Abstract
Aims: Alcoholic cardiomyopathy (ACM) presents as decreased myocardial contractility, arrhythmias and secondary non-ischemic dilated cardiomyopathy leading to heart failure. Mitochondrial dysfunction is known to have a significant role in the development and complications of ACM. This study investigated if chronic ethanol feeding promoted myocardial mitochondrial topoisomerase dysfunction as one underlying cause of mitochondrial DNA (mtDNA) damage and mitochondrial dysfunction in ACM. Methods: The impact of chronic ethanol exposure on the myocardial mitochondria was examined in both neonatal cardiomyocytes using 50 mM ethanol for 6 days and in rats assigned to control or ethanol feeding groups for 4 months. Results: Chronic ethanol feeding led to significant (P < 0.05) decreases in M-mode Fractional Shortening, ejection fraction, and the cardiac output index as well as increases in Tau. Ethanol feeding promoted mitochondrial dysfunction as evidenced by significantly decreased left ventricle cytochrome oxidase activity and decreases in mitochondrial protein content. Both in rats and in cultured cardiomyocytes, chronic ethanol presentation significantly increased mtDNA damage. Using isolated myocardial mitochondria, both mitochondrial topoisomerase-dependent DNA cleavage and DNA relaxation were significantly altered by ethanol feeding. Conclusion: Chronic ethanol feeding compromised cardiovascular and mitochondrial function as a result of a decline in mtDNA integrity that was in part the consequence of mitochondrial topoisomerase dysfunction. Understanding the regulation of the mitochondrial topoisomerases is critical for protection of mtDNA, not only for the management of alcoholic cardiomyopathy, but also for the many other clinical treatments that targets the topoisomerases in the alcoholic patient.
INTRODUCTION
Alcoholism remains a significant health problem and represents the third leading cause of preventable deaths (NIH, 2010). More than 4% of the American adults suffer from alcoholism and ∼10% of those who drink consume half the alcohol sold in the USA (Mack et al., 2010). Approximately 25% of general adult hospital admissions have problems related to chronic alcohol use (i.e. cirrhosis, cardiomyopathy) (Mack et al., 2010). Further, alcoholics are likely to abuse other substances as well as display higher levels of generalized anxiety disorders and posttraumatic stress disorders than the general population (Regier et al., 1990; Mack et al., 2010). Military personnel are at even greater risk for alcohol abuse compared with the general population which is related to the elevated stress that military personnel might face (Bray et al., 2006). Presentation of patients with ACM is similar to patients presenting with a dilated cardiomyopathy (Fauchier et al., 2000; Awtry and Philippides, 2010). Although a J-shaped curve has been identified for average alcohol consumption and cardiovascular heart disease, there are some qualifiers (Corrao et al., 2000; Di Castelnuovo et al., 2006; Costanzo et al. 2010). Even in individuals that have low overall consumption, irregular heavy drinking carries greater risks for cardiovascular disease suggesting that the toxic effects are prolonged (Sempos et al., 2003; Room et al., 2005).
Both acute and chronic ingestion of ethanol is cardio-depressive (Laonigro et al., 2009; Awtry and Philippides, 2010). The toxic effects of alcohol in the cardiovascular system are manifested as decreased myocardial contractility, arrhythmias and secondary non-ischemic dilated cardiomyopathy leading to heart failure (Wilke et al., 1996; Beckemeier and Bora, 1998). There are parallels between alcoholic cardiomyopathy and other cardiomyopathies including diabetes and doxorubicin cardiotoxicity, which are associated with abnormal cardiac function, accelerated apoptosis and loss of cardiac mass (Fiordaliso et al., 2000; Frustaci et al., 2000; Fang et al., 2004, 2005).
Mitochondrial dysfunction has a significant role in the development and complications of alcoholic cardiomyopathy (Kim et al., 2001; Vendemiale et al., 2001; Cahill et al., 2005; Hajnoczky et al., 2005; Piano et al., 2007). Chronic alcohol exposure accelerates mitochondrial dysfunction and apoptosis across different organs including the heart, liver and pancreas (Vendemiale et al., 2001; Cahill et al., 2002; Hajnoczky et al., 2005; Lee et al. 2010). Most studies have independently examined the impact of alcohol use on hepatic mitochondria or examined degradation of cardiac function in response to chronic ethanol exposure (Vendemiale et al., 2001; Cahill et al., 2002; Hajnoczky et al., 2005; Lee et al. 2010). Far fewer studies have examined alcohol's impact on the myocardial mitochondria. Given the near total dependence of the myocardium on aerobic metabolism, this represents a significant gap in our knowledge. Some studies have reported that the deleterious effects of ethanol are a function of increased oxidant stress. It is widely accepted that increased oxidative stress directly attacks mitochondrial DNA (mtDNA), as the sole mechanism for mtDNA damage. Our recent studies in models of diabetes alter that paradigm to include a significant role for mitochondrial topoisomerases in the propagation of mtDNA damage as an underlying cause of diabetic cardiomyopathy (Medikayala et al., 2011; Hicks et al., 2013). These findings are directly applicable to alcohol-induced cardiomyopathies. To date, no reports have examined the impact of ethanol presentation on mitochondrial topoisomerase function in the heart. Thus the present paper seeks to determine if chronic ethanol feeding promotes myocardial mitochondrial topoisomerase dysfunction as one underlying cause of mtDNA damage and mitochondrial dysfunction. Our findings have implications for the management of ACM as well as other clinical treatments that targets the topoisomerases in the alcoholic patient.
METHODS
Male Wistar (3–6 months old) were used throughout this study. Where indicated ethanol feeding was 10% (vol:vol) in drinking water. The efficacy of the ethanol feeding protocol was verified by measurement of blood alcohol levels from blood samples drawn between 0800 and 0900. Alcohol levels were measured using a NAD-ADH protocol as described by the manufacturer (N1760; Sigma-Aldrich, St. Louis, MO, USA). Experimental protocols had institutional approval and animals were maintained in accordance with APS's Guiding Principles in the Care and Use of Animals and the Guide for the Care and Use of Laboratory Animals (National Research Council, revised 1996).
Cell culture
Neonatal cardiomyocytes from 1- to 3-day-old Wistar rats were prepared using collagenase IV, as we have previously described (Edwards et al., 1992, 1994). Following preparation cells were plated overnight LG-DMEM + 10%FBS+ 0.1 mmol/l BrdU+ 1.0 mmol/l d-valine overnight before switching to the experimental media (LG-DMEM + 1% FBS + 1× NEAA +2 mmol/l glutamine), where indicated ethanol was added to the culture media.
