
From a physiological perspective, pyramidal neurons and interneurons can be classified based on the waveform of their action potentials (Mountcastle et al., 1969; McCormick et al., 1985; Kawaguchi and Kubota, 1993, 1997; Kawaguchi and Kondo, 2002; Markham et al., 2004; González-Burgos et al., 2005). More specifically, the waveforms of pyramidal neurons tend to be broader and slower than those seen in the most interneurons. Using this classification, several extracellular-recording studies have been able to elucidate roles of pyramidal neurons and interneurons for visual working memory in the prefrontal cortex (Wilson et al., 1994; Rao et al., 1999; Constantinidis and Goldman-Rakic, 2002; Diester and Nieder, 2008; Hussar and Pasternak, 2012), visual attention in V4 (Mitchell et al., 2007), visual perceptual decision-making in the frontal eye field (Ding and Gold, 2011), motor control in the motor and premotor cortices (Isomura et al., 2009; Kaufman et al., 2010), and auditory processing during the passive listening in the auditory cortex (Atencio and Schreiner, 2008; Sakata and Harris, 2009; Ogawa et al., 2011). Interestingly, most of these studies showed differential roles in pyramidal neurons and interneurons.
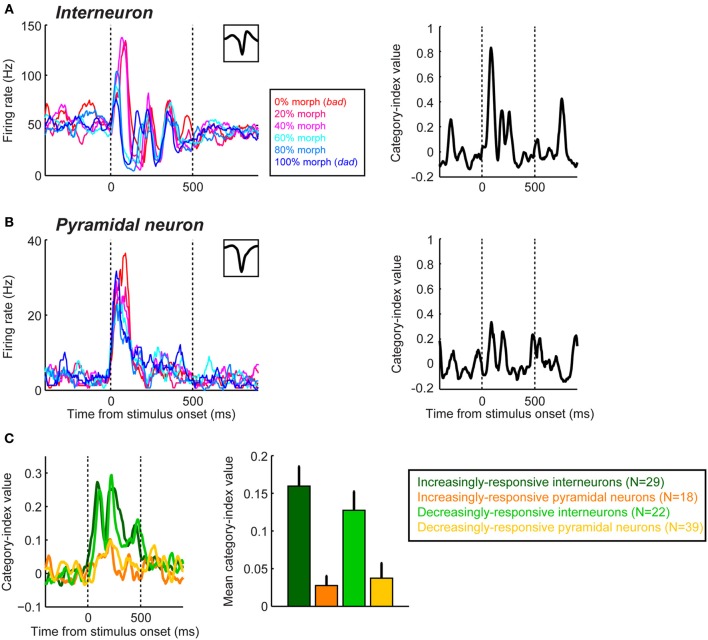
Recently, using differences in the waveform of extracellularly-recorded neurons, we found that putative pyramidal neurons and interneurons in the lateral belt differentially encode and represent auditory categories (Tsunada et al., 2012). Specifically, we found that interneurons, on average, are more sensitive for auditory-category information than pyramidal neurons, although both neuron classes reliably encode category information (Figure 4).
Figure 4.
Category sensitivity in interneurons is greater than that seen in pyramidal neurons during categorization of speech sounds in the auditory cortex. The plots in the left column of panel (A,B) show the mean firing rates of an interneuron (A) and a pyramidal neuron (B) as a function of time and the stimulus presented. The stimuli were two human-speech sounds (“bad” and “dad”) and their morphs. Neural activity is color-coded by morphing percentage of the stimulus as shown in the legend. The inset in the upper graph of each plot shows the neuron's spike-waveform. The right column shows each neuron's category-index values as a function of time. For all of the panels, the two vertical dotted lines indicate stimulus onset and offset, respectively. (C) Population results of category index. The temporal profile (left panel) and mean (right) of the category index during the stimulus presentation are shown. Putative interneurons and pyramidal neurons were further classified as either “increasingly responsive” or “decreasingly responsive” based on their auditory-evoked responses. Error bars represent bootstrapped 95% confidence intervals of the mean. The figure is adopted, with permission, from Tsunada et al. (2012).
Unfortunately, to our knowledge, there have not been other auditory-category studies that have examined the relative category sensitivity of pyramidal neurons vs. interneurons. However, a comparable visual-categorization study on numerosity in the prefrontal cortex (Diester and Nieder, 2008) provides an opportunity to compare results across studies. Unlike our finding, Diester and Nieder found greater category sensitivity for putative pyramidal neurons than for putative interneurons.
