Abstract
Aims: To assess the effectiveness of brief motivational intervention for alcohol and drug use in young adult primary care patients in a low-income population and country. Methods: A randomized controlled trial in a public-sector clinic in Delft, a township in the Western Cape, South Africa recruited 403 patients who were randomized to either single-session, nurse practitioner-delivered Brief Motivational Intervention plus referral list or usual care plus referral list, and followed up at 3 months. Results: Although rates of at-risk alcohol use and drug use did not differ by treatment arm at follow-up, patients assigned to the Brief Motivational Intervention had significantly reduced scores on ASSIST (Alcohol, Smoking and Substance Involvement Screening Test) for alcohol—the most prevalent substance. Conclusion: Brief Motivational Intervention may be effective at reducing at-risk alcohol use in the short term among low-income young adult primary care patients; additional research is needed to examine long-term outcomes.
INTRODUCTION
Tobacco, alcohol and other drug use is a major contributor to the burden of disease and injury around the world, and alcohol use is a particularly detrimental in low and middle income countries (World Health Organization, 2002). This is certainly true of South Africa. Alcohol has been implicated in just under half of all non-natural deaths (Parry et al., 2005), and several South African communities have the world's highest reported prevalence of fetal alcohol syndrome (Viljoen et al., 2005). Cannabis and methaqualone are the most prevalent drugs in South Africa, and the most frequently identified in drug-related arrests, drug-related psychiatric diagnoses and drug-positive trauma patients (Parry et al., 2002). A dramatic surge in methamphetamine use has also been documented (Parry et al., 2004), with increases highest in young people. Alcohol or other drug misuse are also contributing risk factors in each of the ‘colliding epidemics’ (Mayosi et al., 2012) in South Africa of HIV and tuberculosis (Ward et al., 2005; Kalichman et al., 2006; Avalos et al., 2009), chronic illness and mental health problems (Rehm et al., 2009b), injury and violence (Parry et al., 2005; Rehm et al., 2009b), and maternal, neonatal, and child health (Viljoen et al., 2005). Further, in a prior study, we found that the prevalence of risky alcohol and other drug use among public-sector primary health care clinic patients in South Africa was generally higher in adults aged 18–24 versus older adults (Ward et al., 2008), and that such use was strongly related to increased HIV risk behaviors particularly in those aged 18–24 (Ward et al., 2005). Alcohol and drug use, particularly among young adults, is thus an urgent public health priority in South Africa, and internationally. The World Health Organization has adopted a strategy to reduce the harmful use of alcohol globally, and South Africa's Department of Health has a target of a 20% reduction in per capita consumption by 2020 (Mayosi et al., 2012).
Medical settings such as primary care clinics provide an opportunity to screen for and intervene in at-risk drinking and drug use. Access to specialized alcohol and drug treatment programs in South Africa is quite limited, particularly for those who do not have more severe problems (Myers et al., 2010). In this context the public health sector is the main provider of health care and its primary health care clinics are broadly dispersed and located in lower income communities (Health Systems Trust, 2004). Thus, providing screening and intervention for substance use in existing health care settings available in these communities is one of several existing avenues to increase access to services (Weisner and Schmidt, 2001) despite potential challenges of limited time and competing priorities in these settings. In many countries, researchers are emphasizing primary care settings as optimal for brief interventions for substance use (Bertholet et al., 2005). There is also a high prevalence of alcohol and drug misuse among young adults in public-sector primary care clinics in South Africa. In a study of patients in 12 public-sector primary health care clinics in the Western Cape, South Africa, we found a prevalence of at-risk alcohol and drug use of 17 and 8%, respectively, in young adults (aged 18–24 years) (Ward et al., 2008); higher than those in older adults. Similar to findings globally, we also found an increased risk of HIV-risk behaviors for primary care patients with at-risk alcohol and drug use (Avalos et al., 2009), particularly in young adults aged 18–24 (Ward et al., 2005). Those with at-risk drinking or drug use also had high comorbidity, including TB, Hepatitis A and B, and depression and anxiety (Mertens et al., 2009). Alcohol use in South Africa is estimated to be responsible for 939,000 disability-adjusted life-years lost due to TB and HIV/AIDS alone, and is 4–6% of the overall disease burden (Peltzer et al., 2011). Its impact on the burden of disease is largest in low-income countries with relatively high consumption, such as in southern Africa. In some countries, such as South Africa or Nigeria, infectious diseases make up ∼50% of the overall alcohol-attributable disease burden (Rehm et al., 2009a).
Screening and brief interventions are effective for reducing alcohol consumption in primary care patients in high-income countries (Kaner et al., 2007), including in young adults (Grossberg et al., 2004; Jonas et al., 2012). One study found brief interventions effective for those with higher severity alcohol problems in a South African college student population with relatively high socioeconomic status (Pengpid et al., 2013). One randomized study (not limited to young adults) of patients in Cape Town South Africa clinics for sexually transmitted infections found that a behavioral skills intervention was effective in reducing alcohol use in sexual contexts (Kalichman et al., 2007). A multi-country study (both high and low income) of brief intervention for illicit drug use found effectiveness in countries other than the USA but did not focus on young adults, and a large majority of patients in the low-income countries were employed (Humeniuk et al., 2012). Randomized effectiveness studies of brief motivational intervention for either alcohol or drugs are lacking on young adults in public-sector primary care clinics with severely economically disadvantaged patients in either low or high income countries. Because primary care providers in these overwhelmed clinics have significant time constraints (Koopman et al., 2008), alternative delivery models are important to examine. Babor and colleagues found that non-physician providers are effective at delivering brief interventions for alcohol use (Babor et al., 2006) in privately insured US adults. In this study, we examined screening and a brief motivational intervention among young adult (aged 18–24) patients in a public-sector primary health care clinic with high unemployment rates (representative of a majority of South Africa public-sector primary care patients). We randomized patients who screened positive to heavy alcohol or illicit and non-medical drug use (hereafter referred to as ‘drug use’) to a nurse-delivered Brief Motivational Intervention plus a resource list for drinking and drug use problems, or to usual care plus the resource list (minimally enhanced usual care) (Freedland et al., 2011), and examined alcohol and drug use at 3 months. Because of the increasing use of nurse practitioners to deliver primary care in South Africa public-sector health care on a broad scale (Western Cape Department of Health, 2011), employing nurse practitioners for the intervention delivery would have greater potential for implementation, if found effective, than physician-delivered intervention. We hypothesized that patients randomized to Brief Motivational Intervention would have better alcohol and drug use outcomes including lower risky use rates and greater reductions in ASSIST scores, a measure of use and problems that was developed and validated in primary care clinics including within Southern Africa (WHO ASSIST Working Group, 2002).