Cardiovascular function
Echocardiography and pressure–volume loop analysis were used to evaluate cardiovascular function. Echocardiography was assessed using an Accustom Sequoia C256 system as described previously (Kinugawa et al., 2005). In brief, animals were anesthetized using 1% isoflurane/100% O2 metered through an Isotec4 vaporizer (VetEquip, Pleasanton, CA, USA) and maintained on a heated pad throughout the protocol. Once asleep, animals were allow to stabilize for 10 min before baseline measurements were collected. Following this dobutamine was injected (50 µg/kg i.p.) and measurements were collected after 5 min. Pressure–volume loop analysis allows for high fidelity recording of left ventricle function in vivo (Kass et al., 1986). In brief, animals were anesthetized using 3% isoflurane/100% O2 and maintained on a heated pad throughout the protocol. Surgical preparation included: (a) exposing the abdomen to lasso the inferior vena cava just proximal to the insertion of the right renal vein, (b) cannulation of the left external jugular vein for saline or dobutamine infusion, cannulation of the right carotid using a Millar SPR-838 transducer. Once the animal was instrumented, isoflurane was adjusted to 1% and the animal allowed to stabilize for 10 min before baseline occlusion to alter preload was performed. Following this, dobutamine (5.0 µg/kg/min i.v.) was infused and occlusion was performed after 12 min. At the end of the date collection period the isoflurane concentration was increased to 5% and after 5 min the hearts were resected for tissue harvest. Signal acquisition was done using a Millar Pressure–Volume Catheter (PR-838) and signal conditioning performed by the MPVS Ultra® System (Millar, Houston, TX, USA). Subsequent data analysis was performed using LabChart 7 Pro (ADI Instruments, Colorado Springs, CO, USA).
Cellular and mitochondrial function
Cytochrome oxidase (Complex IV) was measured by following the oxidation of reduced cytochrome C at A550, as originally described by Wharton and Tzagoloff (Wharton and Tzagoloff, 1967; Medikayala et al., 2011). Succinate dehydrogenase was determined from the cleavage of the tetrazolium salt MTT (3-4,5-dimethyliazol-2-yl)-2,5-diphenyl tetrazolium bromide (Denizot and Lang, 1986). ATP levels were determined by the CellTiter-Glo luminescent assay (Promega, Madison, WI, USA). Changes in mitochondrial membrane potential were estimated using JC1 (5,5′,6,6′-tetrechloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide). We determined shifts in the distribution of the aggregated (red) JC1 in healthy mitochondria to monomeric (green) JC1 in degenerating mitochondria that exhibited a loss of membrane potential. In brief, cells were incubated with 1 µM JC1 (Anaspec, Fremont, CA, USA) for 30 min at 37°C, before the cells were trypsinized to release them from the plate. Cells were washed twice in filtered (0.2 µ) PBS and resuspended in PBS. The red and green fluorescence was determined using a Guava Flow Cytometer and gated to count cells >10 µ; instrument gains were initially set using unstained cells and the Red:Green ratio was derived from all gated cells >10 µ taking the Red geometric mean: green geometric mean.
Western blot analysis
Western blot analysis on left ventricle homogenates was performed as previously described (Rafalski et al., 2007). The primary antibodies used were COX 4 subunit (1:1000 dilution; Molecular Probes, Grand Island, NY, USA) or VDAC (1:500; Rockland, Gilbertsville, PA, USA). The secondary antibody was an anti-rabbit HRP (1:4000 dilution: Amersham, Buckinghamshire, UK) and used in combination with a Pierce ECL kit. For band quantification, care was taken to ensure that band density remained within the linear range and did not saturate the film, by performing exposures of different times. Band density was quantified using the AlphaEaseFC software (AlphaInnotech, San Leandro, CA, USA).
Mitochondrial isolation
Mitochondria that were devoid of nuclei were isolated by differential centrifugation as we have demonstrated previously (Edwards, 2008; Medikayala et al., 2011). In brief, left ventricles from animals were minced using fine scissors before being put into a dounce homogenizer. Cultured cells were collected in ice-cold PBS and centrifuged (300 g, 5 min at 4°C). The minced tissues or cell pellets were suspended in mitochondrial isolation buffer (250 mmol/l Sucrose, 10 mmol/l Tris-Cl pH 7.5, 1 mmol/l EDTA, 1 mmol/l EGTA, 1.5 mmol/l MgCl2, 10 mmol/l KCl, 1 mmol/l DTT, 1 mmol/l PMSF, 1× protease inhibitors; Sigma P-8340) and homogenized using a dounce homogenizer (10 strokes ‘A’ pestle and 10 strokes ‘B’ pestle). The extracts were then subjected to successive rounds of centrifugation: (a) 300 g, 5 min at 4°C times 3, (b) 1000 g, 5 min at 4°C times 2, (c) 2000 g 5 min at 4°C times 1, (d) 13,000 g, 10 min at 4°C times 3. Mitochondrial pellets were resuspended in buffer (50 mmol/l Tris-Cl pH 7.5, 0.5 mmol/l EDTA, 0.5 mmol/l EGTA, 1 mmol/l DTT, 10% glycerol, 1 × protease inhibitors) and lysed on ice for 30 min before protein concentration was determined by the Bradford method (BioRad, Hercules, CA, USA). Where a nuclei fraction was isolated, the pellet isolated from the first 1000 g spin was used. The cytosolic fraction was derived from the supernatant of the first 13,000 g spin and was cleared of other membrane fractions by an additional centrifugation step (15,000 g, 60 min at 4°C).
mtDNA damage
Total DNA was extracted from cultured cardiomyocytes or 1–5 mg left ventricle using Sigma Extract-n-Amp (Sigma, St. Louis, MO, USA). A LRPCR protocol was used to assess mtDNA damage by real-time QPCR using a Stratagene Mx3000P as we have described previously (Edwards, 2008; Medikayala et al., 2011). In brief, any lesion (strand breaks, base modifications and apurinic sites) will stop a thermostable DNA polymerase capable of generating a long DNA product (Edwards, 2008). This amplification was compared to that of a short PCR (SRPCR) product (150–250 bp) that was unlikely to contain any lesions. A second short range PCR was performed using primers to the genomic gene β-actin and used to derive the mitochondrial copy number. SRPCR was done using a Brilliant QPCR Master Mix, while LRPCR was done using PfuUltra™ II Fusion HS DNA polymerase (Stratagene, La Jolla, CA, USA). The primers for the LRPCR reaction were 5′-GCCAGGACCAAACCTTTGTGTTTA-3′ forward and 5′-GGACTAGCCCCATTCCACTAC-3′ reverse. Primers used for the SRPCR and β-actin PCR reactions were as we have previously described (Edwards, 2008). Quantification of mtDNA damage and mitochondrial copy number were derived by the 2ΔCt method, from the comparison of LRPCR:SRPCR and SRPCR:β-actin, respectively.
