Abstract
Aims: Excessive alcohol consumption is associated with fracture non-union. Canonical Wnt pathway signaling activity regulates normal fracture healing. We previously demonstrated that binge alcohol exposure modulates β-catenin levels in the fracture callus of mice. Here, we sought to determine whether exogenous enhancement β-catenin signaling activity could restore normal fracture healing to binge-exposed mice. Methods: C57BL/6 male mice were exposed to episodic alcohol or saline for 6 total days of alcohol exposure over a 2-week period. Following alcohol exposure, mice were subjected to a stabilized mid-shaft tibia fracture. Beginning 4 days post-injury, mice received daily injections of either lithium chloride or saline subcutaneously. Protein levels of activated, inactivated, and total β-catenin and GSK-3β in fracture calluses were measured at post-injury day 9. Biomechanical strength testing and histology of callus tissue was assessed at post fracture day 14. Results: Binge alcohol was associated with decreased callus biomechanical strength, and reduced cartilaginous callus formation. Alcohol decreased levels of callus-associated activated β-catenin while concomitantly increasing the levels of inactive β-catenin at post-injury day 9. Alcohol also increased callus associated activated GSK-3β at post-injury day 9. Lithium chloride (an inhibitor of GSK-3β) treatment increased activated β-catenin protein levels, significantly decreased activated GSK-3β and restored cartilaginous callus formation and endochondral ossification. Conclusion: These data link alcohol-impaired fracture healing with deregulation of Canonical Wnt signaling activity in the fracture callus. Exogenous activation of the Wnt pathway using LiCl attenuated the damaging effects of binge alcohol exposure on the fracture healing process by modulating canonical Wnt signaling activity.
INTRODUCTION
The impact of alcohol abuse on the skeleton is multifaceted. Excessive alcohol consumption increases the risk of osteopenia, traumatic orthopedic injury (Savola et al., 2005) and complications following injury such as fracture non-union (Janicke-Lorenz and Lorenz, 1984). The association between high rates of binge drinking in adolescent and young adult populations (Miller et al., 2004) and increased risk of motor vehicle-related injury (Quinlan et al., 2005), with resulting orthopedic trauma (Levy et al., 1996), underscores the significance of understanding the consequences of binge alcohol consumption on fracture healing. Up to 10% of bone fracture injuries do not heal normally, leading to the clinically significant problem of fracture non-union (Praemer et al., 1992). Chronic or heavy alcohol consumption in humans has long been associated with delayed or incomplete fracture healing (Kristensson et al., 1980); however, these studies are often confounded by factors such as nutritional status, tobacco use and alcoholism-induced hormonal changes, all of which present difficulties in interpreting the exclusive effects of alcohol exposure on healing bone tissue. The limitations of clinical correlations can be overcome using rodent models of chronic or binge alcohol consumption, which have demonstrated that alcohol inhibits callus formation, decreases the strength of fracture callus tissue and leads to delayed fracture healing (Janicke-Lorenz, 1984; Elmali et al. 2002; Volkmer et al., 2011; Lauing et al., 2012). The mechanisms underlying alcohol-impaired fracture healing are not currently known.
Canonical Wnt signaling plays a pivotal role in fracture healing by promoting the formation of osteoblasts and chondrocytes, the principal cell types involved in formation of the fracture callus. The precise regulation of Wnt/β-catenin signaling has been shown to be essential to promote normal fracture repair (Chen et al., 2007; Kakar et al., 2007; Huang et al., 2011). Temporal regulation of the main effector of the pathway, β-catenin, is essential for chondrocyte development and maturation (Tamamura et al., 2005; Dao et al., 2012) and endochondral bone formation during fracture repair (Huang et al., 2011). Due to the broad range of cellular functions the canonical Wnt pathway is tightly regulated to prevent excessive stimulation and maintain cellular homeostasis. The essential negative regulator of the Wnt/β-catenin pathway is GSK-3β, a serine/threonine kinase that hyper-phosphorylates β-catenin targeting the protein for degradation (Yost et al., 1996). Hypo-phosphorylated β-catenin is able to translocate to the nucleus and activate TCF/LEF-mediated transcription of Wnt target genes (Behrens et al., 1996; Molenaar et al., 1996; Staal et al., 2002).
The canonical Wnt pathway can be indirectly stimulated by using inhibitors of GSK-3β, which results in increased stabilization of β-catenin. Lithium salts, including lithium chloride (LiCl), are effective in preventing GSK-3β-mediated hyperphosphorylation of β-catenin, therefore increasing canonical Wnt signaling. Studies in bone tissue as well as other organ systems have successfully utilized lithium as an inhibitor of GSK-3β to increase canonical Wnt signaling and β-catenin stabilization both in vivo and in vitro (Clement-Lacroix et al., 2005; Nakanishi et al., 2008). Lithium is also used clinically as a psychotropic drug, and patients treated with lithium for long periods of time demonstrate a decreased risk of fracture and increased bone mass (Zamani et al., 2008; Vestergaard, 2009). Activation of canonical Wnt signaling with LiCl during fracture healing must be precisely timed in order to augment fracture repair, suggesting that increasing stabilized, active β-catenin levels too early in the repair process can actually delay or inhibit normal healing (Chen et al., 2007).
Our laboratory previously identified that alcohol exposure targets canonical Wnt-related gene expression in bone tissue (Himes et al., 2008) and recently demonstrated that acute alcohol exposure prior to fracture injury perturbs the normal regulation of β-catenin levels throughout the critical stages of fracture callus formation (Lauing et al., 2012). Alcohol-related deregulation of β-catenin in this study was associated with impaired callus formation, decreased callus biomechanical strength and modulation of β-catenin/TCF-dependent transcription during callus formation. In this study, we utilize a model in which mice receive 2 weeks of binge alcohol exposure prior to bone fracture injury, followed by daily treatment with the GSK-3β inhibitor LiCl, to test whether exogenous enhancement of the canonical Wnt pathway during fracture repair can restore normal bone healing to binge alcohol-exposed mice. We hypothesized that LiCl-mediated suppression of GSK-3β in mice treated with binge alcohol would increase stabilized β-catenin levels in fracture callus, increase callus cartilage and endochondral bone formation and restore callus biomechanical strength to levels observed in saline-treated control mice.