METHODS
Setting
The setting is a large public-sector primary health care clinic, one of 51 in Cape Town, South Africa. The clinic, in the township of Delft, a peri-urban community on the outskirts of Cape Town, was chosen because it is large and its patient population represents approximately equal numbers of the two populations served by these clinics as a whole—black and mixed-ancestry. It is representative of other clinics in the system with regard to high poverty and unemployment rates, high alcohol and drug use prevalence (Ward et al., 2007), and a lack of screening or specific services for alcohol and drugs.
Sample and recruitment
Patients aged 18–24 who visited the clinic between 18 March and 28 November 2008 (N = 2047) were screened by nurse practitioners with one question each for alcohol and drugs. There is a lack of evidence on screening tools in young adults (Jonas et al. 2012; Patton et al., 2014). Based on this and the time pressures of conducting screening in primary care settings we used adaptations of single question screening instruments. We used an adaptation of the single item alcohol screening question (Smith et al., 2009): ‘In the past year, how many times have you had 3 or more drinks on one occasion?’ (for women), or ‘In the past year, how many times have you had 5 or more drinks on one occasion?’ (for men). We adapted the question to ask about ‘3 or more drinks’ for women rather than the standard ‘4 or more’ because rates of non-use of condoms and unplanned pregnancies in South Africa public-sector primary care patients are high (Avalos et al., 2009) and thus intervention at this lower threshold may prevent possible in utero alcohol exposure in a setting with a very high prevalence of fetal alcohol syndrome (Chersich et al., 2012; May et al., 2013). To screen for drug use, we adapted the single question drug screener and asked, and ‘In the past three months, how many times have you used drugs? When I talk about a drug here, I don't mean cigarettes or snuff. I mean drugs like dagga, mandrax, tik, cocaine, heroin, LSD, or sleeping pills or other medication that you have used in a way that it was not prescribed, or not prescribed for you.’ (‘Dagga’ is the South African term for cannabis, ‘mandrax’ for methaqualone and ‘tik’ for methamphetamines—prevalent drugs in South Africa). We have found these questions to be highly sensitive and specific for identifying alcohol misuse as measured by the AUDIT (83% sensitive and 93% specific), and risky drug use as measured by the ASSIST (92% sensitive and 99% specific) in Western Cape public-sector primary care clinic patients aged 18–24 (Mertens et al., 2008).
Those screening positive were assessed by research assistants for other inclusion criteria. Of 2047 assessed, 1478 did not meet inclusion, that is they screened negative (did not answer one or more times to the alcohol question or three or more times to the drug question) (N = 1370), were too ill to participate (N = 22), or had no phone (N = 86) and therefore could not be followed. Research assistants asked those meeting inclusion criteria to be recruited into the study and give informed consent. Those who refused were given an information sheet referring them to services in the Western Cape for alcohol and drug issues—the Cape Town Drug Counseling Centre and SANCA (the South African National Council on Alcoholism). In addition to home address and cellphone (cellphone use is common in South Africa (Kujawski, 2012), including among South Africa's poor (Research ICT Africa and Intelecon, 2012)), participants provided three friend or family contact names and contact information. They also received a business card with study contact information and follow-up interview date.
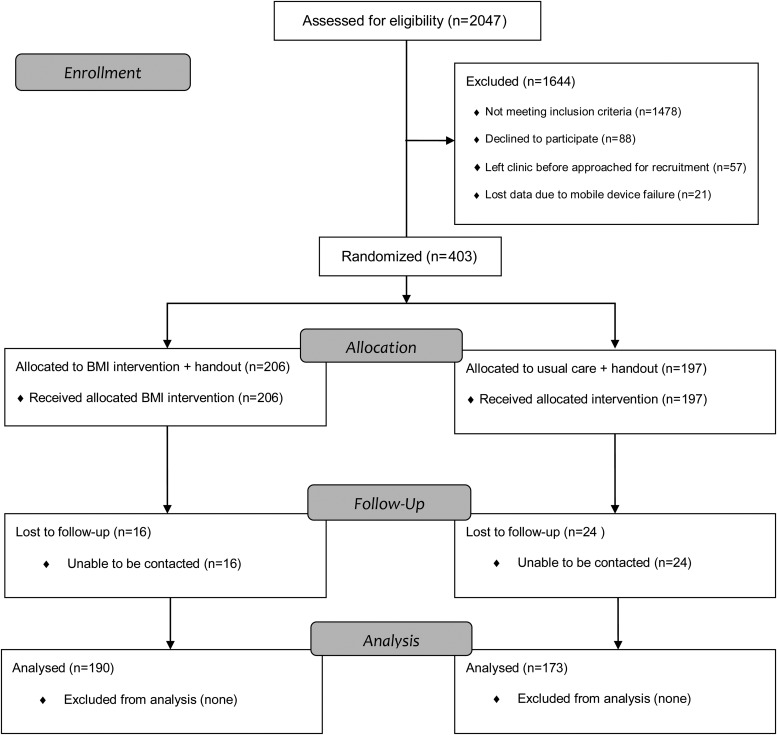
The study recruited 403 patients and implemented the following protocol: patients were interviewed by a research assistant about their demographics, alcohol and drug use and problems, and readiness to change alcohol and drug use (see Measures below). Following the interview, research assistants opened a sealed envelope which contained the randomization result for the patient assigning them to either nurse-practitioner-delivered Brief Motivational Intervention plus a referral resource list for drinking and drug use (Intervention) or minimally enhanced usual care (mEUC) plus resource list. Those assigned to the Intervention were then taken back to the nurse practitioner for Brief Motivational Intervention. Of 403 patients, 206 (51%) were randomized to Intervention and 197 (49%) to mEUC. See Fig. 1 for CONSORT chart.