DNA cleavage assay
DNA cleavage was determined by degradation of linear DNA using a Cy5- fluorescent probe and modified from our previous description (Medikayala et al., 2011). In brief, a linear mtDNA was amplified by a PCR reaction using an internally labeled Cy5-labeled primer: forward: 5′-AAATTTCCCGACACAAAATCTTTCC(Cy5)TCCTAACTAAACCCTCTTTACTTGC-3′ and the reverse primer was: 5′-CTCTTGGTAAGTAAATTTCTTTCTCC-3′ using mtDNA as the template to generate a 1274 bp probe. To perform the cleavage assay, isolated mitochondria (1–10 µg protein) were incubated in buffer (50 mmol/l TrisCl pH 7.5, 100 mmol/l KCl, 0.5 mM EDTA, 0.5 mmol/l DTT, 30 µg/ml BSA) to a final volume of 20 µl. The reaction was cooled on ice and at time zero the labeled DNA was added. The reaction was incubated for 30 min at 37°C to which 6 µl loading buffer was added. The inhibition experiments were performed as described previously where the extracts were incubated (± inhibitor) for 60 min at 37°C before the DNA was added (Medikayala et al., 2011). 7 µl was electrophoresed on a 4% polyacrylamide gel and visualized using a Storm 840 PhosphoImager (Molecular Dynamics, CA, USA). Band density was quantified using the AlphaEaseFC software (AlphaInnotech, San Leandro CA, USA). The DNA relaxation protocol was modified from that described by Low et al. (2003). In brief, mitochondrial extracts (1–10 µg) were incubated in buffer (50 mM TrisCl pH 8.0, 120 mM KCl, 10 mM MgCl2, 0.5 mM ATP, 0.5 mM DTT) and to this 600 ng supercoiled pBR322 plasmid was added to a total volume of 20 µl. The mixture was incubated for 10 min at 37°C and the reaction stopped by the addition of 4 µl of a 5%sarcosine/6× SDS buffer and the reaction placed on ice until 5 µl was loaded onto a 1% agarose/TAE gel. Bands were imaged at 260 nm using a ChemiImager 5550 and density quantified using the AlphaEaseFC software (AlphaInnotech, San Leandro, CA, USA).
Statistical analysis
Where appropriate ANOVA analyses were performed using NCSS Software (NCSS, Kaysville, UT, USA). Post hoc analysis was done using a Fisher's LSD analysis. Values presented are mean ± SEM, and statistical significance was set at P < 0.05, unless otherwise indicated.
RESULTS
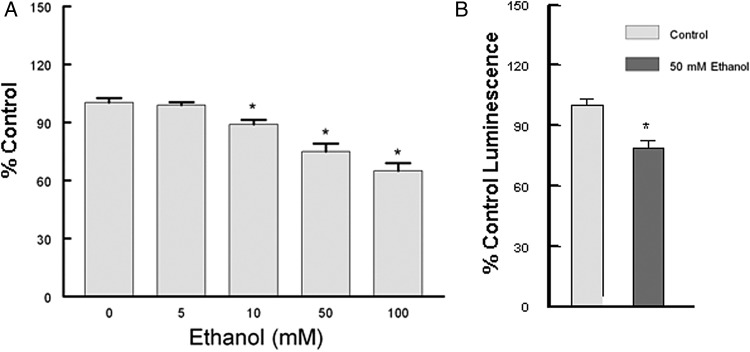
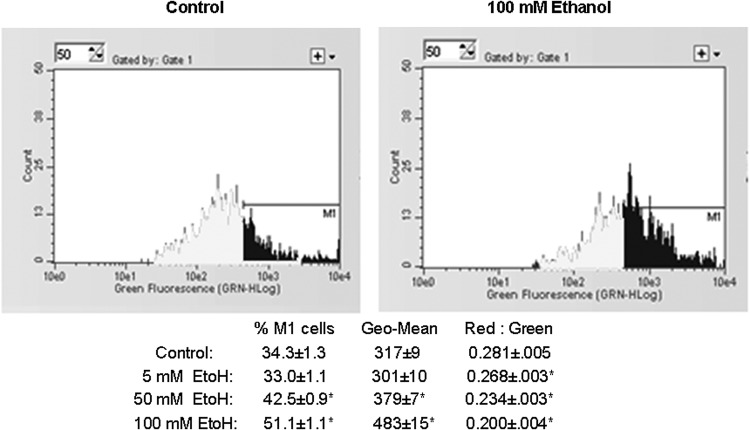
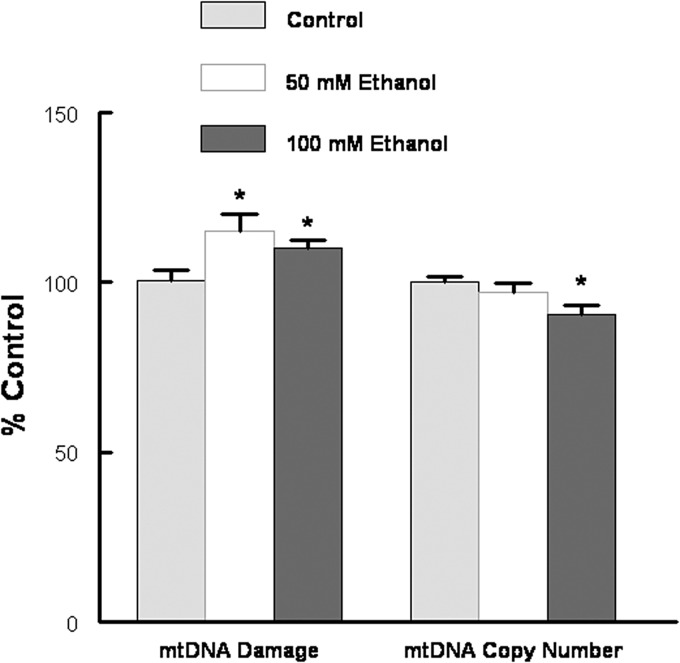
We have investigated the effect of prolonged ethanol exposure on the heart both in vivo by chronic feeding and in vitro by the addition of ethanol to cardiomyocytes in primary culture. Neonatal cardiomyocytes were maintained in the absence or presence of increasing concentrations of ethanol. The media was changed daily as our preliminary experiments indicated that at 100 mM ethanol ∼50% of the ethanol was consumed in a 24 h period (data not shown). Six days of ethanol treatment led to a dose-dependent compromise of mitochondrial function as evidenced by a significant decrease in succinate dehydrogenase activity and decreased ATP levels (Fig. 1). JC1 has been used as an indicator of mitochondrial membrane potential, as depolarization is thought to be an early event of mitochondrial dysfunction (Reers et al., 1995). Using flow cytometry cells were gated to count the larger (>10 µ) cells, and ethanol significantly increased the percentage of depolarized (green) mitochondria as well as the geometric mean of green fluorescence (Fig. 2). Qualitatively similar results were obtained from the analysis of all cells (data not shown). In combination, these results clearly indicate ethanol-induced mitochondrial dysfunction. Concomitant with mitochondrial dysfunction we observed a significant increase in mtDNA damage (Fig. 3). These experiments in cultured cardiomyocytes indicate a direct effect of ethanol on cardiomyocyte metabolism that is not dependent upon signaling from a remote source.
Fig. 1.
Ethanol decreases cardiomyocyte mitochondrial function. (A) Neonatal cardiomyocytes were exposed to increasing ethanol concentrations for 6 days. Succinate dehydrogenase activity was determined by a MTT protocol (Medikayala et al., 2011). (B) Ethanol decreased ATP levels in neonatal cardiomyocytes exposed to 50 mM ethanol for 6 days. Values presented are mean ± SEM and normalized to water fed controls. *P < 0.05 compared with control.
Fig. 2.
Ethanol treatment significantly altered JC1 fluorescence. Following 6 days of ethanol treatment, cardiomyocytes were stained with 1 µM JC1 as described in Methods. Using flow cytometry, cells were gated to count cells >10 µ. Increases in green fluorescence are indicative of mitochondrial depolarization as determined from the percentage of cells in the M1 region (% M1), the geometric mean of all gated cells (Geo-Mean), and the shift in the Red:Green ratios derived from the Red:Green geometric means. Values presented are mean ± SEM. *P < 0.05 compared with water fed control.