MATERIALS AND METHODS
Alcohol administration
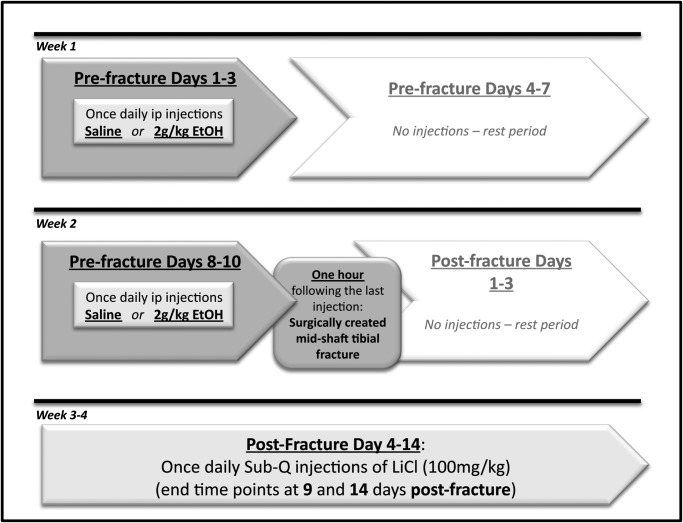
This investigation received full approval from the Loyola University Institutional Animal Care and Use Committee. Our experimental protocol is summarized in Fig. 1. Male C57Bl/6 mice 6–7 weeks of age were obtained from Harlan Laboratories (Indianapolis, IN, USA) and housed in a facility approved by the Institutional Animal Care and Use Committee at Loyola University Medical Center. Animals were randomly assigned to one of four treatment groups: Saline/Saline, Alcohol/Saline, Saline/LiCl or Alcohol/LiCl. The name of each group corresponds to the pre-fracture treatment (saline or alcohol) followed by the post-fracture treatment (saline or lithium chloride). The acute binge alcohol paradigm utilized in all experiments has been described previously (Lauing et al., 2012) and was designed to mimic repeated binge drinking episodes in humans. Briefly, during Week 1 of administration, a single daily intraperitoneal (ip) injection of alcohol given as a 20% (v/v) ethanol/saline solution at a dose of 2 g per kg was given to alcohol-treated mice or an equal volume of sterile isotonic saline was given to control mice once per day for 3 consecutive days. Animals were left undisturbed for the remaining 4 days of the week. The consecutive daily alcohol or saline injections were repeated during Week 2, and 1 h after the sixth and final injection, all mice were subjected to the stabilized tibia fracture surgery described below. Blood alcohol levels averaged ∼200 mg/dl, 1 h post-injection, at the time of fracture surgery.
Fig. 1.
Schematic diagram of the experimental protocol utilized.
Tibia fracture injury
The tibia fracture injury utilized in these studies has been previously described (Lauing et al., 2012). Briefly, 1 h after administration of the final alcohol or saline injection, mice were given an induction dose of anesthesia (0.75 mg/kg ketamine and 0.08 mg/kg xylazine, ip) and the haircoat was removed from the left hind limb of the animal. Mice were given 5 mg/kg of prophylactic gentamicin subcutaneously and anesthetized completely with isoflurane gas. An incision was made over the left stifle, the patellar tendon was exposed and a 27-gauge needle was used to ream a hole through the proximal tibial plateau and into the medullary cavity. The tibia was stabilized by inserting a sterile 0.25 mm diameter stainless steel pin through the reamed hole and down the tibia shaft. Following pin placement, a mid-diaphyseal fracture was created in the tibia using angled bone scissors. Following fracture injury, the wound was irrigated with sterile saline and closed with sutures. Mice were returned to cages on heating pads to recover from surgery and provided with free access to food and water. All animals received post-operative analgesia (buprenorphrine, 0.05 mg/kg, q8 for 24–36 h) for pain control and 1 ml of warmed sterile saline (ip) for resuscitation during the immediate post-surgical period.
Post-fracture lithium chloride administration
Beginning 4 days post fracture until euthanasia at post-fracture days 9 and 14, mice in the Saline/LiCl and Alcohol/LiCl groups received a single daily subcutaneous (Sub-Q) dose 100 mg/kg LiCl (Sigma) dissolved in isotonic sterile. The Saline/Saline and Alcohol/Saline group mice received a subcutaneous injection of an equal volume of sterile isotonic saline. Serum lithium levels were obtained by collecting trunk blood from animals 3 h after lithium administration and submitting serum samples to the clinical laboratory at Loyola University Medical Center for routine serum lithium testing.
Fracture callus histology
Mice were humanely euthanized and the injured and contralateral tibias were harvested from the mice 14 days post-fracture and placed in 10% neutral buffered formalin for 48 h. The tibias were decalcified in 10% EDTA with agitation for 5 days and paraffin-embedded. Five-µm sections were placed onto Superfrost©Plus slides (Fisher Scientific, Pittsburgh, PA, USA) and baked on a 60°C slide warmer overnight. Sections from two mice in each treatment group were stained routinely with hematoxylin and eosin. Fracture callus histological specimens were blinded and scored by an independent, board-certified clinical pathologist (see Results section below for additional details).
Biomechanical testing of fracture callus
Injured and contralateral tibias harvested at 14 days post-fracture were utilized for 4-point bending analysis to examine the effects of alcohol on fracture callus strength. The contralateral tibias served as the uninjured control group. Following removal of the stabilizing stainless pin from the fracture site, the fractured and contralateral tibias were loaded into a customized 4-point bending apparatus and tested at 0.5 mm/s using a materials testing machine (Instron Corporation, Model 5544, Canton, MA, USA). Load-deflection curves were analyzed for maximum load (load to failure). Failure was defined as the point at which a maximal load can no longer be borne by the specimen.
Canonical Wnt signaling assessment
Mice were humanely euthanized and injured tibias were harvested from mice 9 days post-fracture and snap-frozen in liquid nitrogen. Fracture callus tissue was isolated as described previously (Lauing et al., 2012). Briefly, a Dremel cutting tool (Dremel, Inc., Racine, WI, USA) was used to isolate fracture callus tissue, the fragment was pulverized in lysis buffer using a freezer mill (SPEX CertiPrep, Inc., Metuchen, NJ, USA) and total protein was measured. Twenty-five micrograms of total protein from each sample was resolved on 4–20% SDS–PAGE and transferred to PVDF membranes. Membranes were probed with rabbit anti-mouse total β-catenin (Millipore #06-734), non-phospho-β-catenin (Ser33/37/Thr41, Cell Signaling, #4270), phospho-β-catenin (Ser33/37/Thr41, Cell Signaling, #9561), Y216 phospho-GSK-3β (Abcam, ab75745), Ser9 phospho-GSK-3β (Cell Signaling, #9336) and total GSK-3β (Abcam, ab97996). To ensure equal loading of protein, the transferred membranes were Coommassie-stained following total β-catenin detection, and values were normalized to a 60 kDa band on the stained membrane. Densitometric analysis was carried out utilizing the Image Lab software (Bio-Rad, Inc., Hercules, CA, USA) and western blot data were presented as the densitometric ratio of the protein of interest to the Coommassie-stained membrane band.