Fig. 1.
CONSORT Flowchart.
The study was approved by the institutional review boards of the University of Cape Town, the University of California, San Francisco, the Kaiser Foundation Research Institute, and the Department of Health, Provincial Government of the Western Cape, South Africa.
Trial design
This was a single-blinded, parallel-group randomized controlled trial. Research interviewers conducting follow-up were blinded to randomization status.
Intervention
Primary Care Nurse Practitioners were given a 3-day training in Brief Motivational Intervention for alcohol and drug misuse at the Cape Town Drug Counseling Center (CTDCC). The training manual was Rollnick's Health Behavior Change: A Guide for Practitioners (Rollnick et al., 1999). The trainer was an experienced practitioner and trainer. The training was followed by regular supervision meetings (weekly for 6 weeks and monthly thereafter), during which Nurse Practitioners listened to recordings of their interventions with their trainer, who then provided feedback and worked with them to maintain fidelity to the Brief Motivational Interviewing model. The trainer also scored a random selection of these tapes according to the Behavior Change Counseling Index (BECCI), an instrument valid for training in BMI. This ensured the fidelity of the intervention to the brief motivational interviewing model Average intervention length was 10 min.
Follow-up
Fieldworkers contacted patients by phone or in-person to schedule 3-month follow-up interviews. Patients unreachable by phone were located by contacting the three contacts they provided at enrollment. Interviews were conducted in person at the clinic or in the patient's home, according to patient preference. Ninety percent (n = 363) completed the 3-month follow-up. In appreciation, the study offered interviewed patients a voucher for cellphone air-time or for a local supermarket, worth ZAR50 (approximately USD6).
Measures
We developed the questionnaires in English, then translated them into Afrikaans and isiXhosa and checked the quality by back-translation. During a prior pilot study, the wording of many questionnaire measures was modified for cultural relevance (see below) (Ward et al., 2005). All data were gathered by interviewers who conducted interviews using the questionnaires.
Questions on age, race, gender, marital status, education, employment, religion, spoken language, number of children and frequency of participation in religious activity were asked at baseline. From the South African census we used socioeconomic status measures on relative deprivation in urban areas (McIntyre et al., 2002), access to piped water, access to electricity at home, living in formal housing rather than a shack or traditional dwelling, and employment status of the head of household.
Alcohol and drug use
We used the WHO ASSIST (Alcohol, Smoking and Substance Involvement Screening Test) V3 (WHO ASSIST Working Group, 2002) to assess alcohol and drug use which includes cannabis, methaqualone (added to the questionnaire, as ‘mandrax’ is one of the most used drugs in South Africa), cocaine, methamphetamines, inhalants, sedatives, hallucinogens, opiates and ‘other drugs’. We used this instrument for assessment because it was designed for medical settings, and because it was developed and validated in primary care public clinics in high-, middle- and low-income countries including in Southern Africa (WHO ASSIST Working Group, 2002).
The ASSIST includes six questions for each past 3-month substance reported. For each substance, a score of 0–20 is calculated based, which is further categorized into: low- (including no use ever) and medium/high-risk use. Low risk (no intervention needed) for alcohol is 0–10, and for a drug is 0–3. Medium to high risk (intervention or treatment needed) for alcohol is 11 or more, for each drug is 4 or more. For drugs, any past 3-month use or problems results in a score of 4 or higher. Consistent with the ASSIST terminology we define ‘at-risk’ use as scores indicating medium to high risk on the ASSIST instrument.
Heavy drinking was defined as three or more drinks on one occasion (women) or six or more drinks on one occasion (men). We used three or more drinks rather than the standard four or more drinks used for WHO and CDC and NIH definitions of binge drinking because the rate of unplanned pregnancies in South Africa public-sector primary care patients is relatively high and thus intervention at this lower threshold may prevent possible alcohol in utero exposure in a setting with a high prevalence of fetal alcohol syndrome (May et al., 2013). A standard drink in South Africa is 12 g of ethanol.
Readiness to change alcohol and drug use was measured by an adapted version of the SOCRATES V8, (Miller and Tonigan, 1996) which is comprised of 19 Likert-scale questions each for alcohol and drugs. Because the study site was a busy primary care clinic, we collapsed the ‘yes, strongly agree’ and ‘yes, agree’ into one ‘yes’ category, and the ‘no, strongly disagree’ and ‘no strongly disagreed’ into one ‘no’ category. The ‘Recognition’ scale had a score range of 7–21, higher scores indicating greater recognition of use problems. The ‘Ambivalence’ scale had a score range of 4–12, higher scores indicating more openness to contemplation about problem use. The ‘Taking Steps’ scale had a score range of 8–24, higher scores indicating active engagement in efforts to change use.