Fig. 3.
Ethanol treatment increased mtDNA damage. Cardiomyocytes were exposed to 50 or 100 mM ethanol in DMEM/1%FBS for 6 days. mtDNA damage and mitochondrial copy number was assessed by a QPCR protocol as described in Methods. Values are normalized to water fed control and are mean ± SEM. *P < 0.05 compared with the control.
The efficacy of the ethanol feeding protocol was verified by measurement of blood alcohol levels. Water fed animals had a blood alcohol concentration of 6.7 ± 1.9 mg%, while ethanol animals fed had a blood alcohol level of 46.4 ± 1.9 mg%; these values comparable to other studies (Brown et al., 1998).
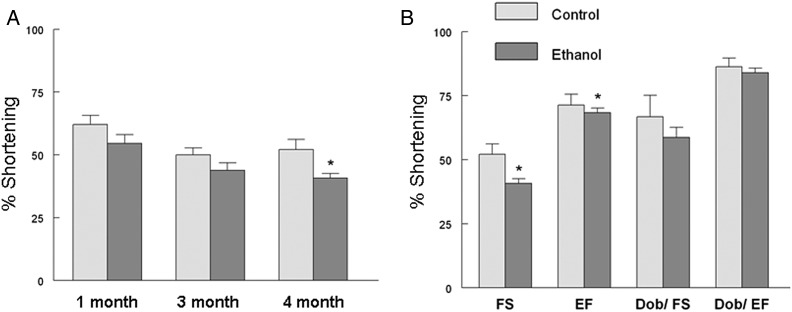
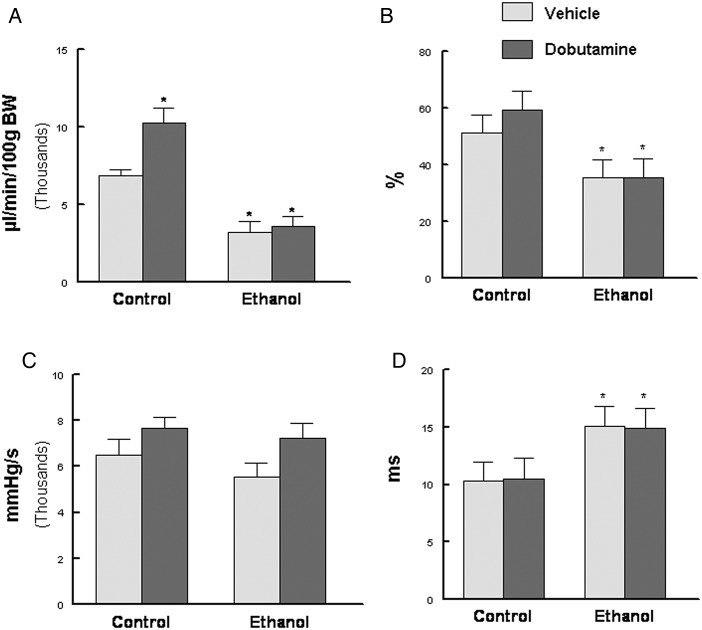
Cardiac function was followed longitudinally by echocardiography. A time-dependent degradation of fractional shortening was observed that was significantly decreased only after 4 months (∼12 human years) of ethanol consumption (Fig. 4A). At 4 months both fractional shortening and ejection fraction were significantly depressed (Fig. 4B). In contrast, following a submaximal (50 µg/kg) injection of dobutamine, no differences were observed between the two groups suggesting the cardiac reserve was not yet significantly compromised (Fig. 4B). By pressure–volume loop analysis, we observed results similar to the echocardiography results as basal function was depressed as evidenced by significant decreases in the cardiac output index and ejection fraction (Fig. 5A and B). Although the cardiac output index was increased in response to dobutamine infusion in the control group, it was blunted in the ethanol fed animals (Fig. 5A). Ethanol feeding also significantly altered Tau (Fig. 5D) suggesting that diastolic function was compromised.
Fig. 4.
Ethanol feeding progressively compromised cardiac function. (A) Basal fractional shortening was measured by M-Mode echocardiography after 1, 3 and 4 months of ethanol feeding. (B) M-Mode analysis echocardiography after 4 months of ethanol feeding. Dobutamine was injected (50 µg/kg i.p) and measurements made after 5 min. Values presented are mean ± SEM *P < 0.05 compared with water fed control animals.
Fig. 5.
Chronic ethanol feeding compromised cardiac function. (A) cardiac output index (µl/min/100 g BW), (B) ejection fraction, (C) dP/dtmax (mmHg/sec), (D) Tau (ms). Animals were anesthetized using 1% isoflurane and a Millar pressure–volume catheter used to make CV functions tests. Dobutamine was infused (5.0 µg/kg/min i.v) and measurements made after 5 min. Values are mean ± SEM; *P < 0.05 compared with control/vehicle animals.
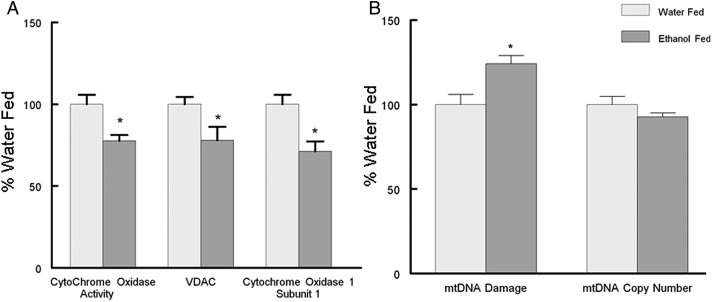
Similar to our results in cardiomyocytes, we observed that chronic ethanol consumption led to the degradation of myocardial mitochondrial function. Ethanol feeding significantly decreased cytochrome oxidase activity that in part was caused by a significant decrease in the mitochondrial encoded Cytochrome Oxidase Subunit 1 (Fig. 6A). Concomitant with these observations we observed a significant increase in mtDNA damage (Fig. 6B), but without changes in mitochondrial copy number.
Fig. 6.
Ethanol feeding induces myocardial mitochondrial dysfunction and mtDNA damage. (A) Left ventricle Cytochrome Oxidase enzyme activity and the mitochondrial proteins VDAC (nuclear encoded) and Subunit 1 Cytochrome Oxidase (mitochondrial encoded) by western blot. (B) Mitochondrial DNA damage was determined by a long range QPCR assay we developed (Edwards, 2008) and is the ratio of LRPCR:SRPCR. mtDNA copy number is the ratio of SRPCR:β-actin QPCR as described in Methods. Values are mean ± SEM and normalized to water fed control animals. *P < 0.05 compared with control animals.