Statistical analysis
Data are expressed as mean ± standard error of the mean. Statistical differences between treatment groups were calculated using One-way ANOVA with Tukey's post hoc testing. A P-value ≤0.05 was noted as statistically significant.
RESULTS
General observations
Animals returned to weight bearing on all four legs immediately following recovery from anesthesia. No differences in animal weights were observed during the experimental period between any of the treatment groups (Supplementary Fig. S1). No evidence of infection was noted at the fracture site in any of the experimental animals. No animals were euthanized during the experimental period for complications resulting from fracture surgery. A small number of fractured tibia specimens collected for histological or biomechanical analysis were removed from the analysis when the specimen did not remain intact at the fracture callus site while removing the stainless steel pin utilized to stabilize the bone during surgery and healing. This fracture callus instability occurred mainly in the alcohol/saline treatment group; fracture callus instability did not impact the use of specimens for molecular analysis.
Serum lithium chloride levels
Mice received daily subcutaneous injections of LiCl beginning at Day 4 post-fracture until humane euthanasia. Serum lithium levels at the time of euthanasia were 1.32 ± 0.39 and 1.33 ± 0.71 mMol/l, in the saline and alcohol groups respectively, which are within the reported therapeutic target range in humans of 0.5–1.5 mMol/l (data not shown). No significant differences in serum lithium levels were noted between the alcohol and saline treatment groups.
Effects of binge alcohol on fracture callus tissue composition
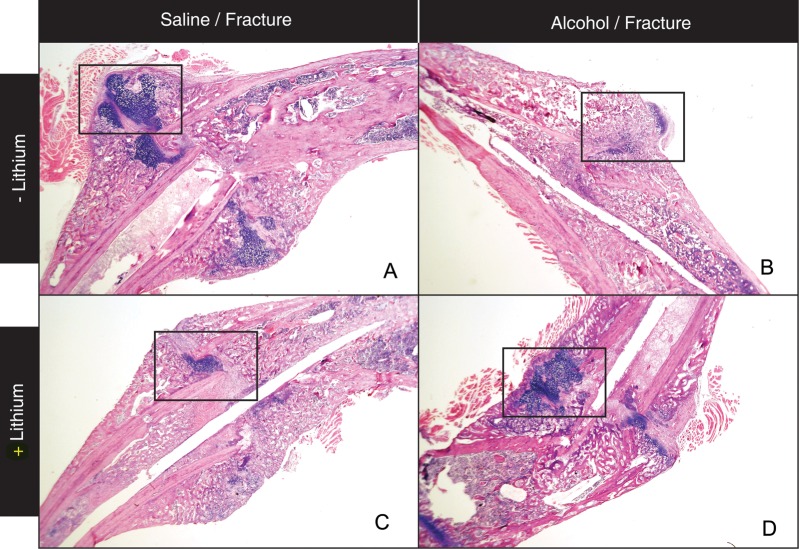
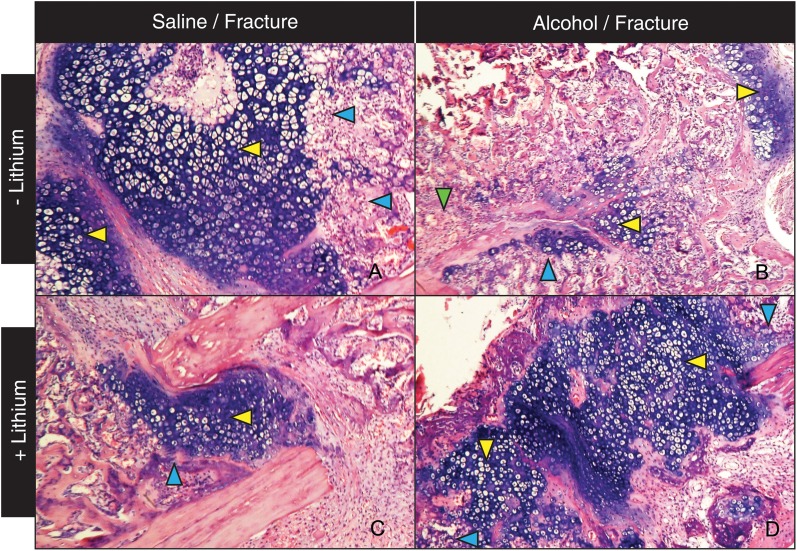
Our lab previously reported that binge alcohol treatment prior to fracture injury decreased callus size, cartilaginous callus formation and inhibited endochondral ossification in the callus (Lauing et al., 2012). Based on these studies, we first examined the effects of LiCl treatment on fracture callus tissue composition in alcohol-exposed mice. Mice received daily subcutaneous injections of LiCl beginning at Day 4 post-fracture, a time point that has previously been reported to improve the fracture repair process (Chen et al., 2007). In external callus tissue from saline/saline control mice, mature bridging callus tissue is present with abundant mature hyaline cartilage (blue stained tissue is within boxed area) at Day 14 post-fracture (Fig. 2A, ×40). Consistent with our previous observations at this time point (Lauing et al. 2012), at higher magnification (×128), we observe that a large percentage of the external cartilaginous callus is composed of hypertrophic chondrocytes (Fig. 3A, yellow arrow) with corresponding endochondral ossification zones evident (3A, blue arrows), supporting that the healing of a stabilized tibia fracture injury in control mice occurs predominantly through endochondral bone formation. In contrast, in the alcohol treatment group, a smaller amount of external callus tissue is present with a minimal cartilaginous component (Fig. 2B, ×40, boxed area). At Higher magnification (×128), minimal chondrocyte hypertrophy (yellow arrow) or associated endochondral bone formation (blue arrow) are observed in the external callus of alcohol-treated mice at Day 14 post-fracture (Fig. 3B). Also consistent with our previous findings, the fracture callus tissue that is present in the alcohol-treated mice appears to be mainly of periosteal origin as this bone is not associated with hypertrophic chondrocytes typically found in zones of endochondral bone formation (Fig. 3B, green arrow). In alcohol-treated mice receiving post-injury LiCl treatment, callus tissue formed is markedly different than that observed in mice receiving alcohol treatment alone (Fig. 2D). We observe a restoration of bridging callus and increased external cartilaginous callus tissue (2D, boxed area). At ×128 magnification, the external callus tissue from the alcohol/LiCl treatment group shows an increase in hypertrophic chondrocytes (Fig. 3D, yellow arrow) with associated zones of endochondral ossification (3D, blue arrow) suggesting that LiCl treatment partially restores normal callus tissue composition to alcohol-treated mice by promoting fracture repair-associated cartilage formation and subsequent endochondral bone ossification. Interestingly, in the group receiving LiCl alone, bridging callus (Fig. 2C), external callus cartilage formation, chondrocyte hypertrophy (Fig. 3C, yellow arrow) and endochondral bone formation (Fig. 3C, blue arrow) appear normal, though at decreased relative amounts compared with saline controls.
