Abstract
While chemotherapy and radiation therapy have been integral components of cancer management for decades, the issues of local recurrence, clinical resistance, and toxicities resulting from these treatment modalities have increased the interest in novel therapeutic approaches that could attenuate tumor progression and prevent recurrences. This Forum highlights current research focused on elucidation of the mechanisms of response to radiation treatment and the development of out-of-the-box therapeutic strategies for cancer treatment with radiation. Experts in the field of radiation research contribute with review articles describing the current knowledge on DNA damage response mechanisms, regulation of signaling involved in the DNA damage response by miRNA, the function of tumor hypoxia in tumor response to radiation, and the role of stem cells in protection of normal tissue against radiation damage. Antioxid. Redox Signal. 21, 218–220.
Introduction
Throughout life, humans face many diseases, each with its own set of treatment challenges impacting survivorship and quality of life. Cancer is one such disease and the American Cancer Society estimates that over 1.6 million new cancer cases will be diagnosed this year alone in the United States. Whereas cancer treatments vary depending on the tumor type, location, and stage, a large majority of cancer patients (approximately two-thirds) undergo radiation therapy during the course of their illness. From a historical perspective, radiation therapy has been used for treating various skin conditions along with cancers of the head, neck, and lymph nodes since the discovery of x-rays in the early 1900s. Reports as early as 1902–1904 document the application of radium in treating pharyngeal carcinomas and delivering radiation through glass tubes placed in close vicinity of the tumors through interstitial brachytherapy (4).
Basic and clinical radiation research over the last 75+ years has produced a significant amount of knowledge that is harnessed today by scientists and clinicians to improve the success of tumor radiation treatment and to mitigate the unwanted damage to normal tissue. Different types of radiation therapies (e.g., external beam radiation therapy, brachytherapy, and systemic radioisotope therapy) and fractionation regimens (e.g., hypofractionation, hyperfractionation, and accelerated fractionation) are currently tested in a variety of cancers under small and large clinical settings (6). Significant advancements in the technologies used in radiation therapies, such as hybrid imaging and image-guided treatment devices, enable a more precise delivery of radiation to the tumor and decreased damage of normal healthy tissue, important factors for long-term survival and quality of life of patients. Nevertheless, the overall therapeutic outcome of radiation therapy remains difficult to predict and is often undermined by tumor cells that resist radiation damage, giving rise to recurrent tumors, for which there are limited treatment options available.
The stochastic nature of radiation interaction with biological molecules has been considered for many years a major bottleneck in the prediction of clinical response to radiation therapy. However, recent studies are beginning to identify logical patterns of response to radiation that are consistent across many cancers (e.g., upregulation of DNA damage response and upregulation of antioxidant systems). These patterns of response often produce or are accompanied by changes in the cellular phenotype (e.g., epithelial/mesenchymal stem cell like) and sensitivity of cells to radiation, chemotherapies, and targeted agents (5, 7). These concepts are represented in this Forum by the research article contributed by Bansal et al., which show the mesenchymal-to-epithelial transition and an increased response to erlotinib (a targeted epidermal growth factor inhibitor) in a matched model of radiation resistance for head and neck cancer. To describe the coordinated patterns of radiation response, one must quantify the broad impact of radiation on the molecular function of cellular components from nucleic acids to proteins and small-molecule metabolites, with a focus on how these individual modifications integrate to determine whether an irradiated cell dies, survives, or is phenotypically modified as a result of the radiation insult. Similarly, intercellular communication is critical to both tumor development and response to therapies, including radiation [e.g., Ref. (2)]. For example, studies focusing on nontargeted effects of radiation have demonstrated the short- and long-range transfer of molecular species from irradiated cells to neighboring cells generating bystander effects. These molecules include reactive oxygen species, reactive nitrogen species, cytokines (IL-6, TNFα), growth factors such as TGFβ, and other molecules (8). In this Forum, Cao et al. show an elegant strategy of exploiting intercellular communication (bystander-like effects) for therapeutic intervention. The authors show that β-lapacone, a radiosensitizer targeting NAD(P)H:quinone oxidoreductase 1 (NQO1)-positive cells, generates cell membrane-permeable H2O2 causing cell death in neighboring NQO1-negative cells. Understanding the players and rules that govern intercellular communication is critical to the design of radiation therapies and for finding new therapeutic interventions for treating radiation-resistant tumors.