Analyses
We used SAS to conduct all quantitative analyses. To examine differences by study arm, we used chi-square tests and Fisher's Exact Tests (where noted and due to very small cell sizes) for categorical variables, and t-tests for continuous variables. We compared changes in ASSIST scores from baseline to follow-up using two-way repeated-measures analysis of variance (ANOVA) and controlling for gender, race, religion and employment status. In order to ensure that baseline differences in ASSIST scores did not explain or affect the results of this comparison, we re-ran the models using a repeated measures mixed-models framework to examine the effects of the explanatory variables on the participants' change in ASSIST scores, while allowing the initial status (i.e. intercept) to vary for every subject. We fitted linear mixed-effects models to examine changes in ASSIST scores between the two groups (Intervention versus mEUC) over time (Hedeker and Gibbons, 2006); results did not differ from the multivariate ANOVA models. We compared the proportion of at-risk alcohol or drug use between the two study arms within the 3-month follow-up sample using chi-square tests and logistic regression analyses. Due to zero or small prevalence of methaqualone and sedative use at follow-up, we were unable to use logistic regression models to examine the relationship of study arm to use of these drugs; we do include these in the bivariate, chi-square analyses. As a post hoc analysis, we also re-ran analyses of significant main effects for women and men separately.
RESULTS
Participant characteristics
Table 1 presents baseline characteristics by treatment arm for the follow-up sample (n = 363). The sample had a mean age of 21 years, and was 48% male, 49% black and 51% mixed-race, with 8% having an education of grade 6 or less, 79% some high school, and 13% having completed high school or some training after school. Seventy-four percent were unemployed, 12% had no piped water in their house, 16% no piped water on site, 6% no electricity at home, 20% lived in a shack or traditional dwelling and 27% lived in a household with an unemployed head of household. Comparisons by treatment arm found only one item—religion—was statistically significantly different.
Table 1.
Demographic characteristics by treatment arms for the follow-up sample (N = 363)
| BMI (n = 190) | Usual care (n = 173) | P-value | |
|---|---|---|---|
| Age in years (%) | |||
| 18 | 14 | 12 | 0.19 |
| 19 | 15 | 11 | |
| 20 | 13 | 13 | |
| 21 | 18 | 10 | |
| 22 | 11 | 16 | |
| 23 | 12 | 16 | |
| 24 | 18 | 21 | |
| Gender (%) | |||
| Male | 44 | 53 | 0.07 |
| Female | 56 | 47 | |
| Race/ethnicity (%) | |||
| Black | 48 | 50 | 0.81 |
| Coloured (mixed-race) | 52 | 50 | |
| Education (%) | |||
| Grade 6 or less | 6 | 9 | 0.17 |
| Some high school | 83 | 75 | |
| Completed high school/Some training after school | 11 | 16 | |
| Employment status (%) | |||
| Full-time/Part-Time | 45 | 55 | 0.09 |
| Student/Homemaker | 25 | 24 | |
| Unemployed | 30 | 21 | |
| Marital status (%) | |||
| Married/Lived as married | 4 | 6 | 0.36 |
| Widowed/separated/divorced | 0 | 0 | |
| Never married/lived as married | 96 | 94 | |
| Social economic status (%) | |||
| No piped water in house | 9 | 16 | 0.05 |
| No piped water on site | 18 | 15 | 0.80 |
| No electricity at home | 5 | 6 | 0.83 |
| Lives in shack or traditional dwelling | 18 | 23 | 0.33 |
| Head of household unemployed | 33 | 34 | 0.74 |
| Religion (%) | |||
| Christian | 94 | 89 | 0.02 |
| Muslim | 3 | 3 | |
| African Traditional | 1 | 7 | |
| Other/None | 2 | 1 | |
| Religious activities (%) | |||
| Never/seldom | 77 | 69 | 0.12 |
| More often | 23 | 31 | |
| Have children (%): | 34 | 35 | 0.75 |
| Language spoken at home (%) | |||
| English | 33 | 43 | 0.06 |
| Africans | 52 | 51 | 0.89 |
| IsiXhosa | 51 | 51 | 0.95 |
| Prevalence of at-risk use (WHO ASSIST) (%) | |||
| Alcohol | 54 | 49 | 0.28 |
| Cannabis | 22 | 19 | 0.41 |
| Methamphetamine | 7 | 12 | 0.11 |
| Methaqualone | 2 | 3 | 0.53a |
| Sedatives | 2 | 0 | 0.06a |
| Baseline ASSIST scores (STD) | |||
| Total | 20.45 (18.02) | 18.81 (15.94) | 0.36 |
| Alcohol | 13.61 (10.28) | 12.03 (10.32) | 0.14 |
| Cannabis | 4.13 (8.80) | 3.60 (8.18) | 0.56 |
| Methamphetamines | 1.88 (7.38) | 2.67 (8.10) | 0.33 |
| Methaqualone | 0.42 (2.90) | 0.54 (3.12) | 0.71 |
| Sedatives | 0.41 (2.71) | 0 (0) | 0.05 |
| Baseline SOCRATES raw score (STD) | |||
| Alcohol Recognition score | 13.12 (4.24) | 12.64 (3.93) | 0.31 |
| Alcohol Ambivalence score | 8.72 (2.73) | 8.85 (2.77) | 0.69 |
| Alcohol Taking Steps score | 18.92 (5.19) | 18.85 (5.04) | 0.89 |
| Drug Recognition score | 16.04 (4.33) | 16 (4.89) | 0.97 |
| Drug Ambivalence score | 9.57 (2.51) | 9.53 (2.57) | 0.94 |
| Drug Taking Steps score | 19.02 (4.89) | 19.84 (5.08) | 0.44 |
Note: Bold P-values indicate differences that are statistically significant at P < 0.05.
aFisher's Exact Test P-value.