Fig. 2.
Representative photomicrographs of H & E stained fracture callus sections at Day 14 post-fracture (×40). (A) Control mouse. (B) Binge alcohol-treated mouse. (C) Lithium chloride-treated mouse. (D) Binge alcohol and lithium chloride-treated mouse. Higher magnification of the boxed areas are seen in Fig. 3.
Fig. 3.
High magnification of fracture callus sections (×128). (A) Control mice show large areas of cartilaginous external callus with abundant hypertrophic chondrocytes and endochondral bone formation. (B) Mice treated with alcohol alone show smaller areas of cartilaginous callus and less evidence of endochondral ossification or hypertrophic chondrocytes. (C) LiCl treatment of saline-treated mice does not affect callus tissue composition. (D) LiCl treatment normalized the histological phenotype of binge alcohol-treated mice, resulting in the reappearance of cartilaginous external callus with associated hypertrophic chondrocytes and zones of endochondral ossification.
We consulted with a pathologist with board certifications in anatomic and clinical pathology to devise a scoring system to quantify the changes in external cartilaginous callus composition associated with alcohol exposure and LiCl treatment described above. This scoring system measured two criteria to evaluate the external fracture that are important histological landmarks for endochondral bone formation normally occurring at 14 days post-injury in the stabilized murine fracture injury model we utilized (Chen et al., 2007); the percent of the external callus comprised of cartilage tissue and the percent of this cartilaginous callus that contained hypertrophic chondrocytes. These two percentage numbers were then multiplied to each other and then by 100 to obtain a final callus score. Fracture callus histological samples were blinded and scored by this independent pathologist using the scoring system. The final callus scores obtained from pathologist for each treatment group validate our initial findings of alcohol-related decreased cartilage formation, which was notably improved with lithium chloride treatment. The final callus scores for each treatment group are shown in Table 1.
Table 1.
Fracture callus histological scoring
| Treatment group | Fracture callus score |
|---|---|
| Control (no treatment) | 36.0 |
| Binge alcohol/saline | 4.0 |
| Saline/lithium chloride | 24.0 |
| Binge alcohol/lithium chloride | 45.0 |
Histological sections of fracture callus from each treatment group (N = 2/group) were blinded and scored by a board-certified pathologist not associated with the study. Callus tissue was scored by determining the percent of the external callus comprised of cartilage tissue and the percent of this cartilage tissue containing hypertrophic chondrocytes. These two percentage numbers were multiplied to each other and then to 100 to obtain a callus score. Numbers obtained were compared with a fracture callus from control animals to assess effects of alcohol and lithium treatment on cartilaginous callus formation.
Effects of binge alcohol and lithium chloride on fracture callus biomechanical strength
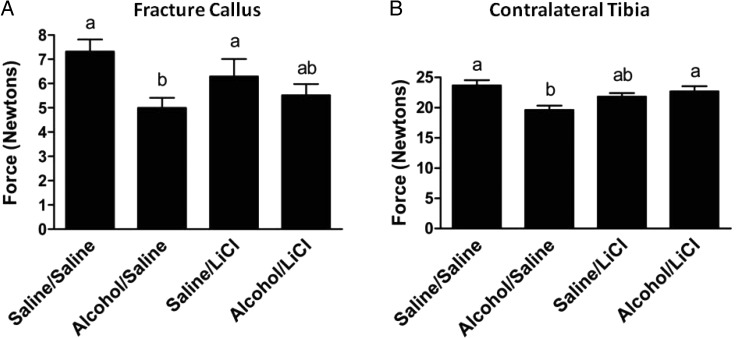
We previously reported that acute alcohol exposure prior to tibia bone injury significantly decreases the biomechanical strength of fracture callus tissue at Day 14 post-injury (Lauing et al., 2012). To examine whether LiCl treatment following binge alcohol exposure affects fracture callus biomechanical parameters, we utilized a four-point bending and biomaterials testing apparatus to measure load-to-failure method as previously described (Hiltunen et al., 1993) of injured and contralateral tibias harvested at Day 14 post-fracture (Fig. 4A). Compared with saline controls, fracture calluses from alcohol-exposed mice show a 32% reduction in biomechanical strength (P < 0.05). The addition of LiCl to the treatment regimen restored the strength of callus tissue from alcohol-treated mice to 88% of the strength observed in saline-treated mice also receiving LiCl. Lithium treatment had no significant effect on the strength of the fracture callus tissue in saline-treated mice.
Fig. 4.
Biomechanical strength of fracture callus tissue. The maximum load sustained of fracture calluses (A) and contralateral tibias (B) from either alcohol or saline-exposed mice, with or without LiCl treatment at 14 days post-fracture. Alcohol exposure significantly reduced bending strength of fracture calluses and contralateral tibias compared with saline-exposed mice. LiCl partially restored fracture callus strength and significantly increased contralateral tibia strength in alcohol-exposed mice. Groups not sharing a letter are significant, P ≤ 0.05 using one-way ANOVA and Tukey's multiple comparison procedure. N = 14–18 mice/treatment group.
In uninjured contralateral tibias, alcohol exposure significantly reduced the four-point bending strength by 17% when compared with saline-treated control uninjured tibias. Following LiCl treatment, the biomechanical strength of the contralateral tibias of binge alcohol-exposed mice was significantly increased by 14% compared with mice receiving binge alcohol treatment alone, while no change in callus strength was observed in saline-treated mice receiving LiCl (Fig. 4B).
Effects of binge alcohol and lithium chloride on the activation state of β-catenin
We previously demonstrated that alcohol-treated mice show a significant decrease in total β-catenin protein expression in fracture callus at Day 9 post-injury (Lauing et al., 2012). Next, we examined whether this alcohol-related decrease in total β-catenin levels corresponds to a decrease in active, hypophosphorylated β-catenin following binge alcohol exposure. We measured levels of both the hypophosphorylated (active) form of β-catenin, which is unphosphorylated at the N-terminal residues S33/S37/T41, as well as the hyperphosphorylated (inactive) form of β-catenin, which is phosphorylated at these residues using western blot analysis of fracture callus protein lysates at Day 9 post-injury.