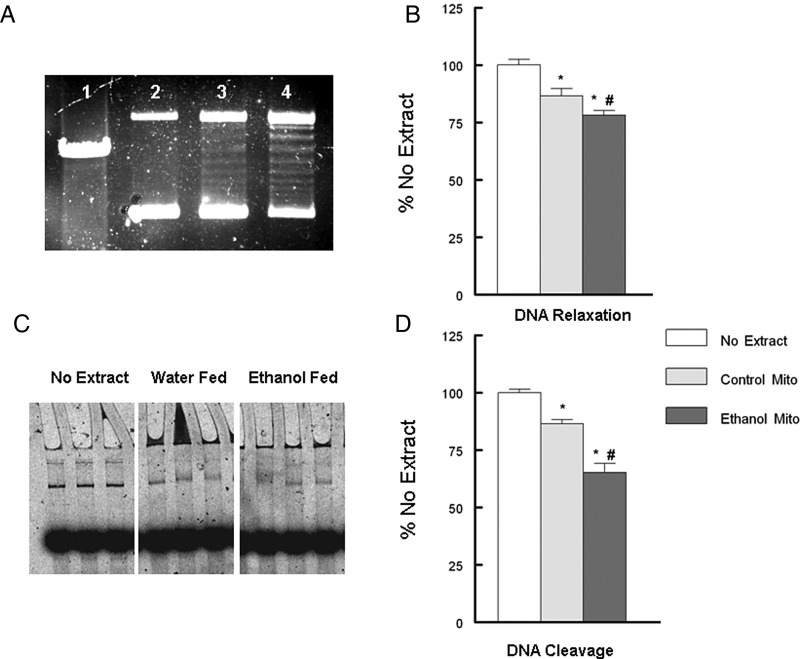
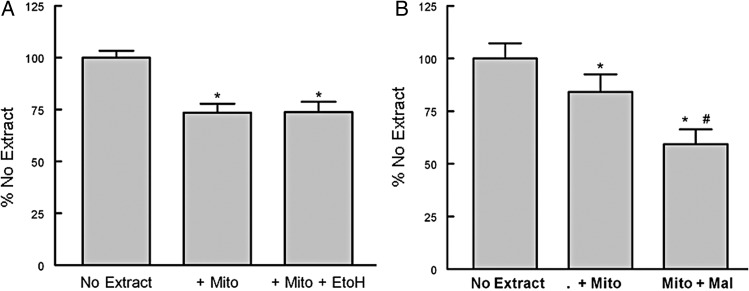
We have previously reported that mitochondrial topoisomerase dysfunction contributed to a decline in mtDNA integrity and mitochondrial competence (Medikayala et al., 2011; Hicks et al., 2013). To evaluate if mitochondrial topoisomerase function was altered by ethanol feeding, isolated left ventricle mitochondrial extracts, devoid of nuclear contaminants, were used. We measured mitochondrial topoisomerase DNA relaxation and DNA cleavage activity (Fig. 7). Incubation of supercoiled DNA with the mitochondrial extracts generated the typical isopane pattern of topoisomerases (Fig. 7A; ln: 3 and 4). Measurement of either mtDNA relaxation or DNA cleavage activity demonstrated that chronic ethanol feeding significantly increased mitochondrial topoisomerase-dependent strand breakage in DNA compared with water fed controls (Fig. 7B and D). To determine if ethanol could directly alter mitochondrial topoisomerase activity, mitochondrial extracts were preincubated with ethanol or maleimide (topoisomerase inhibitor). Acute exposure of ethanol did not significantly alter mitochondrial topoisomerase DNA cleavage (Fig. 8A). In contrast, 1 µM maleimide did significantly increase DNA cleavage of the mitochondrial topoisomerases (Fig. 8B).
Fig. 7.
Ethanol feeding altered myocardial mitochondrial topoisomerase function. (A) DNA relaxation of supercoiled plasmid DNA ln 1: linearized pBR322, ln 2: no extract, ln 3; 5.0 µg mitochondrial extract, ln 4, 10 µg mitochondrial extract. (B) Quantification of DNA relaxation as described in Methods. (C) Representative figure of DNA cleavage assay. (D) Quantification of DNA cleavage as described in Methods. Values are mean ± SEM and normalized to no mito controls. *P < 0.05 compared with no mito, #P < 0.05 compared with control Mito.
Fig. 8.
Ethanol does not directly alter mitochondrial topoisomerase function. (A) Myocardial mitochondria extracts were incubated in the absence or presence of 50 mM ethanol. (B) Myocardial mitochondria extracts were incubated in the absence or presence of 1 µM maleimide. Mitochondrial were incubated for 60 min at 37°C in the absence or presence of the ethanol or maleimide before linear DNA was added and the incubation continued for another 30 min at 37°C. The samples were then placed on ice and loading buffer added and an aliquot run on a 4% polyacrylamide gel. Values are mean ± SEM and normalized to no extract controls. *P < 0.05 compared with no extract, #P < 0.05 compared with + Mito.
DISCUSSION
Both acute and chronic ingestion of ethanol are cardio-depressive (Kim et al., 2001; Vendemiale et al., 2001; Hajnoczky et al., 2005; Laonigro et al., 2009; Larosche et al., 2009; Awtry and Philippides, 2010). Mitochondrial dysfunction has a significant role in the development and complications of alcoholic cardiomyopathy (Kim et al., 2001; Vendemiale et al., 2001; Cahill et al., 2005; Hajnoczky et al., 2005; Piano et al., 2007). This is in addition to the known detrimental effects of long-term ethanol consumption on hepatic mitochondria (Fromenty et al., 1995; Mateos et al., 1995; Mansouri et al., 1997; Cahill et al., 2002; Demeilliers et al., 2002). The major findings of the present study are that increased ethanol presentation, both in vitro and in vivo, significantly elevated mitochondrial topoisomerase DNA cleavage activity that was associated with mtDNA damage and mitochondrial dysfunction.
Presentation of patients with alcoholic cardiomyopathy (ACM) is similar to patients presenting with a dilated cardiomyopathy (Fauchier et al., 2000; Awtry and Philippides, 2010). Lazarevic et al. (2000) observed an increase in isovolumic relaxation time, deceleration time of the early diastolic filling velocity and late diastolic filling velocity (Lazarevic et al., 2000). In the present study, the ethanol-induced increases in Tau are consistent with impaired diastolic function (Fig. 3D). In addition to diastolic impairment, progressive degradation of cardiovascular function has been evidenced by significant decreases in ejection fraction and fractional shortening in patients as well as reduced sensitivity to dobutamine (Segel, 1988; Urbano-Marquez et al., 1989; Kim et al., 2001). These results are consistent with our findings of a reduced response to dobutamine injections on the cardiac output index (Fig. 5).
At the cellular level, many studies have reported that chronic alcohol consumption results in abnormalities in myocardial extracellular and intracellular structures, including the mitochondria (Alexander, 1967; Tsiplenkova et al., 1986; Meehan et al., 1999; Ling et al., 2011). In the present study, ethanol feeding caused a significant decline in mitochondrial function as evidenced by significantly decreased mitochondrial membrane potential, cytochrome oxidase activity, and the mitochondrial proteins, VDAC and cytochrome oxidase subunit 1.