The bases for these different sets of findings are unclear. However, three non-exclusive possibilities may underlie these differences. One possibility may relate to differences in the local-connectivity patterns and interactions between pyramidal neurons and interneurons across cortical areas (Wilson et al., 1994; Constantinidis and Goldman-Rakic, 2002; Diester and Nieder, 2008; Kätzel et al., 2010; Tsunada et al., 2012). Indeed, in the prefrontal cortex, simultaneously recorded (and, hence, nearby) pyramidal neurons and interneurons have different category preferences (Diester and Nieder, 2008). In contrast, in the auditory cortex, simultaneously recorded pairs of pyramidal neurons and interneurons have similar category preferences (Tsunada et al., 2012). Thus, there may be different mechanisms for shaping category sensitivity across cortical areas. Second, the nature of the categorization task may also affect, in part, the category sensitivity of pyramidal neurons and interneurons: our task was a relatively simple task requiring the categorization of speech sounds based primarily on perceptual similarity, whereas Diester and Nieder's study required a more abstract categorization of numerosity. Finally, the third possibility relates to differences between stimulus dynamics: the visual stimuli in the Diester and Nieder's study were static stimuli, whereas our speech sounds had a rich spectrotemporal dynamic structure. To categorize dynamic stimuli, the moment-by-moment features of stimuli need to be quickly categorized. Thus, the greater category sensitivity of interneurons along with their well-known inhibitory influence on pyramidal neurons (Hefti and Smith, 2003; Wehr and Zador, 2003; Atencio and Schreiner, 2008; Fino and Yuste, 2011; Isaacson and Scanziani, 2011; Packer and Yuste, 2011; Zhang et al., 2011) may underlie the neural computations needed to create categorical representations of dynamic stimuli in the auditory cortex.
Conclusions and future directions
Different neural transformations across different scales of neural organization progress during auditory categorization. Along the ascending auditory system in the ventral pathway, there is a progression in the encoding of categories from simple acoustic categories to categories representing abstract information. On the other hand, in local microcircuits within a cortical area, different classes of neurons, pyramidal neurons and interneurons, differentially compute categorical information. The computation is likely dependent upon the functional organization of the cortical area and dynamics of stimuli.
Despite several advances in our understanding of neural mechanism of auditory categorization, there still remain many important questions to be addressed. For example, it is poorly understood how bottom-up inputs from hierarchically lower areas, top-down feedback from higher areas, and local computations interact to form neural representations of auditory categories. Answering this question will provide a more thorough understanding of the information flow in the ventral auditory pathway. Another important question to be tested is what neural circuit mechanisms produce different category sensitivity between pyramidal neurons and interneurons, and functional roles of pyramidal neurons and interneurons in auditory categorization. Relevant to this question, the role that cortical laminae (another key element of local microcircuitry) play in auditory categorization should be also tested. Recent advances in experimental and analysis techniques should enable us to clarify the functional role of different classes of neurons in auditory categorization (Letzkus et al., 2011; Znamenskiy and Zador, 2013) and also test neural categorization across cortical layers (Lakatos et al., 2008; Takeuchi et al., 2011), providing further insights for neural computations for auditory categorization within local microcircuits.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Kate Christison-Lagay, Steven Eliades, and Heather Hersh for helpful comments on the preparation of this manuscript. We also thank Brian Russ and Jung Lee for data collection and Harry Shirley for outstanding veterinary support in our previous experiments. Joji Tsunada and Yale E. Cohen were supported by grants from NIDCD-NIH and the Boucai Hearing Restoration Fund.