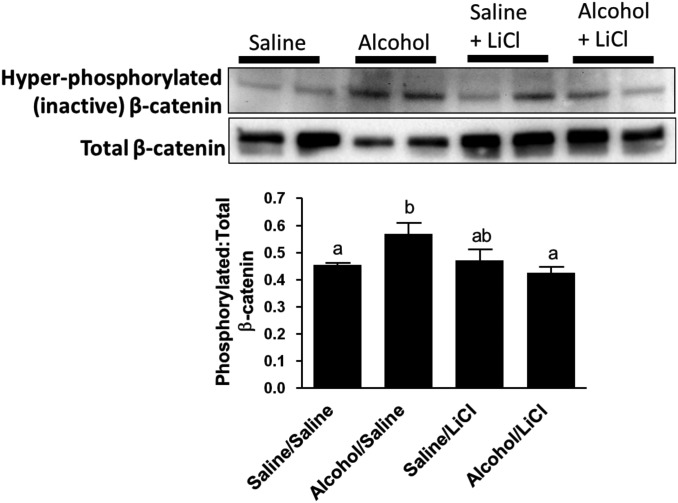
We observed that alcohol-exposed mice exhibited a significant increase (P < 0.05) in the amount of callus-associated hyperphosphorylated β-catenin compared with saline controls, suggesting that a greater proportion of cytosolic β-catenin in alcohol-exposed mice was targeted for degradation. LiCl treatment decreased levels of hyperphosphorylated, inactive β-catenin levels in alcohol-exposed mice to levels observed in saline control mice (Fig. 5).
Fig. 5.
Western blot analysis of fracture callus inactive beta catenin levels. Representative western blots for hyperphosphorylated (inactivated) and total β-catenin protein levels in fracture callus lysates at Day 9 post-fracture. Data are presented as the densitometric ratio of hyperphosphorylated/total β-catenin. Alcohol exposure resulted in a significant increase in the ratio of inactive β-catenin compared with saline-exposed mice. LiCl treatment significantly decreased hyperphosphorylated β-catenin levels to those found in saline-exposed mice. Groups not sharing a letter are significant, P ≤ 0.05 using one-way ANOVA and Tukey's multiple comparison procedure. N = 4–8 mice/treatment group.
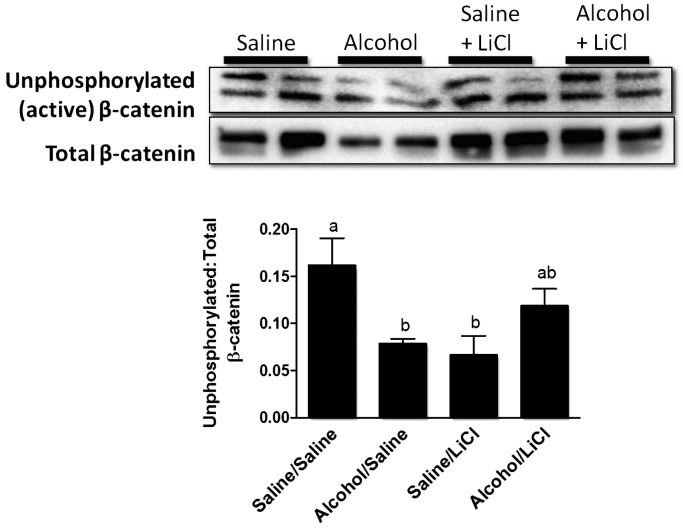
Alcohol exposure also resulted in a significant (48%) decrease (P < 0.05) in the ratio of hypophosphorylated (active) β-catenin to total β-catenin in Day 9 fracture callus lysates compared with saline controls (Fig. 6). A reduction in total β-catenin is also observed in alcohol-treated mice, which parallels our previously published findings at Day 9 post-injury (Lauing et al., 2012). LiCl treatment also increased active, hypophosphorylated β-catenin levels in callus from the alcohol/LiCl group by 52% compared with the group treated with alcohol alone, though this increase was not statistically significant. LiCl treatment caused a significant decrease in levels of active β-catenin in the saline/LiCl group.
Fig. 6.
Western blot analysis of fracture callus active beta catenin levels. Representative western blots for activated and total β-catenin protein levels in fracture callus lysates at Day 9 post-fracture. Data are presented as the densitometric ratio of activated/total β-catenin. Alcohol causes a significant decrease in the ratio of active (hypophosphorylated) β-catenin when compared with saline-exposed mice. LiCl treatment caused a 52% increase in activated β-catenin compared with alcohol treatment alone. Groups not sharing a letter are statistically significant, P ≤ 0.05 using one-way ANOVA and Tukey's multiple comparison procedure. N = 4–8 mice/treatment group.
Effects of binge alcohol and lithium chloride on the activation state of GSK-3β
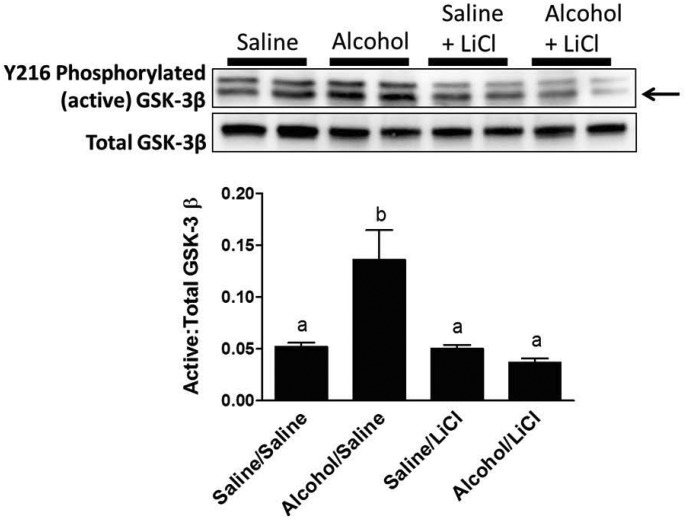
Since GSK-3β is the principal negative regulator of canonical Wnt signaling and lithium potently inhibits this enzyme (Davies et al., 2000), we next investigated whether the reduction in hyperphosphorylated β-catenin levels observed in alcohol-treated mice was accompanied by a corresponding increase in the activation state GSK-3β. We found that callus tissue from alcohol-treated mice did indeed show a significant increase in the ratio of activated, Tyr216-phosphorylated GSK-3β to total GSK-3β at Day 9 post-fracture compared with control animals (Fig. 7). These data parallel the alcohol-induced decrease in activated, unphosphorylated β-catenin at Day 9 post-fracture shown in Fig. 6. LiCl treatment was able to restore callus levels of activated GSK-3β in alcohol-treated mice to control levels observed in the saline-exposed group. LiCl treatment did not result in further decreases in GSK-3β activation in the saline-exposed group.
Fig. 7.
Western blot analysis of fracture callus active GSK 3β levels. Representative western blots for Tyr216-phosphorylated (activated) and total GSK-3β protein levels in fracture callus lysates at Day 9 post-fracture. Data are presented as the densitometric ratio of activated/total GSK-3β. Only the bottom band (arrow) was quantified, as cross-reactivity with the heavier GSK-α also occurs with this antibody. Alcohol exposure resulted in a significant increase in the ratio of activated GSK-3β compared with saline-exposed mice. LiCl treatment significantly decreased activated GSK-3β to levels found in saline-exposed mice. LiCl treatment did not change GSK-3β in the saline-exposed group. Groups not sharing a letter are significant, P ≤ 0.05 using one-way ANOVA and Tukey's multiple comparison procedure. N = 4–8 mice/treatment group.