Loss to follow-up
We successfully followed 90% of the sample. Among the Intervention arm, compared with patients followed up (92%), those not followed had a higher percentage of Christian religious identity (94 versus 81%, respectively), were less likely to have methamphetamine use at baseline and had higher baseline cannabis ASSIST scores than those not followed up. For the mEUC group, compared with patients followed up (88%) those not followed had higher rates of employment and student/homemaker status and lower rates of unemployment. All analyses were replicated controlling for baseline variables on which the non-respondents differed; results remained consistent.
Treatment outcomes
Table 2 presents least square mean baseline and follow-up ASSIST scores by study arm for total substance use, alcohol, cannabis, and methamphetamines and repeated measures ANOVA results, controlling for potential confounders (i.e. gender, race, religion and employment status). P-values are shown for the group by time interactions. (Differences in ASSIST scores between Tables 1 and 2 are due to Table 2 presenting least square means). Results suggested larger baseline to follow-up reductions in ASSIST scores for the Intervention than the mEUC arm for alcohol, which was the most prevalent substance used. Reductions in alcohol ASSIST scores were 38% in the Intervention arm versus 21% in the mEUC arm. In order to examine whether the significant results were due to baseline differences by treatment arm in the ASSIST scores, we replicated these models using repeated measures mixed-models framework, allowing us to examine the effects of the explanatory variables on the participants' change in assist scores, while allowing the initial status (i.e. intercept) to vary for every subject. The pattern of significant results did not change.
Table 2.
Mean ASSIST scores at baseline and follow-up by treatment arm using two-way repeated-measures analysis of variance (ANOVA)a
| Mean baseline scoreb | Mean follow-up scoreb | Mean effect size (% decrease) | Interaction effectc, P | |
|---|---|---|---|---|
| Total ASSIST Score | ||||
| BMI | 21.9 | 13.7 | 37.6 | F = 3.06, P = 0.081 |
| Usual care | 20.3 | 15.1 | 25.5 | |
| Alcohol ASSIST Score | ||||
| BMI | 13.0 | 8.0 | 38.3 | F = 4.79, P = 0.0293 |
| Usual care | 11.5 | 9.1 | 20.9 | |
| Cannabis ASSIST Score | ||||
| BMI | 6.4 | 4.6 | 28.3 | F = 2.54, P = 0.1119 |
| Usual care | 5.7 | 5.2 | 9.8 | |
| Methamphetamine ASSIST Score | ||||
| BMI | 1.5 | 0.7 | 57.2 | F = 1.47, P = 0.2264 |
| Usual care | 2.4 | 0.6 | 76.9 | |
Note: Bold P-values indicate differences that are statistically significant at P < 0.05.
aModels control for gender, race, religion and employment status.
bLeast square mean.
cInteraction of time and treatment arm in predicting a substance involvement score.
Table 3 presents the prevalence of at-risk use of substances (excluding tobacco), alcohol, cannabis, methamphetamines, sedatives and methaqualone at 3-month follow-up by study arm; there were no statistically significant differences. Table 4 presents logistic regression models with at-risk use for each substance as the criterion variables, with treatment arm and potential confounders (i.e. gender, race, religion, employment status and baseline ASSIST score for the relevant substance) as covariates. Odds ratios were in the expected direction for all models except for methamphetamines, but bivariate and logistic regression models did not suggest statistically significant differences between arms in prevalence of at-risk use at follow-up.
Table 3.
Prevalence of at-risk alcohol use, drug use and heavy drinking at 3-month follow-up by study arm
| Study arm |
P-value | ||
|---|---|---|---|
| BMI (%) (n = 190) | Usual care (%) (n = 173) | ||
| Prevalence of at-risk use | |||
| Alcohol use | 33 | 32 | 0.96 |
| Cannabis use | 12 | 14 | 0.62 |
| Methamphetamine use | 5 | 4 | 0.75 |
| Sedative use | 0 | 0 | – |
| Methaqualone use | 1 | 1 | 1.00a |
| Heavy Drinking | 51 | 55 | 0.34 |
aFisher's exact test P-value.
Table 4.
Logistic regression models predicting at-risk alcohol use, cannabis use, methamphetamine use and heavy drinking by treatment arm
| OR for BMI versus usual care | 95% CI | |
|---|---|---|
| At-risk alcohol use | 0.93 | 0.58–1.51 |
| At-risk cannabis use | 0.72 | 0.33–1.59 |
| At-risk methamphetamine use | 1.20 | 0.39–3.65 |
| Heavy drinking | 0.71 | 0.44–1.12 |
Note: Models controlled for gender, race, religion, employment status and baseline ASSIST score for the relevant substance.
As a post hoc analysis, we examined whether the main effects we found on Alcohol ASSIST scores were true for women and men. Although the study was not powered to examine this question, we examined whether the pattern of results was the same for each gender as an exploratory analysis. We found that the treatment effect was more pronounced for women (mean effect size of 59% reduction in Alcohol ASSIST scores for the Intervention arm versus 38% for the mEUC arm; F = 3.20, P = 0.0752) than for men (mean effect size of 31% for the Intervention arm versus 19% for the mEUC arm; F = 1.31, P = 0.2549) (Table 5).
Table 5.
Least Square Mean Alcohol ASSIST scores at baseline and follow-up by treatment arm using two-way repeated-measures analysis of variance (ANOVA)a
| Mean baseline scoreb | Mean follow-up scoreb | Mean effect size (% decrease) | Interaction effectc, P | |
|---|---|---|---|---|
| Women (N = 188) | ||||
| BMI | 11.5 | 4.7 | 59.1 | F = 3.20, P = 0.0752 |
| Usual care | 10.3 | 7.0 | 38.1 | |
| Men (N = 175) | ||||
| BMI | 13.1 | 9.1 | 30.5 | F = 1.31, P = 0.2549 |
| Usual care | 10.4 | 8.4 | 19.2 | |
aModels control for race, religion and employment status.
bLeast square mean.
cInteraction of time and treatment arm in predicting alcohol involvement score.