References
- Altmann C. F., Doehrmann O., Kaiser J. (2007). Selectivity for animal vocalizations in the human auditory cortex. Cereb. Cortex 17, 2601–2608 10.1093/cercor/bhl167 [DOI] [PubMed] [Google Scholar]
- Atencio C. A., Schreiner C. E. (2008). Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons. J. Neurosci. 28, 3897–3910 10.1523/JNEUROSCI.5366-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck B. B., Romanski L. M. (2004). Principal and independent components of macaque vocalizations: constructing stimuli to probe high-level sensory processing. J. Neurophysiol. 91, 2897–2909 10.1152/jn.01103.2003 [DOI] [PubMed] [Google Scholar]
- Belin P., Zatorre R. J., Lafaille P., Ahad P., Pike B. (2000). Voice-selective areas in human auditory cortex. Nature 403, 309–311 10.1038/35002078 [DOI] [PubMed] [Google Scholar]
- Binder J. R., Frost J. A., Hammeke T. A., Bellgowan P. S., Springer J. A., Kaufman J. N., et al. (2000). Human temporal lobe activation by speech and nonspeech sounds. Cereb. Cortex 10, 512–528 10.1093/cercor/10.5.512 [DOI] [PubMed] [Google Scholar]
- Binder J. R., Liebenthal E., Possing E. T., Medler D. A., Ward B. D. (2004). Neural correlates of sensory and decision processes in auditory object identification. Nat. Neurosci. 7, 295–301 10.1038/nn1198 [DOI] [PubMed] [Google Scholar]
- Bizley J. K., Cohen Y. E. (2013). The what, where, and how of auditory-object perception. Nat. Rev. Neurosci. 14, 693–707 10.1038/nrn3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. F., Rieger J. W., Johnson K., Berger M. S., Barbaro N. M., Knight R. T. (2010). Categorical speech representation in human superior temporal gyrus. Nat. Neurosci. 13, 1428–1432 10.1038/nn.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y. E., Hauser M. D., Russ B. E. (2006). Spontaneous processing of abstract categorical information in the ventrolateral prefrontal cortex. Biol. Lett. 2, 261–265 10.1098/rsbl.2005.0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y. E., Russ B. E., Davis S. J., Baker A. E., Ackelson A. L., Nitecki R. (2009). A functional role for the ventrolateral prefrontal cortex in non-spatial auditory cognition. Proc. Natl. Acad. Sci. U.S.A. 106, 20045–20050 10.1073/pnas.0907248106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y. E., Theunissen F., Russ B. E., Gill P. (2007). Acoustic features of rhesus vocalizations and their representation in the ventrolateral prefrontal cortex. J. Neurophysiol. 97, 1470–1484 10.1152/jn.00769.2006 [DOI] [PubMed] [Google Scholar]
- Constantinidis C., Goldman-Rakic P. S. (2002). Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J. Neurophysiol. 88, 3487–3497 10.1152/jn.00188.2002 [DOI] [PubMed] [Google Scholar]
- Desai R., Liebenthal E., Waldron E., Binder J. R. (2008). Left posterior temporal regions are sensitive to auditory categorization. J. Cogn. Neurosci. 20, 1174–1188 10.1162/jocn.2008.20081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt I., Rauschecker J. P. (2012). Phoneme and word recognition in the auditory ventral stream. Proc. Natl. Acad. Sci. U.S.A. 109, E505–E514 10.1073/pnas.1113427109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicarlo J. J., Zoccolan D., Rust N. C. (2012). How does the brain solve visual object recognition? Neuron 73, 415–434 10.1016/j.neuron.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diester I., Nieder A. (2008). Complementary contributions of prefrontal neuron classes in abstract numerical categorization. J. Neurosci. 28, 7737–7747 10.1523/JNEUROSCI.1347-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Gold J. I. (2011). Neural correlates of perceptual decision making before, during, and after decision commitment in monkey frontal eye field. Cereb. Cortex 22, 1052–1067 10.1093/cercor/bhr178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehrmann O., Naumer M. J., Volz S., Kaiser J., Altmann C. F. (2008). Probing category selectivity for environmental sounds in the human auditory brain. Neuropsychologia 46, 2776–2786 10.1016/j.neuropsychologia.2008.05.011 [DOI] [PubMed] [Google Scholar]
- Eckardt W., Zuberbuhler K. (2004). Cooperation and competition in two forest monkeys. Behav. Ecol. 15, 400–411 10.1093/beheco/arh032 [DOI] [Google Scholar]
- Engel L. R., Frum C., Puce A., Walker N. A., Lewis J. W. (2009). Different categories of living and non-living sound-sources activate distinct cortical networks. Neuroimage 47, 1778–1791 10.1016/j.neuroimage.2009.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer C. T., Perez C. A., Carraway R. S., Chang K. Q., Roland J. L., Sloan A. M., et al. (2013). Similarity of cortical activity patterns predicts generalization behavior. PLoS ONE 8:e78607 10.1371/journal.pone.0078607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer C. T., Perez C. A., Chen Y. H., Carraway R. S., Reed A. C., Shetake J. A., et al. (2008). Cortical activity patterns predict speech discrimination ability. Nat. Neurosci. 11, 603–608 10.1038/nn.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S., Armony J. L., Joanette Y., Belin P. (2004). Is voice processing species-specific in human auditory cortex? An fMRI study. Neuroimage 23, 840–848 10.1016/j.neuroimage.2004.09.019 [DOI] [PubMed] [Google Scholar]