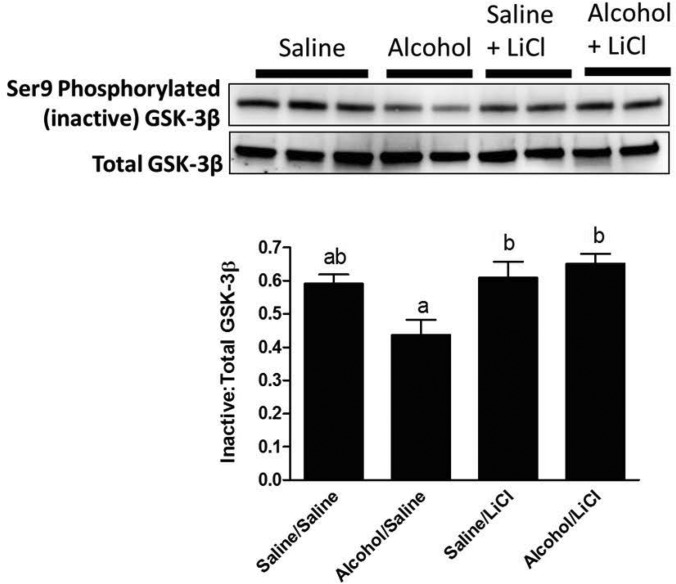
In parallel experiments, alcohol-exposed mice demonstrate significantly decreased levels of deactivated, Ser9-phosphorylated GSK-3β in Day 9 fracture callus tissue compared with controls (Fig. 8). LiCl treatment significantly increased the amount of deactivated GSK-3β in callus from alcohol-exposed mice, revealing that the LiCl dose administered was sufficient to increase the proportion of Ser9-phosphorylated GSK-3β in the fracture callus to levels seen in saline control mice. No effect of LiCl treatment was noted on Ser9-phosphorylation of GSK-3β between the saline-exposed groups.
Fig. 8.
Western blot analysis of fracture callus inactive GSK 3β levels. Representative western blots for Ser9-phosphorylated (inactivated) and total GSK-3β protein levels in fracture callus lysates at Day 9 post-fracture. Data are presented as the densitometric ratio of inactivated/total GSK-3β. Alcohol exposure resulted in a significant decrease in the ratio of inactivated GSK-3β compared with saline-treated mice, while LiCl treatment significantly increased the amount of inactivated GSK-3β to levels found in saline-exposed mice. Groups not sharing a letter are significant, P ≤ 0.05 using one-way ANOVA and Tukey's multiple comparison procedure. N = 4–8 mice/treatment group.
DISCUSSION
We have previously demonstrated that binge alcohol treatment prior to bone fracture injury inhibits normal fracture callus development by suppressing external callus cartilage formation and inhibiting endochondral bone formation, leading to a decrease in the biomechanical strength of fracture callus. These alcohol-related effects on fracture healing were associated with perturbation of the levels of the canonical Wnt pathway protein β-catenin found in the fracture callus (Lauing et al., 2012). Here, we extend those previous findings by demonstrating that a binge alcohol exposure period prior to fracture not only decreases callus-specific total β-catenin levels but proportionately decreases the pool of hypophosphorylated, active β-catenin, increasing the amount of β-catenin targeted for degradation, with a corresponding increase in activated, callus-associated GSK-3β. LiCl treatment was able to attenuate some of these alcohol-related effects on β-catenin signaling by returning callus-associated inactivated β-catenin protein to control levels and by normalizing the activation state of GSK-3β in the callus to control levels in alcohol-exposed mice.
Deregulation of β-catenin activity during fracture repair has been shown to cause delayed healing (Chen et al., 2007) and inhibition of endochondral ossification of the fracture callus (Huang et al., 2011). Strict regulation of Wnt/β-catenin signaling is also essential for normal cartilage formation and for the progression of chondrocytes to hypertrophy (Ryu et al., 2002; Tamamura et al., 2005; Yang et al., 2012). The findings presented here suggest that in alcohol-exposed mice, the absence of callus cartilage tissue and endochondral bone formation is directly linked to the deregulation of callus β-catenin levels, since LiCl treatment was able to restore external callus cartilage, endochondral ossification and to concurrently stabilize β-catenin in alcohol-exposed mice. Chen et al. (2007) also showed that enhancement of the Wnt pathway using LiCl treatment must be precisely timed in order to promote fracture repair. When administered several days following the injury, LiCl treatment enhanced mineralization of the fracture callus; however, administration just prior to fracture resulted in the inhibition of fracture repair and the aggregation of undifferentiated tissue. These data demonstrate the importance of the precise regulation of β-catenin levels during fracture repair. Collectively, these data support our hypothesis that alcohol exposure deregulates levels of stabilized β-catenin, which directly impacts the formation of mature cartilage and new bone in the fracture callus.
We hypothesized that fracture repair parameters would be improved in alcohol-exposed mice by increasing stabilized β-catenin levels through suppression of increased GSK-3β activation with LiCl. Following LiCl treatment in alcohol-exposed mice, we observed a 52% increase in active β-catenin when compared with mice treated with binge alcohol alone. In vitro experiments have shown that a 10-fold increase in unphosphorylated β-catenin following LiCl treatment increases TCF-dependent transcription by over 200-fold (Staal et al., 2002). These results suggest that a modest increase in hypophosphorylated β-catenin, such as that seen in our alcohol-exposed mice receiving LiCl, may be sufficient to cause major changes in β-catenin nuclear localization and Wnt target gene expression and may translate to the restoration of normal healing we observe histologically in the alcohol/LiCl-treated mice.
Histological analysis of fracture callus tissue composition from alcohol-treated mice revealed striking alterations in external callus tissue composition, with binge alcohol-treated animals displaying greatly reduced cartilaginous external callus tissue. LiCl treatment was able to reverse this callus phenotype, restoring normal cartilage content to the external callus of alcohol-exposed animals. These data demonstrate that alcohol negatively impacts fracture repair, at least in part, by targeting the formation of external callus-associated cartilage tissue required for normal endochondral bone formation at the fracture site. This effect on callus-associated cartilage may occur though an alcohol-related inhibition of mesenchymal stem cell differentiation toward an osteochondral lineage at the fracture site, due to the deregulation of normal canonical Wnt signing activity in the callus we demonstrate here. The fact that exogenous activation of β-catenin signaling through LiCl treatment can attenuate the effect of alcohol on external callus cartilage formation supports the supposition that alcohol exposure-related perturbations of canonical Wnt-signaling in callus-associated stem cells are responsible for the inhibition of normal callus formation demonstrated in alcohol-treated mice.