DISCUSSION
This trial examined a single-session brief motivational intervention delivered in public-sector primary care clinics among young adults who screened positive for either binge drinking (5+ for men and 3+ for women) or drug use. Use of alcohol was most common followed by cannabis and methamphetamines—very few patients in the sample had used other drugs (<3%).
Effectiveness
Although we did not find a significant effect of Brief Motivational Intervention on at-risk use of alcohol or marijuana or methamphetamine use at 3-month follow-up, the reductions in ASSIST alcohol involvement scores were significantly larger for the Intervention (effect size for reduction 38%) than mEUC (effect size for reduction 21%). There were not significant reductions in Total ASSIST substance involvement scores or ASSIST scores for cannabis or methamphetamines. Of note, the Least Square mean Alcohol Assist scores were reduced to scores within low risk levels in both arms (8 for Intervention and 9.1 for mEUC) at follow-up; this was not the case for cannabis risk scores which did not differ from baseline.
It is notable that, though the reductions in alcohol ASSIST scores were significantly larger in the Intervention arm, prevalence of at-risk alcohol use and drug use at follow-up did not differ across arm and that reductions in the mEUC arm in both at-risk use and ASSIST scores were significant. This is similar to findings in the broader combined adolescent and young adult literature which finds that brief clinical interventions are most effective for ‘harm reduction’ outcomes, such as reductions in use (Wachtel and Staniford, 2010). In the current study there are several possible explanations for these findings. One likely possibility is that a single-session intervention is not a sufficient ‘dose’ to effect strong and lasting changes. This may be particularly true in economically disadvantaged young adults who have severe economic and life contexts as the patients in the study setting here—there are high rates of unemployment, and two in five lived in shacks or traditional dwellings. Such stressful life circumstances may demand a more intensive or comprehensive intervention. Another possibility frequently mentioned in the SBI literature is assessment or subject reactivity (Walters et al., 2009). The study baseline assessment, which participants in both arms underwent, averaged ∼30 min and included the SOCRATES (Miller and Tonigan, 1996) instrument which is comprised of 19 questions each about readiness to change drinking and drug use, and the full ASSIST, which asks about use and consequences of substance use for eight substances. Further, in attempts to contact and locate patients in both arms for follow-up interviews, patients in both arms were often phoned multiple times or attempts were made to visit them at their homes. The resource list handed out to both arms was a colorful brochure which included the local study name ‘Project MISAY’. This attention may have resulted in subject reactivity that improved outcomes in both study arms. The result may be that participants minimize their substance use in subsequent reporting of outcomes. There is also some evidence that improvement in outcomes as a result of assessment reactivity may be due to participants trying additional risk reduction behaviors (Walters et al., 2009). An alternative explanation is that the reductions found in both groups may be in part a result of regression to the mean (Cunningham, 2006).
This study adds to the very limited literature on the effectiveness of brief interventions for young adults in primary care. Although there is a developing literature on brief intervention among adolescents (Wachtel and Staniford, 2010), fewer studies have specifically focused on young adults who differ from adolescents developmentally in several aspects including their transition into having increasing independence and responsibilities for employment and, for some, child care, as well as transitioning into the legal age of drinking (Chun and Linakis, 2012). Most research on brief interventions for alcohol and drug use in young adults in health care settings has been limited to college student populations which differ greatly in socioeconomic status and life context than the population studied here. However, studies of college students receiving interventions generally have found that brief interventions which use either skills-based or motivational interviewing interventions are effective (Larimer and Cronce, 2007). More specifically, studies of college students receiving interventions in college health care settings have found that single-session brief interventions are effective for reducing alcohol consumption at short-term follow-up (primarily 3 months or fewer). Thus, the current study broadens findings on the short-term effectiveness on improved substance use outcomes of single-session brief intervention to a socioeconomically disadvantaged primary care population. We are not aware of other studies examining this question in young adults beyond the college student population.
The current study had several limitations. As mentioned, the initial assessment was long and we have no minimal assessment group to examine effects of assessment reactivity. This can result in decreases in reported substance use and problems in both the intervention and control groups (Walters et al., 2009). Information used for the analyses here, however, would not have been available in other ways. Medical records at the clinic are very minimal and do not include any of the information we assessed for the study, including socioeconomic status or other background information (other than age and sex), alcohol and drug use and readiness to change alcohol and drug use. Obtaining the information via a written questionnaire would have limited our sample to those with moderate literacy so that it would not have been representative of individuals with low grade levels of reading and writing (low literacy is common in South Africa). In order to collect these assessments and maintain a more representative sample, an interview was necessary. Because this was an exploratory (though randomized) study we included only a 3-month follow-up; it is unknown whether the better alcohol outcomes for the Intervention group were sustained for a longer duration. It is possible that the advantage for the Intervention group may fade when examined at longer follow-up periods, and there is evidence from the screening and brief intervention literature that the effects of brief interventions diminish over time-frames longer than 3-months (Vasilaki et al., 2006). Important strengths of the study are that interventions were delivered by nurse practitioners who delivered the patient exam rather than by research staff, and that we focused on young adults in an understudied population—severely economically disadvantaged individuals.