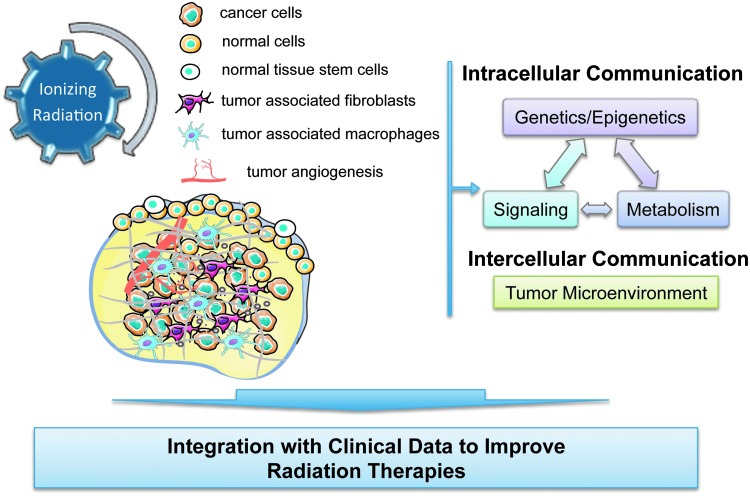
The Forum also includes a series of review articles highlighting current breakthroughs, challenges, and opportunities in cancer radiation treatment. The topics selected connect basic research with preclinical and clinical studies covering exciting areas of radiation research (Fig. 1).
FIG. 1.
Interaction of ionizing radiation with biological systems impacts intracellular and intercellular communication with consequence on the response to radiation therapies. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The interaction of ionizing radiation with DNA remains central to the cellular response to radiation. In this Forum, Santivasi and Xia discuss recent advancements in identifying differences in DNA damage and DNA damage response pathways between tumor and normal tissues that could be exploited to increase the therapeutic index of radiation therapies.
The review by Reisz et al. presents an overview of the effects of ionizing radiation on the broader range of biological molecules, including nucleic acids, proteins, and lipids. The oxidative and reductive chemistries of ionizing radiation interaction with these molecules, along with recent advances in high-throughput technologies for mapping radiation-induced changes in DNA, proteins, and cellular metabolites, are described. A special emphasis is placed on the need for advanced computational tools that would enable the integration of diverse omics data in cancer radiation therapy.
The Forum continues with a review article by Czochor and Glazer focused on the function of miRNAs as critical regulators of signaling pathways induced by radiation. The authors discuss the miRNAs regulating the PI3K/AKT and MAPK signaling and the DNA damage response pathways such as the mechanisms of DNA damage sensing, cell cycle checkpoint activation, DNA double-strand break repair, and apoptosis. There is an increasing recognition of the value of miRNAs in the treatment of a number of diseases, and their function in radiation response points to their potential value in treatment of radiation-resistant tumors. The omics and targeted analysis of miRNA will continue to be critical in the understanding of the mechanisms involved in DNA repair, cell cycle, apoptosis, and senescence, as well as nontargeted radiation-induced effects.
Phenotypic heterogeneity of cancer cells, tumor stroma, tumor vascularization and the interactions among these are widely acknowledged factors in the response to radiation therapies. The communication among tumor components produces dynamic regions within the tumor characterized by low pH, low O2 (hypoxia), and/or phenotypically distinct cancer cells. In this Forum, Lee, Boss, and Dewhirst discuss the implications of tumor hypoxia (chronic or cyclic) as a critical factor in radiation treatment. The authors include a description of current noninvasive imaging techniques for monitoring tumor hypoxia in preclinical and clinical settings such as positron emission tomography, magnetic resonance imaging, optical spectroscopy techniques, phosphorescence lifetime imaging, and photoacoustic tomography.