An N of 2 was utilized for our histological analysis, limiting the interpretation of data to a descriptive analysis of tissue composition and a semi-quantitative analysis of cartilaginous callus area performed by a pathologist. While a larger sample size would have allowed a more rigorous statistical interpretation of these descriptive findings, the alcohol-related inhibition of external cartilaginous callus formation following fracture shown in this study has also been observed in other studies from our laboratory (Volkmer et al., 2011; Lauing et al., 2012) increasing our confidence that this effect of alcohol on callus formation is reproducible. It is well-established that β-catenin signaling regulates cartilage formation in the callus following fracture injury (Huang et al., 2011) and is required for endochondral bone formation (Kitagaki et al., 2003; Tamamura et al., 2005), thus our finding that exogenous activation of Canonical Wnt signaling by LiCl treatment may reverse the alcohol-associated decrease in cartilaginous callus formation and endochondral ossification at the fracture site is both noteworthy and supported by the literature.
While we did observe an improvement in the biomechanical strength of alcohol-exposed mice following LiCl treatment, this change was not statistically different from the mice administered alcohol alone. While LiCl treatment does normalize callus tissue composition in alcohol-treated animals, perhaps other parameters of fracture healing such as callus architecture or ossification of callus tissue are not fully resolved in alcohol-treated mice by exogenous activation of Wnt signaling. Studies utilizing micro computed tomography (micro CT) to analyze fracture callus structure in control and alcohol-treated mice are currently being performed in the laboratory to address these issues.
At Day 9 post-fracture, we observed a significant decrease in activated β-catenin in mice treated with saline and LiCl, when compared with mice exposed to saline alone. This result was unexpected. Since LiCl treatment did not increase activated GSK-3β levels in saline-exposed mice, which would normally lead to decreased levels of activated β-catenin, we can only say that the decrease in hypophosphorylated β-catenin observed in these animals did not occur via affects on GSK-3β activity. We did not observe any significant effects of LiCl administration on histological or biomechanical aspects of fracture healing in saline-exposed mice at Day 14 post-fracture, indicating that the molecular changes we observed did not translate into any callus-specific phenotypic changes caused by LiCl administration in this group. LiCl treatment has previously been demonstrated to increase bone mass in mice (Clement-Lacroix et al., 2005) suggesting that the effects of LiCl on activated β-catenin levels we observed in fracture callus tissue may be transient.
One of the main negative regulators of canonical Wnt/β-catenin signaling is GSK-3β, which when activated by hyperphosphorylation of the S33/S37/T41 residues at the N-terminus of the protein, phosphorylates β-catenin and targets it for degradation (Ikeda et al., 1998). Deregulation of GSK-3β activation state is associated with several pathological disease states (Meijer et al., 2004), demonstrating the potential negative impact of the alcohol-induced activation of GSK-3β we report in this investigation. We observed that alcohol exposure causes an increase in Tyr216-phosphorylated (activated) GSK-3β in fracture callus tissue at Day 9 post-fracture, which also correlates with (a) a significant decrease in activated β-catenin, (b) a significant increase in hyperphosphorylated β-catenin targeted for degradation and (c) a significant decrease in deactivated Ser9-phosphorylated GSK-3β. To our knowledge, this is the first report of GSK-3β deregulation by alcohol exposure, in vivo. In vitro studies have observed similar effects following alcohol exposure, which suppressed nuclear β-catenin levels are seen with a concomitant increase in active GSK-3β (Chen et al., 2010; Vangipuram and Lyman, 2011). Our findings indicate that LiCl was able to abolish the significant increase in activated GSK-3β found in alcohol-exposed mice (and improve stabilized β-catenin levels) while significantly decreasing hyperphosphorylated β-catenin levels. These data support the hypothesis that LiCl is able to enhance β-catenin stabilization though its predicted mechanism of GSK-3β inhibition.
While we focus on GSK3β in this study, it is possible that kinases other than GSK-3β may also play a role in β-catenin stabilization. Protein kinase A (PKA) has been reported to phosphorylate β-catenin, resulting in augmented TCF/LEF transcriptional activation (Taurin et al., 2006). However, the PKA-mediated phosphorylation of β-catenin does not prevent GSK-3β-mediated phosphorylation and subsequent degradation (Taurin et al., 2006). These data suggest that while phosphorylation of β-catenin by other kinases may play a role in enhancing its transcriptional activity, they do not necessarily affect the stabilization of β-catenin. Therefore, in conjunction with our earlier observations, the alcohol-induced deregulation of both GSK-3β and β-catenin levels and subsequent recovery by LiCl treatment described in this study are convincing evidence that the canonical Wnt signaling pathway is targeted by alcohol exposure in bone tissue.
One limitation to the current study was the use of LiCl to activate Canonical Wnt signaling. Although widely used and characterized as a potent GSK-3β inhibitor, lithium has the capability to weakly inhibit several other protein kinases. However, lithium ions have been shown to inhibit the activity of GSK-3β more potently than other cellular kinases, making it an ideal inhibitor of GSK-3β for in vivo use (Davies et al., 2000). Additionally, the dose administered in these studies (100 mg/kg) is well below that which would cause toxicity in mice (Smith, 1978), and we did not observe outward signs of toxicity in animals throughout the duration of treatment.
With respect to the method of alcohol exposure used in this study, our laboratory has utilized ip injection for several years to produce high blood alcohol levels in rodents in an attempt to mimic the episodic binge alcohol consumption patterns common in trauma patients to examine the effects of this alcohol treatment regimen on skeletal integrity and repair. A recent study suggests however that alcohol may exert differential effects on bone when administered by ip injection versus intragastric gavage (Iwaniec and Turner, 2013). Several important differences exist between this study and our own, including the rodent species and gender of animals utilized, the bone integrity-specific parameters examined, the alcohol dosing regimens and peak blood alcohol levels achieved, making it impossible to directly compare the two studies. We have not repeated the current studies with another method of alcohol delivery to date, though previous work from our laboratory suggests that alcohol exposure via ip injection or by liquid diet produces similar deleterious effects on adolescent rat lumbar vertebral bone mineral density and biomechanical strength (Wezeman et al., 2003; Lauing et al., 2008).
In conclusion, we report that binge alcohol exposure significantly modulates activated β-catenin and GSK-3β levels at Day 9 post-fracture. This is associated with decreased biomechanical strength and alterations in fracture callus tissue composition at Day 14 post-fracture. Treatment of binge alcohol-exposed mice with LiCl beginning at 4 days post-fracture improved fracture callus formation, partially restored fracture callus biomechanical strength, increased active β-catenin levels in the callus and decreased levels of callus-associated active GSK-3β to levels observed in callus from control mice. Considering our previous published observations, these data suggest that canonical Wnt signaling is a primary target of alcohol exposure during fracture repair. Further studies will determine if patients at risk for alcohol-related deficiencies in fracture healing or suffering from a non-union related to excessive alcohol consumption may benefit from exogenous enhancement of the canonical Wnt pathway to augment fracture healing.