In both cultured cells and in chronic feeding experiments, elevated ethanol presentation increased mtDNA damage, findings that are consistent with previous reports in alcoholic patients (Teragaki et al., 2000). In contrast, mitochondrial copy number was decreased only in cultured cardiomyocytes exposed to 100 mM ethanol, but no changes were observed in the left ventricle. Previous studies have demonstrated that ethanol feeding significantly decreased mitochondrial DNA and RNA levels as well as decreasing the number of functionally active mitochondrial ribosomes (Kou and Cohen, 1998; Cahill et al., 1999; Cahill and Cunningham, 2000). This suggests that any compensatory response to the decline in cardiovascular function may have been ineffectual as a result of the increase in mtDNA damage or alteration of mtDNA sequence. Although mitochondria are poor at repairing UV-induced damage, they are capable of other forms of DNA damage repair (Clayton et al., 1975; LeDoux et al., 1992; Druzhyna et al., 2008). Thus, the significant increase in mtDNA damage that we observed indicated the rate of mtDNA damage overwhelmed the functional mtDNA repair capability. As we have previously reported, mtDNA damage may also take the form of nucleotide substitutions that significantly alter the coding sequence of the mitochondrial proteins (Hicks et al., 2013). The consequences of mtDNA mutations have been shown to significantly alter OXPHOS assembly (Pello et al., 2008; Gil Borlado et al., 2010). A mutation in a patient in the cytochrome b (A15533G) region resulted in a presentation of lactic acidosis and mild mental decay. Using transmutational cybrids, this group observed significant alterations in the rate of Electron Transport Complex (ETC) assembly (Gil Borlado et al., 2010). Mimicking other mutations common to LHON also significantly altered the rates of ETC assembly (Pello et al., 2008). Another consequence of altered mtDNA sequence is the interdependency of the ETC Complexes for their stability within the mitochondrial membrane (Lamantea et al., 2002; Ugalde et al., 2003; Acin-Perez et al., 2004; Edgar et al., 2009). Collectively these studies point to the critical role of mtDNA fidelity has on the mitochondrial function within the myocardium.
It is widely accepted that mitochondrial-derived oxidant stress is the sole endogenous cause of mtDNA damage. However, a number of reports have found significant mitochondrial dysfunction or mtDNA damage in the absence of oxidant stress (Hiona et al.; Zhang et al., 2000, 2005; Kujoth et al., 2005; Trifunovic et al., 2005). Our recent papers are also at variance with this concept to show that mitochondrial topoisomerase dysfunction promotes mtDNA damage in the diabetic heart (Medikayala et al., 2011; Hicks et al., 2013).
Topoisomerases resolve the topological difficulties of DNA replication by allowing the double helix to pass through itself. In post mitotic cells, the mitochondrial genome is thought to replicate about once a month and resolving the topology of mtDNA replication would be insurmountable in the absence of the topoisomerases (Cortopassi and Wang, 1995). Although topoisomerase activity is essential to the cell and generally protective, it may also be genotoxic (Deweese and Osheroff, 2008). The impact of myocardial mitochondrial topoisomerases on mitochondrial function has been demonstrated on several levels including the use of topoisomerase inhibitors, estimation of molecular weight for DNA cleavage activity and immunoprecipitation of mitochondrial extracts with topoisomerase antibodies (Medikayala et al., 2011; Hicks et al., 2013).
Topoisomerase inhibitors or poisons act via a variety of mechanisms to alter topoisomerase function. Some, such as maleimide, permit DNA binding and cleavage, but not the religation or release of the relaxed DNA structure. This has the consequence of inducing DNA strand breaks (DSB) without a comparable increase in DNA religation. This dysfunction is the underlying basis for their use as antineoplastic or antibiotic drugs. We have previously reported that sobuzoxane and novobiocin (topoisomerase II inhibitors), or hydroxycamptothecin (topoisomerase I inhibitor) exacerbated myocardial mitochondrial topoisomerase-dependent DNA cleavage (Medikayala et al., 2011; Hicks et al., 2013). In the present study, acute exposure to ethanol did not directly alter mitochondrial topoisomerase function, while in contrast maleimide did. That ethanol does not have a direct effect indicates that its effects are mediated through another agent. Chronically elevated ethanol presentation significantly increased both DNA relaxation and DNA cleavage activity of the myocardial mitochondria topoisomerases. As we and others have previously discussed oxidant stress alters topoisomerase function to inhibit the religation step which promotes DNA strand breaks (Li et al., 1999; Medikayala et al., 2011; Hicks et al., 2013). These observations are consistent with ethanol-induced mitochondrial topoisomerase dysfunction resulting in an imbalance between DNA strand breaks and religation. Our findings suggest that alcoholism follows a similar pathophysiologic mechanism in the heart's mitochondria, to that described for diabetes and some topoisomerase inhibitors (Medikayala et al., 2011; Hicks et al., 2013).
SUMMARY
Mitochondrial dysfunction has a significant role in the development and complications of alcoholic cardiomyopathy and the present study reiterates this (Wieland and Lauterburg, 1995; Kim et al., 2001; Pagel et al., 2002; Cahill et al., 2005; Piano et al., 2007; Guiraud et al., 2008). Ethanol feeding compromised mitochondrial function in part as a result of a decline in mtDNA integrity. Separate from a direct impact of oxidative stress on mtDNA, ROS-induced alteration of mitochondrial topoisomerase function appeared to accelerate and propagate an increase in mtDNA damage. This indicates that mitochondrial topoisomerase dysfunction will have important consequences for myocardial function. Topoisomerases are critical for all cells and mitochondria could not survive without them. Topoisomerases are the focal point for many antibiotic and antineoplastic reagents, and their regulation is central to the clinical management of several diseases. These findings have broad implications for the management of the alcoholic patient. Understanding the regulation of the mitochondrial topoisomerases is critical for protection of mtDNA, not only for the management of alcoholic cardiomyopathy, but also for the many other clinical treatments that targets the topoisomerases in the alcoholic patient.
Funding
This work was supported in part by National Institute of Health (HD065551, HL43023) and the New York Medical College Research Endowment Fund.
Conflict of interest statement
None declared.
REFERENCES
- Acin-Perez R, Bayona-Bafaluy MP, Fernandez-Silva P, et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell. 2004;13:805–15. doi: 10.1016/s1097-2765(04)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CS. Electron microscopic observations in alcoholic heart disease. Br Heart J. 1967;29:200–6. doi: 10.1136/hrt.29.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awtry EH, Philippides GJ. Alcoholic and cocaine-associated cardiomyopathies. Prog Cardiovasc Dis. 2010;52:289–99. doi: 10.1016/j.pcad.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Beckemeier M, Bora P. Fatty acid ethyl esters: potentially toxic products of myocardial ethanol metabolism. J Mol Cell Cardiol. 1998;30:2487–94. doi: 10.1006/jmcc.1998.0812. [DOI] [PubMed] [Google Scholar]
- Bray BM, Hourani L, Olmstead K. 2006. 2005 Department of Defence Survey of Heatlh-Related Behaviours among Active Duty Military Personnel RTI International.