- Fecteau S., Armony J. L., Joanette Y., Belin P. (2005). Sensitivity to voice in human prefrontal cortex. J. Neurophysiol. 94, 2251–2254 10.1152/jn.00329.2005 [DOI] [PubMed] [Google Scholar]
- Fino E., Yuste R. (2011). Dense inhibitory connectivity in neocortex. Neuron 69, 1188–11203 10.1016/j.neuron.2011.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D. J., Assad J. A. (2006). Experience-dependent representation of visual categories in parietal cortex. Nature 443, 85–88 10.1038/nature05078 [DOI] [PubMed] [Google Scholar]
- Freedman D. J., Miller E. K. (2008). Neural mechanisms of visual categorization: insights from neurophysiology. Neurosci. Biobehav. Rev. 32, 311–329 10.1016/j.neubiorev.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Freedman D. J., Riesenhuber M., Poggio T., Miller E. K. (2001). Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291, 312–316 10.1126/science.291.5502.312 [DOI] [PubMed] [Google Scholar]
- Gifford G. W., 3rd., Maclean K. A., Hauser M. D., Cohen Y. E. (2005). The neurophysiology of functionally meaningful categories: macaque ventrolateral prefrontal cortex plays a critical role in spontaneous categorization of species-specific vocalizations. J. Cogn. Neurosci. 17, 1471–1482 10.1162/0898929054985464 [DOI] [PubMed] [Google Scholar]
- Gifford G. W., 3rd., Hauser M. D., Cohen Y. E. (2003). Discrimination of functionally referential calls by laboratory-housed rhesus macaques: implications for neuroethological studies. Brain Behav. Evol. 61, 213–224 10.1159/000070704 [DOI] [PubMed] [Google Scholar]
- Giordano B. L., McAdams S., Kriegeskorte N., Zatorre R. J., Belin P. (2013). Abstract encoding of auditory objects in cortical activity patterns. Cereb. Cortex 23, 2025–2037 10.1093/cercor/bhs162 [DOI] [PubMed] [Google Scholar]
- Gold J. I., Shadlen M. N. (2007). The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 10.1146/annurev.neuro.29.051605.113038 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. S. (1995). Cellular basis of working memory. Neuron 14, 477–485 10.1016/0896-6273(95)90304-6 [DOI] [PubMed] [Google Scholar]
- Goll J. C., Crutch S. J., Warren J. D. (2010). Central auditory disorders: toward a neuropsychology of auditory objects. Curr. Opin. Neurol. 23, 617–627 10.1097/WCO.0b013e32834027f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Burgos G., Krimer L. S., Povysheva N. V., Barrionuevo G., Lewis D. A. (2005). Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J. Neurophysiol. 93, 942–953 10.1152/jn.00787.2004 [DOI] [PubMed] [Google Scholar]
- Green D. M., Swets J. A. (1966). Signal Detection Theory and Psychophysics. New York, NY: John Wiley and Sons, Inc [Google Scholar]
- Hackett T. A., Stepniewska I., Kaas J. H. (1998). Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J. Comp. Neurol. 394, 475–495 [DOI] [PubMed] [Google Scholar]
- Hauser M. D. (1998). Functional referents and acoustic similarity: field playback experiments with rhesus monkeys. Anim. Behav. 55, 1647–1658 10.1006/anbe.1997.0712 [DOI] [PubMed] [Google Scholar]
- Hauser M. D., Marler P. (1993a). Food-associated calls in rhesus macaques (Macaca mulatta) 1. Socioecological factors influencing call production. Behav. Ecol. 4, 194–205 10.1093/beheco/4.3.194 [DOI] [Google Scholar]
- Hauser M. D., Marler P. (1993b). Food-associated calls in rhesus macaques (Macaca mulatta) II. Costs and benefits of call production and suppression. Behav. Ecol. 4, 206–212 10.1093/beheco/4.3.206 [DOI] [Google Scholar]
- Hefti B. J., Smith P. H. (2003). Distribution and kinetic properties of GABAergic inputs to layer V pyramidal cells in rat auditory cortex. J. Assoc. Res. Otolaryngol. 4, 106–121 10.1007/s10162-002-3012-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt L. L., Lotto A. J. (2010). Speech perception as categorization. Atten. Percept. Psychophys. 72, 1218–1227 10.3758/APP.72.5.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussar C. R., Pasternak T. (2012). Memory-guided sensory comparisons in the prefrontal cortex: contribution of putative pyramidal cells and interneurons. J. Neurosci. 32, 2747–2761 10.1523/JNEUROSCI.5135-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Zhang R., Zhang Q., Liu Q., Li H. (2012). Neural correlates of audiovisual integration of semantic category information. Brain Lang. 121, 70–75 10.1016/j.bandl.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Scanziani M. (2011). How inhibition shapes cortical activity. Neuron 72, 231–243 10.1016/j.neuron.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y., Harukuni R., Takekawa T., Aizawa H., Fukai T. (2009). Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat. Neurosci. 12, 1586–1593 10.1038/nn.2431 [DOI] [PubMed] [Google Scholar]