Significant research over the past decade has focused on the function of stem cells, both cancerous and from normal tissue, in radiation treatment. The associations among tumor hypoxia, cancer stem cells, and the epithelial-to-mesenchymal transition in radiation resistance have been reviewed recently (7). In this Forum, the focus has been on normal tissue stem cells and the review by Benderitter et al. highlights a number of stem cell-based therapeutic strategies to reduce normal tissue injury associated with radiation therapies. In particular, the review focuses on the reduction and prevention of radiation-induced cognitive impairment in patients diagnosed with brain tumors; xerostomia in head and neck cancer patients; osteoradionecrosis in head and neck cancer, pelvic cancer, or breast cancer patients; skin fibronecrosis, a common side effect of external radiation therapy across cancers; and liver, cardiac, and pelvic damage. The necessary steps of moving the field of stem cell research into viable clinical therapies are discussed along with lessons learned, caveats, and opportunities of this approach.
Together with the research published in the past decades in the area of radiation oncology, the research and review articles included in this Forum highlight the complexity and specificity of the interaction of ionizing radiation with biological matter. The elucidation of temporal and topological organization of these interactions at the cellular, tumor, and organismal level is critical to improve cancer treatment with radiation therapies. Current advancements in technologies and biomedical informatics enable the analysis of biological communication at a level that has not previously been possible. New bioinformatics tools for the unbiased extraction of literature-embedded information are emerging and expected to guide future hypothesis-driven studies (1, 3, 9). Noninvasive imaging reagents, imaging techniques, and computational tools for analysis of imaging data allow for a finer delineation of tumor features that could be exploited for both prediction and monitoring of tumor response to radiation treatment. Coordinated acquisition of multiple level omics, advanced imaging, and short- and long-term clinical data for patients undergoing radiation treatment is critical for establishing new treatment opportunities and improving the outcome of cancer treatment with ionizing radiation. In these integrated basic and clinical studies, analyses of primary patient specimens would be complemented with patient-derived xenografts or primary cell cultures to provide a more detailed view into the changes induced by radiation in tumors during the critical period of treatment when access to patient's tumor may not be possible. While this approach is logistically difficult, requiring close study coordination between a number of clinical teams and laboratory scientists, its rewards are anticipated to be significant and ultimately result in better care for cancer patients.
Abbreviation Used
- IL-6
interleukin 6
- MAPK
mitogen-activated protein kinase
- NQO1
NAD(P)H:quinone oxidoreductase 1
- PI3K
phosphoinositide 3-kinase
- TNFα
tumor necrosis factor alpha
- TGFβ
tumor growth factor beta
Acknowledgment
Financial support was provided by the National Cancer Institute of the National Institutes of Health under award number R01 CA136810 (C.M.F.).
References
- 1.Ananiadou S, Pyysalo S, Tsujii J, and Kell DB. Event extraction for systems biology by text mining the literature. Trends Biotechnol 28: 381–390, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Aravindan N, Aravindan S, Pandian V, Khan FH, Ramraj SK, Natt P, and Natarajan M. Acquired tumor cell radiation resistance at the treatment site is mediated through radiation-orchestrated intercellular communication. Int J Radiat Oncol Biol Phys 88: 677–685, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen AM. and Hersh WR. A survey of current work in biomedical text mining. Brief Bioinform 6: 57–71, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Connell PP, Kron SJ, and Weichselbaum RR. Relevance and irrelevance of DNA damage response to radiotherapy. DNA Repair 3: 1245–1251, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Casal R, Bhattacharya C, Ganesh N, Bailey L, Basse P, Gibson M, Epperly M, and Levina V. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol Cancer 12: 94, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcu LG. Altered fractionation in radiotherapy: from radiobiological rationale to therapeutic gain. Cancer Treat Rev 36: 606–614, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Marie-Egyptienne DT, Lohse I, and Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett 341: 63–72, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Prise KM. and O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 9: 351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu F, Patumcharoenpol P, Zhang C, Yang Y, Chan J, Meechai A, Vongsangnak W, and Shen B. Biomedical text mining and its applications in cancer research. J Biomed Inform 46: 200–211, 2013 [DOI] [PubMed] [Google Scholar]