- Brown RA, Crawford M, Natavio M, et al. Dietary magnesium supplementation attenuates ethanol-induced myocardial dysfunction. Alcohol Clin Exp Res. 1998;22:2062–72. [PubMed] [Google Scholar]
- Cahill A, Cunningham CC. Effects of chronic ethanol feeding on the protein composition of mitochondrial ribosomes. Electrophoresis. 2000;21:3420–6. doi: 10.1002/1522-2683(20001001)21:16<3420::AID-ELPS3420>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Cahill A, Stabley GJ, Wang X, et al. Chronic ethanol consumption causes alterations in the structural integrity of mitochondrial DNA in aged rats. Hepatology. 1999;30:881–8. doi: 10.1002/hep.510300434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill A, Cunningham CC, Adachi M, et al. Effects of alcohol and oxidative stress on liver pathology: the role of the mitochondrion. Alcohol Clin Exp Res. 2002;26:907–15. [PMC free article] [PubMed] [Google Scholar]
- Cahill A, Hershman S, Davies A, et al. Ethanol feeding enhances age-related deterioration of the rat hepatic mitochondrion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1115–23. doi: 10.1152/ajpgi.00193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DA, Doda JN, Friedberg EC. Absence of a pyrimidine dimer repair mechanism for mitochondrial DNA in mouse and human cells. Basic Life Sci. 1975;5B:589–91. doi: 10.1007/978-1-4684-2898-8_26. [DOI] [PubMed] [Google Scholar]
- Corrao G, Rubbiati L, Bagnardi V, et al. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95:1505–23. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- Cortopassi G, Wang E. Modelling the effects of age-related mtDNA mutation accumulation; complex I deficiency, superoxide and cell death. Biochim Biophys Acta. 1995;1271:171–6. doi: 10.1016/0925-4439(95)00025-y. [DOI] [PubMed] [Google Scholar]
- Costanzo S, Di Castelnuovo A, Donati MB, et al. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010;55:1339–47. doi: 10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Demeilliers C, Maisonneuve C, Grodet A, et al. Impaired adaptive resynthesis and prolonged depletion of hepatic mitochondrial DNA after repeated alcohol binges in mice. Gastroenterology. 2002;123:1278–90. doi: 10.1053/gast.2002.35952. [DOI] [PubMed] [Google Scholar]
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–7. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing. Nucleic Acids Res. 2008;37:738–48. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Bagnardi V, et al. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–45. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- Druzhyna NM, Wilson GL, LeDoux SP. Mitochondrial DNA repair in aging and disease. Mech Ageing Dev. 2008;129:383–90. doi: 10.1016/j.mad.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar D, Shabalina I, Camara Y, et al. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009;10:131–8. doi: 10.1016/j.cmet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Edwards JG. Quantification of mitochondrial DNA (mtDNA) damage and error rates by real-time QPCR. Mitochondrion. 2008;9:31–5. doi: 10.1016/j.mito.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JG, Bahl JJ, Flink I, et al. A repressor region in the human beta myosin heavy chain gene that has a partial position dependency. Biochem Biophys Res Commun. 1992;189:504–10. doi: 10.1016/0006-291x(92)91586-f. [DOI] [PubMed] [Google Scholar]
- Edwards JG, Bahl J, Flink I, et al. Thyroid hormone influences expression of the beta myosin heavy chain gene. Biochem Biophys Res Commun. 1994;199:1482–8. doi: 10.1006/bbrc.1994.1398. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–67. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Schull-Meade R, Downey M, et al. Determinants of subclinical diabetic heart disease. Diabetologia. 2005;48:394–402. doi: 10.1007/s00125-004-1632-z. [DOI] [PubMed] [Google Scholar]
- Fauchier L, Babuty D, Poret P, et al. Comparison of long-term outcome of alcoholic and idiopathic dilated cardiomyopathy. Eur Heart J. 2000;21:306–14. doi: 10.1053/euhj.1999.1761. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Li B, Latini R, et al. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II- dependent. Lab Invest. 2000;80:513–27. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- Fromenty B, Grimbert S, Mansouri A, et al. Hepatic mitochondrial DNA deletion in alcoholics: association with microvesicular steatosis. Gastroenterology. 1995;108:193–200. doi: 10.1016/0016-5085(95)90024-1. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–32. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- Gil Borlado MC, Moreno Lastres D, Gonzalez Hoyuela M, et al. Impact of the mitochondrial genetic background in complex III deficiency. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud A, de Lorgeril M, Zeghichi S, et al. Interactions of ethanol drinking with n-3 fatty acids in rats: potential consequences for the cardiovascular system. Br J Nutr. 2008;100:1237–44. doi: 10.1017/S0007114508981472. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Buzas CJ, Pacher P, et al. Alcohol and mitochondria in cardiac apoptosis: mechanisms and visualization. Alcohol Clin Exp Res. 2005;29:693–701. doi: 10.1097/01.alc.0000163493.45344.7a. [DOI] [PubMed] [Google Scholar]
- Hicks S, Labinskyy N, Piteo B, et al. Type II diabetes increases mitochondrial DNA mutations in the left ventricle of the Goto-Kakizaki diabetic rat. Am J Physiol Heart Circ Physiol. 2013;304:H903–15. doi: 10.1152/ajpheart.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiona A, Sanz A, Kujoth GC, et al. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One. 2010;5:e11468. doi: 10.1371/journal.pone.0011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass DA, Yamazaki T, Burkhoff D, et al. Determination of left ventricular end-systolic pressure-volume relationships by the conductance (volume) catheter technique. Circulation. 1986;73:586–95. doi: 10.1161/01.cir.73.3.586. [DOI] [PubMed] [Google Scholar]
- Kim SD, Beck J, Bieniarz T, et al. A rodent model of alcoholic heart muscle disease and its evaluation by echocardiography. Alcohol Clin Exp Res. 2001;25:457–63. [PubMed] [Google Scholar]
- Kinugawa S, Wang Z, Kaminski PM, et al. Limited exercise capacity in heterozygous manganese superoxide dismutase gene-knockout mice: roles of superoxide anion and nitric oxide. Circulation. 2005;111:1480–6. doi: 10.1161/01.CIR.0000159261.11520.63. [DOI] [PubMed] [Google Scholar]
- Kou SY, Cohen NS. Ethanol feeding produces deficiencies in left ventricle total RNA, total DNA and mitochondrial ribosomal RNA. Int J Biochem Cell Biol. 1998;30:475–85. doi: 10.1016/s1357-2725(98)00012-0. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Lamantea E, Carrara F, Mariotti C, et al. A novel nonsense mutation (Q352X) in the mitochondrial cytochrome b gene associated with a combined deficiency of complexes I and III. Neuromuscul Disord. 2002;12:49–52. doi: 10.1016/s0960-8966(01)00244-9. [DOI] [PubMed] [Google Scholar]