- Joassin F., Maurage P., Campanella S. (2011). The neural network sustaining the crossmodal processing of human gender from faces and voices: an fMRI study. Neuroimage 54, 1654–1661 10.1016/j.neuroimage.2010.08.073 [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Hackett T. A. (1999). “What” and “where” processing in auditory cortex. Nat. Neurosci. 2, 1045–1047 10.1038/15967 [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Hackett T. A. (2000). Subdivisions of auditory cortex and processing streams in primates. Proc. Natl. Acad. Sci. U.S.A. 97, 11793–11799 10.1073/pnas.97.22.11793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J. H., Hackett T. A., Tramo M. J. (1999). Auditory processing in primate cerebral cortex. Curr. Opin. Neurobiol. 9, 164–170 10.1016/S0959-4388(99)80022-1 [DOI] [PubMed] [Google Scholar]
- Kätzel D., Zemelman B. V., Buetfering C., Wölfel M., Miesenböck G. (2010). The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat. Neurosci. 14, 100–107 10.1038/nn.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M. T., Churchland M. M., Santhanam G., Yu B. M., Afshar A., Ryu S. I., et al. (2010). Roles of monkey premotor neuron classes in movement preparation and execution. J. Neurophysiol. 104, 799–810 10.1152/jn.00231.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Kondo S. (2002). Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J. Neurocytol. 31, 277–287 10.1023/A:1024126110356 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Kubota Y. (1993). Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J. Neurophysiol. 70, 387–396 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Kubota Y. (1997). GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486 10.1093/cercor/7.6.476 [DOI] [PubMed] [Google Scholar]
- Kluender K. R., Diehl R. L., Killeen P. (1987). Japanese quail can learn phonetic categories. Science 237, 1195–1197 10.1126/science.3629235 [DOI] [PubMed] [Google Scholar]
- Kuhl P. K., Miller J. D. (1975). Speech perception by the chinchilla: voiced-voiceless distinction in alveolar plosive consonants. Science 190, 69–72 10.1126/science.1166301 [DOI] [PubMed] [Google Scholar]
- Kuhl P. K., Miller J. D. (1978). Speech perception by the chinchilla: identification function for synthetic VOT stimuli. J. Acoust. Soc. Am. 63, 905–917 10.1121/1.381770 [DOI] [PubMed] [Google Scholar]
- Kuhl P. K., Padden D. M. (1982). Enhanced discriminability at the phonetic boundaries for the voicing feature in macaques. Percept. Psychophys. 32, 542–550 10.3758/BF03204208 [DOI] [PubMed] [Google Scholar]
- Kuhl P. K., Padden D. M. (1983). Enhanced discriminability at the phonetic boundaries for the place feature in macaques. J. Acoust. Soc. Am. 73, 1003–1010 10.1121/1.389148 [DOI] [PubMed] [Google Scholar]
- Lakatos P., Karmos G., Mehta A. D., Ulbert I., Schroeder C. E. (2008). Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320, 110–113 10.1126/science.1154735 [DOI] [PubMed] [Google Scholar]
- Leaver A. M., Rauschecker J. P. (2010). Cortical representation of natural complex sounds: effects of acoustic features and auditory object category. J. Neurosci. 30, 7604–7612 10.1523/JNEUROSCI.0296-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus J. J., Wolff S. B., Meyer E. M., Tovote P., Courtin J., Herry C., et al. (2011). A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 10.1038/nature10674 [DOI] [PubMed] [Google Scholar]
- Liberman A. M., Cooper F. S., Shankweiler D. P., Studdert-Kennedy M. (1967). Perception of the speech code. Psychol. Rev. 5, 552–563 10.1037/h0020279 [DOI] [PubMed] [Google Scholar]
- Liebenthal E., Binder J. R., Spitzer S. M., Possing E. T., Medler D. A. (2005). Neural substrates of phonemic perception. Cereb. Cortex 15, 1621–1631 10.1093/cercor/bhi040 [DOI] [PubMed] [Google Scholar]
- Liebenthal E., Desai R., Ellingson M. M., Ramachandran B., Desai A., Binder J. R. (2010). Specialization along the left superior temporal sulcus for auditory categorization. Cereb. Cortex 20, 2958–2970 10.1093/cercor/bhq045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N. K., Sheinberg D. L. (1996). Visual object recognition. Annu. Rev. Neurosci. 19, 577–621 10.1146/annurev.ne.19.030196.003045 [DOI] [PubMed] [Google Scholar]
- Lotto A. J., Kluender K. R., Holt L. L. (1997). Perceptual compensation for coarticulation by Japanese quail (Coturnix coturnix japonica). J. Acoust. Soc. Am. 102, 1134–1140 10.1121/1.419865 [DOI] [PubMed] [Google Scholar]