Future research is needed on moderators of intervention effectiveness, including by gender, severity, and life context. Post hoc analyses by gender suggest a greater effect in women than men, but given power constraints of the study we caution against generalization of these findings. The study was not powered to examine whether findings differ by gender, SES, or when excluding high substance problem severity. Given that a reduction in at-risk use of substances was found in both arms, we also suggest that future studies use minimal baseline assessments or add minimal assessment control groups. Another consideration for future research in South Africa and other settings where nurses are in high demand is the difficulty in retaining nurses. This may suggest that alternative provider types—perhaps peer counselors—be considered as a more feasible alternative, though effectiveness of intervention delivered by peer counselors in this population needs study. Yet, in this nurse-based service, it is useful to know whether the providers who most commonly treat primary care patients are effective at delivering interventions, which this study suggests is true with regard to short-term outcomes.
Funding
The study was funded by the National Institute on Drug Abuse R21DA022557.
Conflict of interest statement
None declared.
Acknowledgements
We dedicate this manuscript with gratitude and sorrow to Dr Alan Flisher, whose untimely death prevented him from contributing as an author. This study could not have been accomplished without his leadership, wisdom and scientific contributions. We thank the patients and staff of the Metro District Health Services in Cape Town. We are grateful to the research assistants, Lesley Arendse, Wendy de Booy, Vuyisa Dumile, Nokuthula Kulati, and Rodney Stoffberg, and project coordinator Lynn Hendricks for patient recruitment, participant tracking and follow-up interviewing and coordination. We thank the nurse practitioners Sr Yvette MacDonald, Sr Winifred Mgudlwa, and Sr Zoliswa Ngqiniso. We also thank the University of Cape Town's University Research Committee for their support. We thank Agatha Hinman for editorial assistance with the manuscript.
REFERENCES
- Avalos LA, Mertens J, Ward CL, et al. Stressors, substance use and sexual risk behaviors among primary care patients in Cape Town, South Africa. AIDS Behav. 2009;14:359–70. doi: 10.1007/s10461-009-9525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Dauser D, et al. Brief interventions for at-risk drinking: patient outcomes and cost-effectiveness in managed care organizations. Alcohol Alcohol. 2006;41:624–31. doi: 10.1093/alcalc/agl078. [DOI] [PubMed] [Google Scholar]
- Bertholet N, Daeppen JB, Wietlisbach V, et al. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165:986–95. doi: 10.1001/archinte.165.9.986. [DOI] [PubMed] [Google Scholar]
- Chersich MF, Urban M, Olivier L, et al. Universal prevention is associated with lower prevalence of fetal alcohol spectrum disorders in Northern Cape, South Africa: a multicentre before–after study. Alcohol Alcohol. 2012;47:67–74. doi: 10.1093/alcalc/agr145. [DOI] [PubMed] [Google Scholar]
- Chun TH, Linakis JG. Interventions for adolescent alcohol use. Curr Opin Pediatr. 2012;24:238–42. doi: 10.1097/MOP.0b013e32834faa83. [DOI] [PubMed] [Google Scholar]
- Cunningham JA. Regression to the mean: what does it mean? Alcohol Alcohol. 2006;41:580. doi: 10.1093/alcalc/agl039. [DOI] [PubMed] [Google Scholar]
- D'Amico EJ, Hunter SB, Miles JN, et al. A randomized controlled trial of a group motivational interviewing intervention for adolescents with a first time alcohol or drug offense. J Subst Abuse Treat. 2013;45:400–8. doi: 10.1016/j.jsat.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland KE, Mohr DC, Davidson KW, et al. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosom Med. 2011;73:323–35. doi: 10.1097/PSY.0b013e318218e1fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg PM, Brown DD, Fleming MF. Brief physician advice for high-risk drinking among young adults. Ann Fam Med. 2004;2:474–80. doi: 10.1370/afm.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J., editor. Health Systems Trust. The National Primary Health Care Facilities Survey 2003. Durban: Health Systems Trust and Department of Health; 2004. [Google Scholar]
- Hedeker D, Gibbons R. Longitudinal Data Analysis. Hoboken, NJ: John Wiley and Sons; 2006. [Google Scholar]
- Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957–66. doi: 10.1111/j.1360-0443.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Garbutt JC, Brown JM, et al. Screening, behavioral counseling, and referral in primary care to reduce alcohol misuse. 2012. July 10 Comparative Effectiveness Review No. 64. Available at http://www.effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=1135. 19 December 2013, date last accessed. [PubMed]
- Kalichman SC, Simbayi LC, Jooste S, et al. Sensation seeking, alcohol use, and sexual behaviors among sexually transmitted infection clinic patients in Cape Town, South Africa. Psychol Addict Behav. 2006;20:298–304. doi: 10.1037/0893-164X.20.3.298. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Vermaak R, et al. HIV/AIDS risk reduction counseling for alcohol using sexually transmitted infections clinic patients in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2007;44:594–600. doi: 10.1097/QAI.0b013e3180415e07. [DOI] [PubMed] [Google Scholar]
- Kaner E, Beyer F, Dickinson H, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004148.pub3. CD004148. [DOI] [PubMed] [Google Scholar]
- Koopman FA, Parry CD, Myers B, et al. Addressing alcohol problems in primary care settings: a study of general medical practitioners in Cape Town, South Africa. Scand J Public Health. 2008;36:298–302. doi: 10.1177/1403494808086914. [DOI] [PubMed] [Google Scholar]
- Kujawski M. 2012. Finally some 2012 Statistics for the African Mobile Phone Market. Public Sector Marketing 2.0 Available at http://www.mikekujawski.ca/2012/05/30/finally-some-2012-statistics-for-the-african-mobile-phone-market/ 19 December 2013, date last accessed.