- Laonigro I, Correale M, Di Biase M, et al. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11:453–62. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- Larosche I, Choumar A, Fromenty B, et al. Prolonged ethanol administration depletes mitochondrial DNA in MnSOD-overexpressing transgenic mice, but not in their wild type littermates. Toxicol Appl Pharmacol. 2009;234:326–38. doi: 10.1016/j.taap.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Lazarevic AM, Nakatani S, Neskovic AN, et al. Early changes in left ventricular function in chronic asymptomatic alcoholics: relation to the duration of heavy drinking. J Am Coll Cardiol. 2000;35:1599–606. doi: 10.1016/s0735-1097(00)00565-9. [DOI] [PubMed] [Google Scholar]
- LeDoux SP, Wilson GL, Beecham EJ, et al. Repair of mitochondrial DNA after various types of DNA damage in Chinese hamster ovary cells. Carcinogenesis. 1992;13:1967–73. doi: 10.1093/carcin/13.11.1967. [DOI] [PubMed] [Google Scholar]
- Lee JH, Nguyen KH, Mishra S, et al. Prohibitin is expressed in pancreatic beta-cells and protects against oxidative and proapoptotic effects of ethanol. FEBS J. 2010;277:488–500. doi: 10.1111/j.1742-4658.2009.07505.x. [DOI] [PubMed] [Google Scholar]
- Li TK, Chen AY, Yu C, et al. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev. 1999;13:1553–60. doi: 10.1101/gad.13.12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Li-Jun Z, Feng-min Z, et al. Tenascin-x facilitates myocardial fibrosis and cardiac remodeling through transforming growth factor beta1 and peroxisome proliferator activated receptor gamma in alcoholic cardiomyopathy. Chin Med J. 2011;124:390–5. [PubMed] [Google Scholar]
- Low RL, Orton S, Friedman DB. A truncated form of DNA topoisomerase IIbeta associates with the mtDNA genome in mammalian mitochondria. Eur J Biochem. 2003;270:4173–86. doi: 10.1046/j.1432-1033.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- Mack AH, Harrington AL, Frances RJ. Clinical Manual for Treatment of Alcoholism and Addictions. Arlington: American Psychiatric Publishing; 2010. [Google Scholar]
- Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96–102. doi: 10.1016/s0168-8278(97)80286-3. [DOI] [PubMed] [Google Scholar]
- Mateos A, Orfao A, Almeida A, et al. Effect of ethanol consumption on adult rat liver mitochondrial populations analyzed by flow cytometry. Alcohol Clin Exp Res. 1995;19:1327–30. doi: 10.1111/j.1530-0277.1995.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Medikayala S, Piteo B, Zhao X, et al. Chronically elevated glucose compromises myocardial mitochondrial DNA integrity by alteration of mitochondrial topoisomerase function. Am J Physiol Cell Physiol. 2011;300:C338–48. doi: 10.1152/ajpcell.00248.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan J, Piano MR, Solaro RJ, et al. Heavy long-term ethanol consumption induces an alpha- to beta-myosin heavy chain isoform transition in rat. Basic Res Cardiol. 1999;94:481–8. doi: 10.1007/s003950050164. [DOI] [PubMed] [Google Scholar]
- NIH. FY 2010 President's Budget Request for NIAAA. 2010. Senate Subcommittee on Labor-HHS-Education Appropriations.
- Pagel PS, Krolikowski JG, Kehl F, et al. The role of mitochondrial and sarcolemmal K(ATP) channels in canine ethanol-induced preconditioning in vivo. Anesth Analg. 2002;94:841–8. doi: 10.1097/00000539-200204000-00012. table of contents. [DOI] [PubMed] [Google Scholar]
- Pello R, Martin MA, Carelli V, et al. Mitochondrial DNA background modulates the assembly kinetics of OXPHOS complexes in a cellular model of mitochondrial disease. Hum Mol Genet. 2008;17:4001–11. doi: 10.1093/hmg/ddn303. [DOI] [PubMed] [Google Scholar]
- Piano MR, Geenen DL, Schwertz DW, et al. Long-term effects of alcohol consumption in male and female rats. Cardiovasc Toxicol. 2007;7:247–54. doi: 10.1007/s12012-007-9002-y. [DOI] [PubMed] [Google Scholar]
- Rafalski K, Abdourahman A, Edwards JG. Early adaptations to training: upregulation of α-myosin heavy chain gene expression. Med Sci Sports Exerc. 2007;39:75–82. doi: 10.1249/01.mss.0000240324.08406.3d. [DOI] [PubMed] [Google Scholar]
- Reers M, Smiley ST, Mottola-Hartshorn C, et al. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–17. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–8. [PubMed] [Google Scholar]
- Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–30. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- Segel LD. The development of alcohol-induced cardiac dysfunction in the rat. Alcohol. 1988;23:391–401. doi: 10.1093/oxfordjournals.alcalc.a044834. [DOI] [PubMed] [Google Scholar]
- Sempos CT, Rehm J, Wu T, et al. Average volume of alcohol consumption and all-cause mortality in African Americans: the NHEFS cohort. Alcohol Clin Exp Res. 2003;27:88–92. doi: 10.1097/01.ALC.0000046597.92232.73. [DOI] [PubMed] [Google Scholar]
- Teragaki M, Takeuchi K, Toda I, et al. Point mutations in mitochondrial DNA of patients with alcoholic cardiomyopathy. Heart Vessels. 2000;15:172–5. doi: 10.1007/pl00007268. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Hansson A, Wredenberg A, et al. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci USA. 2005;102:17993–8. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiplenkova VG, Vikhert AM, Cherpachenko NM. Ultrastructural and histochemical observations in human and experimental alcoholic cardiomyopathy. J Am Coll Cardiol. 1986;8(1 Suppl A):22A–32A. doi: 10.1016/s0735-1097(86)80025-0. [DOI] [PubMed] [Google Scholar]
- Ugalde C, Triepels RH, Coenen MJ, et al. Impaired complex I assembly in a Leigh syndrome patient with a novel missense mutation in the ND6 gene. Ann Neurol. 2003;54:665–9. doi: 10.1002/ana.10734. [DOI] [PubMed] [Google Scholar]
- Urbano-Marquez A, Estruch R, Navarro-Lopez F, et al. The effects of alcoholism on skeletal and cardiac muscle. N Engl J Med. 1989;320:409–15. doi: 10.1056/NEJM198902163200701. [DOI] [PubMed] [Google Scholar]
- Vendemiale G, Grattagliano I, Altomare E, et al. Mitochondrial oxidative damage and myocardial fibrosis in rats chronically intoxicated with moderate doses of ethanol. Toxicol Lett. 2001;123:209–16. doi: 10.1016/s0378-4274(01)00401-5. [DOI] [PubMed] [Google Scholar]
- Wharton D, Tzagoloff A. Assay of cytochrome oxidase. Methods Enzymol. 1967;10:245–7. [Google Scholar]
- Wieland P, Lauterburg BH. Oxidation of mitochondrial proteins and DNA following administration of ethanol. Biochem Biophys Res Commun. 1995;213:815–9. doi: 10.1006/bbrc.1995.2202. [DOI] [PubMed] [Google Scholar]
- Wilke A, Kaiser A, Ferency I, et al. Alcohol and myocarditis. Herz. 1996;21:248–57. [PubMed] [Google Scholar]
- Zhang D, Mott JL, Chang SW, et al. Construction of transgenic mice with tissue-specific acceleration of mitochondrial DNA mutagenesis. Genomics. 2000;69:151–61. doi: 10.1006/geno.2000.6333. [DOI] [PubMed] [Google Scholar]
- Zhang D, Mott JL, Chang SW, et al. Mitochondrial DNA mutations activate programmed cell survival in the mouse heart. Am J Physiol Heart Circ Physiol. 2005;288:H2476–83. doi: 10.1152/ajpheart.00670.2004. [DOI] [PubMed] [Google Scholar]