- Mann V. A. (1980). Influence of preceding liquid on stop-consonant perception. Percept. Psychophys. 28, 407–412 10.3758/BF03204884 [DOI] [PubMed] [Google Scholar]
- Markham H., Toledo-Rodriguez M., Wang Y., Gupta A., Silberberg G., Wu C. (2004). Interneuron of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. (1985). Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 54, 782–806 [DOI] [PubMed] [Google Scholar]
- Merchant H., De Lafuente V., Pena-Ortega F., Larriva-Sahd J. (2012). Functional impact of interneuronal inhibition in the cerebral cortex of behaving animals. Prog. Neurobiol. 99, 163–178 10.1016/j.pneurobio.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Mesgarani N., Cheung C., Johnson K., Chang E. F. (2014). Phonetic feature encoding in human superior temporal gyrus. Science 343, 1006–1010 10.1126/science.1245994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesgarani N., David S. V., Fritz J. B., Shamma S. A. (2008). Phoneme representation and classification in primary auditory cortex. J. Acoust. Soc. Am. 123, 899–909 10.1121/1.2816572 [DOI] [PubMed] [Google Scholar]
- Miller C. T., Cohen Y. E. (2010). Vocalization processing, in Primate Neuroethology, eds Ghazanfar A., Platt M. L. (Oxford, UK: Oxford University Press; ), 237–255 10.1093/acprof:oso/9780195326598.003.0013 [DOI] [Google Scholar]
- Miller E., Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Miller E. K. (2000). The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 1, 59–65 10.1038/35036228 [DOI] [PubMed] [Google Scholar]
- Miller E. K., Freedman D. J., Wallis J. D. (2002). The prefrontal cortex: categories, concepts, and cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 29, 1123–1136 10.1098/rstb.2002.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K., Nieder A., Freedman D. J., Wallis J. D. (2003). Neural correlates of categories and concepts. Curr. Opin. Neurobiol. 13, 198–203 10.1016/S0959-4388(03)00037-0 [DOI] [PubMed] [Google Scholar]
- Mitchell J. F., Sundberg K. A., Reynolds J. H. (2007). Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 5, 131–141 10.1016/j.neuron.2007.06.018 [DOI] [PubMed] [Google Scholar]
- Mottonen R., Calvert G. A., Jaaskelainen I. P., Matthews P. M., Thesen T., Tuomainen J., et al. (2006). Perceiving identical sounds as speech or non-speech modulates activity in the left posterior superior temporal sulcus. Neuroimage 30, 563–569 10.1016/j.neuroimage.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Talbot W. H., Sakata H., Hyvarinen J. (1969). Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J. Neurophysiol. 32, 452–484 [DOI] [PubMed] [Google Scholar]
- Nieder A. (2012). Supramodal numerosity selectivity of neurons in primate prefrontal and posterior parietal cortices. Proc. Natl. Acad. Sci. U.S.A. 109, 11860–11865 10.1073/pnas.1204580109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourski K. V., Reale R. A., Oya H., Kawasaki H., Kovach C. K., Chen H., et al. (2009). Temporal envelope of time-compressed speech represented in the human auditory cortex. J. Neurosci. 29, 15564–15574 10.1523/JNEUROSCI.3065-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J., Boecker H., Drzezga A., Haslinger B., Hennenlotter A., Roettinger M., et al. (2006). Vowel sound extraction in anterior superior temporal cortex. Hum. Brain Mapp. 27, 562–571 10.1002/hbm.20201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J., Leaver A. M., Van Meter J., Rauschecker J. P. (2010). Segregation of vowels and consonants in human auditory cortex: evidence for distributed hierarchical organization. Front. Psychol. 1:232 10.3389/fpsyg.2010.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J., Zimmermann J., Van Meter J., Rauschecker J. P. (2007). Multiple stages of auditory speech perception reflected in event-related FMRI. Cereb. Cortex 17, 2251–2257 10.1093/cercor/bhl133 [DOI] [PubMed] [Google Scholar]
- Ogawa T., Riera J., Goto T., Sumiyoshi A., Noaka H., Jerbi K., et al. (2011). Large-scale heterogeneous representation of sound attributes in rat primary auditory cortex: from unit activity to population dynamics. J. Neurosci. 31, 14639–14653 10.1523/JNEUROSCI.0086-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl F. W., Scheich H., Freeman W. J. (2001). Change in pattern of ongoing cortical activity with auditory category learning. Nature 412, 733–736 10.1038/35089076 [DOI] [PubMed] [Google Scholar]