Supplementary material
Supplementary material is available at Alcohol and Alcoholism online.
Funding
The NIH, National Institute on Alcohol Abuse and Alcoholism grants R01 AA016138 (J.J.C.), R21 AA021225 (J.J.C.), F32 019613 (K.L.L.) and T32 013527 supported this work.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgements
The authors thank Drs Michael Viglione and Sherri Yong for their invaluable assistance with fracture callus histology descriptions and scoring criteria. Their expert assistance in this area greatly improved our work.
References
- Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Lin AC, et al. Beta-catenin plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Shankar K, et al. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res. 2010;25:1117–27. doi: 10.1002/jbmr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA. 2005;102:17406–11. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DY, Jonason JH, Zhang Y, et al. Cartilage-specific β-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J Bone Miner Res. 2012;27:1680–94. doi: 10.1002/jbmr.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, et al. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmali N, Ertem K, Ozen S, et al. Fracture healing and bone mass in rats fed on liquid diet containing ethanol. Alcohol Clin Exp Res. 2002;26:509–13. [PubMed] [Google Scholar]
- Hiltunen A, Vuorio E, Aro HT. A standardized experimental fracture in the mouse tibia. J Orthop Res. 1993;11:305–12. doi: 10.1002/jor.1100110219. [DOI] [PubMed] [Google Scholar]
- Himes R, Wezeman FH, Callaci JJ. Identification of novel bone-specific molecular targets of binge alcohol and ibandronate by transcriptome analysis. Alcohol Clin Exp Res. 2008;32:1167–80. doi: 10.1111/j.1530-0277.2008.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhang X, Du K, et al. Inhibition of β-catenin signaling in chondrocytes induces delayed fracture healing in mice. J Orthop Res. 2011;30:304–10. doi: 10.1002/jor.21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17:1371–84. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniec UT, Turner RT. Intraperitoneal injection of ethanol results in drastic changes in bone metabolism not observed when ethanol is administered by oral gavage. Alcohol Clin Exp Res. 2013;37:1271–7. doi: 10.1111/acer.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke-Lorenz J, Lorenz R. Alcoholism and fracture healing. Arch Orthop Trauma Surg. 1984;103:286–9. doi: 10.1007/BF00387336. [DOI] [PubMed] [Google Scholar]
- Kakar S, Einhorn TA, Vora S, et al. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007;22:1903–12. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- Kitagaki J, Iwamoto M, Liu JG, et al. Activation of beta-catenin-LEF/TCF signal pathway in chondrocytes stimulates ectopic endochondral ossification. Osteoarthritis Cartilage. 2003;11:36–43. doi: 10.1053/joca.2002.0863. [DOI] [PubMed] [Google Scholar]
- Kristensson H, Lundén A, Nilsson BE. Fracture incidence and diagnostic roentgen in alcoholics. Acta Orthop Scand. 1980;51:205–7. doi: 10.3109/17453678008990787. [DOI] [PubMed] [Google Scholar]
- Lauing KL, Himes R, Rachwalski M, et al. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol. 2008;42:649–55. doi: 10.1016/j.alcohol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauing KL, Roper PM, Nauer RK, et al. Acute alcohol exposure impairs fracture healing and deregulates β-catenin signaling in the fracture callus. Alcohol Clin Exp Res. 2012;36:2095–103. doi: 10.1111/j.1530-0277.2012.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RS, Hebert CK, Munn BG, et al. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10:21–7. doi: 10.1097/00005131-199601000-00004. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Phamacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–80. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, et al. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–9. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Miller JW, Gfroerer JC, Brewer RD, et al. Prevalence of adult binge drinking: a comparison of two national surveys. Am J Prev Med. 2004;27:197–204. doi: 10.1016/j.amepre.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Nakanishi R, Akiyama H, Kimura H, et al. Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass. J Bone Miner Res. 2008;23:271–7. doi: 10.1359/jbmr.071007. [DOI] [PubMed] [Google Scholar]
- Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: The American Academy of Orthopaedic Surgeons; 1992. pp. 85–124. [Google Scholar]
- Quinlan KP, Brewer RD, Siegel P, et al. Alcohol-impaired driving among U.S. adults, 1993–2002. Am J Prev Med. 2005;28:346–50. doi: 10.1016/j.amepre.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Kim SJ, Kim SH, et al. Regulation of the chondrocyte phenotype by beta-catenin. Development. 2002;129:5541–50. doi: 10.1242/dev.129.23.5541. [DOI] [PubMed] [Google Scholar]
- Savola O, Niemelä O, Hillbom M. Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol Alcohol. 2005;40:269–73. doi: 10.1093/alcalc/agh159. [DOI] [PubMed] [Google Scholar]
- Smith DF. Lithium chloride toxicity and pharmacodynamics in inbred mice. Acta Pharmacol Toxicol (Copenh) 1978;43:51–4. doi: 10.1111/j.1600-0773.1978.tb02231.x. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Noort Mv M, Strous GJ, et al. Wnt signals are transmitted though N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–8. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamura Y, Otani T, Kanatani N, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–95. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Qin Y, et al. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–6. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- Vangipuram SD, Lyman WD. Ethanol affects differentiation-related pathways and suppresses Wnt signaling protein expression in human neural stem cells. Alcohol Clin Exp Res. 2011;36:788–97. doi: 10.1111/j.1530-0277.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- Vestergaard P. Fracture risks of antidepressants. Expert Rev Neurother. 2009;9:137–41. doi: 10.1586/14737175.9.1.137. [DOI] [PubMed] [Google Scholar]
- Volkmer DL, Sears B, Lauing KL, et al. Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. J Orthop Trauma. 2011;25:516–21. doi: 10.1097/BOT.0b013e31821f65cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezeman FH, Juknelis D, Frost N, et al. Spine bone mineral density and vertebral body height are altered by alcohol consumption in growing male and female rats. Alcohol. 2003;31:87–92. doi: 10.1016/j.alcohol.2003.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zou Y, Guo XM, et al. Temporal activation of β-catenin signaling in the chondrogenic process of mesenchymal stem cells affects the phenotype of the cartilage generated. Stem Cells Dev. 2012;21:1966–76. doi: 10.1089/scd.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zamani A, Omrani GR, Nasab MM. Lithium's effect on bone mineral density. Bone. 2008;44:331–4. doi: 10.1016/j.bone.2008.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.