- Larimer ME, Cronce JM. Identification, prevention, and treatment revisited: individual-focused college drinking prevention strategies 1999–2006. Addict Behav. 2007;32:2439–68. doi: 10.1016/j.addbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37:818–30. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayosi BM, Lawn JE, van Niekerk A, et al. Health in South Africa: changes and challenges since 2009. Lancet. 2012;380:2029–43. doi: 10.1016/S0140-6736(12)61814-5. [DOI] [PubMed] [Google Scholar]
- McIntyre D, Muirhead D, Gilson L. Geographic patterns of deprivation in South Africa: Informing health equity analyses and public resource allocation strategies. Health Policy Plan. 2002;17(Suppl):30–9. doi: 10.1093/heapol/17.suppl_1.30. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Ward CL, Flisher AJ, et al. Development of a brief alcohol and drug screener in public sector Cape Town primary health care clinics. 10th annual meeting of the International Society of Addiction Medicine; Cape Town, South Africa. 2008. [Google Scholar]
- Mertens JR, Flisher AJ, Ward CL, et al. Medical conditions of hazardous drinkers and drug users in primary care clinics in Cape Town, South Africa. J Drug Issues. 2009;39:889–1014. doi: 10.1177/002204260903900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: the Stages of Change Readiness and Treatment Eagerness (SOCRATES) Psychol Addict Behav. 1996;10:81–9. [Google Scholar]
- Myers BJ, Louw J, Pasche SC. Inequitable access to substance abuse treatment services in Cape Town, South Africa. Subst Abuse Treat Prev Policy. 2010;5:28. doi: 10.1186/1747-597X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry CD, Bhana A, Pluddemann A, et al. The South African Community Epidemiology Network on Drug Use (SACENDU): description, findings (1997–99) and policy implications. Addiction. 2002;97:969–76. doi: 10.1046/j.1360-0443.2002.00145.x. [DOI] [PubMed] [Google Scholar]
- Parry CD, Myers B, Pluddemann A. Drug policy for methamphetamine use urgently needed. S Afr Med J. 2004;94:964–5. [PubMed] [Google Scholar]
- Parry CD, Pluddemann A, Steyn K, et al. Alcohol use in South Africa: findings from the first Demographic and Health Survey (1998) J Stud Alcohol. 2005;66:91–7. doi: 10.15288/jsa.2005.66.91. [DOI] [PubMed] [Google Scholar]
- Patton R, Deluca P, Kaner E, et al. Alcohol Screening and Brief Intervention for Adolescents: The How, What and Where of Reducing Alcohol Consumption and Related Harm Among Young People. Alcohol Alcohol. 2014;49:207–12. doi: 10.1093/alcalc/agt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauw I. (Nov 28, 2011) Tik: Is your child at risk? health24. Available at http://www.health24.com/Mental-Health/Tik-is-your-child-at-risk-20120721. (19 December 2013, date last accessed)
- Peltzer KK, Naidoo PP, Matseke GG, et al. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary care clinics in South Africa: a cluster randomized controlled trial protocol. BMC Public Health. 2011;11:394. doi: 10.1186/1471-2458-11-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengpid S, Peltzer K, van der Heever H, et al. Screening and brief interventions for hazardous and harmful alcohol use among university students in South Africa: results from a randomized controlled trial. Int J Environ Res Public Health. 2013;10:2043–57. doi: 10.3390/ijerph10052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Anderson P, Kanteres F, et al. 2009a. Alcohol, social development and infectious disease. Available at http://www.sahealthinfo.net/admodule/Stockholm.pdf. (19 December 2013, date last accessed)
- Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009b;373:2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Research ICT Africa and Intelecon. 2012. Mobile usage at the base of the pyramid in South Africa. Available at http://media.kiva.org/labs/mobile/2012_South_Africa_BoP_Study.pdf. (19 December 2013, date last accessed)
- Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. New York: Churchill Livingstone; 1999. [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783–8. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: A meta-analytic review. Alcohol Alcohol. 2006;41:328–35. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- Viljoen DL, Gossage JP, Brooke L, et al. Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol. 2005;66:593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel T, Staniford M. The effectiveness of brief interventions in the clinical setting in reducing alcohol misuse and binge drinking in adolescents: a critical review of the literature. J Clin Nurs. 2010;19:605–20. doi: 10.1111/j.1365-2702.2009.03060.x. [DOI] [PubMed] [Google Scholar]
- Walters ST, Vader AM, Harris TR, et al. Reactivity to alcohol assessment measures: an experimental test. Addiction. 2009;104:1305–10. doi: 10.1111/j.1360-0443.2009.02632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CL, Mertens JR, Flisher AJ, et al. Substance abuse and HIV risk behaviours amongst primary health care service users in Cape Town. S Afr Psychiatry Rev. 2005;8:160–5. [Google Scholar]
- Ward CL, Mertens JR, Flisher AJ, et al. Substance abuse in South African primary care clinics. Poster presented at the 11th Biennial Conference of the Society for Community Research and Action; Pasadena. 2007. [Google Scholar]
- Ward CL, Mertens JR, Flisher AJ, et al. Prevalence and correlates of substance use among South African primary care clinic patients. Subst Use Misuse. 2008;43:1395–410. doi: 10.1080/10826080801922744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisner C, Schmidt LA. Rethinking access to alcohol treatment. Recent Dev Alcohol. 2001;15:107–36. doi: 10.1007/978-0-306-47193-3_7. [DOI] [PubMed] [Google Scholar]
- Western Cape Department of Health. 2011. 2020. The future of health care in the Western Cape. A draft framework for dialogue. Available at http://www.westerncape.gov.za/other/2011/12/healthcare_2020_-_9_december_2020.pdf. (March 3, 2014, date last accessed)
- WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Development, reliability and feasibility. Addiction. 2002;97:1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2002. The World Health report 2002. Reducing risks, promoting healthy life. Available at http://www.who.int/whr/2002/en/whr02_en.pdf. (19 December 2013, date last accessed)