- Osada T., Adachi Y., Kimura H. M., Miyashita Y. (2008). Towards understanding of the cortical network underlying associative memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2187–2199 10.1098/rstb.2008.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer A. M., Yuste R. (2011). Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J. Neurosci. 14, 13260–13271 10.1523/JNEUROSCI.3131-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore R. E., Li X. F., Layer J. K. (1990). Categorical perception of nonspeech chirps and bleats. Percept. Psychophys. 48, 151–156 10.3758/BF03207082 [DOI] [PubMed] [Google Scholar]
- Perrodin C., Kayser C., Logothetis N. K., Petkov C. I. (2011). Voice cells in the primate temporal lobe. Curr. Biol. 21, 1408–1415 10.1016/j.cub.2011.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov C. I., Kayser C., Steudel T., Whittingstall K., Augath M., Logothetis N. K. (2008). A voice region in the monkey brain. Nat. Neurosci. 11, 367–374 10.1038/nn2043 [DOI] [PubMed] [Google Scholar]
- Plakke B., Diltz M. D., Romanski L. M. (2013a). Coding of vocalizations by single neurons in ventrolateral prefrontal cortex. Hear. Res. 305, 135–143 10.1016/j.heares.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakke B., Hwang J., Diltz M. D., Romanski L. M. (2013b). The role of ventral prefrontal cortex in auditory, visual and audiovisual working memory, in Society for Neuroscience, Program No. 574.515 Neuroscience Meeting Planner (San Diego, CA: ). [Google Scholar]
- Plakke B., Ng C. W., Poremba A. (2013c). Neural correlates of auditory recognition memory in primate lateral prefrontal cortex. Neuroscience 244, 62–76 10.1016/j.neuroscience.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Poremba A., Bigelow J., Rossi B. (2013). Processing of communication sounds: contributions of learning, memory, and experience. Hear. Res. 305, 31–44 10.1016/j.heares.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. G., Williams G. V., Goldman-Rakic P. S. (1999). Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J. Neurophysiol. 81, 1903–1915 [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P. (1998). Cortical processing of complex sounds. Curr. Opin. Neurobiol. 8, 516–521 10.1016/S0959-4388(98)80040-8 [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P. (2012). Ventral and dorsal streams in the evolution of speech and language. Front. Evol. Neurosci. 4:7 10.3389/fnevo.2012.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P., Scott S. K. (2009). Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat. Neurosci. 12, 718–724 10.1038/nn.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P., Tian B. (2000). Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc. Natl. Acad. Sci. U.S.A. 97, 11800–11806 10.1073/pnas.97.22.11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P., Tian B., Hauser M. (1995). Processing of complex sounds in the macaque nonprimary auditory cortex. Science 268, 111–114 10.1126/science.7701330 [DOI] [PubMed] [Google Scholar]
- Recanzone G. H., Cohen Y. E. (2010). Serial and parallel processing in the primate auditory cortex revisited. Behav. Brain Res. 5, 1–6 10.1016/j.bbr.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski L. M., Averbeck B. B. (2009). The primate cortical auditory system and neural representation of conspecific vocalizations. Annu. Rev. Neurosci. 32, 315–346 10.1146/annurev.neuro.051508.135431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski L. M., Bates J. F., Goldman-Rakic P. S. (1999a). Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 403, 141–157 [DOI] [PubMed] [Google Scholar]
- Romanski L. M., Tian B., Fritz J., Mishkin M., Goldman-Rakic P. S., Rauschecker J. P. (1999b). Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 2, 1131–1136 10.1038/16056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ B. E., Ackelson A. L., Baker A. E., Cohen Y. E. (2008a). Coding of auditory-stimulus identity in the auditory non-spatial processing stream. J. Neurophysiol. 99, 87–95 10.1152/jn.01069.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ B. E., Lee Y.-S., Cohen Y. E. (2007). Neural and behavioral correlates of auditory categorization. Hear. Res. 229, 204–212 10.1016/j.heares.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Russ B. E., Orr L. E., Cohen Y. E. (2008b). Prefrontal neurons predict choices during an auditory same-different task. Curr. Biol. 18, 1483–1488 10.1016/j.cub.2008.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S., Harris K. D. (2009). Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron 64, 404–418 10.1016/j.neuron.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner C. E. (1998). Spatial distribution of responses to simple and complex sounds in the primary auditory cortex. Audiol. Neurootol. 3, 104–122 10.1159/000013785 [DOI] [PubMed] [Google Scholar]
- Selezneva E., Scheich H., Brosch M. (2006). Dual time scales for categorical decision making in auditory cortex. Curr. Biol. 16, 2428–2433 10.1016/j.cub.2006.10.027 [DOI] [PubMed] [Google Scholar]
- Sinnott J. M., Brown C. H. (1997). Perception of the American English liquid vertical bar ra-la vertical bar contrast by humans and monkeys. J. Acoust. Soc. Am. 102, 588–602 10.1121/1.419732 [DOI] [PubMed] [Google Scholar]
- Staeren N., Renvall H., De Martino F., Goebel R., Formisano E. (2009). Sound categories are represented as distributed patterns in the human auditory cortex. Curr. Biol. 19, 498–502 10.1016/j.cub.2009.01.066 [DOI] [PubMed] [Google Scholar]
- Steinschneider M. (2013). Phonemic representations and categories, in Neural Correlates of Auditory Cognition, eds Cohne Y. E., Popper A. N., Fay R. R. (New York, NY: Springer; ), 151–191 10.1007/978-1-4614-2350-8_6 [DOI] [Google Scholar]
- Steinschneider M., Fishman Y. I., Arezzo J. C. (2003). Representation of the voice onset time (VOT) speech parameter in population responses within primary auditory cortex of the awake monkey. J. Acoust. Soc. Am. 114, 307–321 10.1121/1.1582449 [DOI] [PubMed] [Google Scholar]
- Steinschneider M., Nourski K. V., Kawasaki H., Oya H., Brugge J. F., Howard M. A. (2011). Intracranial study of speech-elicited activity on the human posterolateral superior temporal gyrus. Cereb. Cortex 10, 2332–2347 10.1093/cercor/bhr014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider M., Volkov I. O., Fishman Y. I., Oya H., Arezzo J. C., Howard M. A., 3rd. (2005). Intracortical responses in human and monkey primary auditory cortex support a temporal processing mechanism for encoding of the voice onset time phonetic parameter. Cereb. Cortex 15, 170–186 10.1093/cercor/bhh120 [DOI] [PubMed] [Google Scholar]
- Takeuchi D., Hirabayashi T., Tamura K., Miyashita Y. (2011). Reversal of interlaminar signal between sensory and memory processing in monkey temporal cortex. Science 331, 1443–1447 10.1126/science.1199967 [DOI] [PubMed] [Google Scholar]
- Tian B., Rauschecker J. P. (2004). Processing of frequency-modulated sounds in the lateral auditory belt cortex of the rhesus monkey. J. Neurophysiol. 92, 2993–3013 10.1152/jn.00472.2003 [DOI] [PubMed] [Google Scholar]
- Tsunada J., Lee J. H., Cohen Y. E. (2011). Representation of speech categories in the primate auditory cortex. J. Neurophysiol. 105, 2634–2646 10.1152/jn.00037.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunada J., Lee J. H., Cohen Y. E. (2012). Differential representation of auditory categories between cell classes in primate auditory cortex. J. Physiol. 590, 3129–3139 10.1113/jphysiol.2012.232892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lancker D. R., Canter G. J. (1982). Impairment of voice and face recognition in patients with hemispheric damage. Brain Cogn. 1, 185–195 [DOI] [PubMed] [Google Scholar]
- Van Lancker D. R., Cummings J. L., Kreiman J., Dobkin B. H. (1988). Phonagnosia—a dissociation between familiar and unfamiliar voices. Cortex 24, 195–209 10.1016/S0010-9452(88)80029-7 [DOI] [PubMed] [Google Scholar]
- Warren J. D., Scott S. K., Price C. J., Griffiths T. D. (2006). Human brain mechanisms for the early analysis of voices. Neuroimage 31, 1389–1397 10.1016/j.neuroimage.2006.01.034 [DOI] [PubMed] [Google Scholar]
- Wehr M. S., Zador A. (2003). Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 27, 442–446 10.1038/nature02116 [DOI] [PubMed] [Google Scholar]
- Werner S., Noppeney U. (2010). Distinct functional contributions of primary sensory and association areas to audiovisual integration in object categorization. J. Neurosci. 30, 2662–2675 10.1523/JNEUROSCI.5091-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., O'Scalaidhe S. P., Goldman-Rakic P. S. (1994). Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 91, 4009–4013 10.1073/pnas.91.9.4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. I., Zhou Y., Tao H. W. (2011). Perspectives on: information and coding in mammalian sensory physiology: inhibitory synaptic mechanisms underlying functional diversity in auditory cortex. J. Gen. Physiol. 138, 311–320 10.1085/jgp.201110650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamenskiy P., Zador A. M. (2013). Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485 10.1038/nature12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Züberbuhler K. (2000a). Causal cognition in a non-human primate: field playback experiments with Diana monkeys. Cognition 76, 195–207 10.1016/S0010-0277(00)00079-2 [DOI] [PubMed] [Google Scholar]
- Züberbuhler K. (2000b). Interspecies semantic communication in two forest primates. Proc. R. Soc. Lond. B Biol. Sci. 267, 713–718 10.1098/rspb.2000.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbuhler K., Seyfarth R. M. (1997). Diana monkey long-distance calls: messages for conspecifics and predators. Anim. Behav. 53, 589–604 10.1006/anbe.1996.0334 [DOI] [Google Scholar]